Abstract
Tumor necrosis factor alpha (TNF-α) has been demonstrated to inhibit steroidogenesis in Leydig cells at the transcriptional level of steroidogenic enzymes. However, the molecular mechanism of this observed gene repression is not well understood. We now demonstrate that nuclear factor κB (NF-κB) activated by TNF-α inhibits the transactivation of orphan nuclear receptors, which regulate the expression of steroidogenic-enzyme genes. TNF-α treatment suppressed the luteinizing-hormone-induced or Nur77/SF-1-stimulated promoter activity of steroidogenic-enzyme genes in Leydig cells. The TNF-α-mediated gene suppression was blocked by treatment with an inhibitor of NF-κB. In addition, overexpression of the p65 (RelA) subunit of NF-κB showed the same effect as TNF-α and inhibited Nur77 transactivation, suggesting the involvement of NF-κB activation in the observed gene repression. Physical association of Nur77 with p65 was revealed by mammalian two-hybrid, GST pull-down, and coimmunoprecipitation analyses. The NF-κB inhibition of Nur77 transactivation was likely due to the competition of p65 for Nur77 binding with coactivators. Finally, chromatin immunoprecipitation assays revealed that TNF-α treatment caused the recruitment of NF-κB to the promoter of the steroidogenic-enzyme P450c17 gene, supporting the hypothesis that the TNF-α-mediated gene repression involves NF-κB inhibition of the transcriptional activity of Nur77 and other orphan nuclear receptors. These findings provide a molecular mechanism underlying the inhibition of testicular steroidogenesis by proinflammatory cytokines.
It has been suggested that proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 1 (IL-1), have an inhibitory role in gonadal functions, particularly in the steroidogenesis of Leydig cells. Elevated TNF-α and IL-1 levels have been observed in human patients with critical illness, burns, and sepsis (5, 6, 10) who experience depressed gonadal functions with low serum testosterone levels (54, 58). Furthermore, administration of TNF-α to healthy men and rats causes a decrease in serum testosterone levels (37, 52), while treatment with TNF-α or IL-1 causes an inhibition of steroidogenesis in cultured Leydig cells (reviewed in reference 18).
Steroidogenesis in Leydig cells is initiated with cholesterol transfer into the mitochondria, which is mediated by the steroidogenic acute regulatory (StAR) protein. In the mitochondria, cholesterol is converted to pregnenolone by the cholesterol side chain cleavage enzyme (P450scc). Pregnenolone is then converted sequentially to progesterone by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD), to 17α-hydoxyprogesterone and then to androstenedione by 17α-hydroxylase/C17-20 lyase (P450c17), and finally to testosterone by 17β-hydroxyateroid dehydrogenase. Steroidogenesis in Leydig cells is primarily regulated by the pituitary gonadotropin luteinizing hormone (LH) through the production of the intracellular second messenger cyclic AMP (cAMP). cAMP stimulates steroidogenesis by increasing the expression of steroidogenic-enzyme genes (reviewed in reference 43).
Proinflammatory cytokines, such as TNF-α, IL-1, and IL-6, inhibit testicular Leydig cell steroidogenesis at the level of gene expression of different steroidogenic enzymes (reviewed in reference 18). For example, TNF-α decreases the expression of P450scc, P450c17, and 3β-HSD in primary cultures of mouse Leydig cells (59, 60) and decreases the expression of StAR in primary cultures of porcine Leydig cells (34). However, the molecular mechanisms of gene repression by proinflammatory cytokines are not well understood.
The expression of steroidogenic-enzyme genes is mainly regulated at the transcriptional level. Previous studies have suggested that the orphan nuclear receptor Nur77, as well as SF-1, regulates the expression of steroidogenic-enzyme genes (38, 42, 46, 61). Nur77, which is also known as NGFI-B, TR3, and NAK-1, is a member of the Nur77 gene family, which contains the orphan nuclear transcription factors Nurr-1 and NOR-1. These factors are highly homologous in the zinc finger DNA-binding domain, moderately homologous in the ligand-binding domain, and somewhat divergent in the N terminus (12). The Nur77 family members regulate the steroidogenic-enzyme genes encoding steroid 21α-hydroxylase, P450c17, and 20α-hydroxysteroid dehydrogenase (51, 57, 62). A Nur77-binding site within the rat P450c17 gene promoter has been identified (62). Although Nur77 coregulators are not well identified, recent studies have shown that steroid receptor coactivator 1 (SRC-1) family gene products and SMRT (silencing mediator of retinoid and thyroid receptors) regulate the transactivation of Nur77 through direct protein-protein interactions (32, 48).
TNF-α and IL-1 are known to activate nuclear factor κB (NF-κB). NF-κB is a pivotal transcription factor that governs the expression of early-response genes involved in numerous cellular responses to a wide range of signals. The major form of NF-κB is a heterodimer of the p65 (RelA) and p50 subunits. In the inactivated state, NF-κB is sequestered in the cytoplasm through its association with the inhibitor protein IκB. Activation of the NF-κB signaling cascade results in the phosphorylation and subsequent degradation of IκB, which allows the translocation of NF-κB to the nucleus (8). Previous studies have shown that NF-κB interacts with other proteins. While NF-κB transactivation function is modulated by proteins such as C/EBP, SRC-1, glucocorticoid receptor, and retinoic acid X receptor (35, 39, 40, 50), NF-κB is able to repress the activities of other transcription factors, such as steroid receptors (20, 35, 36, 41).
In the present study, we investigate the molecular mechanism by which TNF-α inhibits steroidogenesis in testicular Leydig cells. Using mice and Leydig cell lines, we demonstrate that TNF-α exerts its inhibitory action on steroidogenesis at least through activated NF-κB, which inhibits the transactivation of the orphan nuclear receptors Nur77 and SF-1. Such an inhibitory effect is mediated in part by the competition of the p65 subunit of NF-κB for Nur77 binding with its coactivators. These findings provide a mechanistic explanation for the long-standing observation that proinflammatory cytokines suppress the expression of testicular steroidogenic enzymes.
MATERIALS AND METHODS
Animals.
FVB mice were purchased from a commercial supplier (Daehan Laboratories, Daejon, Korea). Ten-week-old male mice were injected intraperitoneally with recombinant mouse TNF-α (Pierce Biotechnology, Inc.) at 50 μg/kg of body weight for 6 h. The animals were kept in a cage with water and chow available and were maintained under controlled conditions (12-h light and dark photoperiods; 50% humidity; 22°C). The ethical treatment of animals was carried out according to National Institutes of Health standards.
Plasmids.
The mammalian expression vectors of p65, p50, and IκB and the glutathione S-transferase (GST)-p65, GST-p50, and Gal4-p65 constructs were described previously (39). The mammalian expression vector of SF-1 and the reporter SFRE-Luc were previously described (22). Mammalian expression constructs of Nur77, Nurr-1, NOR-1, and Nur77ΔAF2 and the reporter plasmids NurRE-Luc and NBRE-tk-Luc were previously described (48, 49). GST-Nur77 and Gal4-Nur77 were constructed by cloning the full-length cDNA of Nur77 into pGEX-4T-2 and pCMX-Gal4N, respectively. VP16-Nur77 full-length and deletion mutants were constructed by cloning the full-length cDNA of Nur77 or its deletion mutants into pCMX-VP16-N. GST-Nur77N and GST-Nur77C constructs were obtained by cloning the N-terminal (amino acids 1 to 254) and C-terminal (amino acids 255 to 597) regions of Nur77 into pGEX-4T-1. Mouse StAR(−2200/+3)-Luc and StAR cDNA as a probe were previously described (26). The rat P450c17-luciferase expression constructs P450c17(−1561/+1)-Luc, TK32-Luc, WT(−447/-399)P450c17-Luc, and Mut(−447/-339Δ2)P450c17-Luc were previously described (14, 62). Mouse 3β-HSD(−4700/+40)-Luc and 3β-HSD cDNA were kindly donated by A. H. Payne (Stanford University). Rat P450c17 cDNA and P450scc for probes were amplified from a rat testis cDNA library by PCR using forward (5′-GGCATAGAGACAACTACCAC-3′; nucleotides 946 to 965) and reverse (5′-AAACCTCTGCAGTAGCAAGG-3′; nucleotides 1408 to 1428) primers for P450c17 (GenBank accession number M31681) and forward (5′-CCACTTCCTGAGGGAGAACG-3′; nucleotides 203 to 222) and reverse (5′-TTCCAGCTCTGCAATCCGCC-3′; nucleotides 1405 to 1424) primers for P450scc (GenBank accession number J05156).
Cell culture.
R2C cells were maintained in F10 medium supplemented with 15% horse serum and 2.5% fetal bovine serum, and K28 cells were maintained in Dulbecco's minimum essential medium supplemented with 15% fetal bovine serum. HeLa and CV-1 cells were maintained in Dulbecco's minimum essential medium supplemented with 10% fetal bovine serum. All cells were cultured at 37°C in 5% CO2.
Transient-transfection assays.
Transfections were carried out using the Effectene (Qiagen) transfection reagent for HeLa and CV-1 cells and the Lipofectamine Plus (Invitrogen) transfection reagent for K28 and R2C cells, according to the manufacturers' specifications. Cells were plated in 24-well plates and transfected with the appropriate amounts of expression plasmids, a reporter plasmid, and the control lacZ expression plasmid pCMVβ (Clontech). The total amount of DNA was kept constant by adding appropriate amounts of pcDNA3 empty vector. Typically, for 24-well plates, 200 and 400 ng of DNA per well were used for Effectene and Lipofectamine Plus transfection reagents, respectively. The cells were treated with 10 ng of TNF-α (Endogen)/ml for 24 h after 24 h following transfection. The cells were treated with trichostatin A (TSA) for 20 h before being harvested. The cells were lysed 42 to 48 h after transfection using lysis buffer containing 0.1% Triton X-100 and 0.2 M Tris-HCl (pH 8.0). Luciferase and β-galactosidase activities were assayed as described previously (27). The levels of luciferase activity were normalized to the lacZ expression. Transfections were done in duplicate for each construct, and all values represent the mean ± standard deviation of at least three independent transfection experiments.
Northern blot analysis.
Total RNA was prepared from testes and R2C cells using Tri Reagent (Molecular Research Center, Inc.). Twenty micrograms of total RNA was separated on a 1.2% denaturing agarose gel, transferred onto Zeta-probe nylon membrane (Bio-Rad), and immobilized by UV cross-linking. The membrane was hybridized with a random-primed 32P-labeled StAR, P450scc, P450c17, or 3β-HSD cDNA probe as described previously (27). The membrane was reprobed for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a loading control.
GST pull-down assay.
GST, GST-p65, GST-Nur77, GST-Nur77N(AF-1), and GST-Nur77C(DBD/LBD/AF2) fusion proteins were expressed in Escherichia coli BL21 cells and isolated with glutathione-Sepharose-4B beads (Amersham Bioscience AB). The immobilized GST fusion proteins were then incubated with [35S]methionine-labeled proteins produced by in vitro translation using the TNT-coupled transcription-translation system (Promega). The binding reactions were carried out in 250 μl of GST binding buffer (20 mM Tris-HCl at pH 7.9, 150 mM NaCl, 10% glycerol, 0.05% NP-40, 5 mM MgCl2, 0.5 mM EDTA, 1 mM dithiothreitol [DTT], and 1.5% bovine serum albumin) for 4 h at 4°C. The beads were washed three times with 1 ml of GST binding buffer and one time with phosphate-buffered saline containing protease inhibitors. Bound proteins were eluted by adding 20 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and analyzed by SDS-PAGE and autoradiography (27).
Coimmunoprecipitation.
In vivo coimmunoprecipitation assays were performed with rat Leydig R2C cells. The exponentially growing R2C cells were cultured in F10 culture medium for 1, 3, or 6 h in the presence of 10 ng of TNF-α/ml. The cells were harvested with RIPA cell lysis buffer (50 mM Tris-HCl at pH 7.5, 50 mM NaCl, 1 mM EGTA, 1% Triton X-100, 50 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 1 μg of aprotinin/ml, 0.1 μg of leupeptin/ml, 1 μg of pepstatin/ml, 0.1 mM phenylmethylsulfonyl fluoride, and 1 mM DTT). The whole-cell lysate (200 μg) was incubated with 8 μg of anti-Nur77 (Santa Cruz Biotechnology) or anti-SF-1 antibody (a kind gift from K. Morohashi, National Institute for Basic Biology, Okazaki, Japan) for 4 h at 4°C and incubated for another 4 h after the addition of 20 μl of a protein A-agarose bead slurry (Invitrogen). The agarose beads were then washed four times with RIPA buffer at 4°C. Bound proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Sigma Chemical Co.). The blots were analyzed using anti-Nur77, anti-p65, anti-SRC-1 (Santa Cruz Biotechnology), anti-HDAC4, anti-HDAC1 (Zymed Laboratories Inc.), and anti-SF-1 antibodies and then detected with an ECL kit (Amersham Pharmacia) (48).
ChIP assay.
Testes from adult male mice injected with TNF-α were dissected out, chopped, and cross-linked with 1% formaldehyde for 15 min at room temperature on a rotating platform. After 15 min, the cross-linking reaction was stopped by the addition of glycine to a final concentration of 0.125 M for 5 min at room temperature (56). R2C cells were treated with 10 ng of TNF-α/ml for 3, 6, or 12 h or untreated and cross-linked with 1% formaldehyde. After incubation of the samples with TSE I (100 mM Tris-HCl at pH 9.4 and 10 mM DTT) for 20 min at 30°C, the cells were washed and processed for chromatin immunoprecipitation (ChIP) assays as previously described (47). Anti-Nur77, anti-SF-1, anti-p65, anti-p50 (Santa Cruz Biotechnology), or anti-SRC-1 antibody was used for immunoprecipitation. Immunoprecipitated DNA and input sheared DNA were subjected to PCR using either rat P450c17 primer pairs (sense, 5′-GATCTGAATGGCTCCTATGC-3′, and antisense, 5′-ATCCTCCCAGAGGCAAATGC-3′), which amplify a 253-bp region (−533 to −281) spanning the Nur77/SF-1 binding site of the rat P450c17 gene promoter, or mouse P450c17 primer pairs (sense, 5′-TTTCAGGGGCCAGAAGGTG-3′, and antisense, 5′-TCCTCCCAGAGGCAAATGC-3′), which amplify a 541-bp region (−837 to −296) spanning putative Nur77 and SF-1 binding sites of the mouse P450c17 gene promoter. As a negative control, PCRs were done using actin primer pairs (sense, 5′-GAGACCTTCAACACCCCAGCC-3′, and antisense, 5′-CCGTCAGGCAGCTCATAGCTC-3′) which amplify a 362-bp region spanning exon 4 of the β-actin gene.
RIA.
Testosterone and progesterone concentrations were measured by radioimmunoassay (RIA). Dissected testes were homogenized in phosphate-buffered saline (0.01 M; pH 7.2), and steroid was extracted three times with 3 volumes of diethyl ether. The exponentially growing R2C cells seeded onto a 24-well culture plate (8 × 104/well) were cultured for 24 h in F10 medium supplemented with 15% charcoal-stripped fetal bovine serum. Culture medium was collected for RIA at the appropriate time points after changing to fresh medium containing 10 ng of TNF-α/ml. The culture media were assayed directly without further purification. Each treatment group contained duplicate cultures, and each experiment was repeated at least twice. Assay procedures were followed as previously described (1, 24), using labeled testosterone ([1,2,6,7-3H]testosterone; 96 Ci/mol) and progesterone ([1,2,6,7-3H]progesterone; 96 Ci/mol) obtained from NEN.
RESULTS
TNF-α inhibits the expression of steroidogenic enzyme genes in R2C Leydig cells and mouse testis.
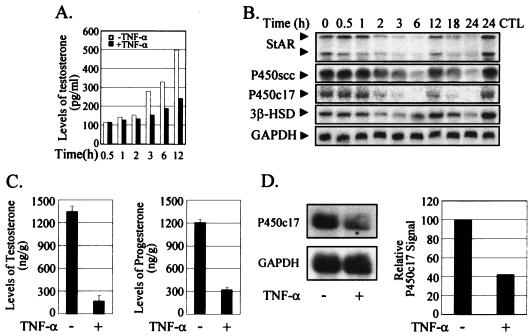
TNF-α has been shown to inhibit steroidogenesis in primary Leydig cells at the level of steroidogenic-enzyme gene expression (18). We confirmed this observed inhibition in the R2C rat Leydig cell line, which is constitutively steroidogenic (44). The testosterone level of the cultured medium became lower than that of the control after 1 to 2 h of treatment with TNF-α and reached only ∼50% of the control level after 12 h of treatment (Fig. 1A). This inhibition of testosterone biosynthesis was accompanied by decreased transcript levels of steroidogenic enzymes, such as StAR, P450scc, P450c17, and 3β-HSD (Fig. 1B). The transcript levels went down after 2 h of treatment with TNF-α but recovered almost fully at ∼12 h of treatment. Interestingly, the transcript levels decreased again with a longer TNF-α treatment, indicating a biphasic pattern of regulation. There was little change in the transcript levels of steroidogenic enzymes in the non-TNF-α-treated control cells through the time course of the experiment (data not shown). The biphasic effect of TNF-α on NF-κB activation, at the levels of both NF-κB nuclear translocation-DNA-binding and transactivation function, has been reported (15, 19, 25), although the biological significance of the biphasic profile of NF-κB activation remains poorly understood.
FIG. 1.
Inhibition of the expression of steroidogenic-enzyme genes by TNF-α in R2C Leydig cells and mouse testis. (A) TNF-α inhibited the biosynthesis of testosterone in R2C cells. Exponentially growing R2C cells were exposed to fresh medium containing 10 ng of TNF-α/ml, and the culture medium was collected at the indicated times for the measurement of testosterone levels by RIA. −, without; +, with. (B) TNF-α suppressed the expression of steroidogenic-enzyme genes in R2C cells. R2C cells were collected at the indicated times after treatment with 10 ng of TNF-α/ml, and total RNAs were prepared for Northern blot analysis. The expression of GAPDH was used as an internal control. CTL, control without the treatment of TNF-α. (C) TNF-α inhibited testicular steroidogenesis in mice. Adult male mice were injected intraperitoneally with TNF-α at 50 μg/kg for 6 h, and the testes were dissected out for measurement of testosterone and progesterone levels by RIA. (D) TNF-α suppressed expression of the P450c17 gene in mouse testis. Total RNAs were prepared from the testes of TNF-α-treated mice (panel C) for Northern blot analysis. The P450c17 mRNA signal was quantified and normalized by the GAPDH level in each sample. The error bars indicate standard deviations.
We also confirmed the TNF-α inhibition of testicular steroidogenesis and steroidogenic-enzyme expression in animals. Six hours after the administration of TNF-α to adult mice, testicular testosterone and progesterone levels were reduced to ∼10 and 25% of the untreated-control levels, respectively (Fig. 1C). This TNF-α inhibition of steroidogenesis was also accompanied by a decreased transcript level of P450c17 in the testis, as in R2C Leydig cells (Fig. 1D).
TNF-α suppresses the promoter activity of steroidogenic-enzyme genes.
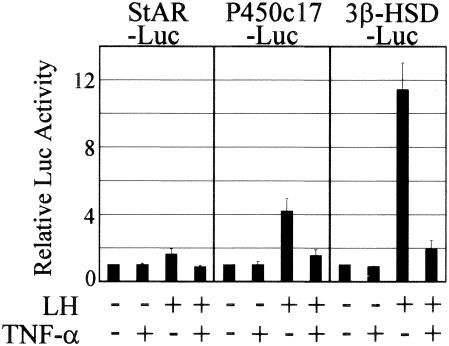
We then tested the effect of TNF-α on the transcription of steroidogenic-enzyme genes by transiently transfecting K28 mouse Leydig cells, which were transfected with reasonable efficiency, with StAR-Luc, P450c17-Luc, or 3β-HSD-Luc, a luciferase reporter construct containing a promoter of the steroidogenic-enzyme genes. The LH-induced activation of the promoters was suppressed almost to basal levels by TNF-α treatment (Fig. 2). These results suggest that TNF-α signaling represses the promoter activity of steroidogenic-enzyme genes, resulting in the reduction of their transcript levels.
FIG. 2.
Suppression of promoter activities of steroidogenic-enzyme genes by TNF-α. TNF-α inhibited the LH-induced promoter activities of StAR, P450c17, and β-HSD genes. K28 cells were transiently transfected with the indicated luciferase reporter genes and treated (+) with 100 ng of LH/ml and 10 ng of TNF-α/ml for 24 h. The error bars indicate standard deviations.
TNF-α suppresses steroidogenic-enzyme gene promoters activated by orphan nuclear receptors.
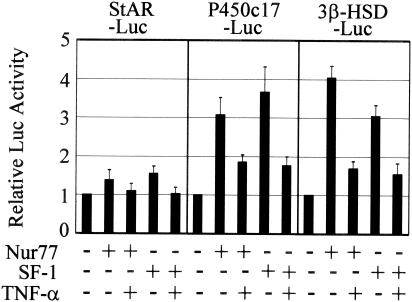
Previous studies have shown that the gene expression of steroidogenic enzymes in Leydig cells is regulated by orphan nuclear receptors, such as SF-1 and Nur77 (38, 42, 46, 61). To investigate whether TNF-α signaling affects the promoter activation of steroidogenic-enzyme genes by orphan nuclear receptors, K28 Leydig cells were cotransfected with Nur77 or SF-1 expression plasmid, along with each of the reporter constructs, StAR-Luc, P450c17-Luc, and 3β-HSD-Luc, and treated with TNF-α or untreated (Fig. 3). The overexpression of Nur77 or SF-1 increased promoter activity from the P450c17-Luc and 3β-HSD-Luc constructs and only slightly increased activity from the StAR-Luc construct. TNF-α treatment suppressed the activation of the steroidogenic-enzyme gene promoters by the orphan nuclear receptors Nur77 and SF-1. These results suggest that TNF-α signaling may affect the transcriptional activities of Nur77 and SF-1 on steroidogenic-enzyme gene promoters in Leydig cells.
FIG. 3.
TNF-α suppression of promoter activities of steroidogenic-enzyme genes. K28 Leydig cells were cotransfected with (+) 20 ng of Nur77 or SF-1 expression plasmid along with 140 ng of the indicated reporter construct, StAR-Luc, P450c17-Luc, or 3β-HSD-Luc. The cells were then treated with 10 ng of TNF-α/ml for 24 h or left untreated, and luciferase activity was measured. The error bars indicate standard deviations.
NF-κB is involved in the TNF-α-mediated suppression of steroidogenic enzyme gene promoters activated by orphan nuclear receptors.
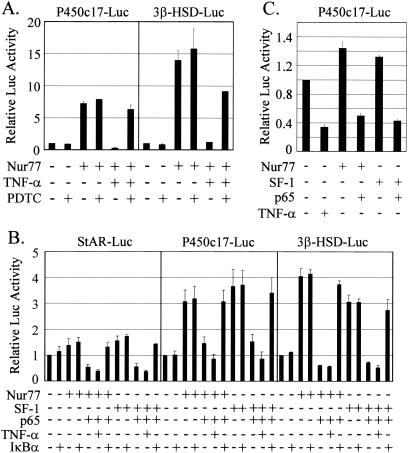
TNF-α activates the transcription factor NF-κB, its major downstream effector. Furthermore, it was previously demonstrated that the p65 subunit of NF-κB interacts with SF-1 protein and inhibits SF-1 transactivation to regulate gene expression (22). Thus, we first investigated the involvement of NF-κB in the TNF-α-mediated suppression of steroidogenic enzyme gene promoters using pyrrolidine dithiocarbamate (PDTC), an antioxidant that has been widely used as an NF-κB inhibitor (4). PDTC treatment efficiently blocked the TNF-α downregulation of the Nur77-stimulated P450c17 and 3β-HSD gene promoters (Fig. 4A). Furthermore, coexpression of the p65 subunit with the orphan nuclear receptor Nur77 or SF-1 in K28 Leydig cells reduced luciferase activity from the StAR, 3β-HSD, and P450c17-Luc reporters to below or near basal levels (Fig. 4B). Concurrent treatment of TNF-α with the coexpression of p65 caused a further inhibition of the promoter activities. The p65-mediated gene repressions were almost fully recovered by coexpression of IκB, the inhibitor of NF-κB. The same inhibitions of P450c17 gene promoter activity by TNF-α and the p65 subunit were observed in R2C cells (Fig. 4C). In R2C cells, overexpression of Nur77 or SF-1 further activated the P450c17 gene promoter to a small extent, which was probably due to the constant high activation of the promoter in the constitutively steroidogenic R2C cells. Together, these results suggest a role for the p65 subunit of NF-κB in the TNF-α-mediated suppression of Nur77- and SF-1-stimulated transcriptional activities of the steroidogenic-enzyme gene promoters.
FIG. 4.
Involvement of NF-κB in the TNF-α-mediated suppression of steroidogenic-enzyme gene promoters. (A) Inhibition of TNF-α-mediated transcriptional repression by PDTC. HeLa cells were transiently transfected with (+) 10 ng of Nur77 expression vector and 70 ng of the indicated reporter and treated with 10 ng of TNF-α/ml and/or 100 μg of PDTC/ml, an inhibitor of NF-κB, for 24 h, and luciferase activity was measured. (B) Effect of the p65 subunit of NF-κB on Nur77/SF-1-mediated transcription. K28 Leydig cells were cotransfected with the indicated combination of expression vectors (20 ng of Nur77 and SF-1 and 100 ng of p65 and IκB), together with 140 ng of the indicated reporter. The cells were then treated with 10 ng of TNF-α/ml or left untreated. (C) Effects of TNF-α and the p65 subunit of NF-κB on the P450c17 gene promoter in R2C cells. R2C Leydig cells were cotransfected with the indicated combinations of expression vectors (100 ng of Nur77 and SF-1 and 100 ng of p65), together with 150 ng of the indicated reporter. The cells were treated with 10 ng of TNF-α/ml or left untreated. The error bars indicate standard deviations.
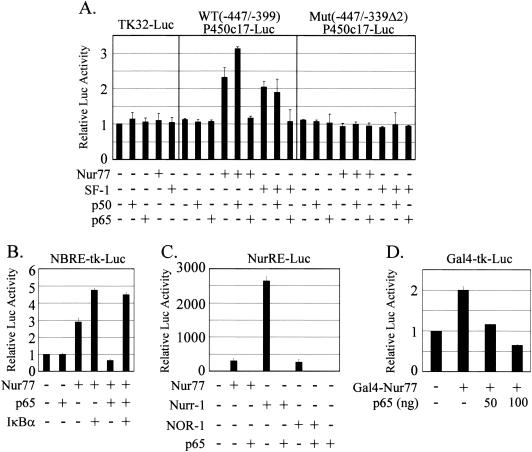
The p65 subunit of NF-κB functionally interacts with Nur77 protein.
A Nur77/SF-1 binding site has been defined in the rat P450c17 promoter (62). To examine whether the p65 subunit of NF-κB functionally interacts with Nur77/SF-1 protein on the P450c17 promoter, the Nur77 or SF-1 expression plasmid was cotransfected into K28 Leydig cells with or without the p65 expression vector and a reporter plasmid containing the defined promoter, TK32-Luc—WT(−447/-399)P450c17-Luc, containing the Nur77/SF-1 binding site upstream of TK32-Luc, or Mut(−447/-339Δ2)P450c17-Luc, in which the Nur77/SF-1 site was mutated (Fig. 5A). Expression of the control reporter TK32-Luc was unaffected by the coexpression of Nur77 or SF-1, as well as the p50 or p65 subunit. However, the expression of WT(−447/-399)P450c17-Luc was stimulated by the coexpression of either Nur77 or SF-1, as expected. Coexpression of p65 suppressed the Nur77- or SF-1-stimulated promoter activity to basal levels. On the other hand, coexpression of the p50 subunit of NF-κB did not inhibit the Nur77/SF-1-stimulated promoter activity. Rather, it appeared to increase the Nur77-stimulated activity but not the SF-1-stimulated activity. Although p50 homodimers were shown to act as repressors, there are several reports that have suggested that in certain cases the p50 homodimer can transactivate gene expression, especially after interaction with particular nuclear proteins (7, 11, 13, 21). Expression of either the p65 or p50 subunit alone did not affect the expression of WT(−447/-399)P450c17-Luc. The promoter activation by Nur77/SF-1 and suppression by p65 were not observed with the Mut(−447/-339Δ2)P450c17-Luc reporter (62).
FIG. 5.
Functional interaction of the p65 subunit of NF-κB with Nur77 protein. (A) Involvement of the Nur77/SF-1 binding site of the P450c17 gene promoter in p65-mediated downregulation. K28 Leydig cells were cotransfected with (+) 20 ng of the Nur77 or SF-1 expression plasmid, together with 100 ng of p65 and/or p50 expression vector. The reporter plasmids containing a defined promoter, TK-32-Luc, WT(−447/−339)P450c17-Luc, and Mut(−447/−339Δ2)P450c17-Luc, were tested. (B) Functional interaction of p65 with Nur77. HeLa cells were cotransfected with the Nur77 expression plasmid and the reporter NBRE-tk-Luc containing the Nur77 (NGFI-B) binding sequence of the POMC gene, together with p65 and/or IκB expression vector. (C) Functional interaction of p65 with other Nur77 family proteins. HeLa cells were cotransfected with 10 ng of the Nur77, Nurr-1, or NOR-1 expression plasmid and 70 ng of the reporter NurRE-Luc containing the consensus sequence of Nur77 binding elements with or without 100 ng of p65 expression vector. (D) Interaction of p65 with the Gal4-Nur77 protein. CV-1 cells were cotransfected with 10 ng of Gal4-fused Nur77 expression plasmid and 70 ng of the reporter Gal4-tk-Luc, along with or without p65 expression vector. The error bars indicate standard deviations.
To confirm the functional interaction between Nur77 and the p65 subunit of NF-κB, HeLa cells were cotransfected with the Nur77 and p65 expression plasmid, along with the reporter NBRE-tk-Luc containing the Nur77 (NGFI-B) binding sequence of the POMC gene (Fig. 5B). As expected, the coexpression of p65 inhibited the transactivation of Nur77, which was efficiently reversed by coexpression of IκB, the inhibitor of NF-κB. Together, these results suggest that the p65 subunit of NF-κB activated by TNF-α suppresses Nur77/SF-1 transactivation on the promoters of steroidogenic-enzyme genes, probably through protein-protein interaction, thus inhibiting gene expression.
Because Nur77 family genes show structural similarity and exert functional redundancy (9, 12), we also examined the p65-mediated transactivation inhibition with Nurr-1 and NOR-1, members of the Nur77 gene family (33, 49) (Fig. 5C). Nurr-1 and NOR-1, as well as Nur77, activated the expression of the reporter NurRE-Luc containing the consensus sequence of Nur77 binding elements, but the activation by Nurr-1 was much greater than the activation by Nur77 or NOR-1. P65 completely inhibited the activation of Nur77-, Nurr-1-, and NOR-1-stimulated gene transcription.
To confirm that the p65-mediated gene suppression worked through interactions with the Nur77 protein rather than through a direct interaction with DNA, Nur77 was tethered to DNA by using a Gal4-fused Nur77 and the reporter Gal4-tk-Luc. The Gal4-Nur77 fusion protein induced the expression of the Gal4 luciferase reporter construct by approximately twofold, and p65 coexpression inhibited reporter expression (Fig. 5D), indicating the functional interaction of p65 with Nur77.
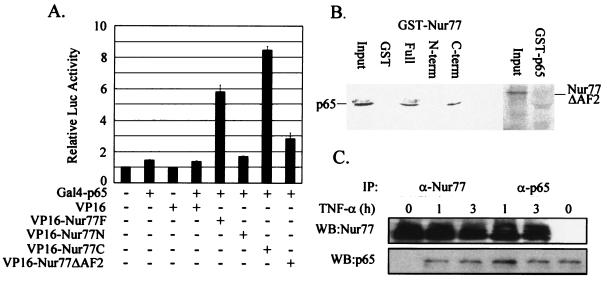
Nur77 directly interacts with the p65 subunit of NF-κB through its C-terminal region in vivo.
To verify that the functional interaction between p65 and Nur77 involves their physical association, we performed mammalian two-hybrid analysis using p65 fused to the Gal4 DNA-binding domain (Gal4-p65) and Nur77 fused to the VP16 activation domain (VP16-Nur77F) and using Gal4-tk-Luc as a luciferase reporter (Fig. 6A). Gal4-p65 itself weakly induced luciferase expression but strongly induced reporter expression with the coexpression of VP16-Nur77F, indicating a physical association between p65 and full-length Nur77. The Nur77 domain responsible for its interaction with p65 was investigated by the transfection of Nur77 deletion mutants fused to the VP16 activation domain together with Gal4-p65 (Fig. 6A). The C terminus of Nur77 (amino acids 297 to 597) (VP16-Nur77C) induced luciferase activity to a level comparable to that induced by the full-length Nur77 (VP16-Nur77F), whereas the N terminus of Nur77 (amino acids 1 to 296) (VP16-Nur77N) and the AF-2 domain deletion mutant (amino acids 586 to 597) (VP16-Nur77ΔAF2) did not.
FIG. 6.
Direct interaction of Nur77 with the p65 subunit of NF-κB through its C-terminal region in vivo. (A) Nur77 association with p65. CV-1 cells were cotransfected with 10 ng of Gal4-p65 and 100 ng of VP16-Nur77 full-length or deletion mutant expression plasmids, along with 70 ng of the reporter Gal4-tk-Luc. Luciferase activity was measured 48 h after transfection. (B) Nur77 directly interacted with p65 through its C-terminal region in GST pull-down assays. [35S]methionine-labeled p65 produced by in vitro translation was allowed to bind the GST fusion protein of Nur77 (Full) or its deletion mutants (N-term and C-term) (left), while [35S]methionine-labeled Nur77ΔAF2 was allowed to bind the GST fusion protein of p65 (right). (C) Nur77 association with p65 upon TNF-α treatment in vivo. R2C cells were treated with TNF-α and were harvested at the indicated times for coimmunoprecipitation (IP) experiments using anti-Nur77 (α-Nur77) and anti-p65 (α-p65) antibodies. WB, Western blotting. The error bars indicate standard deviations.
The direct physical interaction between p65 and Nur77 was assessed by GST pull-down analysis. [35S]methionine-labeled p65 produced by in vitro translation was incubated with the GST fusion protein of Nur77 (full length) or its deletion mutants, and [35S]methionine-labeled Nur77ΔAF2 was incubated with the GST fusion protein of p65 (Fig. 6B). p65 interacted with both full-length GST-Nur77 and its C terminus but did not interact with the GST-Nur77 N terminus, while GST-p65 did not interact with Nur77ΔAF2. These results are consistent with the luciferase data from the mammalian two-hybrid analysis (Fig. 6A) and together suggest that Nur77 directly interacts with p65 through the C-terminal region of Nur77. The data further suggest that the AF2 domain is especially important for the interaction.
To determine whether endogenous Nur77 protein interacts with p65 in vivo, coimmunoprecipitation experiments were carried out using R2C cells treated with TNF-α or untreated (Fig. 6C). Whole-cell extracts were prepared after 1- and 3-h treatments with TNF-α, and immunoprecipitations were carried out using anti-Nur77 and anti-p65 antibodies. Western blot analysis of the immunoprecipitated material revealed that Nur77 was physically associated with p65 in vivo but only when the cells were treated with TNF-α.
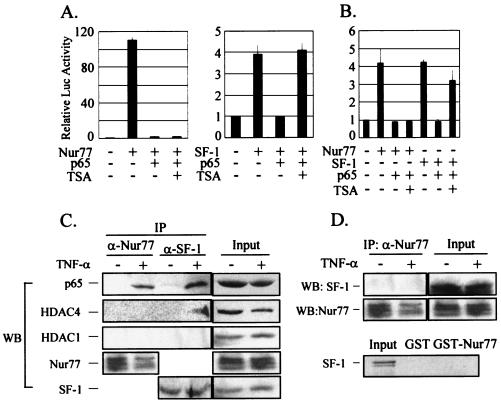
HDAC activity is not involved in NF-κB repression of Nur77 transactivation.
To explore how the p65 subunit of NF-κB affects Nur77 function, we first assessed the involvement of histone deacetylases (HDACs) using the HDAC inhibitor TSA. In HeLa cells, the transactivation of Nur77 was inhibited by p65 coexpression, but the repressed Nur77 activity was not recovered by TSA treatment (Fig. 7A). On the other hand, the SF-1 activity repressed by p65 was recovered by TSA treatment, as previously reported (22). We confirmed the TSA insensitivity of the NF-κB repression of Nur77 transactivation with a native steroidogenic-enzyme P450c17 gene promoter. As shown in Fig. 7B, the repression of Nur77 transactivation by p65 coexpression was not affected by TSA treatment, whereas the repressed SF-1 activity was recovered to ∼80%. These results suggest that HDACs are not involved in the NF-κB repression of Nur77 transactivation in the steroidogenic-enzyme gene promoters, whereas they probably are involved in the NF-κB repression of SF-1 transactivation.
FIG. 7.
NF-κB repression of Nur77 transactivation in an HDAC-independent manner. (A) HDAC activity was not involved in the p65 repression of Nur77 transactivation. HeLa cells were transfected with (+) 10 ng of Nur77 and/or 100 ng of p65 expression vector, together with 70 ng of a NurRE-Luc reporter construct (left), or were transfected with 10 ng of SF-1 and/or 100 ng of p65 expression vector, together with 70 ng of a SFRE-Luc reporter (right). After 24 h of transfection, the cells were treated with 100 nM TSA for 24 h or left untreated. (B) HDAC activity was not involved in the p65 repression of Nur77-mediated transcription of the P450c17 gene promoter. K28 Leydig cells were cotransfected with the indicated combinations of expression vectors (20 ng of Nur77 and SF-1 and 100 ng of p65), together with 140 ng of P450c17 gene promoter-Luc reporter. (C) Nur77 did not recruit HDACs in response to TNF-α in vivo. R2C cells were treated with TNF-α for 6 h and were harvested for coimmunoprecipitation (IP) experiments using anti-Nur77 and anti-SF-1 antibodies. Western blot (WB) analyses of the immunoprecipitates were performed using anti-p65 (α-p65), anti-HDAC4, anti-HDAC1, anti-Nur77, and anti-SF-1 antibodies. (D) SF-1 did not interact with Nur77. R2C cells were treated with TNF-α for 6 h and were harvested for coimmunoprecipitation experiments using anti-Nur77 antibody. (Top) Western blots were analyzed with anti-SF-1 and anti-Nur77 antibodies. (Bottom) [35S]methionine-labeled SF-1 produced by in vitro translation was allowed to bind the GST fusion protein of Nur77. The error bars indicate standard deviations.
To verify further that NF-κB represses Nur77 transactivation in an HDAC-independent manner, we carried out coimmunoprecipitation experiments using R2C cells treated with TNF-α or untreated (Fig. 7C). Whole-cell extracts were prepared after a 6-h treatment with TNF-α, and immunoprecipitations were carried out using anti-Nur77 and anti-SF-1 antibodies. Western blot analysis of the immunoprecipitated material revealed that, in response to TNF-α, the Nur77-p65 complex did not recruit HDACs, whereas the SF-1-p65 complex recruited HDAC4 but not HDAC1, as previously reported (22).
Because Nur77 and SF-1 have been shown to bind and act through the same site in the P450c17 gene promoter, we investigated the possibility that they interact with each other to heterodimerize. Coimmunoprecipitation and GST pull-down experiments showed that Nur77 and SF-1 interacted neither in vivo nor in vitro (Fig. 7D).
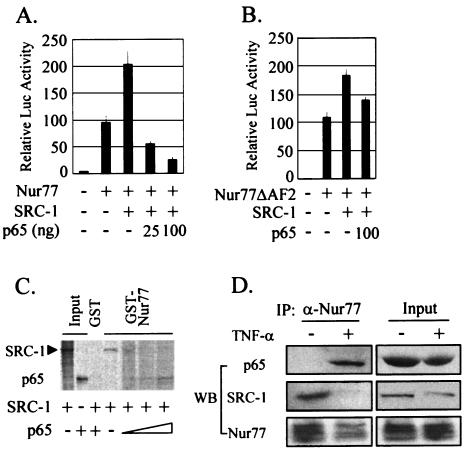
The p65 subunit of NF-κB competes with SRC-1, a Nur77 coactivator, for Nur77 binding.
HDAC-independent repression of Nur77 transactivation by NF-κB then let us test any alteration of Nur77 DNA-binding activity as an alternative mechanism. Electrophoretic mobility shift assays were done with whole-cell lysates from R2C cells treated with TNF-α, or untreated, using an NBRE oligomer duplex as a probe. The results revealed no difference between the DNA-binding activities of Nur77 in the two extracts (data not shown).
Because the p65 suppression of Nur77 transactivation included neither recruitment of HDACs nor alteration of the Nur77 DNA-binding activity, we hypothesized that p65 might interfere with the interaction between Nur77 and its coactivators. To test this hypothesis, HeLa cells were cotransfected with Nur77 and SRC-1 expression plasmids along with or without p65 (Fig. 8A). SRC-1 has been shown to act as a Nur77 coactivator that binds to the AF-1 domain of Nur77 (32). As expected, the expression of SRC-1 with Nur77 induced Nur77 transcriptional activity and coexpression of p65 blocked SRC-1 enhancement of Nur77 transactivation. SRC-1 also increased the transcriptional activity of Nur77ΔAF2, which has the AF2 domain, which is important for the interaction between Nur77 and p65, deleted. The enhanced transactivation of Nur77ΔAF2 was not inhibited by p65 (Fig. 8B).
FIG. 8.
The p65 subunit of NF-κB competes with the Nur77 coactivator SRC-1 for Nur77 binding. (A) p65 coexpression blocked the SRC-1 enhancement of Nur77 transactivation. HeLa cells were cotransfected with (+) the indicated expression vectors (5 ng of Nur77, 25 ng of SRC-1, and 25 or 100 ng of p65) and 50 ng of the reporter NurRE-Luc. (B) Effect of SRC-1 on transactivation of Nur77ΔAF2, a deletion mutant of the AF2 domain. HeLa cells were cotransfected with the indicated expression vectors (5 ng of Nur77ΔAF2, 25 ng of SRC-1, and 100 ng of p65) and 50 ng of the reporter NurRE-Luc. (C) p65 interfered with the binding of the coactivator SRC-1 to Nur77 in GST pull-down assays. [35S]methionine-labeled SRC-1 was allowed to bind GST-Nur77 in the presence of increasing amounts of [35S]methionine-labeled p65. (D) p65 competed with SRC-1 for Nur77 binding in response to TNF-α in vivo. R2C cells were treated with TNF-α for 6 h and were harvested for coimmunoprecipitation (IP) experiments using anti-Nur77 antibody. Western blot (WB) analyses of the immunoprecipitates were performed using anti-p65 (α-p65), anti-SRC-1, and anti-Nur77 antibodies. The error bars indicate standard deviations.
Competition of SRC-1 with p65 for Nur77 binding was subsequently investigated by GST pull-down assays (Fig. 8C). [35S]methionine-labeled SRC-1 produced by in vitro translation was allowed to bind the GST fusion protein of Nur77 in the presence of increasing amounts of [35S]methionine-labeled p65. SRC-1 directly interacted with Nur77, as previously reported (55). The binding of SRC-1 to Nur77 decreased as the binding of p65 to Nur77 increased in a dose-dependent manner.
Competition of SRC-1 with p65 for Nur77 binding in vivo was also examined by coimmunoprecipitation experiments using R2C cells that were treated with TNF-α or untreated (Fig. 8D). Whole-cell extracts were prepared after 6 h of treatment with TNF-α, and immunoprecipitations were carried out using anti-Nur77 antibody. Western blot analysis of the immunoprecipitated material revealed that, in response to TNF-α, the p65 subunit of NF-κB was recruited to Nur77, while SRC-1 lost its association with Nur77. Together, these results suggest that p65 inhibits the transactivation of Nur77 through interference in coactivator binding to Nur77.
NF-κB is recruited by Nur77 to the p450c17 gene promoter upon TNF-α signaling, causing SRC-1 dissociation.
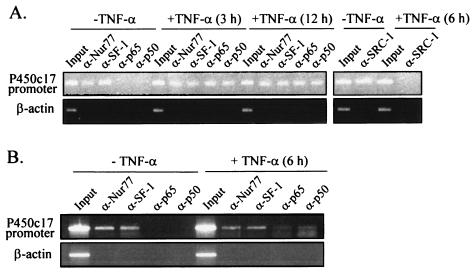
To examine whether TNF-α causes NF-κB recruitment onto and SRC-1 dissociation from the P450c17 gene promoter, we performed ChIP assays with R2C cells treated with TNF-α for 3, 6, and 12 h (Fig. 9A) or untreated. We observed that without TNF-α treatment, Nur77, but neither the p65 nor the p50 subunit of NF-κB, became associated with the P450c17 gene promoter. However, TNF-α treatment resulted in the recruitment of NF-κB to the P450c17 gene promoter, given that both p65 and p50 became associated with the endogenous P450c17 gene promoter. SF-1 showed behavior similar to that of Nur77 and was associated with the P450c17 gene promoter in the absence or presence of TNF-α. Meanwhile, SRC-1, which was associated with the P450c17 gene promoter in the absence of TNF-α, was dissociated by TNF-α treatment. No signal was detected from the control PCR for nonspecific immunoprecipitation using primers specific to the β-actin coding region.
FIG. 9.
NF-κB is recruited to the promoter of the p450c17 gene, along with the orphan nuclear receptors, upon TNF-α signaling in both R2C Leydig cells and mouse testis. (A) ChIP assays were done with R2C cells treated with (+) 10 ng of TNF-α/ml for the indicated times or left untreated. (B) ChIP assays were done with testes dissected from male mice that had been injected with TNF-α at 50 μg/kg for 6 h. Anti-Nur77, anti-SF-1, anti-p65, anti-p50, and anti-SRC-1 antibodies were used for immunoprecipitations. The immunoprecipitates were analyzed by PCR using a pair of specific primers spanning a region containing the Nur77/SF-1 binding site of the P450c17 gene promoters. A control PCR for nonspecific immunoprecipitation was done using primers specific for the β-actin coding region.
NF-κB recruitment onto the P450c17 gene promoter upon TNF-α signaling was also verified in animals. We performed ChIP assays with testes dissected from adult mice that had been administered TNF-α for 6 h and obtained results similar to those with R2C cells (Fig. 9B). Altogether, the results suggest that NF-κB, when activated by TNF-α signaling, is recruited to the P450c17 gene promoter through Nur77-DNA interactions, and this NF-κB(p65)-Nur77 interaction displaces a coactivator-Nur77 interaction, resulting in the downregulation of P450c17 expression.
DISCUSSION
Over the past several years, it has been proposed that Leydig cells and macrophages in the interstitial tissue of the testis are functionally associated (reviewed in reference 18). Macrophages within the testis have been shown to produce cytokines, such as TNF-α, in response to immune challenge or chronic inflammatory disease and thereafter inhibit steroidogenesis in Leydig cells (37). The inhibition of steroidogenesis by cytokines, such as IL-1 and TNF-α, has also been described in Leydig cell lines (17, 18, 39, 60). TNF-α has been shown to inhibit the LH- or cAMP-activated gene expression of steroidogenic enzymes at the transcriptional level (60). The present study also demonstrates a similar reduction of steroidogenic-enzyme transcripts by TNF-α in R2C rat Leydig cells and animals. Such TNF-α-mediated gene repression of steroidogenic enzymes is likely due to the influence of TNF-α signaling on promoter activity. Testosterone production, steroidogenic-enzyme mRNA expression, and transcriptional activation of steroidogenic-enzyme genes are repressed by TNF-α treatment.
The present study demonstrates that NF-κB activation by TNF-α is important to the repression of steroidogenic-enzyme genes in Leydig cells. An earlier work also reported that TNF-α-activated protein kinase C (PKC) represses the cAMP-stimulated steroidogenesis and expression of the P450c17 gene and that treatment with calphostin C, an inhibitor of PKC, overcomes the inhibitory effect of TNF-α (30). Because PKC has been shown to mediate the activation of NF-κB in many systems (2, 16, 23, 29, 53), it is possible that TNF-α-activated PKC also activates NF-κB in Leydig cells to downregulate the expression of steroidogenic-enzyme genes. However, we cannot rule out the possibility that TNF-α-activated PKC and NF-κB signaling pathways are independently involved in the inhibition of P450c17 gene expression by TNF-α.
Previous studies have shown that the orphan nuclear receptor SF-1 mediates the transcription of steroidogenic enzymes in Leydig cells (28, 61). Another orphan nuclear receptor, Nur77, has also been reported to regulate the transcription of the P450c17 gene in testicular Leydig cells (62). The present study shows that the promoters of StAR, 3β-HSD, and P450c17 genes are also activated by the expression of Nur77 and that these activations are similar in magnitude to activation by SF-1. These data therefore suggest the involvement of Nur77, as well as SF-1, in the transcriptional regulation of these genes in Leydig cells. Furthermore, the NF-κB-mediated suppression of steroidogenic-enzyme genes seems to be through both Nur77 and SF-1, although the downstream-acting modes are distinct. The NF-κB-mediated repression of Nur77 transactivation includes competition of the p65 subunit of NF-κB with Nur77 coactivators for Nur77 binding but not the recruitment of HDACs. On the other hand, the NF-κB-mediated repression of SF-1 transactivation includes the recruitment of HDACs, as previously reported with the MIS gene promoter (22). In the case of the P450c17 gene promoter, whether Nur77 or SF-1 binds to the defined Nur77/SF-1 binding site in vivo may depend on the developmental and physiological conditions of testicular Leydig cells. Alternatively, Nur77 and SF-1 may competitively bind to the site.
Although initial reports suggested that Nur77 binding and SF-1 binding were distinct (57), our data indicate that Nur77 and SF-1 bind to and elicit an activity on the same DNA element in the P450c17 gene promoter (38, 62). In addition, a recent study has shown that Nur77 and SF-1 bind to the same two binding sites within the promoter of the mouse GnRH receptor gene in vitro (45). These data suggest that Nur77 and SF-1 may share some binding elements in some genes while having distinct binding elements in others. Hence, it is possible that Nur77 binds to only a subset of SF-1 binding sites, and vice versa.
The coregulators of Nur77 are unclear. Recent studies have shown that SRC-1 family members activate the transactivation of Nur77 through protein-protein interaction with the N-terminal AF-1 region of Nur77 (32). In the present study, we demonstrate that the p65 subunit of NF-κB, which binds to the C-terminal region of Nur77, competes with SRC-1 for Nur77 binding and blocks the SRC-1 enhancement of Nur77 transactivation. Intramolecular interactions between the N- and C-terminal regions of Nur77 have been demonstrated previously (55). Thus, p65 binding to the C-terminal region of Nur77 may block the recruitment of SRC-1 to the N-terminal region. Similar observations have been reported for the androgen receptor (AR), which also exhibits N-C domain interactions. For example, SMRT, which binds to the ligand-binding domain of AR, competes with TIF2, which binds to the N-terminal region and the DNA-binding domain, to regulate the transcriptional activity of AR (3, 31).
In summary, we have demonstrated that TNF-α-activated NF-κB inhibits the transactivation of orphan nuclear receptors, Nur77 and SF-1, thereby resulting in the downregulation of steroidogenic-enzyme gene expression in Leydig cells. Such an inhibitory action is most likely mediated in two distinct ways, competition of the p65 subunit with the Nur77 coactivators for Nur77 binding and recruitment of HDACs by p65 that becomes associated with SF-1. These findings may provide the molecular mechanism underlying the inhibition of testicular steroidogenesis by proinflammatory cytokines, which are produced by macrophages within the testis in response to immune challenge or chronic inflammatory disease.
Acknowledgments
We thank A. H. Payne and K. Morohashi for providing 3β-HSD constructs and anti-SF-1 antibody, respectively.
This work was supported by a Korea Research Foundation Grant (KRF-2002-070-C0007). C. Y. Hong and J. H. Park were supported by a Brain Korea 21 Research Fellowship in 2003.
REFERENCES
- 1.Ahn, R. S., M. S. Yoo, and H. B. Kwon. 1999. Evidence for two-cell model of steroidogenesis in four species of amphibian. J. Exp. Zool. 284:91-99. [Google Scholar]
- 2.Bearz, A., G. Tell, A. Colombatti, S. Formisano, and C. Pucillo. 1998. Fibronectin binding promotes a PKC-dependent modulation of NF-kappa B in human T cells. Biochem. Biophys. Res. Commun. 243:732-737. [DOI] [PubMed] [Google Scholar]
- 3.Berrevoets, C. A., P. Doesburg, K. Steketee, J. Trapman, and A. O. Brinkmann. 1998. Functional interactions of the AF-2 activation domain core region of the human androgen receptor with the amino-terminal domain and with the transcriptional coactivator TIF2 (transcriptional intermediary factor 2). Mol. Endocrinol. 12:1172-1183. [DOI] [PubMed] [Google Scholar]
- 4.Bian, X., A. W. Opipari, Jr., A. B. Ratanaproeksa, A. E. Boitano, P. C. Lucas, and V. P. Castle. 2002. Constitutively active NFκB is required for the survival of S-type neuroblastoma. J. Biol. Chem. 277:42144-42150. [DOI] [PubMed] [Google Scholar]
- 5.Calandra, T., J. D. Baumgartner, G. E. Grau, M. M. Wu, P. H. Lambert, J. Schellekens, J. Verhoef, M. P. Glauser, et al. 1990. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. J. Infect. Dis. 161:982-987. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, J. G., R. G. Thompkins, J. A. Gelfand, H. R. Michie, G. G. Stanford, J. W. M. van der Meer, S. Endres, G. Lonnemann, J. Corsetti, B. Chernow, D. W. Wilmore, S. M. Wolff, J. F. Burke, and C. Dinarello. 1990. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 161:79-84. [DOI] [PubMed] [Google Scholar]
- 7.Cha-Molstad, H., A. Agrawal, D. Zhang, D. Samols, and I. Kushner. 2000. The Rel family member P50 mediates cytokine-induced C-reactive protein expression by a novel mechanism. J. Immunol. 165:4592-4597. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, L. E., F. K. Chan, D. Cado, and A. Winoto. 1997. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J. 16:1865-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damas, P., A. Reuter, P. Gysen, J. Demonty, M. Lamy, and P. Franchimont. 1989. Tumor necrosis factor and interleukin-1 serum levels during severe sepsis in humans. Crit. Care Med. 17:975-978. [DOI] [PubMed] [Google Scholar]
- 11.Dechend, R., F. Hirano, K. Lehmann, V. Heissmeyer, S. Ansieau, F. G. Wulczyn, C. Scheidereit, and A. Leutz. 1999. The Bcl-3 oncoprotein acts as a bridging factor between NF-κB/Rel and nuclear co-regulators. Oncogene 18:3316-3323. [DOI] [PubMed] [Google Scholar]
- 12.Enmark, E., and J. A. Gustafsson. 1996. Orphan nuclear receptors—the first eight years. Mol. Endocrinol. 10:1293-1307. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, T., G. P. Nolan, H. C. Liou, M. L. Scott, and D. Baltimore. 1993. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 7:1354-1363. [DOI] [PubMed] [Google Scholar]
- 14.Givens, C. R., P. Zhang, S. R. Bair, and S. H. Mellon. 1994. Transcriptional regulation of rat cytochrome P450c17 expression in mouse Leydig MA-10 and adrenal Y-1 cells: identification of a single protein that mediates both basal and cAMP-induced activities. DNA Cell Biol. 13:1087-1098. [DOI] [PubMed] [Google Scholar]
- 15.Haddad, J. J. 2002. Recombinant TNF-alpha mediated regulation of the I kappa B-alpha/NF-kappa B signaling pathway: evidence for the enhancement of pro- and anti-inflammatory cytokines in alveolar epithelial cells. Cytokine 17:301-310. [DOI] [PubMed] [Google Scholar]
- 16.Hah, N., and S. T. Lee. 2003. An absolute role of the PKC-dependent NF-κB activation for induction of MMP-9 in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 305:428-433. [DOI] [PubMed] [Google Scholar]
- 17.Hales, D. B. 1992. Interleukin-1 inhibits Leydig cell steroidogenesis primarily by decreasing 17 alpha-hydroxylase/C17-20 lyase cytochrome P450 expression. Endocrinology 131:2165-2172. [DOI] [PubMed] [Google Scholar]
- 18.Hales, D. B. 2002. Testicular macrophage modulation of Leydig cell steroidogenesis. J. Reprod. Immunol. 57:3-18. [DOI] [PubMed] [Google Scholar]
- 19.Han, Y., and A. R. Brasier. 1997. Mechanism for biphasic rel A. NF-κB1 nuclear translocation in tumor necrosis factor alpha-stimulated hepatocytes. J. Biol. Chem. 272:9825-9832. [DOI] [PubMed] [Google Scholar]
- 20.Heck, S., K. Bender, M. Kullmann, M. Gottlicher, P. Herrlich, and A. C. B. Cato. 1997. I κB alpha-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO J. 16:4698-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heissmeyer, V., D. Krappmann, F. G. Wulczyn, and C. Scheidereit. 1999. NF-κB p105 is a target of IκB kinases and controls signal induction of Bcl-3-p50 complexes. EMBO J. 18:4766-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong, C. Y., J. H. Park, K. H. Seo, J.-M. Kim, S. Y. Im, J. W. Lee, H.-S. Choi, and K. Lee. 2003. Expression of MIS in the testis is downregulated by TNF-α through the negative regulation of SF-1 transactivation by NF-κB. Mol. Cell. Biol. 23:6000-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, W. C., J. J. Chen, H. Inoue, and C. C. Chen. 2003. Tyrosine phosphorylation of I-κB kinase alpha/beta by protein kinase C-dependent c-Src activation is involved in TNF-alpha-induced cyclooxygenase-2 expression. J. Immunol. 170:4767-4775. [DOI] [PubMed] [Google Scholar]
- 24.Kwon, H. B., Y. K. Lim, M. J. Choi, and R. S. Ahn. 1989. Spontaneous mutation of follicular oocyte in Rana dybowskii in vitro: seasonal influences, progesterone production and involvement of cAMP. J. Exp. Zool. 252:190-199. [DOI] [PubMed] [Google Scholar]
- 25.Ladner, K. J., M. A. Caligiuri, and D. C. Guttridge. 2003. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J. Biol. Chem. 278:2294-2303. [DOI] [PubMed] [Google Scholar]
- 26.Lee, H.-K., M.-S. Yoo, H.-S. Choi, H.-B. Kwon, and J. Soh. 1999. Retinoic acids up-regulate steroidogenic acute regulatory protein gene. Mol. Cell. Endocrinol. 148:1-10. [DOI] [PubMed] [Google Scholar]
- 27.Lee, Y. S., H.-J. Kim, H. J. Lee, J. W. Lee, S.-Y. Chun, S.-K. Ko, and K. Lee. 2002. Activating signal cointegrator 1 is highly expressed in murine testicular Leydig cells and enhances the ligand-dependent transactivation of androgen receptor. Biol. Reprod. 67:1580-1587. [DOI] [PubMed] [Google Scholar]
- 28.Leers-Sucheta, S., K.-I. Morohashi, J. I. Mason, and M. H. Melner. 1997. Synergistic activation of the human type II 3β-hydroxysteroid dehydrogenase/δ5-δ4 isomerase promoter by the transcription factor steroidogenic factor-1/adrenal 4-binding protein and phorbol ester. J. Biol. Chem. 272:7960-7967. [DOI] [PubMed] [Google Scholar]
- 29.Leitges, M., L. Sanz, P. Martin, A. Duran, U. Braun, J. F. Garcia, F. Camacho, M. T. Diaz-Meco, P. D. Rennert, and J. Moscat. 2001. Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol. Cell 8:771-780. [DOI] [PubMed] [Google Scholar]
- 30.Li, X., G. L. Youngblood, A. H. Payne, and D. B. Hales. 1995. Tumor necrosis factor-alpha inhibition of 17 alpha-hydroxylase/C17-20 lyase gene (Cyp17) expression. Endocrinology 136:3519-3526. [DOI] [PubMed] [Google Scholar]
- 31.Liao, G., L. Y. Chen, A. Zhang, A. Godavarthy, F. Xia, J. C. Ghosh, H. Li, and J. D. Chen. 2003. Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J. Biol. Chem. 278:5052-5061. [DOI] [PubMed] [Google Scholar]
- 32.Maira, M., C. Martens, E. Batsche, Y. Gauthier, and J. Drouin. 2003. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol. Cell. Biol. 23:763-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maira, M., C. Martens, A. Philips, and J. Drouin. 1999. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol. Cell. Biol. 19:7549-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauduit, C., F. Gasnier, C. Rey, M. A. Chauvin, D. M. Stocco, P. Louisot, and M. Benahmed. 1998. Tumor necrosis factor-alpha inhibits Leydig cell steroidogenesis through a decrease in steroidogenic acute regulatory protein expression. Endocrinology 139:2863-2868. [DOI] [PubMed] [Google Scholar]
- 35.McKay, L. I., and J. A. Cidlowski. 1998. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol. Endocrinol. 12:45-56. [DOI] [PubMed] [Google Scholar]
- 36.McKay, L. I., and J. A. Cidlowski. 1999. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr. Rev. 20:435-459. [DOI] [PubMed] [Google Scholar]
- 37.Mealy, K., B. Robinson, C. F. Millette, J. Majzoub, and D. W. Wilmore. 1990. The testicular effects of tumor necrosis factor. Ann. Surg. 211:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mellon, S. H., N. A. Compagnone, and P. Zhang. 1998. Orphan receptors, proto-oncogenes and other nuclear factors regulate P450C17 gene transcription. Endocr. Res. 24:505-513. [DOI] [PubMed] [Google Scholar]
- 39.Na, S. Y., B. Y. Kang, S. W. Chung, S. J. Han, X. Ma, G. Trinchieri, S. Y. Im, J. W. Lee, and T. S. Kim. 1999. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J. Biol. Chem. 274:7674-7680. [DOI] [PubMed] [Google Scholar]
- 40.Na, S. Y., S. K. Lee, S. J. Han, H. S. Choi, S. Y. Im, and J. W. Lee. 1998. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor κB-mediated transactivations. J. Biol. Chem. 273:10831-10834. [DOI] [PubMed] [Google Scholar]
- 41.Palvimo, J. J., P. Reinikainen, T. Ikonen, P. J. Kallio, A. Moilanen, and O. A. Janne. 1996. Mutual transcriptional interference between RelA and androgen receptor. J. Biol. Chem. 271:24151-24156. [DOI] [PubMed] [Google Scholar]
- 42.Parker, K. L., and B. P. Schimmer. 1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr. Rev. 18:361-377. [DOI] [PubMed] [Google Scholar]
- 43.Payne, A. H., and P. J. O'Shaughnessy. 1996. Structure, function and regulation of steroidogenic enzymes in the Leydig cell, p. 259-285. In A. H. Payne, M. P. Hardy, and L. D. Russell (ed.), The Leydig cell. Cache River Press, Vienna, Ill.
- 44.Rao, R. M., Y. Jo, S. Leers-Sucheta, H. S. Bose, W. L. Miller, S. Azhar, and D. M. Stocco. 2003. Differential regulation of steroid hormone biosynthesis in R2C and MA-10 Leydig tumor cells: role of SR-B1-mediated selective cholesteryl ester transport. Biol. Reprod. 68:114-121. [DOI] [PubMed] [Google Scholar]
- 45.Sadie, H., G. Styger, and J. Hapgood. 2003. Expression of the mouse gonadotropin-releasing hormone receptor gene in alpha T3-1 gonadotrope cells is stimulated by cyclic 3′,5′-adenosine monophosphate and protein kinase A, and is modulated by steroidogenic factor-1 and Nur77. Endocrinology 144:1958-1971. [DOI] [PubMed] [Google Scholar]
- 46.Sadovsky, Y., and C. Dorn. 2000. Function of steroidogenic factor 1 during development and differentiation of the reproductive system. Rev. Reprod. 5:136-142. [DOI] [PubMed] [Google Scholar]
- 47.Shang, Y., X. Hu, J. Direnzo, M. A. Lazar, and M. Brown. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843-852. [DOI] [PubMed] [Google Scholar]
- 48.Sohn, Y. C., E. Kwak, Y. Na, J. W. Lee, and S.-K. Lee. 2001. Silencing mediator of retinoid and thyroid hormone receptors and activating signal cointegrator-2 as transcriptional coregulators of the orphan nuclear receptor Nur77. J. Biol. Chem. 276:43734-43739. [DOI] [PubMed] [Google Scholar]
- 49.Song, K. H., J. I. Park, M. O. Lee, J. Soh, K. Lee, and H. S. Choi. 2001. LH induces orphan nuclear receptor Nur77 gene expression in testicular Leydig cells. Endocrinology 142:5116-5123. [DOI] [PubMed] [Google Scholar]
- 50.Stein, B., P. C. Cogswell, and A. S. Baldwin, Jr. 1993. Functional and physical associations between NF-kappa B and C/EBP family members: a Rel domain-bZIP interaction. Mol. Cell. Biol. 13:3964-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stocco, C. O., L. F. Lau, and G. Gibori. 2002. A calcium/calmodulin-dependent activation of ERK1/2 mediates JunD phosphorylation and induction of nur77 and 20α-hsd genes by prostaglandin F2α in ovarian cells. J. Biol. Chem. 277:3293-3302. [DOI] [PubMed] [Google Scholar]
- 52.van der Poll, T., J. A. Romijn, E. Endert, and H. P. Sauerwein. 1993. Effects of tumor necrosis factor on the hypothalamic-pituitary-testicular axis in healthy men. Metabolism 42:303-307. [DOI] [PubMed] [Google Scholar]
- 53.Vielma, S. A., G. Krings, and M. F. Lopes-Virella. 2003. Chlamydophila pneumoniae induces ICAM-1 expression in human aortic endothelial cells via protein kinase C-dependent activation of nuclear factor-κB. Circ. Res. 92:1130-1137. [DOI] [PubMed] [Google Scholar]
- 54.Vogel, A. V., G. T. Peake, and R. T. Rada. 1985. Pituitary-testicular axis dysfunction in burned men. J. Clin. Endocrinol. Metab. 60:658-665. [DOI] [PubMed] [Google Scholar]
- 55.Wansa, K. D., J. M. Harris, and G. E. Muscat. 2002. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J. Biol. Chem. 277:33001-33011. [DOI] [PubMed] [Google Scholar]
- 56.Wells, J., and P. J. Farnham. 2002. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods 26:48-56. [DOI] [PubMed] [Google Scholar]
- 57.Wilson, T. E., A. R. Mouw, C. A. Weaver, J. Milbrandt, and K. L. Parker. 1993. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21-hydroxylase. Mol. Cell. Biol. 13:861-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woolf, P. D., W. R. Hamill, J. V. McDonald, L. A. Lee, and M. Kelly. 1985. Transient hypogonadotropic hypogonadism caused by critical illness. J. Clin. Endocrinol. Metab. 60:444-450. [DOI] [PubMed] [Google Scholar]
- 59.Xiong, Y., and D. B. Hales. 1993. The role of tumor necrosis factor-alpha in the regulation of mouse Leydig cell steroidogenesis. Endocrinology 132:2438-2444. [DOI] [PubMed] [Google Scholar]
- 60.Xiong, Y., and D. B. Hales. 1997. Differential effects of tumor necrosis factor-alpha and interleukin-1 on 3 beta-hydroxysteroid dehydrogenase/delta 5→delta 4 isomerase expression in mouse Leydig cells. Endocrine 7:295-301. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, P., and S. H. Mellon. 1996. The orphan nuclear receptor steroidogenic factor-1 regulates the cyclic adenosine 3′,5′-monophosphate-mediated transcriptional activation of rat cytochrome P450c17 (17 alpha-hydroxylase/c17-20 lyase). Mol. Endocrinol. 10:147-158. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, P., and S. H. Mellon. 1997. Multiple orphan nuclear receptors converge to regulate rat P450c17 gene transcription: novel mechanisms for orphan nuclear receptor action. Mol. Endocrinol. 11:891-904. [DOI] [PubMed] [Google Scholar]