Abstract
The detection of gene mutations in patients with congenitally missing teeth is not very complicated however proving causality is often quite difficult. Here we report the detection of a substitution mutation, A42P, within the prodomain of bone morphogenetic protein-4 (BMP4) in a small family with tooth agenesis and describe a functional alteration that may be responsible for the tooth phenotype. Since BMP4 is essential for the development of teeth but also for many other organs, it would be of considerable interest to find a BMP4 mutation that is only associated with tooth agenesis. Our in vitro investigations revealed that the A42P mutation did neither affect processing and secretion of BMP4 nor alter functional properties like the induction of alkaline phosphatase or signaling through Smad1/5/8 phosphorylation by the mature BMP4 ligand. However immunofluorescent staining revealed that the prodomains of BMP4 which harbor the A42P substitution, form fibrillar structures around transfected cells in culture and that this fibrillar network is significantly decreased when mutant prodomains are expressed. Our finding suggests that in vivo, BMP4 prodomain behavior might also be altered by the mutation and could influence storage or transport of mature BMP4 in the extracellular matrix of the developing tooth.
Keywords: Tooth agenesis, BMP4 prodomain mutation, functional analysis
Tooth agenesis is the most common congenital malformation in humans with a prevalence ranging from 1.6 to 9.6% excluding third molars (1, 2). It can also occur as part of a syndrome. Although tooth agenesis can be caused by environmental factors such as infection (3), trauma, toxin or radiation, the main cause is believed to be genetic, however, for the majority of tooth agenesis families the causative gene(s) is still unknown.
Genes that have been associated with human non-syndromic tooth agenesis include WNT10A (4, 5), AXIN2 (6), EDA pathway genes (7), MSX1 and PAX9 (8). In mice, tooth development is arrested at the bud stage in the absence of either Msx1 (9) or Pax9 (10) transcription factors, both of which have been described as upstream effectors of bone morphogenetic protein 4 (BMP4) expression in dental mesenchyme. BMP4, a member of the transforming growth factor-β (TGF-β) superfamily, first detected in the presumptive dental epithelium and then shifts to the dental mesenchyme where it plays a critical role in mediating epithelial-mesenchymal interactions during early tooth development (11) driving bud to cap stage transition and formation of the enamel knot signaling center. This role of BMP4 and its functional connection with two well established tooth agenesis genes makes it an ideal candidate gene for tooth agenesis however so far no tooth agenesis causing BMP4 mutations have been described although several BMP4 mutations were found in patients with microphthalmia, polydactyly, kidney cysts and orofacial clefts (12, 13).
BMP4 protein is synthesized as a large precursor which dimerizes and undergoes one to two proteolytic cleavages to release the C-terminal active ligand as well as the N-terminal prodomains (14). The cleavages are carried out by FURIN and/or other members of the proprotein convertase (PC) family (15). The initial main BMP4 cleavage occurs in the trans-Golgi network (TGN) at a multibasic motif (-RXKR-, termed the S1 site) to generate the active C-terminal ligand which stays noncovalently associated with the N-terminal prodomains. A second cleavage can occur at an upstream motif (-RXXR-, the S2 site) in a tissue-specific manner. Mice carrying a point mutation that prevents S2 processing show severe loss of BMP4 activity in some tissues, such as testes and germ cells (16).
Upon secretion of the processed BMP4-prodomain complex, the prodomains can bind to fibrillins in the extracellular matrix (17, 18) presumably for storage or transport purposes. In analogy to the role of the similarly structured TGF-β prodomains, the Bmp4 prodomains have also been suggested to function as a shield between ligand and receptor (19). Although the function of the BMP4 prodomain is not quite clear yet, mutations mapped to this area were found to be associated with congenital anomalies of the kidney and urinary track (20).
Here we report our findings of a novel BMP4 prodomain missense mutation (c.124 G > C; Ala42Pro) in a small family where the mother and daughter presented with missing premolars. We analyzed the effects of this mutation on the processing and secretion of proBMP4 as well as on functional properties of mature BMP4 in order to document any difference between wild type and mutant that could reasonably play a role in the causation of the observed dental phenotype.
Material and Methods
Sample collections and mutation analysis
Ninety unrelated Caucasian people with congenital tooth agenesis consented to participate in this IRB approved study and donated cheek swab or saliva samples for DNA extraction. The exons and adjacent intron regions of Msx1, Msx2, Pax9, Axin2, Wnt10a, Lef1, Bmp4 and EDA pathway genes were PCR amplified and sequenced. Sequence traces were visually inspected for heterozygosity and searched against the reference sequence database for mismatches using BLAST (NCBI). For every SNP, allele frequencies were compared between the experimental group and the normal Caucasian control populations in the NCBI SNP database and in the NHLBI Exome sequencing database.
In silico analysis of the BMP4 mutation
The potential impact of the A42P mutation on BMP4 function was evaluated by the PolyPhen, (Polymorphism Phenotyping; http://genetics.bwh.harvard.edu/pph/) program and the SIFT (Sorting Intolerant from Tolerant; (http://sift.bii.a-star.edu.sg/) program. Both programs make predictions about the impact of amino acid substitutions on protein function based on sequence homologies, physical properties of amino acids, interruption of local structural and functional motives or overall structure and function.
Construction of expression plasmids and site-directed mutagenesis
Since human and mouse BMP4 protein sequences share over 97% identity, mouse BMP4 cDNA was used for cloning into the mammalian expression vector pcDNA3. The identified mutation was introduced into the BMP4 cDNA by a site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Sequence encoding the FLAG tag was inserted in-frame in the N-terminal side of BMP4 prodomain (SHAS[FLAG]LIPE) while sequence encoding the HA tag was inserted in-frame in the N-terminal side of mature BMP4 (-RAKRSPKH[HA]HPQR-). The specific sites of tag insertion were described previously (21–23). The primers used are available on request. All constructs were sequenced entirely to confirm the correct reading frame and point mutation.
Cell culture, Transient transfection and Western blot analysis
Cos-7 cells, a mesenchymal cell line and C2C12 cells which can be specifically used for testing Bmp2/4 activity, were obtained from the American Type Culture Collection (ATCC). Both cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Cellgro, Manassas, VA, USA). Plasmids of both wild-type and mutant BMP4 were transfected into cells using FuGENE6 Transfection Reagent (Roche Diagnosis, Mannheim, Germany). The empty expression vector was used as a control. Seventy-two hours after transfection, culture media were collected and the cells were lysed in immunoprecipitation assay buffer (50 mM Tris-Cl, PH 8, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS) with protease inhibitors. The lysate was cleared by centrifugation and protein content was quantified by Pierce BCA Protein Assay (Thermo Scientific, Rockford, IL, USA). The same amount of protein (20 μg) was loaded for wild-type and mutant. Proteins were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Blots were immunolabeled with mouse monoclonal anti-HA (Covance; 1:2000) and then followed by secondary labeling with HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Dallas, TX, USA; 1:1000) and ECL Chemiluminescent Detection (Thermo Scientific, Rockford, IL, USA).
Alkaline phosphatase assay
In response to BMP treatments, C2C12 mouse myoblast cells display an increase in alkaline phosphatase (24). To determine the function of the mutant BMP4, C2C12 cells were plated in a 12-well plate in DMEM supplemented with 10% fetal bovine serum. Cells were allowed to grow for 12–18 h, and then transfected with wild-type and mutant BMP4 constructs (without tag). Empty vector was used as control. Seventy-two hours after transfection, alkaline phosphatase activity was analyzed according to the manufacturer’s instruction using the Alkaline Phosphatase Colorimetric Assay Kit (ab83369, Abcam, Cambridge, MA, USA). All experiments were carried out in triplicate and repeated three times. Two-sample t tests were employed to evaluate the difference.
Detection of the phosphorylated Smad1/5/8 complex
C2C12 cells were transfected with either wild-type or mutant BMP4 constructs (without tag) using FuGENE6 Transfection Reagent (Roche Diagnosis), with the empty vector as control. Total cell lysates were harvested 72 h after transfection and Western-blot analyses were performed to analyze the levels of phosphorylated Smad1/5/8 complex (Cell Signaling Technology, Danvers, MA, USA, 1:1000). Beta-actin was used as a loading control. Quantification was carried out using Image J software and normalized by Beta-actin signal.
Immunofluorescence microscopy
Different amounts of wild-type and mutant BMP4 expression plasmids with a FLAG tag in the prodomain were transfected into Cos-7 cells. Cells were allowed to grow to confluence (72 hrs after transfection). The cells were then fixed with 100% methanol for 10 min at room temperature, permeabilized with 0.1% Triton X-100 in PBS for 3 min and blocked overnight with 1% goat serum + 0.05% NaN3 in PBS. Cell samples were incubated with mouse anti-Flag antibodies (Stratagene, La Jolla, CA, USA) at room temperature for 1 h, followed by incubation with Alexa 555-conjugated goat anti-mouse IgG antibody (Invitrogen, Eugene, OR, USA) for 1 h. The nuclei were counterstained with DAPI. Immunofluorescently stained cells were examined under a Nikon Eclipse TE2000-U fluorescence microscope (Nikon, Tokyo, Japan) and a Leica TCS SP5-II upright microscope equipped with confocal fixed stage system (Leica DM 6000 CFS, Wetzlar, Germany). Normal mouse IgG was used as negative control. The experiment was repeated 3 times.
Results
The A42P mutation
Among all the genes we tested, only a single BMP4 missense mutation was identified in one of the 90 tooth agenesis patients. This mutation resulted in an alanine to proline substitution at codon 42 (A42P) in the amino acid sequence (Fig. 1A). The same mutation was subsequently identified in the proband’s affected mother. The mother has only maxillary missing premolars while both maxillary and mandibular second premolars are missing in the daughter. But both of them have only tooth phenotype without any other phenotypic manifestations. The father was unaffected and had no mutation. Other family members were not available for further analysis of a genotype-phenotype correlation. The A42P mutation is located in a highly conserved region of the prodomain of mammalian BMP4 protein (Fig. 1B) and had not been reported at the time of detection. The mutation was scored at 0.523 (“possibly damaging”) using the PolyPhen program or 0.33 (“tolerated”) using the SIFT program. Predictably, any BMP4 mutation associated with such a mild phenotype cannot be expected to be severely disruptive of BMP4 structure and function.
Fig. 1.
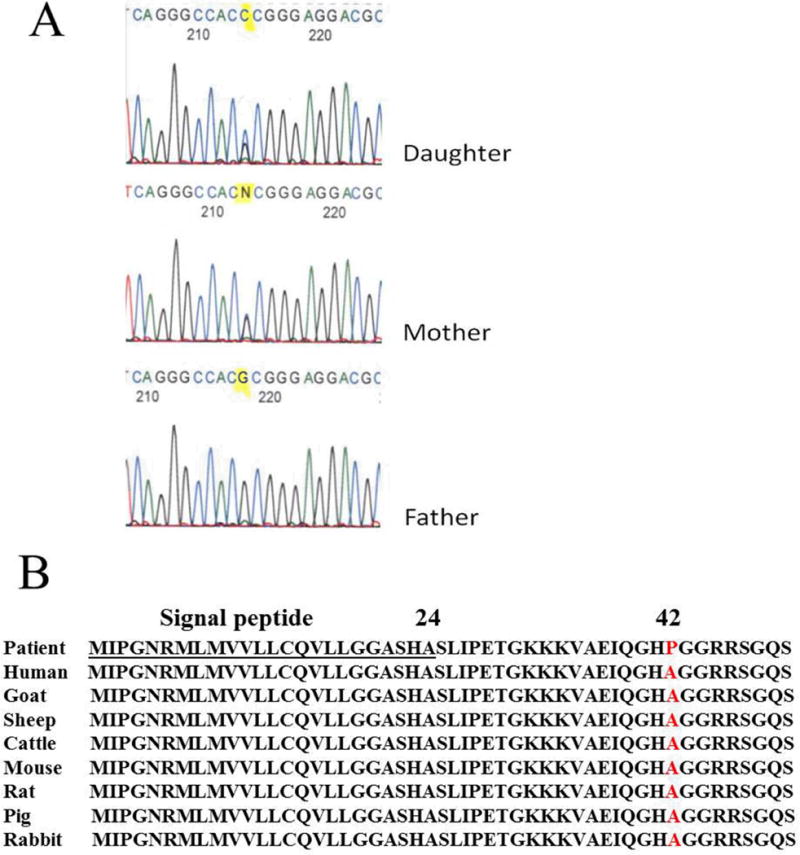
BMP4 (G to C) mutation identified in a nuclear family. (A) Sequencing results showing the normal father (bottom) and the mutated alleles in mother and daughter. (B) A line-up of BMP4 sequences of patients and different species. Note that the mutation is located in a highly conserved region of the prodomain of the mammalian BMP4 protein (red).
Effect of A42P on BMP4 processing and secretion
To determine whether the A42P mutation would affect the BMP4 processing and secretion, the wild-type and mutant BMP4 expression constructs with HA tag were transfected into Cos-7 cells. Western-blot analysis with an HA antibody showed that both wild-type and mutant BMP4 have approximately the same amount of proBMP4 as well as mature BMP4 in cell lysates and in the culture media (Fig. 2A).
Fig. 2.
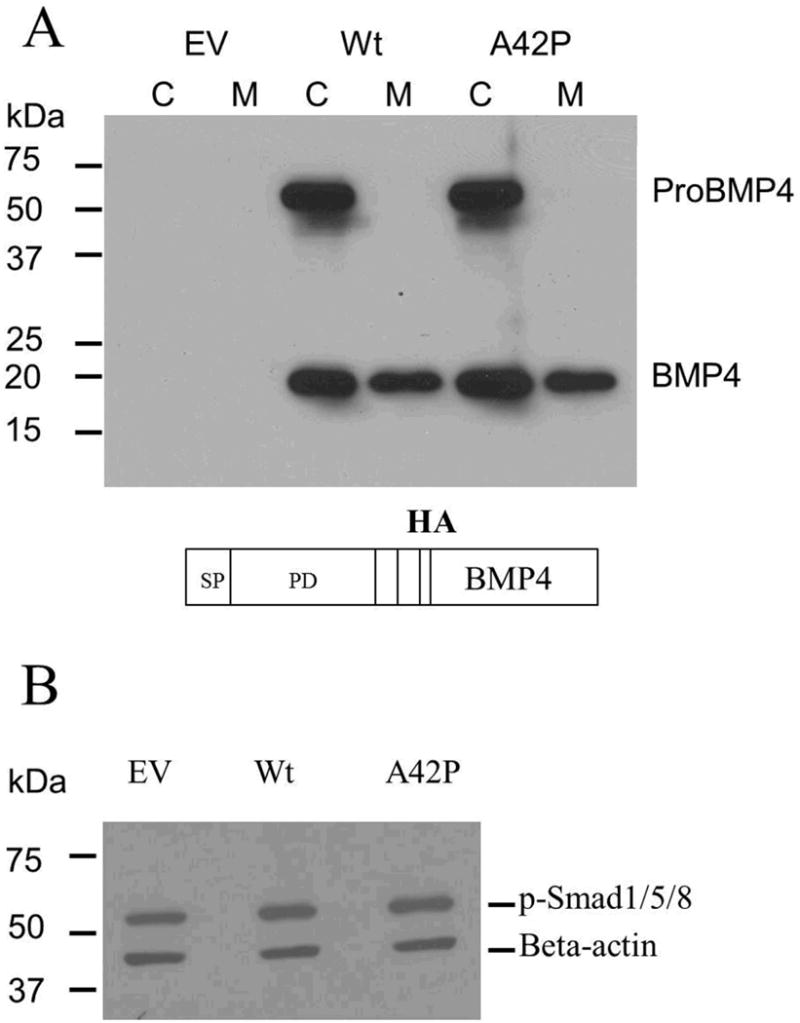
Effect of the A42P mutation on proBMP4 processing and secretion, as well as signaling transduction. (A) Western-blot analysis with anti-HA showed that there was no apparent difference in ProBMP4 and BMP4 levels in cell lysates and in the medium from Cos-7 cells transfected with HA-tagged wild-type or mutant BMP4 expression constructs. A schematic representation of the construct used in this experiment was shown at the bottom. (B) Western-blot analysis showed that wild-type and mutant BMP4 induced a comparable increase in the levels of phospho-Smad 1/5/8 in the C2C12 cell lysates. Empty vector of pcDNA3 was used as control and beta-actin was used as a loading control. Quantification using Image J software showed 1.39 and 1.46 fold increases of phosphorylated Smad1/5/8 for wild-type and A42P, respectively, normalized by empty vector. C, cell lysate; M, culture medium; EV, empty vector; Wt, wild-type; SP, signal peptide; PD, prodomain; BMP4, mature, ligand. All experiments were repeated 3 times.
Effect of Ala42Pro on BMP4 function
Alkaline phosphatase activity analysis showed that C2C12 cells transfected with either wild-type or mutant BMP4 displayed an increase in alkaline phosphatase activity, compared with control (P ≤ 0.001). However, no significant difference was found between wild-type and mutant BMP4 (P > 0.05, data not shown). Consistently, Western-blot analysis showed that mutant BMP4 triggered the phosphorylation of Smad1/5/8, the direct downstream targets of BMP4 signaling, to the same extent as the wild-type BMP4 (Fig. 2B). These observations indicate that the function of the mature BMP4 ligand was not affected by the prodomain mutation.
Immunolocalization of BMP4 prodomains
Constructs expressing Flag-tagged wild-type or mutant BMP4 were transfected into Cos-7 cells at different dosage (1.2 μg/ml, 0.8 μg/ml, 0.4 μg/ml). Immunofluorescent staining revealed a fibrillar distribution of BMP4 prodomains in the extracellular space around cells transfected with wild-type BMP4, suggesting that BMP4 prodomains can form or bind extracellular fibrillar structures. A similar, but less abundant fibrillar prodomain network was observed around cells transfected with mutant BMP4 plasmid DNA at a concentration of 0.4 μg/ml (Fig. 3). At higher concentrations of plasmid DNA (1.2 μg/ml, 0.8 μg/ml), the fibrillar prodomain staining showed no apparent difference between wild-type and the mutant. These findings suggest that the A42P mutation may have a subtle effect on BMP4 prodomain attachment, storage or transport in the extracellular matrix (ECM), possibly in a dose dependent manner.
Fig. 3.
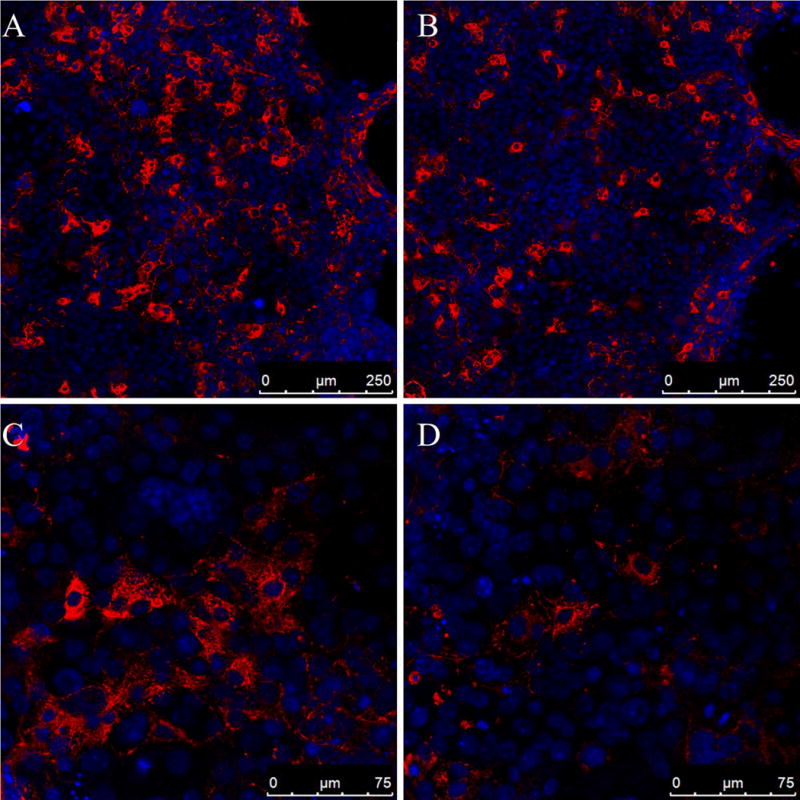
Immunofluorescence staining showed that the fibrillar distribution of BMP4 prodomains was affected by the A42P mutation at a low amount of transfected plasmid DNA (0.4 μg/ml). Constructs expressing Flag-tagged wild-type or mutant BMP4 were transfected into Cos-7 cells. Three days later, immunofluorescent staining was performed to detect proBMP4 or BMP4 prodomain with anti-FLAG antibodies; DAPI was used to stain nuclei. Confocal images revealed less fibrillar BMP4 prodomain structure around cells transfected with the A42P mutant (B, D) compared to the wild-type (A, C) in three independent experiments.
Discussion
It has been known for two decades that BMP4 plays a key role in the signaling interactions between tooth bud epithelium and mesenchyme during tooth morphogenesis (11); however, tooth agenesis-causing BMP4 mutations have never been reported. Because of its universal function in embryonic development, mutations located in the Bmp4 ligand itself cause quite severe phenotypes (12) and are not likely to be found in selective tooth agenesis. In this study we provided evidence that mutations in the BMP4 prodomain may result in or contribute to such less severe hypodontia phenotypes.
Expression constructs with an HA tag inserted in the mature BMP4 region enabled us to track the mature BMP4 ligand and a FLAG tag in the prodomain region made it possible to detect the prodomain fragments while both tags mark the full length BMP4 protein, proBMP4. The A42P mutation neither affected the processing and secretion of BMP4 nor the functionality of the mature BMP4 ligand. Although both wild-type and mutant BMP4 prodomains can form a fibrillar structure once they are secreted from the cells, this fibrillar structure is less abundant in the mutant samples at a low concentration of transfected plasmid DNA suggesting that the distribution of BMP4 prodomains in the ECM in vivo may also be affected by the A42P mutation in a dose dependent manner.
The function of BMP4 prodomains has been largely uncharacterized until recently when it was reported that BMP4 prodomains have a high affinity to the N-terminal region of Fibrillin-1, a major component of the extracellular matrix (18). It would be interesting to investigate if BMP4 prodomains also interact with some of the more tooth bud specific ECM proteins. This indicates that BMP4 prodomains may have the function of attaching to the microfibrils in the ECM for storage or transport of the mature BMP4 dimers. In addition, a recent structural study about the role of prodomains in members of the Tgf-beta family has revealed that the prodomains play a role in preventing premature receptor ligand interaction (19).
Different tissues have different sensitivity to BMP4 dosage. Tissues such as testes and germ cells are more sensitive to BMP4 dosage while other tissues like the limb, dorsal vertebrae and kidney may not be affected by subtle loss of BMP4 activity (17). The developing tooth organ is also sensitive to BMP4 dosage (25); therefore, the A42P caused reduction in storage capability of mature BMP4 in the ECM could be the reason for the tooth agenesis phenotype.
Rare genetic variants are much more likely to have phenotypic consequences than the more common polymorphisms (26) however proving the functional significance of a rare mutation is not an easy task especially when extended family data are not available. When we first discovered the A42P mutation in this tooth agenesis family of Turkish ancestry, it was a new mutation and only several months later A42P appeared in the NHLBI Exome SNP database with an allele frequency of about 0.05% in Caucasians and 0 in African Americans. Since tooth agenesis is quite common and no phenotype data are available for the genotypes represented in the NHLBI SNP database there was no reason to assume that the A42P is a completely neutral mutation. The location of A42P in the N-terminal α1 helix of the prodomain, an area which has been shown to participate in the formation of a “straitjacket” shielding the TGF-beta ligand from receptor recognition (19) and the amino acid change from alanine to proline which is likely to introduce a conformational change in the protein structure, suggests that A42P could contribute to the observed tooth agenesis phenotype.
Acknowledgments
The authors are indebted to the individuals that participated in this study. We would like to thank Alexandre R. Vieira (Department of Oral Biology, School of Dental Medicine, University of Pittsburgh) and his collaborators, Figen Seymen (Pedodontics Department, Istanbul University), Asli Patir (Pedodontics Department, Medipol Istanbul University) and Mine Yildirim (Pedodontics Department, Istanbul University) for providing DNA samples and clinical phenotypes. This work was supported by grants from the National Institute for Dental and Craniofacial Research (R01 DE 019471-01 to RDS and GM; ARRA Supplements: R01 DE 019471-0S1209 to RDS and R01 DE 0194710-01S309 to RDS, GM and ARV). Yanyu Huang was supported by The China Scholarship Council.
Footnotes
Conflict of interest – The authors report no conflict of interest
References
- 1.Vastardis H. The genetics of human tooth agenesis: new discoveries for understanding dental anomalies. Am J Orthod Dentofacial Orthop. 2000;117:650–656. [PubMed] [Google Scholar]
- 2.Matalova E, Fleischmannova J, Sharpe PT, Tucker AS. Tooth Agenesis: from Molecular Genetics to Molecular Dentistry. J Dent Res. 2008;87:617–623. doi: 10.1177/154405910808700715. [DOI] [PubMed] [Google Scholar]
- 3.Gullikson JS. Tooth morphology in rubella syndrome children. ASDC J Dent Child. 1975;42:479–482. [PubMed] [Google Scholar]
- 4.van den Boogaard MJ, Creton M, Bronkhorst Y, van der Hout A, Hennekam E, Lindhout D, Cune M, Ploos van Amstel HK. Mutations in WNT10A are present in more than half of isolated hypodontia cases. J Med Genet. 2012;49:327–331. doi: 10.1136/jmedgenet-2012-100750. [DOI] [PubMed] [Google Scholar]
- 5.Kantaputra P, Sripathomsawat W. WNT10A and isolated hypodontia. Am J Med Genet A. 2011;155A:1119–1122. doi: 10.1002/ajmg.a.33840. [DOI] [PubMed] [Google Scholar]
- 6.Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mues GI, Griggs R, Hartung AJ, Whelan G, Best LG, Srivastava AK, D’Souza R. From ectodermal dysplasia to selective tooth agenesis. Am J Med Genet A. 2009;149A:2037–2041. doi: 10.1002/ajmg.a.32801. [DOI] [PubMed] [Google Scholar]
- 8.Kapadia H, Mues G, D’Souza R. Genes affecting tooth morphogenesis. Orthodont Craniofac Res. 2007;10:237–244. doi: 10.1111/j.1601-6343.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- 10.Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 12.Reis LM, Tyler RC, Schilter KF, Abdul-Rahman O, Innis JW, Kozel BA, Schneider AS, Bardakjian TM, Lose EJ, Martin DM, Broeckel U, Semina EV. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet. 2011;130:495–504. doi: 10.1007/s00439-011-0968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki S, Marazita ML, Cooper ME, Miwa N, Hing A, Jugessur A, Natsume N, Shimozato K, Ohbayashi N, Suzuki Y, Niimi T, Minami K, Yamamoto M, Altannamar TJ, Erkhembaatar T, Furukawa H, Daack-Hirsch S, L’Heureux J, Brandon CA, Weinberg SM, Neiswanger K, Deleyiannis FW, de Salamanca JE, Vieira AR, Lidral AC, Martin JF, Murray JC. Mutations in BMP4 are associated with subepithelial, microform, and overt cleft lip. Am J Hum Genet. 2009;84:406–411. doi: 10.1016/j.ajhg.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995;210:670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y, Jean F, Thomas G, Christian JL. BMP4 is proteolytically activated bu furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998;17:4735–4743. doi: 10.1093/emboj/17.16.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, Christian JL. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 2006;133:1933–1942. doi: 10.1242/dev.02368. [DOI] [PubMed] [Google Scholar]
- 17.Sopory S, Nelsen SM, Degnin C, Wong C, Christian JL. Regulation of Bone Morphogenetic Protein-4 Activity by Sequence Elements within the Prodomain. J Biol Chem. 2006;281:34021–34031. doi: 10.1074/jbc.M605330200. [DOI] [PubMed] [Google Scholar]
- 18.Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of Bone Morphogenetic Protein Growth Factor Complexes to Fibrillin. J Biol Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabatabaeifar M, Schlingmann KP, Litwin M, Emre S, Bakkaloglu A, Mehls O, Antignac C, Schaefer F, Weber S, Group ET. Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr Nephrol. 2009;24:2361–2368. doi: 10.1007/s00467-009-1287-6. [DOI] [PubMed] [Google Scholar]
- 21.Constam DB, Robertson EJ. Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol. 1999;144:139–149. doi: 10.1083/jcb.144.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Y, Hackenmiller R, Berg L, Jean F, Nakayama T, Thomas G, Christian JL. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev. 2001;15:2797–2802. doi: 10.1101/gad.940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem. 2009;284:27157–27166. doi: 10.1074/jbc.M109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Selever J, Murali D, Sun X, Brugger SM, Ma L, Schwartz RJ, Maxson R, Furuta Y, Martin JF. Threshold-specific requirements for Bmp4 in mandibular development. Dev Biol. 2005;283:282–293. doi: 10.1016/j.ydbio.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Q, Ge D, Maia JM, Zhu M, Petrovski S, Dickson SP, Heinzen EL, Shianna KV, Goldstein DB. A genome-wide comparison of the functional properties of rare and common genetic variants in humans. Am J Hum Genet. 2011;88:458–468. doi: 10.1016/j.ajhg.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


