Abstract
Objective
To evaluate the effect of ambient bright light therapy on agitation among institutionalized persons with dementia.
Methods
High intensity, low glare ambient lighting was installed in activity and dining areas of a state psychiatric hospital unit in North Carolina and a dementia-specific residential care facility in Oregon. The study employed a cluster-unit crossover design involving four ambient lighting conditions: AM bright light, PM bright light, All Day bright light, and Standard light. Sixty-six older persons with dementia participated. Outcome measures included direct observation by research personnel and completion by staff caregivers of the 14-item, short form of the Cohen-Mansfield Agitation Inventory (CMAI).
Results
Analyses of observational data revealed that for participants with mild/moderate dementia, agitation was higher under AM light (p=0.003), PM light (p<0.001), and All Day light (p=0.001) than Standard light. There also was a trend toward severely demented participants being more agitated during AM light than Standard light (p=0.053). Analysis of CMAI data identified differing responses by site: the North Carolina site significantly increased agitation under AM light (p=0.002) and PM light (p=0.013) compared with All Day light while in Oregon, agitation was higher for All Day light compared to AM light (p=0.030). In no comparison was agitation significantly lower under any therapeutic condition, in comparison to Standard lighting.
Conclusions
Ambient bright light is not effective in reducing agitation in dementia and may exacerbate this behavioral symptom.
Keywords: Alzheimer’s disease, dementia, light therapy, agitation, behavioral symptoms
Introduction
Dementia-related agitated behavioral symptoms occur in up to 93 percent of nursing home residents and 42 percent of assisted living residents and are associated with negative outcomes for both residents and caregivers (Cohen-Mansfield and Bilig, 1986; Gruber-Baldini et al., 2004; Burgio et al., 1988). Current treatment relies largely on medications, despite limited effectiveness and significant adverse reactions (Sink et al., 2005; Ray et al., 2009). Nonpharmacological interventions yield mixed results and are often labor intensive (Snowden et al., 2003). Safer, more effective treatments are needed to reduce agitation.
Increased agitation is associated with sleep disturbance, circadian disruptions, and shorter daylight hours, suggesting a link between agitation and circadian cycles (Ancoli-Israel et al., 2003; Bliwise et al., 1989; Cohen-Mansfield and Marx, 1990; Martin et al., 2000; Skjerve et al., 2004). Daily exposure to bright light and bright light therapy (BLT) is a minimally invasive intervention to entrain the circadian system. It causes few side effects and has effectively treated sleep disorders in adults without dementia (Kryger et al., 1989; Chesson et al., 1999).
Studies, mostly involving small samples, have examined the efficacy of BLT on agitation among people with dementia (Ancoli-Israel et al., 2003; Haffmans et al., 2001; Lovell et al., 1995; Lyketsos, et al., 1999; Mishima et al., 1998; Satlin et al., 1992; Skjerve et al., 2004; Van Someren et al., 1997). Overall, results have been mixed, but this could be attributable to differences in timing (e.g., morning versus early evening), lighting intensity, duration of daily exposure, trial duration, and outcome measure (Lovell et al., 1995; Lyketsos et al., 1999; Mishima et al., 1998; Satlin et al., 1992; Haffmans et al., 2001; Ancoli-Israel et al., 2003; Skjerve et al., 2004). In most studies, BLT was delivered using light boxes or similar devices that require subjects to keep their eyes oriented toward the light for specified time periods, and study personnel sat with subjects to ensure adherence, possibly producing an unmeasured social effect on agitation. A Cochrane review concluded there was insufficient evidence of the efficacy of BLT in reducing agitation among people with dementia (Forbes et al., 2004). Since this review, Riemersma-van der Lek et al. (2008) found that all day light combined with melatonin reduced agitation, suggesting the need for further study of BLT and agitation.
Because of the prevalence and impact of agitation in this population, the need for nonpharmacological approaches and conflicting evidence on the effect of BLT, we conducted a cluster-unit crossover trial of BLT, delivered using ambient lighting installed in public spaces of two dementia care settings, and measuring agitation both by staff report and by direct observation.
Methods
The study was conducted in two residential care settings that primarily serve people with dementia: two units of a 32-bed acute care Geropsychiatry Institute in a North Carolina (NC) psychiatric hospital, and a 24-bed unit in a private, dementia-specific, residential care facility in Oregon (OR). The study was approved by Institutional Review Boards of the University of North Carolina-Chapel Hill School of Medicine, the state psychiatric hospital in NC, and Oregon Health & Science University.
Lighting Intervention
The intervention consisted of four lighting conditions, each presented during multiple three week intervention periods: AM bright light (7–11 AM); PM bright light (4–8 PM); All Day bright light (7 AM – 8 PM); and Standard light (i.e. the baseline condition). The three-week duration for each trial was chosen because studies of human circadian systems indicate that entrainment occurs within 1–2 weeks. Treatment sequences were predetermined by the study statistician to achieve: a) a balance between temporally adjacent treatment pairs (to reduce potential carryover effects); b) even distribution of all treatment conditions across seasons (to minimize potential bias from differences in daylight exposure); and c) assignment of all four conditions as equally as possible within each study unit. There were 22 intervention periods in NC and 8 in OR. A computerized system maintained lighting intensity at 2,000 – 3,000 lux during BLT hours, and at 500–600 lux (industry minimum standard lighting for task areas) during the remainder of daylight hours and the entire Standard lighting period. The treatment areas were restricted to the common areas of each unit.
Subjects
All residents with a dementia diagnosis residing in the study units were eligible for recruitment. Designated family members or guardians provided proxy consent. Exclusion criteria included proliferative diabetic retinopathy, moderate or severe macular degeneration, and absence of a lens in one or both eyes. Study units had been selected such that they were in facilities with multiple units, so that people who were ineligible or declined to participate could move if desired to another unit.
Sixty-six residents participated in 338 three-week intervention periods. Recruitment rates for eligible subjects were 90% in NC and 93% in OR. In NC, where short-term residency was common, participants were enrolled an average of 4.2 (range 1–16; median 3) intervention periods. In OR, the majority (75%) of participants were enrolled in all 8 intervention periods (average 7.4; range 3–8).
Measures and Data Collection
Demographic, health, and medication information were abstracted from medical records. Cognitive status was measured using the Minimum Data Set Cognition Scale (MDS-COGS), and Mini-Mental State Examination (MMSE) (Hartmaier et al., 1994; Folstein et al., 1975). Daily sunrise and sunset times were obtained from the US Naval Observatory for both study sites (United State Naval Observatory). These times were used to calculate average day length and rate of change of day length in minutes per day for each period for each site.
The name, dose, and schedule of every medication administered for each day of study participation were obtained from medical records. A composite measure of analgesic and sedative medication use was calculated to yield an overall estimate of daily sedative and analgesic loads for each participant (Sloane et al., 2008).
Observational data on participant agitation were collected by trained research assistants blinded to study aims during the third week of each treatment period. Hourly observations were made between 6 AM and 8 PM for a total of 48 hours of observations. During these observations, the presence or absence of the following eight agitated behaviors was rated: crying, loud verbal excess, non-loud verbal excess, pacing, physical aggression, repetitive mannerisms, rummaging, and socially inappropriate behaviors. These observed behaviors were used to construct a single dichotomous "any agitation" variable, which became the outcome for statistical analyses. There was 86% overall agreement between raters for any agitation, with a kappa of 0.47 (95% CI 0.29–0.65), indicating moderate reliability (Landis and Koch, 1977). If a participant was not in a treatment area, agitation status was coded as missing for that observation. Exposure to each light condition was estimated based on each participant’s location during observation periods.
Informant ratings of participant agitation were obtained by having staff caregivers on all three shifts complete the short form of the Cohen-Mansfield Agitation Inventory (CMAI) during week three of each intervention period (Werner et al., 1994). Staff caregivers rated the frequency of each of 14 behaviors during the preceding two weeks on a 5-point scale (1=never to 5=several times an hour or continuously for > 30 minutes). These were summed to construct an overall CMAI score (range 14–70) for each shift and intervention period.
Statistical Analysis
The characteristics of study participants at the two sites were compared using t-tests for continuous data and Fisher’s Exact Test for categorical data. To assess effects of BLT on observed agitation, a logistic mixed model was estimated (McCulloch and Searle, 2001). The dependent variable was any of the eight observed agitated behaviors. The analytical model included normally distributed resident random effects, fixed effects for treatment conditions, and additionally adjusted for site, gender, dementia severity (mild/moderate or severe/very severe), site-specific average day length and rate of change of day length over the 3-week intervention period, daily sedative load, daily analgesic load, and time of day of measurement (8AM-noon, noon-4PM, 4PM-8PM). As there was a significant treatment-by-dementia severity interaction (p=0.019), this effect also was included in the model, and treatment effects were calculated separately for mild/moderate and severe/very severe dementia.
To assess associations between BLT and informant-rated agitation, a linear mixed model including resident and staff caregiver random effects was employed (McCulloch and Searle, 2001). The dependent variable was the overall CMAI score. This analytical approach accounted for repeated measures and clustering by staff caregivers. Preliminary analyses indicated a strong treatment-by-site interaction (p=0.008) and a statistically significant treatment-by-shift interaction when the sites were combined (p=0.015). Therefore, separate linear mixed models (without interactions) were computed for each site. The linear mixed models additionally adjusted for gender, dementia severity (mild/moderate, severe, very severe), staff shift, site-specific average day length and rate of change of day length over the 3-week intervention period, analgesic score, and sedative score.
Results
Participant characteristics are presented in Table 1. The majority (68%) had severe or very severe cognitive impairment. Participants in OR tended to be older, female, white, more educated, more functionally impaired (with the exception of incontinence), and on fewer sedative medications than participants in North Carolina.
Table 1.
Characteristics of Study Participants, by Site
| Characteristic | North Carolina (N = 46) |
Oregon (N = 20) |
p-value |
|---|---|---|---|
| n (%) or Mean (SD) | |||
| Age | p=0.002 | ||
| < 65 years | 6 (13%) | 0 (0) | |
| 65 – 79 years | 24 (52%) | 4 (20%) | |
| ≥ 80 years | 16 (35%) | 16 (80%) | |
| Gender | p<0.001 | ||
| Male | 34 (74%) | 1 (5%) | |
| Female | 12 (26%) | 19 (95%) | |
| Race | p=0.013 | ||
| African American | 16 (35%) | 1 (5%) | |
| White | 30 (65%) | 19 (95%) | |
| Educational attainment* | p=0.107 | ||
| ≥ 12th grade | 17 (49%) | 10 (77%) | |
| < 12th grade | 18 (51%) | 3 (23%) | |
| Marital status* | p=0.038 | ||
| Never married | 6 (14%) | 1 (5%) | |
| Married | 11 (25%) | 3 (15%) | |
| Widowed | 16 (36%) | 15 (75%) | |
| Divorced/separated | 11 (25%) | 1 (5%) | |
| Functional status† | |||
| Need assistance in bathing | 26 (58%) | 18 (90%) | p=0.011 |
| Need assistance in locomotion | 6 (13%) | 6 (30%) | p=0.162 |
| Need assistance in eating | 10 (22%) | 6 (30%) | p=0.538 |
| Incontinent of urine | 25 (54%) | 2 (10%) | p<0.001 |
| Cognitive impairment‡ | p=0.365 | ||
| Mild | 3 (7%) | 0 (0%) | |
| Moderate | 10 (22%) | 8 (40%) | |
| Severe | 22 (48%) | 9 (45%) | |
| Very severe | 11 (24%) | 3 (15%) | |
| Medication use | |||
| One or more antidepressants§ | 23 (50%) | 14 (70%) | p=0.180 |
| One or more anxiolytics§ | 14 (30%) | 2 (10%) | p=0.118 |
| One or more antipsychotics§ | 32 (70%) | 8 (40%) | p=0.031 |
| Sedative load∥ | 10.5 (10.8) | 4.5 (3.6) | p=0.001 |
| Analgesic load∥ | 2.8 (3.4) | 2.4 (3.4) | p=0.656 |
Data on educational status were missing for 11 subjects in North Carolina and 7 in Oregon; data on marital status was missing for 2 in North Carolina.
Data on bathing functional status were missing on 1 subject in North Carolina. A study participant was defined as needing assistance if the staff nurse informant rated the individual as needing limited or full assistance. Residents were considered to be incontinent of urine if they were incontinent ≥ 2 times/ week. Percentages sum to >100 because items are not mutually exclusive.
Based on MDS-COGS score,28 with mild=0–1, moderate=2–4, severe=5–8 and very severe=9–10. For 3 residents for whom MDS-COGS was unavailable, cognitive impairment was based on MMSE29 score and consultation with hospital psychologist.
Use of a given medication class required a study participant to have received a medication in that class for the majority (11 or more days) of at least one 3-week intervention period. Percentages sum to >100 because drug categories are not mutually exclusive.
Daily sedation loads for each resident were calculated based on dosage(s) received and drug potency (scored as 0, 1, 3, or 6), summed across all medications a given resident received. Means (SD) shown here are based on daily loads averaged across all intervention periods for a given resident. Analgesic load was calculated in same way except that drug potencies were scored as 0, 1, 3, 6, 9. See further details in text.
At both sites, light intensity during the three BLT conditions at multiple measurement points was largely within the targeted range (X= 2,535 lux [NC] and 2,638 lux [OR]). During the Standard condition, mean light intensity was 617 lux (NC) and 591 lux (OR). Mean exposure to BLT was estimated, based on observational data, as 2.64, 2.87, and 8.40 hours daily during the AM, PM, and All Day BLT, respectively. Exposure to the lighting conditions was not statistically different by site (p=0.791) or dementia severity (p=0.292).
Observed agitation
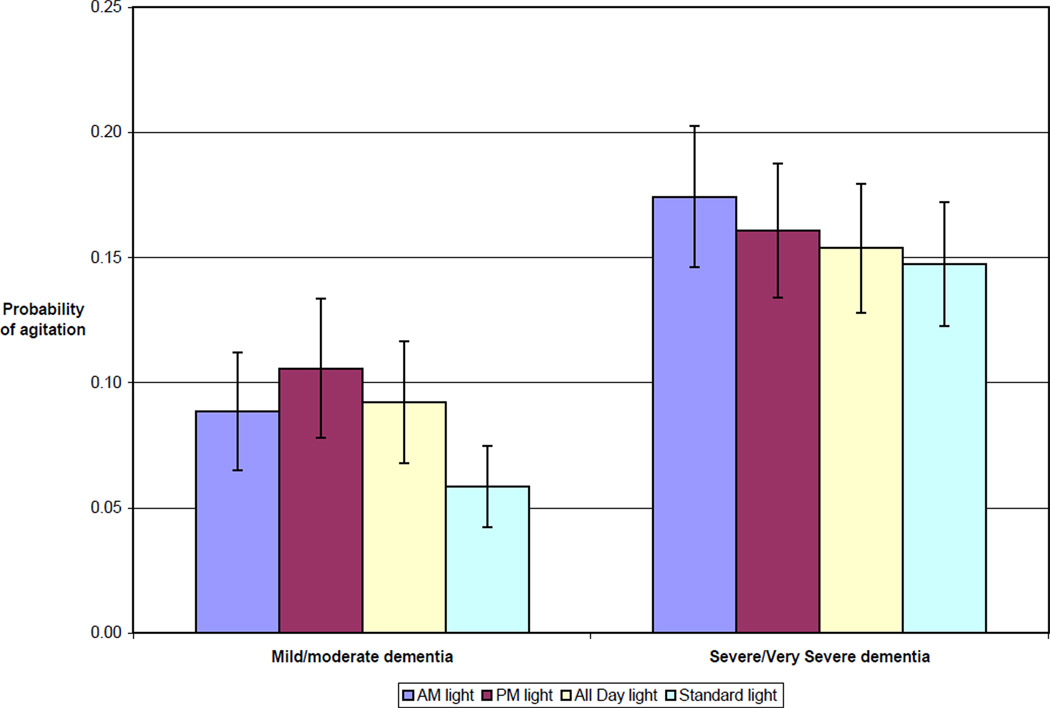
Table 2 contains results from the logistic mixed model for observed agitation. For people with mild/moderate dementia, levels of agitation were higher during AM light (p=0.003), PM light (p=<0.001) and All Day light (p=0.001) than during Standard light. The model-adjusted probability of agitation for an average resident participant with mild/moderate dementia was highest during PM light (0.106, SE 0.028) and lowest during Standard light (0.059, SE 0.016; Figure 1). Severely demented participants were marginally more likely to be agitated during AM light than Standard light (0.174 vs. 0.148; p=0.055). Agitation increased for all participants under all 4 conditions as the day progressed, with highest agitation levels between 4 PM and 8 PM (p=<0.001).
Table 2.
Independent Associations between Observed Agitation and Lighting Treatment, Resident Characteristics, and Selected Environmental Factors: Results of a Logistic Mixed Model (N=66 residents, 10,001 observations)*
| Variable | Odds Ratio (95% CI) for Observed Agitation |
p-value | |
|---|---|---|---|
| Treatment Pair Comparisons† | |||
| Mild/moderate dementia | <0.001† | ||
| AM light vs. Standard | 1.57 | (1.18, 2.08) | 0.003 |
| PM light vs. Standard | 1.90 | (1.42, 2.55) | <0.001 |
| All Day vs. Standard | 1.63 | (1.22, 2.18) | 0.001 |
| AM light vs. PM light | 0.82 | (0.64, 1.07) | 0.143 |
| AM light vs. All Day light | 0.96 | (0.74, 1.24) | 0.747 |
| PM light vs. All Day light | 1.17 | (0.90, 1.52) | 0.257 |
| Severe/very severe dementia | 0.247‡ | ||
| AM light vs. Standard | 1.22 | (1.00, 1.49) | 0.055 |
| PM light vs. Standard | 1.11 | (0.90, 1.36) | 0.328 |
| All Day vs. Standard | 1.05 | (0.86, 1.29) | 0.638 |
| AM light vs. PM light | 1.10 | (0.90, 1.34) | 0.342 |
| AM light vs. All Day light | 1.16 | (0.95, 1.42) | 0.147 |
| PM light vs. All Day light | 1.06 | (0.86, 1.29) | 0.602 |
| Oregon site | 1.43 | (0.61, 3.34) | 0.411 |
| Female gender | 1.47 | (0.68, 3.18) | 0.337 |
| Mild/moderate dementia (Standard light) | 0.36 | (0.18, 0.72) | 0.005§ |
| Time of day (Reference: 8 AM – Noon) | <0.001∥ | ||
| Noon – 4 PM | 1.26 | (1.10, 1.44) | 0.002 |
| 4 PM – 8 PM | 1.81 | (1.58, 2.07) | <0.001 |
| Day length (hrs) | 1.03 | (0.98, 1.08) | 0.259 |
| Rate of change of day length (min/day) | 0.98 | (0.94, 1.02) | 0.237 |
| Daily sedative load (per 10 units) | 1.04 | (0.96, 1.14) | 0.349 |
| Daily analgesic load (per 10 units) | 1.33 | (0.99, 1.77) | 0.061 |
Because a significant (p=0.019) dementia severity-by-treatment interaction effect was present, the mixed model estimates separate treatment effects for mild/moderate and severe/very severe dementia. The mixed model includes resident random effect and all fixed effects shown; thus, all values are adjusted for other variables shown. The estimate (SE) for the resident variance is 1.271 (0.282), p<0.001.
AM light = 7AM – 11AM high intensity light; PM light = 4 PM – 8 PM high intensity light; All Day light = 7 AM – 8 PM high intensity light; Standard = recommended minimum Standard lighting for task areas for older persons (500–600 lux)
P-value for overall F test (3 df) for lighting treatment (for mild/moderate and severe/very severe dementia respectively).
P-value for mild/moderate vs. severe/very severe dementia is 0.030 for AM light, 0.178 for PM light, and 0.103 for All Day light.
P-value for overall F test (2 df) for time of day; the p-value for Noon-4PM vs. 4–8 PM is <0.001.
Figure 1.
Probability of observing agitation for an average* resident, by dementia severity and treatment condition
*Average resident defined as one with a value of 0 for the resident random effect
Staff reported agitation
Table 3 provides results from site-specific linear mixed models for CMAI total scores. In NC, mean adjusted agitation scores were significantly higher for AM compared with All Day (2.41 points; p<.002), and for PM compared with All Day light (2.09 points; p=0.013). Scores were marginally lower for All Day compared with Standard light (1.53 points; p=0.064). Also, there was a significant effect for daylight rate of change of day length (p=0.008); in spring, when days were getting longer, there was more agitation. Finally, staff caregivers on the evening shift reported higher agitation levels than did staff on the day (3.49 points, p=0.021) or night shifts (3.63 points, p= 0.036).
Table 3.
Independent Associations Between Reported Agitation Scores on the Cohen-Mansfield Agitation Inventory and Lighting Treatment, Resident Characteristics, and Selected Environmental Factors: Results of a Linear Mixed Model (N=66 residents, 1,010 observations)*
| Variable | North Carolina | Oregon | ||||
|---|---|---|---|---|---|---|
| Estimate | (95% CI) | p-value | Estimate | (95% CI) | p-value | |
| Intercept | 24.47 | (17.77, 31.16) | <0.001 | 12.82 | (3.49, 22.15) | 4.762 |
| Treatment Pair Comparison† | 0.011‡ | 0.157‡ | ||||
| AM light vs. Standard | 0.89 | (−0.64, 2.41) | 0.255 | −0.91 | (−2.29, 0.47) | 0.196 |
| PM light vs. Standard | 0.56 | (−1.06, 2.18) | 0.497 | −0.05 | (−1.49, 1.40) | 0.952 |
| All Day vs. Standard | −1.53 | (−3.14, 0.08) | 0.064 | 0.72 | (−0.78, 2.21) | 0.349 |
| AM light vs. PM light | 0.32 | (−1.24, 1.88) | 0.685 | −0.87 | (−2.44, 0.71) | 0.283 |
| AM light vs. All Day light | 2.41 | (0.93, 3.89) | 0.002 | −1.63 | (−3.09, –0.16) | 0.030 |
| PM light vs. All Day light | 2.09 | (0.44, 3.73) | 0.013 | −0.76 | (−2.05, 0.53) | 0.248 |
| Female gender | 3.61 | (−0.59, 7.81) | 0.093 | 3.85 | (−3.56, 11.25) | 0.309 |
| Mild/moderate dementia | −3.42 | (−7.26, 0.43) | 0.083 | −2.67 | (−6.13, 0.79) | 0.131 |
| Staff shift (Ref: night shift) | 0.040∥ | 0.883∥ | ||||
| Day shift | 0.14 | (−2.96, 3.23) | 0.930 | −0.42 | (−2.91, 2.08) | 0.742 |
| Evening shift | 3.63 | (0.24, 7.01) | 0.036 | 0.34 | (−3.22, 3.90) | 0.852 |
| Day length (hrs) | −0.22 | (−0.67, 0.24) | 0.348 | 0.22 | (−0.30, 0.73) | 0.407 |
| Rate of change of day length (min/day) | 0.53 | (0.14, 0.92) | 0.008 | 0.28 | (−0.15, 0.71) | 0.202 |
| Sedative medication score | −0.05 | (−0.11, 0.01) | 0.080 | 0.37 | (0.02, 0.71) | 0.037 |
| Analgesic medication score | 0.12 | (−0.12, 0.35) | 0.326 | 0.12 | (−0.27, 0.52) | 0.539 |
Because a significant (p<0.001) site-by-treatment interaction effect was present, mixed models were estimated separately for the North Carolina and Oregon study sites. Each analysis included resident and staff random effects and all fixed effects shown; thus, all values are adjusted for other variables shown. The estimate (SE) for the resident variance is 27.71 (6.98), p<0.001 for NC and 12.30 (4.55), p=0.003 for OR; for the staff variance it is 17.14 (6.31), p=0.003 for NC and 6.75 (4.09), p=0.049 for OR; residual variance is 33.25 (2.14), p<0.001 for NC and 15.72 (1.11), p<0.001 for OR.
AM light = 7AM – 11AM high intensity light; PM light = 4 PM – 8 PM high intensity light; All Day light = 7 AM – 8 PM high intensity light; Standard = recommended minimum Standard lighting for task areas for older persons (500–600 lux)
P-value for overall F test (3 df) for lighting treatment (for NC and OR respectively)
P-value for overall F test (2 df) for staff shift; the p-value for day vs. evening shift is 0.021 for NC and 0.653 for OR.
In OR, reported agitation was higher for All Day than AM light (1.63 points, p=0.030). Although differences were small and not significant for other comparisons, the direction of treatment effects was generally opposite those observed in NC, with higher levels for All Day than for Standard or PM light. The only other significant association found was of sedative medication use with decreased agitation (p=0.037).
Discussion
The relationship between bright light therapy and agitation in dementia has been controversial for over a decade (Ancoli-Israel et al., 2003; Haffmans et al., 2001; Lovell et al., 1995; Lyketsos, et al., 1999; Martin et al., 2000; Mishima et al., 1998; Satlin et al., 1992; Skjerve et al., 2004; Van Someren et al., 1997, Riemersma-van der Lek et al. 2008). This study, which evaluated multiple dosage regimens, achieved target exposures, and controlled for many possible confounding factors, failed to demonstrate an ameliorative effect of bright light on agitation. When agitation was evaluated through observation, participants with mild/moderate dementia in both study sites were significantly more agitated under all three BLT conditions in comparison to Standard conditions, and severely demented participants were marginally more agitated during AM BLT. When evaluated through staff caregiver reports using the CMAI, agitation was not significantly lower under any BLT condition compared with Standard light. Therefore, we conclude that BLT alone did not reduce agitation and indeed may increase agitation risk in certain individuals with dementia. These results confirm the findings of two earlier, smaller, controlled trials (Ancoli-Israel et al., 2003; Lyketsos, et al., 1999). These results are consistent with those obtained in a large, double-blinded, placebo-controlled trial in which only subjects who received melatonin in combination with light exhibited reduced agitation; subjects in the light only condition of this study did not display significantly reduced agitation (Riemersma-van der Lek et al., 2008).
Our two measures of assessing agitation were complementary. Differences in results could, therefore, be due to the fact that they measured different aspects of agitation (Ancoli-Israel et al., 2003; Sloane et al., 1998; Thorpe et al., 2000). The CMAI relies on caregiver recall of a two week period and is well suited for measuring low frequency and severe behaviors, which makes it relatively insensitive to changes or differences. Thus, the relative lack of significant effects by light therapy on agitation as measured using the CMAI could have been due to either lack of effect on severe behavioral symptoms or lack of sensitivity of the measure. Observational methods, on the other hand, are less useful for rare behaviors but are more sensitive to differences in the frequency of common, more subtle behaviors, and so the increases in observed agitation during periods of bright light exposure suggest a true effect.
Prior studies indicating reduced agitation with light exposure used light boxes as the treatment modality. Often, research staff attended the intervention, providing prompting to keep participants seated in front of the light. These social exchanges could have independently improved study participant agitation (Marx et al., 1989; Roth et al., 2002). Therefore, our treatment method – which provided target exposure levels without adding a potentially confounding staff effect – represents a more valid method of studying the effects of light, a factor supporting the validity of our conclusions.
A likely explanation for these findings is that light provides sensory stimulation, and in persons with dementia this stimulation can engender agitation. Rationale exists for this hypothesis, as a popular method of treating dementia is the creation of low or controlled stimulus units to control behavioral symptoms (Hall et al., 1995). However, there were no significant differences in observed alertness across the four conditions (OR p=0.168; NC p=0.082). Thus we do not consider increased activation to be a factor in agitation level. Improvements in sleep reported in a previous paper (Sloane et al., 2007) may have been associated with agitation. However, we found no significant relationship between hours of sleep and reported (p=0.426) or observed (p=0.104) agitation. An alternative explanation would be that the effects noted in this study were indirect ones, due to the influence of light on depression; this appears unlikely in these data, because concomitant analyses in this trial demonstrated no change in depressive symptoms (Hickman et al., 2007)).
Our study has several limitations. There are some indications in the literature that BLT effects vary for persons with vascular dementia and Alzheimer’s disease, however, in our study a specific dementia diagnosis often was not available (Mishima et al., 1998). Other unmeasured differences may also be a limitation; for example, the instruments measured only negative behaviors and there may have been undetected improvements in unmeasured adaptive behaviors, although previous studies suggest BLT does not impact such behaviors (Thorpe et al., 2000). We did not look at change in falls, which are often associated with agitation (Marx et al., 1990) or at changes in involvement in activities for person who were agitated. Furthermore, we may have underestimated a treatment effect if persons who responded favorably to the BLT treatment were differentially discharged because their agitation had improved; this possibility is considered very unlikely because the Oregon facility, which experienced virtually no participant attrition, did not show marked differences from the North Carolina facility in sub-analyses. Additionally, if this bias was present, it would have been reduced by use of the mixed model analytic strategy.
A strength of the study was the cross-over experimental design and statistical analysis using mixed effects models that effectively handled imbalance in the number of observations observed across study participants, particularly evident in the NC site. The effectiveness of the study design in achieving its goals is manifested in the final data set of 338 observations corresponding to so many resident-periods being distributed almost evenly across treatments and subjects: 52 different residents were observed for a total of 91 resident-periods under condition A; 46 residents for a total of 81 resident-periods under condition B; 47 residents for a total of 81 resident-periods under condition C; and 48 residents for a total of 85 resident-periods under condition D. The mixed effects models accounted for substantial intra-resident correlation effectively weighting observations so that a few NC residents with many periods would not have undue influence on model estimates. With respect to assessing treatment differences, bias due to participant withdrawal is considered unlikely because study withdrawal was mainly due to administrative reasons and not believed to be due to lighting conditions.
In conclusion, BLT does not appear promising as a treatment for agitation and indeed, may exacerbate it. Lack of efficacy may be due to absence of direct links between light, circadian rhythms and agitation, or to a need to include other treatments such as melatonin. Whatever the mechanism, BLT alone appears unlikely to reduce agitation in unselected institutional populations with dementia. Thus, this study adds support to evidence that bright light is not effective for reducing agitation among people with dementia.
Key points.
Bright light therapy is not associated with improvement in behavioral symptoms among persons with dementia
Acknowledgements
This research was supported by grants R01AT00202 and R01AT00212-AS from the National Center for Complementary and Alternative Medicine, and Senior Academic Leadership Award K02 AG00970 from the National Institute on Aging.
The authors acknowledge the following individuals and organizations for their professional, financial, or in-kind assistance to the project: Advance Transformer Company; Better Bricks Daylighting Laboratories; Cascade Lighting; Center of Design for an Aging Society; Collins & Aikman; C.F Stinson; DeaMor; Encompass Lighting Technology; Energy Trust of Oregon, Inc; General Electric Lighting; Interface Engineering; Johnson Air Products; Kalwall; Lighting Design Laboratory; Lithonia Lighting; LRS Architects; Lutron; MechoShade Systems; Northwest Energy Efficiency Alliance; Ocean Optics; Oregon Department of Energy; Pacific Coast Air Balancing; Platt Electric; Seabold Construction; and SPI Lighting.
Sponsor’s Role: The funding agencies had no role in study design, methods, data analysis, or manuscript preparation. No author had any conflict of interest.
Footnotes
None of the authors have a conflict of interest or any financial agreement with any company that gave support to this research.
Contributor Information
Ann Louise Barrick, Department of Psychology, University of North Carolina at Chapel Hill and Central Regional Hospital.
Philip D. Sloane, Cecil G. Sheps Center for Health Services Research and Department of Family Medicine, University of North Carolina at Chapel Hill.
Christianna S. Williams, Abt Associates Inc., Durham, NC.
C. Madeline Mitchell, Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill.
Bettye Rose Connell, Rehabilitation Research and Development Center of Excellence, Atlanta Veterans Affairs Medical Center, Division of Geriatrics, Emory University, Atlanta, GA.
Wendy Wood, Department of Occupational Therapy, Colorado State University.
Susan E. Hickman, School of Nursing, Oregon Health & Science University, Portland, Oregon, Indiana University, Indianapolis, Indiana.
John S. Preisser, School of Public Health, University of North Carolina at Chapel Hill.
Sheryl Zimmerman, Cecil G. Sheps Center for Health Services Research and School of Social Work, University of North Carolina at Chapel Hill.
References
- Ancoli-Israel S, Martin JL, Gehrman P, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer's disease. American Journal of Geriatric Psychiatry. 2003;11(2):194–203. [PubMed] [Google Scholar]
- Bliwise DL, Carroll JS, Dement WC. Apparent seasonal variation in sundowning behavior in a skilled nursing facility. Sleep Research. 1989;18:408. [Google Scholar]
- Burgio LD, Butler F, Engel BT. Nurses' attitudes towards geriatric behavior problems in long-term care settings. Clinical Gerontologist. 1988;7(3/4):23–34. [Google Scholar]
- Chesson AL, Jr, Littner M, Davila D, et al. Practice parameters for the use of light therapy in treatment of sleep disorders. Sleep. 1999;22(5):641–660. doi: 10.1093/sleep/22.5.641. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Billig N. Agitated behaviors in the elderly. I. A conceptual review. Journal of the American Geriatrics Society. 1986;34(10):711–721. doi: 10.1111/j.1532-5415.1986.tb04302.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Marx MS. The relationship between sleep disturbances and agitation in a nursing home. Journal of Aging and Health. 1990;2(1):42–57. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state." A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes D, Morgan DG, Bangma J, Peacock S, Pelletier N, Adamson J. Light therapy for managing sleep, behaviour, and mood disturbances in dementia (Electronic version) Cochrane Database Systematic Review. 2004;Vol 2 doi: 10.1002/14651858.CD003946.pub2. [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Boustani M, Sloane PD, Zimmerman S. Behavioral symptoms in residential care/assisted living facilities: Prevalence, risk factors, and medication management. Journal of the American Geriatrics Society. 2004;52(10):1610–1617. doi: 10.1111/j.1532-5415.2004.52451.x. [DOI] [PubMed] [Google Scholar]
- Haffmans PM, Sival RC, Lucius SA, Cats Q, van Gelder L. Bright light therapy and melatonin in motor restless behaviour in dementia: A placebo-controlled study. Geriatric Psychiatry. 2001;16(1):106–110. doi: 10.1002/1099-1166(200101)16:1<106::aid-gps288>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Hall GR, Gerdner L, Zwygart-Stauffacher M, Buckwalter KC. Principles of nonpharmacological management: Caring for people withe Alzheimer's disease using conceptual model. Psychiatric Annals. 1995;25(7):432–440. [Google Scholar]
- Hartmaier SL, Sloane PD, Guess HA, Koch GG. The MDS Cognition Scale: A valid instrument for identifying and staging nursing home residents with dementia using the minimum data set. Journal of the American Geriatrics Society. 1994;42(11):1173–1179. doi: 10.1111/j.1532-5415.1994.tb06984.x. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Barrick AL, Williams CS, et al. The effect of ambient bright light therapy on depressive symptoms in persons with dementia. Journal of the American Geriatrics Society. 2007;55(11):1817–1824. doi: 10.1111/j.1532-5415.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- Kryger MH, Roth T, Carskadon M. Circadian rhythms in humans. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 2nd ed. Philadelphia: W.B. Saunders; 1989. [Google Scholar]
- Landis JR, Koch GG. The measurement of observor agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- Lovell BB, Ancoli-Israel S, Gevirtz R. Effect of bright light treatment on agitated behavior in institutionalized elderly subjects. Psychiatry Research. 1995;57(1):7–12. doi: 10.1016/0165-1781(95)02550-g. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. International Journal of Geriatric Psychiatry. 1999;14(7):520–525. [PubMed] [Google Scholar]
- Martin J, Marler M, Shochat T, Ancoli-Israel S. Circadian rhythms of agitation in institutionalized patients with Alzheimer's disease. Chronobiology International. 2000;17(3):405–418. doi: 10.1081/cbi-100101054. [DOI] [PubMed] [Google Scholar]
- Marx MS, Cohen-Mansfield J, Werner P. Agitation and falls in institutionalized elderly persons. Journal of Applied Gerontology. 1990;9:106–117. doi: 10.1177/073346489000900109. [DOI] [PubMed] [Google Scholar]
- Marx MS, Werner P, Cohen-Mansfield J. Agitation and touch in the nursing home. Psychology Reports. 1989;64(3):1019–1026. doi: 10.2466/pr0.1989.64.3c.1019. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR. Generalized , linear, and mixed models. New York: John Wiley & Sons; 2001. [Google Scholar]
- Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer's type. Chronobiology International. 1998;15(6):647–654. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- Riemersma-van Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, Van Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. Journal of the American Medical Association. 2008;299(22):2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- Roth DL, Stevens AB, Burgio LD, Burgio KL. Timed-event sequential analysis of agitation in nursing home residents during personal care interactions with nursing assistants. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57(5):461–468. doi: 10.1093/geronb/57.5.p461. [DOI] [PubMed] [Google Scholar]
- Satlin A, Volicer L, Ross V, Herz L, Campbell S. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer's disease. American Journal of Psychiatry. 1992;149(8):1028–1032. doi: 10.1176/ajp.149.8.1028. [DOI] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: A review of the evidence. Journal of the American Medical Association. 2005;293(5):596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- Skjerve A, Bjorvatn B, Holsten F. Light therapy for behavioral and psychological symptoms of depression. International Journal of Geriatric Psychiatry. 2004;19(6):516–522. doi: 10.1002/gps.1087. [DOI] [PubMed] [Google Scholar]
- Skjerve A, Holsten F, Aarsland D, Bjorvatn B, Nygaard HA, Johansen IM. Improvement in behavioral symptoms and advance of activity acrophase after short-term bright light treatment in severe dementia. Psychiatry and Clinical Neurosciences. 2004;58(4):7–12. doi: 10.1111/j.1440-1819.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- Sloane P, Ivey J, Roth M, Roederer M, Williams CS. Accounting for the sedative and analgesic effects of medication during patient participation in clinical research studies: Measurement development and application to a sample of institutionalized geriatric patients. Contemporary Clinical Trials. 2008;29(2):140–148. doi: 10.1016/j.cct.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane PD, Mitchell CM, Phillips C, Burker E, Commander C, Long K. Environmental correlates of resident agitation on Alzheimer's disease special care units. Journal of the American Geriatrics Society. 1998;46(2):862–869. doi: 10.1111/j.1532-5415.1998.tb02720.x. [DOI] [PubMed] [Google Scholar]
- Sloane PD, Williams CS, Mitchell CM, et al. High intensity environmental light in dementia: Impact on sleep and activity. Journal of the American Geriatrics Society. 2007;55(10):1524–1533. doi: 10.1111/j.1532-5415.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- Snowden M, Sato K, Roy-Barne P. Assessment and treatment of nursing home residents with depression or behavioral symptoms associated with dementia: A review of the literature. Journal of the American Geriatrics Society. 2003;51(9):1305–1317. doi: 10.1046/j.1532-5415.2003.51417.x. [DOI] [PubMed] [Google Scholar]
- Thorpe L, Middleton J, Russell G, Stewart N. Bright light therapy for demented nursing home patients with behavioral disturbance. American Journal of Alzheimer's Disease and Other Dementias. 2000;15(1):18–26. [Google Scholar]
- United States Naval Observatory. Table of sunrise/sunset for an entire year. http://aa.usno.navy.mil/data/docs/RS_OneYear.php.
- Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biological Psychiatry. 1997;41(9):955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- Wayne RA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. New England Journal of Medicine. 2009;360:225–35. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Cohen-Mansfield J, Koroknay V, Braun J. The impact of a restraint-reduction program on nursing home residents. Geriatric Nursing. 1994;15(3):142–146. doi: 10.1016/s0197-4572(09)90040-4. [DOI] [PubMed] [Google Scholar]