Abstract
In vitro studies were performed to characterize the relative performance of candidate receptors to target microparticles to inflammatory markers on vascular endothelium. To model the interactions of drug-bearing micro-particles or imaging contrast agents with the vasculature, 6 micron polystyrene particles bearing antibodies, peptides, or carbohydrates were perfused over immobilized E- or P-selectin in a flow chamber. Microparticles conjugated with HuEP5C7.g2 (HuEP), a monoclonal antibody (mAb) specific to E- and P-selectin, supported leukocyte-like rolling and transient adhesion at venular shear rates. In contrast, microparticles conjugated with a higher affinity mAb specific for P-selectin (G1) were unable to form bonds at venular flow rates. When both HuEP and G1 were conjugated to the microparticle, HuEP supported binding to P-selectin in flow which allowed G1 to form bonds leading to stable adhesion. While the microparticle attachment and rolling performance was not as stable as that mediated by the natural ligands P-selectin Glycoprotein Ligand-1 or sialyl Lewisx, HuEP performed significantly better than any previously characterized mAb in terms of mediating micro-particle binding under flow conditions. HuEP may be a viable alternative to natural ligands to selectins for targeting particles to inflamed endothelium.
Keywords: HuEP, P-selectin, E-selectin, microparticles, rolling, drug-targeting
Introduction
In an immune response, leukocytes are selectively recruited to the endothelium though specialized adhesion molecules called selectins. Selectins are glycoproteins whose expression is restricted to the vasculature and are highly specific markers for thrombosis and inflammation. Drug-bearing microparticles can be delivered to inflamed endothelium by selectively targeting selectins (Bendas et al., 1999), and this approach may represent an attractive pathway to deliver anti-inflammatory therapeutics. Since selectin expression is restricted to the vascular endothelium, circulating leukocytes, and platelets; selectins may serve as targets for drugs against other vascular disorders such as atherosclerosis or thrombosis.
Antibodies have been shown to inhibit inflammation in animal models by targeting surface expressed E- and P-selectin (Winn et al., 1998). Since E- and P-selectin can work cooperatively together in a number of inflammatory conditions (Eppihimer et al., 1996; Labow et al., 1994), the blocking of both selectins is preferred for the therapeutic treatment of such conditions and can be done via a cross-reactive monoclonal antibody (mAb) such as HuEP5C7.g2 (HuEP) (Berg et al., 1995; He et al., 1998). As a targeting modality, antibodies are attractive because they have relatively high affinity for specific antigens, and can be easily functionalized for conjugation to other molecules or supports.
HuEP is a humanized, complementary determining region-grafted version of a murine antibody, mEP-5C7, incorporating human γ2 (IgG2 Ab) and κ light chain constant region, which retains specificity for both E- and P-selectin, binding to a common epitope on both selectins (Blackwell et al., 2001; He et al., 1998). Humanized antibodies contain 90% human sequences, and only ~10% mouse sequences, which reduces immunogenicity while retaining the antibody specificities (Co and Queen, 1991). When coated on nanospheres, HuEP supports binding to CHO-E and CHO-P cells under simulated in vivo flow conditions (Blackwell et al., 2001). The binding epitope for the mAb EP-5C7 has been mapped to be near the junction between the lectin and EGF domains for E-selectin (Tsurushita et al., 1998).
Microparticles coated with natural adhesion ligands such as P-selectin glycoprotein ligand-1 (PSGL-1) effectively target selectin expressing surfaces and support tethering and rolling to cellular monolayer surfaces and cell-free systems (Cummings, 1999; Goetz et al., 1997; Lawrence et al., 1997; Park et al., 2002; Sakhalkar et al., 2003). Even isolated carbohydrate structures such as sialyl Lewisx (sLex) have demonstrated the ability to support rolling under shear stress (Beauharnois et al., 2005; Brunk and Hammer, 1997; Greenberg et al., 2000; Rodgers et al., 2000; Zou et al., 2005). In this study, microparticles coated with a panel of mAb having a known specificity to the lectin domain of P- and E-selectin were tested for their response under flow conditions and compared to natural ligands. Compared to a panel of mAb specific for selectins as well as other reports in the literature (Chen et al., 1997), HuEP performed dramatically better in terms of supporting microparticle adhesion in flow, or tethering, on both P-selectin and E-selectin under shear stress. HuEP’s performance advantage in flow over many other antibodies characterized to date offers an alternative to natural ligands in selectin targeting systems.
Materials and Methods
Antibodies and Protein Substrates
HuEP, a humanized murine mEP-5C7 IgG2 P- and E-selectin blocking mAb, was developed as previously described (He et al., 1998). The function blocking anti-human P-selectin (CD62P) mAb, G1, was purchased from Ancell (Bayport, MN). Anti-human E-selectin (CD62E) blocking mAb HAE-1f was purchased from Ancell. Anti-human E-selectin (CD62E) blocking mAb BBIG-E4 was purchased from R&D Systems (Minneapolis, MN). P-selectin blocking mAb WAPS12.2 was purified by Dr. Richard Lawson from ATTC. Biotin N-hydroxisuccinimide (NHS) ester, purchased from Sigma (St. Louis, MO), was used to biotinylate the antibodies in phosphate buffer solution (PBS) (Biowhittaker, Walkerville, MD). The ester was dissolved in DMSO at a concentration of 4.5 mg/mL for each 2 mg of antibody to be biotinylated as previously described (Hermanson, 1996; Melnikova et al., 2000). The pH was adjusted to 8.0 with 1 μL Triethylamine (TEA), and reacted for 2 h at 25°C. Any unbound biotin and byproducts were removed by dialysis in PBS using a Spectra/Por Microdialyzer and Spectra/Por 7 10 kDa MWCO membrane (Spectrum, Gardania, CA) for 2.5 h at 4°C. The amount of biotin bound to the antibody was determined using avidin-HABA (2-(4′-hydroxyazonbenzene) benzoic acid, Pierce, Rockford, IL) displacement method (Takalkar et al., 2004). Recombinant human P-selectin IgG and recombinant human E-selectin IgG, with the transmembrane and cytoplasmic domain replaced with an Fc tail, were purchased from R&D Systems. PSGL-1 was purified from harvested HL-60 cells homogenized in 20 nM Tris, 140 mM NaCl, 0.025% sodium azide (TSA buffer) with 5 mM EDTA, and 1% Triton ×−100 in the presence of protease inhibitors 10 μM leupeptin and 0.1 U/mL aprotinin adjusted to pH 8.0. The cell lysate was passed twice over a column of CNBr-activated Sepharose 4B (Pharmacia AB, Uppsala, Sweden) coupled to KPL-1 (1.3 mg/mL). The column was washed (30 column volumes) with TSA adjusted to pH 7.4 and contained 1% octylglucopyranoside (OG, Sigma). PSGL-1 was eluted from the column using TSA, pH 12.0, with 1% OG, and neutralized with 10% (V/V) 1 M Tris, pH 4.0, 1% OG (Park et al., 2002). Biotinylated Sialyl Lewisx(sLex) was purchased from Glycotech (Gaithersburg, MD).
Preparation of Selectin Substrates
Plastic polystyrene culture dishes (85 mm, Corning, Corning, NY) were rinsed with alternating washes of ethanol and deionized water, and then dried. Recombinant P-selectin or E-selectin was adsorbed onto the culture dishes in a 1 cm diameter area at a range of concentrations from 0.5 ng/μL to 4 ng/μL in PBS (Biowhittaker). The plates were incubated for 2 h at room temperature. After adsorption, the plates were washed with PBS containing 0.05% Tween 20 (BioRad, Hercules, CA). Finally, the plates were blocked against non-specific adhesion with 1% w/v of casein protein in PBS (Pierce) overnight at 4°C. The plates were used as the lower wall of the parallel plate flow chamber and mounted over an inverted phase-contrast microscope (Diaphot-TMD; Nikon, Garden City, NJ).
Selectin Site Density Determination
The site density of the selectin surface was determined by time-resolved fluorescence detection of Europium (Eu)-labeled streptavidin (PerkinElmer Wallac, Turku, Finland) (Suonpaa et al., 1992). Culture dishes (35 mm) (Nunc, Roskilde, Denmark) were prepared in an identical fashion as the polystyrene plates. Biotinylated G1 antibody (1 μg/mL) in 2 mM CaCl2 PBS was added to the dish and allowed to incubate for 1 h. Any unbound antibody was washed out with 0.05% Tween in PBS. Europium-labeled streptavidin at a concentration of 1 μg/mL was added to the dishes and allowed to incubate for 30 min at room temperature. The dishes were then washed with Tween-PBS. Finally, 600 μL of DELFIA enhancer solution (Perkins Elmer Wallac) was added and allowed to incubate for 5 min at room temperature. The enhancer solution was aliquoted into a 96-well plate to measure the time-resolved fluorescence at 360 nm excitation and 610-nm emission with a detection interval of 250–1250 μs on a SPECTRAmax Gemini xS dual-scanning microplate spectrofluorometer (Molecular Devices, Sunnyville, CA).
Preparation of the Targeting Microbeads
Polybead polystyrene 6.0 micron microspheres, (Polyscience, Warrington, PA) were washed in 1 mL of 0.1 M borate buffer (Polyscience) twice. PSGL-1, at a concentration of 1 μg per 2 × 107 microbeads in PBS, was incubated with the microbeads for 3 h at room temperature with end-to-end rotation. After incubation, the functionalized microbeads were washed with 0.5% Tween-20 and blocked against non-specific interactions with casein overnight at 4°C with end-to-end rotation. The microbeads were stored in PBS with 2 mM CaCl2 at a concentration of 5 × 105 beads/mL.
Polybead streptavidin-coated 6.0 micron microspheres (Polyscience) were used to conjugate the biotinylated antibodies in an oriented configuration on the surface of the beads (Korpela, 1984). From prepared stock, sLex and the biotinylated mAbs: HuEP, G1, HAE, WAPS, and BBIG, were incubated with the streptavidin beads at 1 μg per 2 × 106 microbeads in PBS for 2 h at room temperature with end-to-end mixing. After incubation, the functionalized microbeads were washed with 0.5% Tween-20, and blocked against non-specific interactions with casein overnight at 4°C with end-to-end rotation. The microbeads were stored in PBS at a concentration of 5 × 105 beads/mL. In the stock with microbeads containing mAb’s HAE and G1, 2 mM CaCl2 was added.
For multi-targeted microbeads, Polybead streptavidin-coated 6.0 micron microspheres were conjugated with equal molar amounts of HuEP and G1 at a total concentration of 1 μg per 2 × 106 microbeads for 3 h at room temperature with end-to-end rotation. After incubation, the functionalized microbeads were washed with 0.5% Tween-20 and blocked against non-specific interactions with casein overnight at 4°C with end-to-end rotation. The microbeads were stored in PBS with 2 mM CaCl2 at a concentration of 5 × 105 beads/mL.
Determination of Glycoprotein and Antibody Concentration on the Microbead Surface
The functionalized microparticles were subjected to the same time-resolved fluorescence detection of Europium (Eu)-labeled streptavidin assay as the surface site density measurements (Suonpaa et al., 1992). The microbead concentration and surface area of the population of functionalized microbeads were determined using a Coulter counter and a Bright-Line hemacytometer (Hausser Scientific; Horsham, PA). The amount of antibody on the microbead surface was calculated from the europium fluorescence results, the fluorescence of the secondary antibody binding, and the concentration of the microbead.
Epitope Specificity
Various concentrations of calcium chloride (CaCl2) (1 μM to 2 mM) were added to a stock of HuEP microbeads and G1 microbeads before being drawn into the flow chamber on a surface of P-selectin with a high site density (~300 sites/μm2). After 2 min of settling and contact time, flow was introduced at a wall shear rate of 100 s−1. The population of adhered microbeads remaining was recorded for each mAb-selectin pair at each CaCl2 concentration.
A concentration of 1 μg/mL of the mAbs G1, HAE, WAPS, and BBIG, respectively, was drawn though the flow chamber and allowed to incubate statically for 30 min to test their inhibition of HuEP binding to P- and E-selectin. After the incubation time, the flow chamber was washed with PBS to remove any unbound antibodies. Microbeads functionalized with HuEP were then perfused though the flow chamber, and after a settling and contact time of 10 min, flow was introduced at a wall shear rate of 100 s−1 for 1 min.
Parallel Plate Flow Chamber Assay
The adhesion experiments of the HuEP beads were done through a parallel plate flow chamber (Glycotech; Gaithers-burg, MD). The site densities of the selectin surfaces used ranged from 20 to 300 sites/μm2, where noted. This set up was positioned on an inverted phase-contrast microscope (Diaphot-TMD; Nikon, Garden City, NY) equipped with a Photron FastCam Camera R2 model 1 K (Photron USA, Inc., San Diego, CA) allowing high resolution time recordings. The microbeads were perfused through the flow chamber at wall shear rates of 10–200 s−1 by a Harvard Apparatus PHD2000 Syringe Pump (Instech Labs, Inc., Plymouth Meeting, PA). PBS media (~1 cP) was drawn from the stock solution through the flow chamber into a 10 mL glass “gas-tight” syringe (Hamilton, Reno, NV). The field of view was set at a 20× objective and a frame rate of 250 frames per second to measure bond lifetimes and distances between adhesion events. Standard video frame rates of 30 fps were used in all other assays unless indicated.
Microbead Attachment Rate and Detachment to the Substrate
The microbeads (G1, WAPS, HAE, BBIG, HuEP, PSGL-1, and sLex) were drawn into the flow chamber at various shear rates from 10 s−1 to 300 s−1 for 5 min. The rate of initial microbead attachment in this field of view during this time was assessed. To determine the detachment of the microbe-ads under shear stress, the microbeads were drawn into the flow chamber and allowed to settle and adhere to the substrate surface for 5 min under no flow conditions. After 5 min, blank PBS was drawn through the flow chamber at 10 s−1 wall shear rate to remove unbound beads and beads in the tubing. The total number of adhered microbeads was counted. Flow was then introduced to the system and increased every 30 s.
Video Imaging and Data Analysis
Video imagery of the rolling interactions was digitally recorded to the HDD of a Windows xP computer (Dell, Inc., Round Rock, TX) in avi video format via the FastCam Motion Capture software. The interaction data: microbead accumulation, microbead detachment, rolling velocities, paused times, and skip distance between adhesion events was determined using a computer tracking algorithm coded in JAVA for the ImageJ software (NIH, Washington D.C.), which uses a cross-correlation interpolation that allow for subpixel resolution of changes in position to identify the bead in consecutive image frames (Cheezum et al., 2001). Interaction events were recorded as occurring when the microbead motion across the field of view stopped. In the event of “rolling,” transient interactions between the antibody and its receptor forming and breaking bonds under shear stress occurs. The stop and start motions of rolling can be divided into two separate regimes: stop time and step distance. A stop is defined as any event falling 3 standard deviations away from a defined threshold velocity. The hydrodynamic velocity is defined as the averaged velocity of a non-interacting bead of similar size traveling near the wall under shear. For a 6-μm diameter microbead with a separation distance of 100 nm, ranged from 1.6 μm/s at 10 s−1 wall shear rate to 160 μm/s at 100 s−1 wall shear rate (Goldman et al., 1967). The step distance is the distance traveled between the paused times of defined interactions.
In order to determine the attachment rate (number of microbeads/time/area), the number of adhering microbeads in each field of view, a 0.307 mm2 area, were counted for 5 min at each shear rate. To determine the overall initial tether rate of the targeting microbeads, no distinction was made between rolling and firmly adhering beads in the calculation of the attachment rate. Only adhesion events originating in the field of view are counted. Rolling microbeads that enter the field as rolling microbeads are discounted. To determine the rolling flux of the microbeads, the number of rolling microbeads in the field of view was counted for 2 min. Microbeads were counted as rolling if they exhibited transient interactions or continuous rolling at a velocity less than the threshold free-stream velocity. Firm attachment flux was measured by counting the number of non-moving microbeads at the end of the recording period. Non-moving beads were defined as any microbeads remaining stationary for greater than 60 s. The count number is divided by the total time elapsed and the area of the field of view.
Statistics
All differences were evaluated by student’s t-test. P <0.05 was considered statistically significant. All error bars represent standard deviations. For the non-linear fitting, chi squared distribution was used to evaluate the statistical significance of observed values to theoretical calculations.
Results
Characterization of Selectin Surface
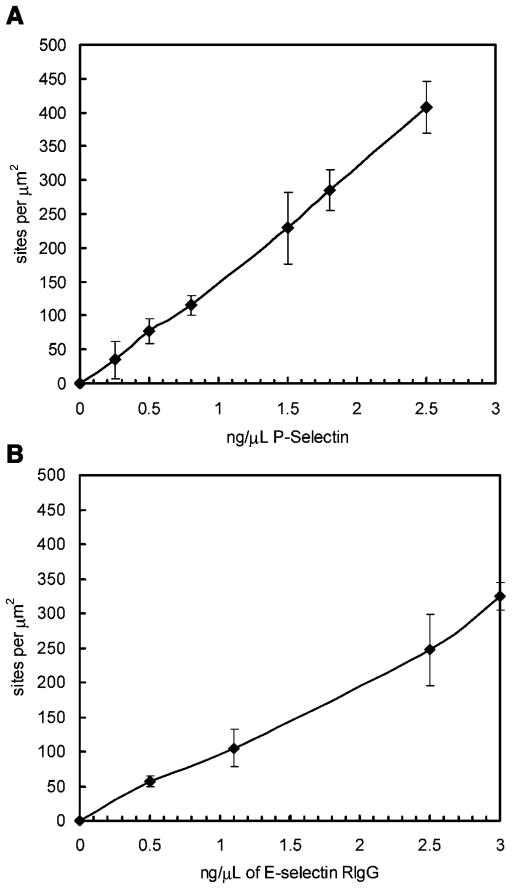
From the europium assay, a titration response of adsorbed selectin onto a plastic surface was measured from a range of 0 sites/μm2 to 400 sites/μm2 (Fig. 1). The surface site densities for P-selectin was determined to be approximately 20 molecules/μm2, 115 ±14 molecules/μm2, and 300 molecules/μm2 when 0.16 ng/μL, 0.85 ng/μL, and 1.88 ng/μL was adsorbed to the dish surface, respectively. The surface site densities for E-selectin was determined to be approximately 20 molecules/μm2, 105 ±27 molecules/μm2, and 325 ±20.35 molecules/μm2 when 0.16 ng/μL, 1.10 ng/μL, and 3 ng/μL was adsorbed to the dish surface, respectively.
Figure 1.
Site density of P-selectin RIgG (A) and E-selectin RIgG (B) on the parallel plate flow chamber plastic slide as a function of incubated concentration. N = 3 ±SEM.
Characterization of the Antibody Microbead
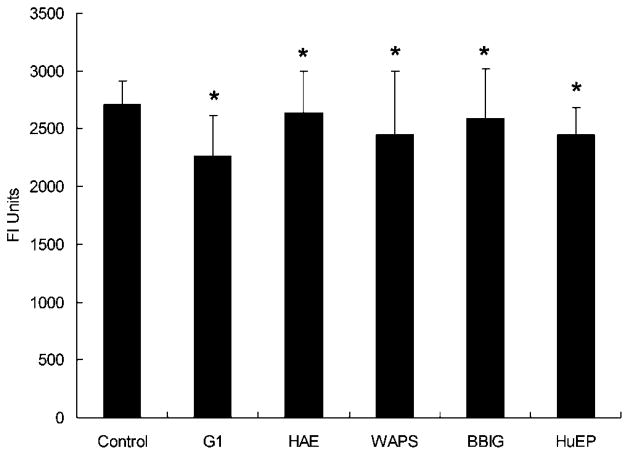
Flow cytometry using FITC-tagged secondary antibodies confirmed the presence of the adsorbed HuEP mAbs on the microbeads. From europium fluorescence assays, the number of HuEP antibodies per microbead was calculated to be (250 ±10) ×103, or ~2200 HuEP antibodies per μm2 of microbead surface. The antibody distribution on the surface of the microbead comes out to be 1 antibody molecule for every 450 nm2 (Fig. 2).
Figure 2.
Europium fluorescence assay measurement results displaying the presence of G1, HAE, WAPS, BBIG, and HuEP on the microbeads. From Europium fluorescence results and compared to flow cytometry calibration measurements, approximately 2200 HuEP antibodies per μm2 of microbead surface were calculated to be the surface density. Thus, the antibody distribution on the surface of the microbead yielded 1 antibody molecule for every 450 nm2. Similar site densities from the other antibodies tested achieved comparable site density measurements. *P >0.2 compared to far left control.
A concentration of PSGL-1 of 0.01 μg/mL will adsorb onto a polystyrene microbead at a site density of ~95 sites/μm2 (Park et al., 2002). To maintain comparable site density measurements, PSGL-1 was adsorbed onto the microbeads at a 25-fold higher concentration to yield similar site density of the antibodies. While not measured in this study, the saturation site density of a biotinylated sLex to an avidin functionalized polystyrene bead has been reported to be approximately 1,300 molecules/μm2 (Brunk et al., 1996).
Antibody Binding Epitope Characterization
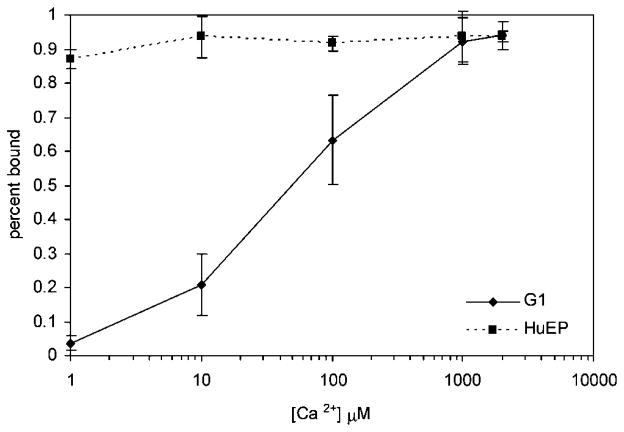
The mAb G1 is a function blocking antibody that binds to the calcium region of the lectin domain of P-selectin (Geng et al., 1990, 1991; Johnston et al., 1989). The binding epitope of EP5C7, the murine mAb that HuEP is based upon, maps to the lectin domain near the junction to the EGF domain. EP5C7 binds to both E- and P-selectin, suggesting that an overlapping epitope exists (Berg et al., 1995). HuEP is assumed to bind in a similar fashion. The dependency on calcium on HuEP and G1 binding to P-selectin was measured (Fig. 3). With increasing concentrations of Ca2+ added, the G1 functionalized microbeads responds to calcium and reaches a peak binding percentage at 1 mM, while HuEP functionalized microbeads show no effect to the increasing amount of Ca2+ in the system.
Figure 3.
Ca2+ dependence of antibody binding to P-selectin. P-selectin was immobilized on a surface at a site density of ~300 sites/μm2. Microbeads adsorbed with G1 antibody and HuEP antibody, respectively, were allowed to settle via gravity to the surface and contact the surface for 2 min. The indicated amount of CaCl2 was added to the bead solution for each run. After settling time passed, flow at a wall shear rate of 100 s−1 was introduced for 1 min. During that time, the number of firmly attached beads was counted against the total population of the beads before flow was introduced.
By introducing the competitive antibody to the selectin surface before drawing in the targeting beads, the specificity of the binding epitope of HuEP was confirmed in the microbead system. Corroborating with published results (Berg et al., 1995), antibodies WAPS and BBIG prevented the binding of HuEP-microbeads to the P- and E-selectin surfaces. Additionally, G1 and HAE did not prevent the binding of HuEP-microbeads to the selectin surface (Table I). The data further supports the idea that HuEP binds to an epitope on the lectin domain that is separate from the Ca2+ region of the selectin and that surface immobilization does not alter specificity.
Table I.
Competitive Antibody Binding
| MAb competitor | Binding | Ability to block HuEP binding to
|
|
|---|---|---|---|
| P-selectin | E-selectin | ||
| G1 | P-selectin | − | − |
| HAE | E-selectin | − | − |
| WAPS12.2 | P-selectin | + | − |
| BBIG-E4 | E-selectin | − | + |
Table of competitive antibody binding against HuEP microbeads to map specific adhesion epitope. All antibodies are function blocking against the respective selectin target. The ability to block HuEP binding to P-selectin or E-selectin is indicated by a “+” and measured by introducing the competing antibody to the selectin surface before introducing microbeads coated with HuEP.
Characterization of Antibody-Conjugated Microbead Accumulation
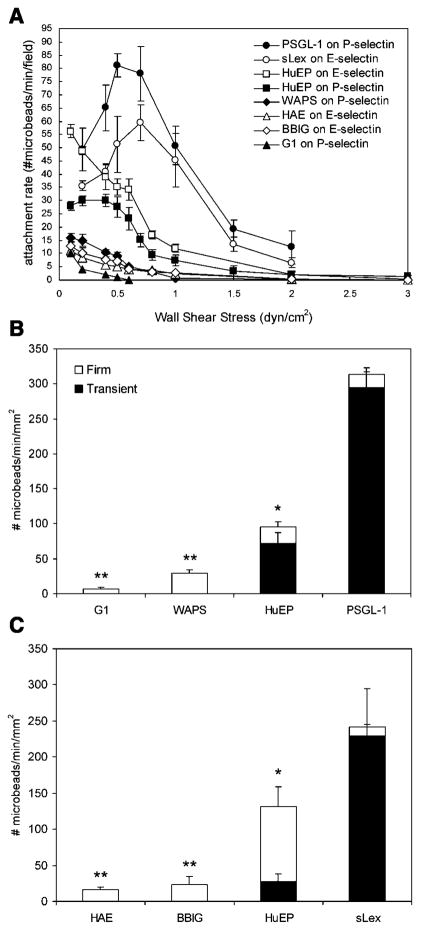
The accumulation of microbeads functionalized with HuEP, other selectin binding antibodies, PSGL-1, and sLex was studied under several flow regimes (Fig. 4A). Microbeads with PSGL-1, a natural ligand, and sLex, a minimal selectin recognition motif, were used as positive controls to assess the tethering performances of the antibody-coated microbe-ads to P-selectin and E-selectin, respectively. The HuEP microbeads on E-selectin show a higher affinity to binding under increasing shear stress when compared to P-selectin. HuEP bound significantly better than the G1 and WAPS antibodies, HAE, and BBIG antibodies under shear stress when compared to their respective ligands. The WAPS and BBIG antibodies showed a modest increase in the rate of accumulation when compared to the calcium-dependent antibodies G1 and HAE. The majority of previously reported antibodies do not support transient adhesion interactions at near venular flow rates, nor display rolling-like behavior (Chen et al., 1997; Marshall et al., 2003). However, as the HuEP microbeads experienced shear stress against a P-selectin surface, they were able to bind and detach fast enough to enable rolling behavior. HuEP showed a higher accumulation to E-selectin versus P-selectin for firm adhesion, but much of the adhesion interactions of HuEP to P-selectin were rolling interactions. The optimal shear rate for rolling interactions appeared to occur at around 50 s−1 wall shear rate. PSGL-1 and sLex microbeads displayed a significantly greater attachment rate compared to the panel of antibodies. While the attachment rates of PSGL-1 and sLex microbeads were greater, they displayed very few stationary interactions as indicated by a larger rolling flux over firm adhesion flux (Fig. 4B, C).
Figure 4.
Attachment rate as a function of shear stress. A: The accumulation of microbeads in the field of view. G1, HAE, WAPS, and BBIG did not display any transient interactions under shear stress. B: Rolling and firm adhesion flux for HuEP, G1, WAPS, and PSGL-1-coated microbeads on P-selectin at 50 s−1 wall shear rate. C: Rolling and firm attachment flux for HuEP, HAE, BBIG, and sLex coated microbeads on E-selectin at a wall shear rate of at 50 s−1. The E- and P-selectin surface site density was set at 100 sites/μm2. Accumulations and fluxes shown are the result of at least two independent measurements. *P <0.05 compared to the far right control. **P <0.05 compared to the HuEP microbeads.
Characterization of Antibody-Conjugated Microbead Detachment
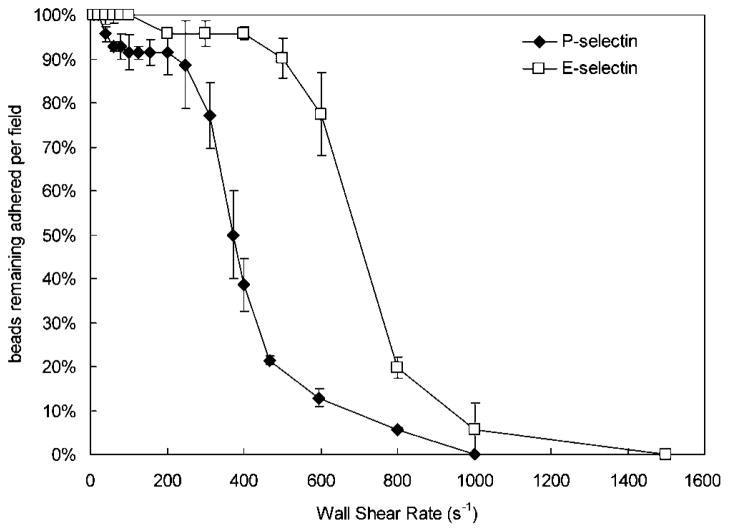
The adhesion strength of the antibody-conjugated microbead under wall shear stress can be determined through detachment kinetics. Microbead detachment was determined by increasing the flow every 30 s and counting the number of microbeads remaining. The percentage of bound beads under increasing wall shear rates was measured for microbeads functionalized with HuEP on P-selectin and E-selectin (Fig. 5). HuEP displayed a significant enhancement to its adhesion to E-selectin over P-selectin at higher wall shear rates. Half-maximal detachment for HuEP on P-selectin was achieved at a wall shear rate of approximately 500 s−1, while on E-selectin, half-maximal detachment was achieved at approximately 700 s−1. The detachment of the antibodies G1, WAPS, HAE, and BBIG, up until about 500 s−1 wall shear rate, were relatively comparable. At wall shear rate greater than 500 s−1, a higher percentage of G1 microbeads were able to resist shear forces resulting in a competitive binding advantage over WAPS, HAE, and BBIG (not shown).
Figure 5.
Detachment of HuEP-adsorbed microbeads resulting from periodic (every 30 s) incremental increases of the shear rate on a P-selectin (closed diamonds) and an E-selectin (open squares) surface with density of 100 sites/μm2.
Shear Stress Dependence
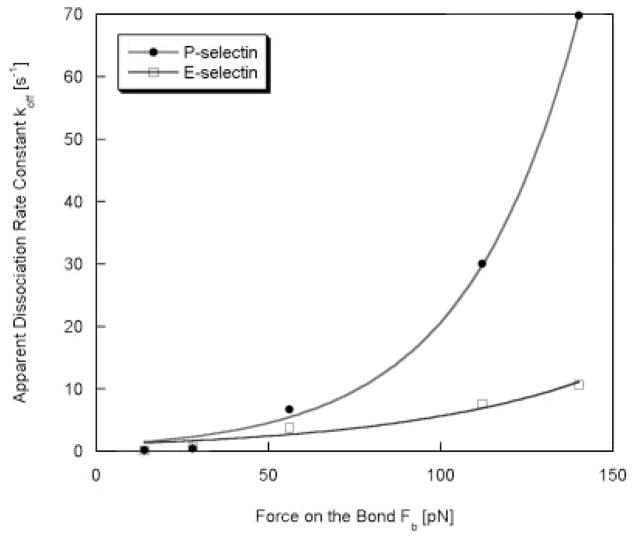
The adhesion characteristics of HuEP on E- and P-selectin under increasing shear stresses were assessed in a bond lifetime assay previously used to characterize selectin bond strength (Park et al., 2002). The distribution of bond lifetimes can be fit to a first order bimolecular reaction model to produce the dissociation rate constant of a reaction under wall shear stress. From the Bell model, a relationship between static bond force, paused lifetime and shear stress can be measured for the HuEP-coated microbeads (Fig. 6) (Alon et al., 1995; Bell, 1978; Smith et al., 1999). The molecular kinetics under shear stress are modulated by forces on the particle, which depend on both flow rates and binding geometry. To determine effects of force on the dissociation rate constant (koff), the data were fit into the Bell model yielding a relationship between force (Fb), dissociation rate, and wall shear stress (Bell, 1978). Nonlinear fitting calculated by the Leverburg–Marquardt algorithm yielded an unstressed dissociation rate constant of koff° = 0.99 ± 0.22 s−1 for P-selectin (χ2 = 7.05), and koff° = 1.03 ± 0.47 s−1 for E-selectin (χ2 = 4.41). A reactive bond compliance (σ) for HuEP binding to P-selectin was solved to be 1.22 ± 0.07 Angstroms and 0.68 ± 0.14 Angstroms for HuEP binding to E-selectin. The bond compliance values of HuEP illustrated that HuEP was approximately three times more force-sensitive than P-selectin–PSGL-1 bonds (σ = 0.37 Angstroms) and E-selectin–sLex bonds (σ = 0.25 Angstroms) (Alon et al., 1995; Chang and Hammer, 2000; Park et al., 2002) and six times more force-sensitive than P-selectin–G1 bonds (σ = 0.24 Angstroms) (Marshall et al., 2003).
Figure 6.
The distribution of paused lifetimes were used to calculate the bond dissociation constants (koff) of microparticles and plotted as a function of the force on the bond. The bond lifetime, microbead bond geometry, and shear parameters were fit into the Bell model to calculate the bond’s reactive compliance under wall shear rate from the relationship between force and bond lifetimes. HuEP interacting with an E-selectin surface (white), HuEP interacting with a P-selectin surface (black). The dissociation constant values were measured at wall shear rates of 10 s−1 to 100 s−1. The non-linear curve fit line was evaluated from Levenberg–Marquardt algorithm.
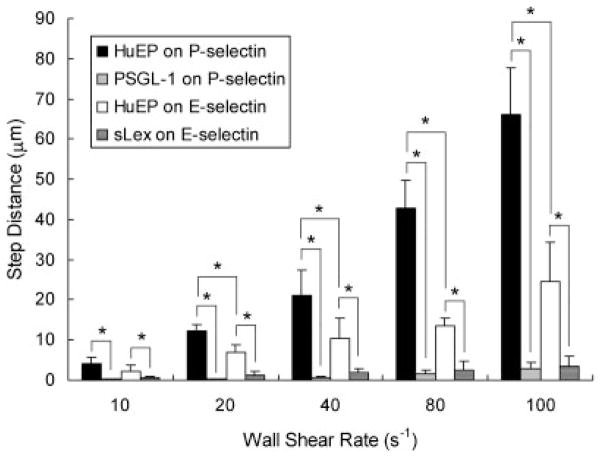
Microparticle dynamic adhesion can be characterized as a sequence of pauses and the distance traveled between pauses, or step distance (Smith et al., 2004). The step distance represents the likelihood of a repeated interaction following bonds breaking. From a range of wall shear rates of 10 s−1 to 100 s−1, the step distances of HuEP on P- and E-selectin were compared to PSGL-1 and sLex (Fig. 7). HuEP adhesion to E-selectin demonstrated an increased likelihood of a repeated interaction over P-selectin adhesion. Under increasing wall shear rates, the step sizes of HuEP increased from small rolling-like step distances to larger transient-like distances. Comparatively, the very small step sizes of PSGL-1 and sLex microbeads under increasing wall shear stress demonstrated an ability to roll on the surface.
Figure 7.
The averaged step distances between skips for microparticles with HuEP interacting with an E-selectin surface (white), HuEP interacting with a P-selectin surface (black), PSGL-1 interacting with a P-selectin surface (gray), and sLex interacting with an E-selectin surface (dark gray). The E- and P-selectin site density was set at 100 sites/μm2. The step distance was defined as the distance traveled between paused events. *P <0.05.
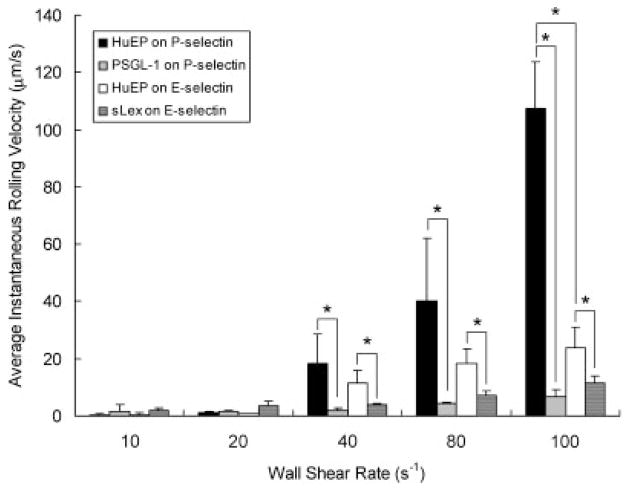
The combination of step distance and koff constants controls the translocation velocity, or rolling, of microbeads in flow. From 10 s−1 to 100 s−1 wall shear rates, the rolling velocity of microbeads with HuEP, PSGL-1, and sLex were measured (Fig. 8). For HuEP on P-selectin, the dissociation rate constant and step distance rise rapidly with shear rates which resulted in an increased rolling velocity. Microbeads with PSGL-1 and sLex exhibited a much slower overall rolling velocity than HuEP microbeads, suggesting more avid bonds under shear stress.
Figure 8.
The averaged rolling velocities of microparticles with HuEP interacting with an P-selectin surface (black), HuEP interacting with a E-selectin surface (white), PSGL-1 interacting with P-selectin (gray), and sLex on E-selectin (dark gray) under wall shear rates of 10 s−1 to 100 s−1. No rolling interactions for mAb G1, HAE, WAPS, and BBIG. *P <0.05.
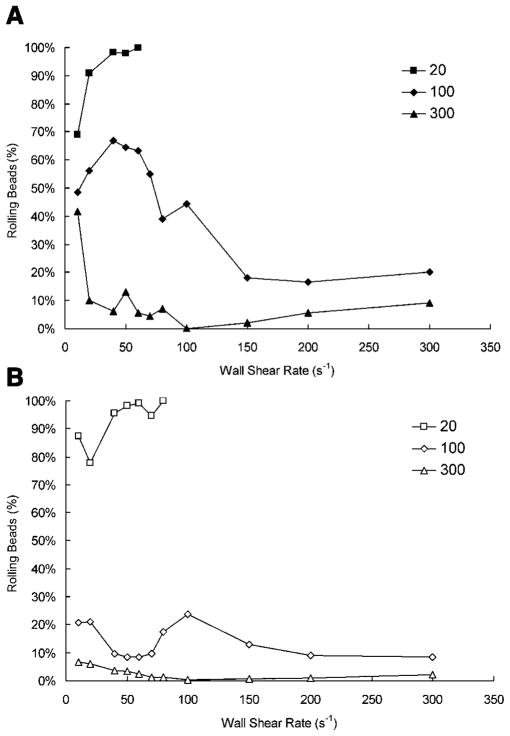
From (Fig. 9) the effect that selectin site density has on HuEP rolling was quantified. At low site densities (20 sites/μm2), almost all the adhering microbeads demonstrated transient rolling interactions. However, at such low site densities, microbead accumulation to the surface was extremely low and at a wall shear rate of 100 s−1, no accumulation was observed. At a high site density of 300 sites/μm2, stationary interactions dominated the adhesion behavior in both P-and E-selectin, with few to no rolling interactions. Optimal rolling behaviors appeared to be located around the site density of 100 sites/μm2. While E-selectin did elicit a stronger binding to HuEP, P-selectin induced more rolling microbeads under comparable wall shear rates. It is clear that the number of beads rolling on P-selectin when compared to E-selectin was vastly greater from ~20 s−1 to ~60 s−1 wall shear rate. This is no surprise as within this range of shear rates, HuEP exhibited optimal rolling numbers on P-selectin. When the site densities of the selectin substrate were increased from 20 sites/μm2 to 300 sites/μm2, a sharp decline in rolling population percentage coupled with an increase in firm adhesion of microbeads was observed.
Figure 9.
The effect of site density on rolling of HuEP microbeads on P- and E-selectin. Site densities of the selectin substrate were varied from 20 sites/μm2 to 300 sites/μm2. A: Percentage of bound HuEP microbeads that rolled on P-selectin. B: Percentage of bound HuEP microbeads that rolled on E-selectin.
Dual Antibody Microbead Targeting
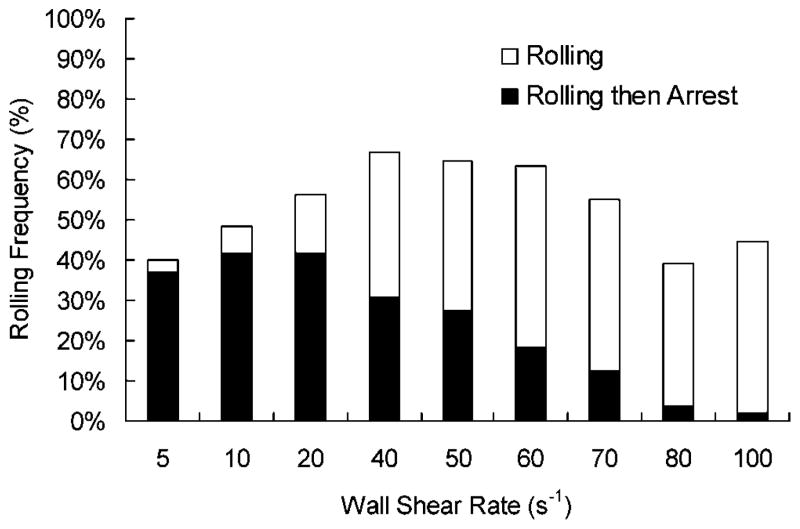
Equal molar amounts of the mAbs G1 and HuEP were coated to microbeads to create a dual-targeting micro-particle. Under a range of wall shear rates, the effect that G1 adds to the overall microbead adhesion dynamics of HuEP selectin interactions were observed (Fig. 10). The initial tethering behavior of the dual-targeted microbeads followed a similar adhesion profile as HuEP microbeads. As the microbeads moved faster over the P-selectin surface, the ability of G1 to induce arrest was decreased.
Figure 10.
Rolling frequency of two antibody-targeted microbead. Microbeads were conjugated with equal molar amounts of HuEP and G1 and drawn through the flow chamber. The frequency of rolling beads under shear stress was counted, and from that population, the percent of rolling beads that then firmly arrested to the surface were marked as arrested beads. The inclusion of G1 did not significantly alter the initial tethering events of the microbeads to P-selectin.
Discussion
Drug targeting is often cited as a solution to the toxic and negative side effects that accompany systematic drug administrations in intravascular pharmaceuticals (Allen, 2002; Allen and Cullis, 2004; Gregoriadis, 1977; Sapra et al., 2005; Torchilin, 2000). There are several levels at which a drug can be targeted: to organ or tissue, to a specific cell type, or against a specific molecular pathway. Ideally, some combination of all three would be expected to maximize therapeutic impact. To target an organ or tissue experiencing adverse inflammatory reactions, the first step to achieve high but localized concentrations of a therapeutic drug is to selectively deliver drug carriers to specific sites on the vascular endothelium (Bendas et al., 1999; Hajitou et al., 2006; Spragg et al., 1997). For maximum effectiveness in localizing intravascular drug carriers, the endothelium should report to some extent the condition of the tissue. Such is the case with inflammation where the vascular endothelium transiently expresses unique receptors to recruit leukocytes (Somers et al., 2000).
The choice of targeting markers to specific cell types is often restricted to cell-surface glycoproteins that can be recognized by mAbs. In this study, we investigated the adhesion dynamics of a panel of mAb-coated microparticles designed to target endothelial selectins and compared their performance to natural ligands used by leukocytes to home to sites of tissue inflammation. Antibodies are an attractive choice for targeting because of their generally high affinity for specific antigens and ease of biotinylation (Storm et al., 1996). These properties have resulted in their use in a number of drug targeting strategies in microparticle and microbubble systems to target and image inflamed endothelium in vitro and in vivo conditions (Dickerson et al., 2001; Lindner et al., 2001; Sakhalkar et al., 2003, 2005).
The motivation for analyzing the performance of targeting monoclonal antibodies presented on micron-sized particles was twofold. For one, we wished to assess their relevance for both targeted ultrasound imaging of contrast agents and drug carrying microparticles. Since the circulatory system is an important pathway of administration, any targeting of specific sections of vasculature would require a microparticle to attach to the vessel wall under flow conditions that generate significant shear forces (Eniola and Hammer, 2005; Patil et al., 2001). Second, the microparticle platform reduces the relative effects of non-specific surface forces on the binding probability of immobilized receptors by the amplification of fluid shear forces (Langer and Tirrell, 2004; Leckband and Israelachvili, 2001). Comparison under flow conditions thereby allowed us to focus on the molecularly-specific effects of forces and short contact times on the targeting reaction (Simon and Goldsmith, 2002).
Binding of HuEP nanoparticles to cellular monolayers expressing E-selectin and P-selectin indicates that the common recognition epitope is accessible when presented on the cell surface (Blackwell et al., 2001). The binding of HuEP to E- and P-selectin maps to an epitope that is located in an overlapping region on the lectin domain near the junction to the EGF domain. Competitive inhibition of HuEP microparticle binding by the mAb WAPS on P-selectin and BBIG on E-selectin suggested they recognized similar epitopes. The mAb G1, which maps to the Ca2+-dependent region of the lectin domain observed to participate in PSGL-1/P-selectin rolling, did not inhibit HuEP adhesion (Somers et al., 2000). Since tethering, transient adhesion, and rolling are seen only with HuEP among mAb WAPS and BBIG, which bind within the overlapping epitope region, the ability to tether in flow and mediate rolling is not likely to be solely dependent upon the structure and location of the monoclonal binding site.
Selection of a candidate vascular targeting ligand based solely on considerations of affinity would guarantee a high degree of specific binding, a highly desirable characteristic of a targeting ligand. However, achieving therapeutic accumulation of micro- or nanoparticles at a site of inflammation or vascular damage may depend, in part, on the probability of tethering in flow. In previous studies, mAb-coated microparticles generally formed only stable bonds at low shear rates. Exceptions have been observed in the case of several carbohydrate binding IgM antibodies that have been shown to facilitate rolling of leukocytes at venular shear rates but near-stasis levels were required before binding interactions could take place (Chen et al., 1997; Pierres et al., 1994; Takalkar et al., 2004; Tempelman and Hammer, 1994). HuEP is the first protein-binding antibody reported that can tether and mediate transient rolling on selectin expressing surfaces at venular shear rates. It is of interest that the best performing mAb supports microparticle rolling in flow.
Based on its superior performance relative to other mAbs specific to selectins, the mAb HuEP appeared to have the potential to serve as an intravascular targeting ligand. In contrast, the microbeads that presented mAbs G1, HAE, WAPS, and BBIG were capable of forming stable bonds only at lower shear rates, though all interactions were highly specific. While the tethering performance of HuEP microbeads was not as robust as that of PSGL-1 or sLex microbeads, HuEP performed significantly better than the other panel of mAbs investigated in terms of mediating microparticle tethering under flow conditions. Since mAbs such as G1 have high affinity towards selectins, our observations suggest that kinetic properties may be more important than overall affinity in predicting the ability of antibodies to mediate microparticle tethering under shear flow.
The lifetime of stressed bonds is an important factor in regulating the detachment of microbeads from the surface (Kuo et al., 1997; Kuo and Lauffenburger, 1993; Saterbak et al., 1993). A low detachment rate indicates an increased bond lifetime and is suggestive of higher bond avidity. The adhesion dynamics of the mAb-coated microbeads correlates with the affinity behavior of the antibody–antigen bond. From published competitive antibody binding assays and Scatchard Plot analysis, the affinity (reported as avidity) of HuEPC7.g4, a similar short-circulating antibody with an IgG4 tail, for P-selectin and E-selectin was reported as 1.5 E 8 M−1 and 3.3 E 8 M−1, respectively (He et al., 1998). For HuEP microbeads, the distance between tethering events was 2.5-fold less for E-selectin than on P-selectin surfaces suggesting a higher rate of bond formation. In addition, the wall shear rate to achieve a half-maximal detachment from E-selectin was 1.5-fold higher compared to P-selectin suggesting higher affinity. The majority of the tethering events observed in HuEP microbead adhesion to E-selectin were stable bonds, with approximately 25% slow rolling. Slower rolling velocities and increased bond lifetimes suggested a lower stressed dissociation rate constant than P-selectin. While firm adhesion dominated the adhesion behavior, unlike G1, HuEP to E-selectin was able to initiate enhanced adhesive interactions under flow conditions.
Microparticle rolling on vascular endothelium could be useful in targeting by allowing increased opportunity to survey the surface of the blood vessel wall for signals. The ability of HuEP in vitro to mediate rolling-type adhesions may be a partial consequence of its relatively high bonding rate suggested from microparticle adhesion dynamics. Leukocyte and selectin functionalized microparticle rolling can be dynamically characterized by the relative rates of association and dissociation (Chen et al., 1997; Smith et al., 2004). Selectin molecular bonds are characterized by high association rates and dissociation rates; much higher than the standard antibody–antigen reaction (Mehta et al., 1998; Nicholson et al., 1998; Ushiyama et al., 1993). It has been proposed that high association and dissociation rates are required for leukocyte rolling (Lawrence and Springer, 1991; Tempelman et al., 1994). The small step distances and slow rolling velocities of PSGL-1 and sLex microbeads are suggestive of high rates of association and dissociation, and resulted in more stable rolling than observed on HuEP microbeads. Consistent with previous theoretical analyses, fast association rate appeared important for initiating rolling behavior in flow conditions (Chang and Hammer, 2000; Hammer and Lauffenburger, 1987; Kuo and Lauffenburger, 1993).
The rolling and transient events observed and measured for HuEP may not be entirely dependant upon the molecular kinetics of binding; mechanical force may be important as well. Although the bond compliance of HuEP to P-selectin and E-selectin is four times that of the natural ligands, it is apparently low enough to facilitate rolling and transient adhesion under shear. The dependency of koff to force is described as reactive compliance. If the koff is unresponsive to force, then the bond is described as having low compliance and is mechanically stable (Chen et al., 1997). Theoretical models have suggested that reactive compliance correlate positively with rolling adhesion (Hammer and Apte, 1992; Torzeren and Ley, 1992). Therefore, higher bond compliance mediates faster rolling and reduces the rolling stability by narrowing the range of shear stresses that support microparticle rolling adhesion. For HuEP, E-selectin binding is less compliant, making the bond stronger-and it is not the best roller. This supports the findings that E-selectin rolling is more stable at higher shear stresses and correlates with the molecular biochemistry.
Rolling of microparticles may allow scanning of stretches of endothelium, yet effective targeting may additionally require a robust stop signal. Therefore, a more complete targeted microparticle system might include a high affinity antibody, such as G1, as a secondary targeting antibody. Such a cascading system would work analogously to the leukocyte adhesion cascade by the HuEP interaction slowing the microparticle in flow conditions enough to allow the slower binding, but higher affinity G1 to attach and induce firm arrest in a similarly adhesive phenotype as has been previously described for a sLex/ICAM antibody dual targeting system (Bhatia et al., 2003). The initial tethering of the dual antibody microbeads followed a similar adhesion profile as HuEP microbeads, in which the initial accumulation of microbeads appeared dominated by HuEP binding. Thus, the inclusion of G1 may not influence the initial tethering that leads to transient rolling, consistent with the two-step adhesive mechanism used by leukocytes. At higher shear rates, the ability of G1 to induce firm arrest after rolling is greatly diminished. This suggests that it may be critical to slow the microbead below a threshold velocity to allow the arresting mAb enough time to bind. Both PSGL-1 and sLex support higher tether frequency and slower rolling and would be predicted to have superior performance compared to HuEP alone.
In summary, this study has made the first step in characterizing the tethering and rolling of targeting antibodies under flow conditions. The investigation of the dual specificity of HuEP to both P- and E-selectin under physiological flow conditions reveals an attractive modality to target selectin expressing surfaces with one antibody. While the tethering and rolling performance of HuEP is not as robust and stabile as PSGL-1 or sLex, it is significantly better than comparative antibodies; enough to mediate transient rolling in flow. The adhesion capabilities of HuEP have applications in multi-staged targeting of selectin expressing endothelium. Glycoproteins are expensive and often require extensive post-translational modifications for activity. HuEP, however, is a novel and attractive alternative to natural ligands of selectins that retains dual selectin specificity. As a substitute in targeting selectin expressing surfaces, HuEP has the potential to be a key moiety in intravascular targeting.
Acknowledgments
This work was supported by the National Institute of Health grant R01HL54614 (to MBL), the Bioengeneering Research Partnership grant HL64381 (to MBL), and the Established Investigator Award from the American Heart Association (to DJG).
References
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nature Rev Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374(6522):539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- Beauharnois ME, Lindquist K, Marathe D, Vanderslice P, Xia J, Matta KL, Neelamegham S. Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry. 2005;44:9507–9519. doi: 10.1021/bi0507130. [DOI] [PubMed] [Google Scholar]
- Bell GI. Models for the specific adhesion of cells to cells. Science. 1978 May;200(12):618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Bendas G, Krause A, Bakowsky U, Vogel J, Rothe U. Targetability of novel immunoliposomes prepared by a new antibody conjugation technique. Int J Pharm. 1999;181(1):79–93. doi: 10.1016/s0378-5173(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Berg E, Fromm C, Melrose J, Tsurushita N. Antibodies cross-reactive with E- and P-selectin block both E- and P-selectin functions. Blood. 1995;85(1):31–37. [PubMed] [Google Scholar]
- Bhatia SK, King MR, Hammer DA. The state diagram for cell adhesion mediate by two receptors. Biophys J. 2003;84(4):2671–2690. doi: 10.1016/S0006-3495(03)75073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell JE, Dagia HM, Dickerson JB, Berg EL, Goetz DJ. Ligand coated nanosphere adhesion to E- and P-selectin under static and flow conditions. Ann Biomed Eng. 2001;29:523–533. doi: 10.1114/1.1376697. [DOI] [PubMed] [Google Scholar]
- Brunk DK, Hammer DA. Quantifying rolling adhesion with a cell-free assay: E-Selectin and its carbohydrate ligands. Biophys J. 1997;72:2820–2833. doi: 10.1016/S0006-3495(97)78924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk DK, Goetz DJ, Hammer DA. Sialyl LewisX/E-selectin-mediated rolling in a cell-free system. Biophys J. 1996;71:2902–2907. doi: 10.1016/S0006-3495(96)79487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-C, Hammer D. Adhesive dynamics simulations of Sialyl-LewisX/E-selectin-mediated rolling in a cell-free system. Biophys J. 2000;79(4):1891–1902. doi: 10.1016/S0006-3495(00)76439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheezum MK, Walker WF, Guilford WH. Quantitative comparison of algorithms for tracking single fluorescent particles. Biophys J. 2001;81(4):2378–2388. doi: 10.1016/S0006-3495(01)75884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Alon R, Fuhlbrigge RC, Springer TA. Rolling and transient tethering of leukocytes on antibodies reveal specializations of selectins. PNAS. 1997;94(7):3172–3177. doi: 10.1073/pnas.94.7.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co MS, Queen C. Humanized antibodies for therapy. Nature. 1991;351(6326):501–502. doi: 10.1038/351501a0. [DOI] [PubMed] [Google Scholar]
- Cummings RD. Structure and function of the selectin ligan PSGL-1. Braz J Med Biol Res. 1999;32(5):519–528. doi: 10.1590/s0100-879x1999000500004. [DOI] [PubMed] [Google Scholar]
- Dickerson JB, Blackwell JE, Ou JJ, Patil VRS, Goetz DJ. Limited adhesion of biodegradable microspheres to E- and P-selectin under flow. Biotechnol Bioeng. 2001;73(6):500–509. doi: 10.1002/bit.1085. [DOI] [PubMed] [Google Scholar]
- Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: Effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–670. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79(3):560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- Geng J-G, Bevilacquat MP, Moore KL, Mclntyre TM, Prescott SM, Kim JM, Bliss GA, Zimmerman GA, McEver RP. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Geng J, Moore K, Johnson A, McEver R. Neutrophil recognition requires a Ca(2+)-induced conformational change in the lectin domain of GMP-140. J Biol Chem. 1991;266(33):22313–22318. [PubMed] [Google Scholar]
- Goetz DJ, Greif DM, Ding H, Camphausen RT, Howes S, Comess KM, Snapp KR, Kansas GS, Luscinskas FW. Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P-and E- selectin. J Cell Biol. 1997;137(2):509–519. doi: 10.1083/jcb.137.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AJ, Cox RG, Brenner H. Slow Viscous motion of a sphere parallel to a plane wall—II Couette flow. Chemical Eng Sci. 1967;22:653–660. [Google Scholar]
- Greenberg AW, Brunk DK, Hammer DA. Cell-free rolling mediated by L-selectin and sialyl LewisX reveals the shear threshold effect. Biophys J. 2000 Nov;79:2391–2402. doi: 10.1016/S0006-3495(00)76484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriadis G. Targeting of drugs. Nature. 1977;265(5593):407–411. doi: 10.1038/265407a0. [DOI] [PubMed] [Google Scholar]
- Hajitou A, Pasqualini R, Arap W. Vascular targeting: Recent advances and therapeutic perspectives. Trends Cardiovasc Med. 2006;16(3):80–88. doi: 10.1016/j.tcm.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer DA, Apte SM. Simulation of cell rolling and adhesion on surfaces in shear flow: General results and analysis of selectin-mediated neutrophil adhesion. Biophys J. 1992;63(1):35–57. doi: 10.1016/S0006-3495(92)81577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer DA, Lauffenburger DA. A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J. 1987;52:475–487. doi: 10.1016/S0006-3495(87)83236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X-Y, Xu Z, Melrose J, Mullowney A, Vasquez M, Queen C, Vexler V, Klingbeil C, Co MS, Berg EL. Humanization and pharmacokinetics of a monoclonal antibody with specificity for both E- and P-selectin. J Immunol. 1998;160(2):1029–1035. [PubMed] [Google Scholar]
- Hermanson GT. Bioconjugate Techniques. San Diego, CA: Academic Press; 1996. [Google Scholar]
- Johnston GI, Cook RG, McEver RP. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: Sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Korpela J. Avidin: A high affinity biotin-binding protein, as a tool and subject of biological research. Med Biol. 1984;62:5–26. [PubMed] [Google Scholar]
- Kuo SC, Lauffenburger DA. Relationship between receptor/ligand binding affinity and adhesion strength. Biophys J. 1993;65(5):2191–2200. doi: 10.1016/S0006-3495(93)81277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SC, Hammer DA, Lauffenburger DA. Free in PMC Simulation of detachment of specifically bound particles from surfaces by shear flow. Biophys J. 1997;73:517–531. doi: 10.1016/S0006-3495(97)78090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow MA, Norton CR, Rumberger JM, Lombard-Gillooly KM, Shuster DJ, Hubbard J, Bertko R, Knaack PA, Terry RW, Harbison ML, et al. Characterization of E-selectin-deficient mice: Demonstration of overlapping function of the endothelial selectins. Immunity. 1994;1(8):709–720. doi: 10.1016/1074-7613(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer T. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell. 1991;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Kansas GS, Kunkel EJ, Ley K. Threshold levels of fluid shear promote leukcyte adhesion through selectins (CD62L, P, E) J Cell Biol. 1997 Feb 10;136(3):717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband D, Israelachvili JN. Intermolecular forces in biology. Q Rev Biophys. 2001;34(2):105–267. doi: 10.1017/s0033583501003687. [DOI] [PubMed] [Google Scholar]
- Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury With microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423(6936):190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- Mehta P, Cummings RD, McEver RP. Affinity and kinetic analysis of P-selectin Binding to P-selectin glycoprotein ligand-1. J Biol Chem. 1998 Dec 4;49:32506–32513. doi: 10.1074/jbc.273.49.32506. [DOI] [PubMed] [Google Scholar]
- Melnikova YI, Odintsov SG, Kravchuk ZI, Kartsev SP. Antigen-Binding Activity of Monoclonal Antibodies after Incubation with Organic Solvents. Biochemistry. 2000;65(11):1256–1265. [PubMed] [Google Scholar]
- Nicholson MW, Barclay AN, Singer MS, Rosen SD, van der Merwe PA. Affinity and kinetic analysis of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273(2):763–770. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- Park EYH, Smith MJ, Stropp ES, Snapp KR, DiVietro JA, Walker WF, Schmidtke DW, Diamond SL, Lawrence MB. Comparison of PSGL-1 microbead and neutrophil rolling: Microvillus elongation stabilizes P-selectin bond clusters. Biophys J. 2002 Apr;82:1835–1847. doi: 10.1016/S0006-3495(02)75534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil VRS, Campbell CJ, Yun YH, Slack SM, Goetz DJ. Particle diameter influences adhesion under flow. Biophys Jl. 2001;80(4):1733–1743. doi: 10.1016/s0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierres A, Tissot O, Malissen B, Bongrand P. Dynamic adhesion of CD8-positive cells to antibody-coated surfaces: The initial step is independent of microfilaments and intracellular domains of cell-binding molecules 10.1083/jcb.125.4.945. J Cell Biol. 1994;125(4):945–953. doi: 10.1083/jcb.125.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SD, Camphausen RT, Hammer DA. Sialyl LeweisX-mediated, PSGL-1-Independent Rolling Adhesion on P-Selectin. Biophys J. 2000;79:694–706. doi: 10.1016/S0006-3495(00)76328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc Natl Acad Sci USA. 2003;100(26):15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhalkar HS, Hanes J, Fu J, Benavides U, Malgor R, Borruso CL, Kohn LD, Kurjiaka DT, Goetz DJ. Enhanced adhesion of ligand-conjugated biodegradable particles to colitic venules. FASEB J. 2005:04-2668fje. doi: 10.1096/fj.04-2668fje. [DOI] [PubMed] [Google Scholar]
- Sapra P, Pradeep T, Allen TM. Ligand-targeted liposomes for cancer treatment. Curr Drug Deliv. 2005;2(4):369–381. doi: 10.2174/156720105774370159. [DOI] [PubMed] [Google Scholar]
- Saterbak A, Kuo SC, Lauffenburger DA. Heterogeneity and probabilistic binding contributions to receptor-mediated cell detachment kinetics. Biophys J. 1993;65:243–252. doi: 10.1016/S0006-3495(93)81077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann Biomed Eng. 2002;30(3):315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Berg EL, Lawrence MB. A direct comparison of selectin-mediated transient, adhesive events using high temporal resolution. Biophys J. 1999;77:3371–3383. doi: 10.1016/S0006-3495(99)77169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Smith BRE, Lawrence MB, Snapp KR. Functional anaylsis of the combined role of the o-linked branching enzyme core 2 b1-6-N-glucosaminyltransferase and dimerization of P-selectin glycoprtein ligand-1 in rolling on P-selectin. J Biol Chem. 2004;279(21):21984–21991. doi: 10.1074/jbc.M402731200. [DOI] [PubMed] [Google Scholar]
- Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLeX and PSGL-1. Cell. 2000;103(3):467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Spragg DD, Alford DR, Greferath R, Larsen CE, Lee K-D, Gurtner GC, Cybulsky MI, Tosi PF, Nicolau C, Gimbrone MA., Jr Immuno-targeting of liposomes to activated vascular endothelial cells: A strategy for site-selective delivery in the cardiovascular system. PNAS. 1997;94(16):8795–8800. doi: 10.1073/pnas.94.16.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D, Loos M, Kaul M. Biotinylation of proteins via amino groups can induce binding to U937 cells, HL-60 cells, monocytes and granulocytes. J Immunol Methods. 1996;199:87–99. doi: 10.1016/s0022-1759(96)00172-x. [DOI] [PubMed] [Google Scholar]
- Suonpaa M, Markela E, Stahlberg T, Hemmila I. Europium-labelled streptavidin as a highly sensitive universal label: Indirect time-resolved immunofluorometry of FSH and TSH. J Immunol Methods. 1992;149(2):247–253. doi: 10.1016/0022-1759(92)90256-s. [DOI] [PubMed] [Google Scholar]
- Takalkar AM, Klibanov AL, Rychak JJ, Lindner JR, Ley K. Binding and Detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Release. 2004;96:473–482. doi: 10.1016/j.jconrel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Tempelman LA, Hammer DA. Receptor-mediated binding of IgE-sensitized rat basophilic leukemia cells to antigen-coated substrates under hydrodynamic flow. Biophys J. 1994;66(4):1231–1243. doi: 10.1016/S0006-3495(94)80907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempelman LA, Park S, Hammer DA. Motion of Model Leukocytes near a Wall in Simple Shear Flow. Biotechnol Prog. 1994;10(1):97–108. doi: 10.1021/bp00025a012. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11(Supplement 2):S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Torzeren A, Ley K. How do selectins mediate leukocyte rolling in venules? Biophys Jl. 1992;62(3):700–709. doi: 10.1016/S0006-3495(92)81660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurushita N, Fu H, Melrose J, Berg EL. Epitope mapping of mouse monoclonal antibody EP-5C7 which neutralizes both human E- and P-selectin. Biochem Biophys Res Commun. 1998;242(1):197–201. doi: 10.1006/bbrc.1997.7942. [DOI] [PubMed] [Google Scholar]
- Ushiyama S, Laue T, Moore K, Erickson H, McEver R. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993;268(20):15229–15237. [PubMed] [Google Scholar]
- Winn R, Vedder N, Ramamoorthy C, Sharar S, Harlan J. Endothelial and leukocyte adhesion molecules in inflammation and disease. Blood Coagul Fibrinolysis Suppl. 1998;2:S17–23. [PubMed] [Google Scholar]
- Zou X, Shinde Patil VR, Dagia NM, Smith LA, Wargo MJ, Interliggi KA, Lloyd CM, Tees DFJ, Walcheck B, Lawrence MB, et al. PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. Am J Physiol Cell Physiol. 2005;289(2):C415–424. doi: 10.1152/ajpcell.00289.2004. [DOI] [PubMed] [Google Scholar]