Abstract
AIM: To investigate the presence of small intestinal villous atrophy in celiac disease patients from quantitative analysis of videocapsule image sequences.
METHODS: Nine celiac patient data with biopsy-proven villous atrophy and seven control patient data lacking villous atrophy were used for analysis. Celiacs had biopsy-proven disease with scores of Marsh II-IIIC except in the case of one hemophiliac patient. At four small intestinal levels (duodenal bulb, distal duodenum, jejunum, and ileum), video clips of length 200 frames (100 s) were analyzed. Twenty-four measurements were used for image characterization. These measurements were determined by quantitatively processing the videocapsule images via techniques for texture analysis, motility estimation, volumetric reconstruction using shape-from-shading principles, and image transformation. Each automated measurement method, or automaton, was polled as to whether or not villous atrophy was present in the small intestine, indicating celiac disease. Each automaton’s vote was determined based upon an optimized parameter threshold level, with the threshold levels being determined from prior data. A prediction of villous atrophy was made if it received the majority of votes (≥ 13), while no prediction was made for tie votes (12-12). Thus each set of images was classified as being from either a celiac disease patient or from a control patient.
RESULTS: Separated by intestinal level, the overall sensitivity of automata polling for predicting villous atrophy and hence celiac disease was 83.9%, while the specificity was 92.9%, and the overall accuracy of automata-based polling was 88.1%. The method of image transformation yielded the highest sensitivity at 93.8%, while the method of texture analysis using subbands had the highest specificity at 76.0%. Similar results of prediction were observed at all four small intestinal locations, but there were more tie votes at location 4 (ileum). Incorrect prediction which reduced sensitivity occurred for two celiac patients with Marsh type II pattern, which is characterized by crypt hyperplasia, but normal villous architecture. Pooled from all levels, there was a mean of 14.31 ± 3.28 automaton votes for celiac vs 9.67 ± 3.31 automaton votes for control when celiac patient data was analyzed (P < 0.001). Pooled from all levels, there was a mean of 9.71 ± 2.8128 automaton votes for celiac vs 14.32 ± 2.7931 automaton votes for control when control patient data was analyzed (P < 0.001).
CONCLUSION: Automata-based polling may be useful to indicate presence of mucosal atrophy, indicative of celiac disease, across the entire small bowel, though this must be confirmed in a larger patient set. Since the method is quantitative and automated, it can potentially eliminate observer bias and enable the detection of subtle abnormality in patients lacking a clear diagnosis. Our paradigm was found to be more efficacious at proximal small intestinal locations, which may suggest a greater presence and severity of villous atrophy at proximal as compared with distal locations.
Keywords: Automata, Celiac disease, Small intestine, Videocapsule, Villous atrophy
Core tip: Videocapsule endoscopy images from celiac disease patients and controls were extracted from video clips and compared using image processing. The image processor consists of 24 automated measurements, or automata. The values of these automata were polled for yes or no vote, which depended on a predetermined threshold value set for each measurement. The polling process predicted whether the patient had celiac disease, based on majority vote from the 24 automata. Celiac patients with even subtle villous atrophy were distinguished from controls by this method. For 16 patients, the overall sensitivity, specificity, and accuracy of the method was 83.9%, 92.9%, and 88.1%, respectively.
INTRODUCTION
Videocapsule endoscopy has been used in clinical practice for over 10 years as a way to visualize the entire small intestine in patients with known or suspected celiac disease, inflammatory bowel disease, and other diseases where lesions are likely to be present in this region[1-5]. The capsule is swallowed and then provides two high resolution images per second from all regions of the gastrointestinal system, including distal areas where standard endoscopy cannot be used. Based on findings that show the videocapsule to be helpful in the identification of abnormalities consistent with celiac disease[6-9], there is increasing use in clinical practice.
When villous atrophy is present in the small intestine, as confirmed using standard endoscopy with biopsy, abnormalities are often evident in the endoscopic images including fissuring, mosaic pattern, and scalloping of mucosal folds[10-14]. These abnormalities are often patchy in location, being interspersed with more normal-appearing mucosal surface. We have developed quantitative analyses of videocapsule endoscopy images[15-19]. In these studies, it was hypothesized that patchy small intestinal abnormality would result in quantitative differences in the digital images. In patient image sequences with substantial heterogeneity, caused by visually evident abnormalities including fissuring, mosaic pattern, and scalloping of mucosal folds in celiac patients, the image texture, which is the pixel-to-pixel variability in brightness level, would be expected to increase. Furthermore, it was hypothesized that in celiac patients, small bowel regions with villous atrophy may have abnormal motility, manifested as changes in oscillations in videocapsule image brightness. These hypotheses were validated in our initial studies[15-19], and it was determined that patients with active celiac disease vs controls could be classified using threshold levels of the quantitative parameters used to measure image texture and oscillations in image brightness levels.
Although in prior work, classification of celiac vs control image sequences was done using several variables and multidimensional nonlinear discriminant functions[15-19], development of such functions without user intervention is computationally intensive. Herein, a means to automatically classify celiac vs control image sequences using quantitative texture and oscillation variables is described using automata-based polling[20].
MATERIALS AND METHODS
Clinical procedure and data acquisition
Retrospective videocapsule endoscopy data was obtained from 9 celiac patients and 7 control patients. In all except one patient, six biopsy specimens were obtained during endoscopy and then analyzed using light microscopy. In one hemophiliac patient, biopsies were not obtained. The celiac patients had recently begun a gluten-free diet, except for one patient with hemophilia and positive anti-endomysial antibody who had not yet started the diet. These patients had a diagnostic biopsy with Marsh grade II-IIIC lesions, and positive serology for celiac disease upon diagnosis. These patients were still considered to have active celiac disease due to the fact that a period of months is often needed for the diet to cause a reduction in small intestinal villous atrophy[1-4].
For the videocapsule endoscopy study, informed consent was obtained. Exclusion criteria were age under 18 years, history of or suspected small bowel obstruction, dysphagia, presence of electromedical implants, previous gastrointestinal surgery, pregnancy, or nonsteroidal anti-inflammatory drug use during the previous month. For analysis, only complete videocapsule endoscopy studies, reaching the colon, were used. The study was approved by the Internal Review Board at Columbia University Medical Center, with all patients being evaluated from May 1, 2008 to July 31, 2009.
The PillCam videocapsule (ver. SB2, 2007, Given Imaging, Yoqneam, Israel)[21] was used for imaging. The device includes a recorder unit, battery pack, wireless interface, and real-time viewer. The capsule acquires digital image frames at a 2/second rate, with a resolution of 576 × 576 pixels, and it is a single-use pill-sized device[21]. For each patient undergoing the procedure, abdominal leads were placed on the upper, mid, and lower abdomen, and a belt containing the data recorder was positioned at waist level. All subjects swallowed the videocapsule with radio transmitter in the early morning with approximately 200 cc’s of water and 80 mg simethicone after an overnight fast without bowel preparation. Subjects were allowed to drink water two hours after capsule ingestion, and to eat a light meal four hours after capsule ingestion. The recorder was then removed, and the data downloaded to a HIPAA-compliant PC-based computer console equipped with RAPID software (ver. 5, 2008, Given Imaging, Yoqneam, Israel). The RAPID software was used for review and clinical report generation during the videocapsule endoscopy studies. Videos were interpreted by three experienced gastroenterologists. Selected video clips, 200 image frames in length (100 s of data), were exported to external media without patient identifiers for quantitative analysis. Images were acquired immediately distal to the pylorus corresponding to the proximal duodenum (location 1). The total small bowel transit time of the videocapsule was divided into tertiles. Video clips were also acquired from each of the three tertiles for each patient (locations 2, 3, and 4, roughly corresponding to the distal duodenum, the jejunum, and the proximal ileum, respectively).
Data preprocessing
From each color videoclip, 200 grayscale images (i.e., 256 brightness levels, 0 = black, 255 = white) were extracted using Matlab Ver. 7.7, 2008 (The MathWorks, Natick, MA, United States). The image data were ported into software created by the authors, which was coded using the Intel Visual Fortran Compiler (ver. 9.0, 2005, Intel Corporation, Santa Clara, CA, United States).
Implementation of automata-based polling
A procedure termed automata-based polling[20] was implemented for analysis of videocapsule images. Automata are defined as functional nodes in a computational network, and they are used for quantitative analysis of a physiological system. At each node, a calculation is done based on a predefined set of rules and equations. Quantitative measurements devised previously were used to develop the network of automata for polling[15-19]. The following methods were used:
Texture analysis: Texture analysis[15] is based on measurement in 10 × 10 subimage regions from each 576 × 576 pixel image in the sequence, excluding edge pixels. Texture was defined by the measurement of standard deviation in pixel grayscale level in each subimage. The average grayscale level (brightness) and the standard deviation in grayscale level (image texture) of each subimage were averaged for all subimages in each image. The mean in brightness and texture over 200 frames (100 s) were used as automata measurements. It was expected that brightness would decrease with increase in abnormal features due to villous atrophy in active celiac images. Similarly, it was expected that texture would increase in celiac patient images due to heterogeneity in the mucosal surface characteristics. In Figure 1 are shown examples of control and celiac images in color. The control images have uniform texture, no fissures, and minute protrusions. There are few folds and no scalloping of folds. In contrast, the celiac images have variable texture, presence of fissuring, and a mosaic pattern of large protrusions. There are more folds and scalloping of folds.
Figure 1.
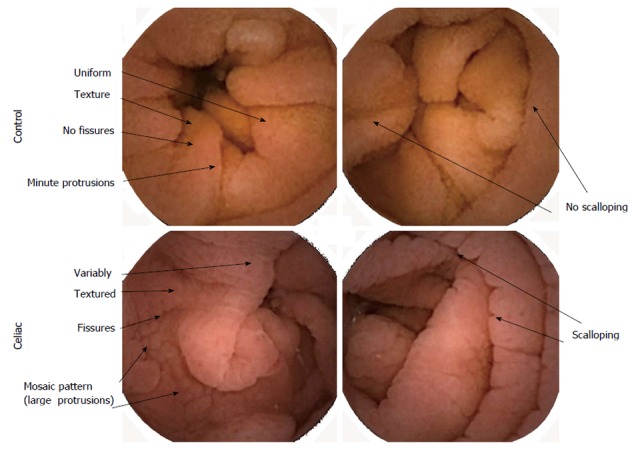
Color images from videocapsule device. Differences in celiac images where villous atrophy is present are shown vs control. Control images have a more uniform texture at the mucosal surfaces. Celiac surfaces have a rougher appearance, with more fissuring, large protrusions, and scalloping along the folds.
A third parameter was derived from the variability in frame-to-frame mean image brightness over the sequence of 200 images. The largest peak in the frequency spectrum of this measurement is termed the dominant frequency[15]. Its inverse, the dominant period, was used as a third automaton measurement, and it is reflective of oscillations in image brightness over the 200 image sequence. Thus three automata were developed from the texture analysis method. Mean values for celiac and control from the prior study[15] were used to develop threshold levels for classification. The threshold levels used were the midpoints between celiac and control data. An input videoclip sequence with a measured value closer to the celiac mean as compared with the threshold level would be counted as a vote for celiac. An input videoclip sequence with a measurement value closer to the control mean as compared with the threshold level would be counted as a vote for control.
Extraction from texture subbands: These measurements are made by plotting the brightness and texture of individual subimages. In the scatterplot, subimage values for all 200 images are included. The scatterplot is then divided into subbands. This is shown in Figure 2. Subband 1 is defined as including those subimages having a texture of 0-10 as measured by the standard deviation in pixel brightness. The average texture and the average brightness level within this subband, shown as hatched lines in the figure, along with the number of subimage values contained in the subband, are used as measurement values. This is repeated for subband 2 (texture of 10-20 as measured by the standard deviation in pixel brightness) and subband 3 (texture of 20-30 as measured by the standard deviation in pixel brightness). Thus there are 9 measurements in all, 8 of which were used for automata. The texture for subband 3 was not used as an automaton, since the mean values from previous data for celiac and controls overlapped. Threshold levels for classification of celiacs vs controls were determined from previous data[16]. The scatterplots of Figure 2 suggest that celiacs tend to have greater texture in the 0-10 subband, which is the subband consisting of most of the subimages. Also, as shown in Figure 2, celiacs tend to have less total pixels in the 0-10 subband but more total pixels in the 10-20 and 20-30 subbands, suggesting that celiac subimages have greater texture variability (their presence is at the higher variability subbands) as compared with controls.
Figure 2.
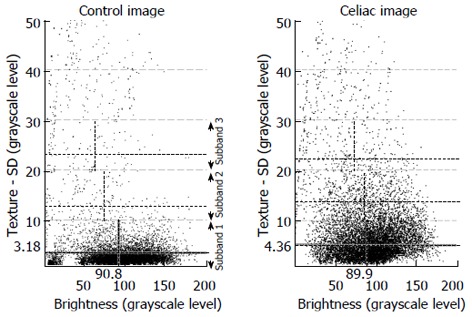
The method of using subbands in the standard deviation of image texture. Subbands are shown to the right of the control scatterplot. Mean values for each of the two variables are noted as hatched lines in each subband. The subband characteristics differ in celiac vs control videoclip series. In the control scatterplot the points are concentrated at low values of standard deviation in texture. In the celiac scatterplot the points are concentrated at higher values of standard deviation in texture. Thus the celiac image data has a greater variability of pixel gray level, which can be used to distinguish it from the control image data.
Motility estimation: The darkest 10000 pixels per image were selected as an approximation of the view along the luminal axis of the small intestine. Variation in the centroid of this region (mean pixel location along the x and y axes), and variation in the maximum width of the region, were used as estimates of motility. The threshold values for the three automata to distinguish celiac from control data were based on a prior study[17]. In the prior study it was found that celiacs tend to have more variability in the x and y position of the lumen, perhaps due to irregular motility.
Volumetric method: Two-dimensional images were converted to three dimensions using shape-from-shading principles[18]. The third dimension is formed according to the grayscale level of each pixel. The principle is shown in Figure 3. Darker pixels and darker image regions are at greater depth along the z-axis in the three-dimensional representation at right, while brighter pixels and image regions are at shallower depth. Examples of corresponding locations in two and three dimensions are noted by asterisks. The text at left in the two-dimensional image is shown for reference in the three-dimensional representation. Based on the three-dimensional structure, a syntax was developed to detect and measure luminal wall protrusions. Protrusions were quantified according to their height, width, and number per image. Means and standard deviations for each 200 image sequence were used as automata values. Threshold values for polling were obtained from a prior study[18]. In this study it was shown that active celiac patients tend to have less protrusions per image, and that these protrusions are greater in width and height, and in the standard deviation, or variability, in the width and height dimensions[18]. This may be due the blunted and perhaps clumped nature of villi when there is atrophy.
Figure 3.
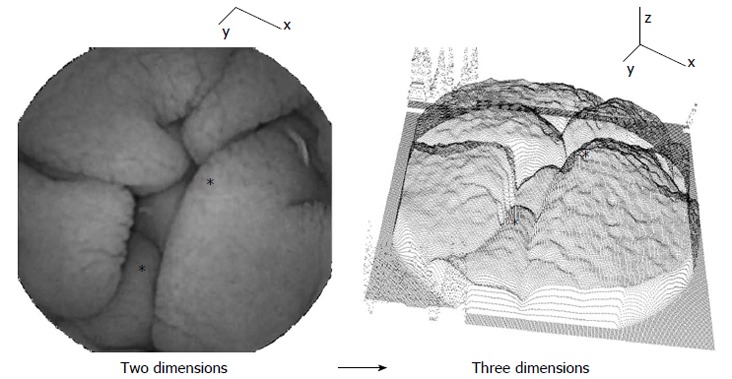
Image transformation from two to three dimensions. Coordinate axes are shown for reference. Examples of corresponding landmark locations are noted by asterisks. The gray levels of the two-dimensional endoscopic image at left are converted to a depth along the z axis in the three-dimensional projection at right. The characteristics of the surface protrusions in the three-dimensional image are used for distinguishing celiac from control patient data.
Image transformation to basis images: Transformed images contain salient features from the sequence of original videocapsule images and are termed basis images[19]. The purpose of transformation is to retain repetitive components in the sequence of images, while removing noise and extraneous substances such as air bubbles and opaque fluids[19]. Analysis of basis images rather than the original images makes the measurements more robust to features that were not a part of the actual luminal surface. Examples of basis images are shown in Figure 4. Note that the control basis images appear smoother and with less change in content as compared with the celiac basis images. The control basis contains more uniform homogeneous structure. The celiac basis images vary substantially in content and brightness. These basis images are indicative of the original salient content in each series of images. The parameters measured from basis images were the mean texture and standard deviation in brightness over the basis image series. The dominant period was also measured, as calculated from the original 200 image sequence. A separate spectrum was constructed for each x, y pixel location (576 × 576 pixels in total)[19]. These separate spectra were then averaged to form a mean spectrum from which the dominant period was determined. An example is shown in Figure 5. Control spectra generated from image series acquired from locations 3 and 4 have highest peak (dominant period) at 4.0-4.5 s. Celiac spectra generated from image series acquired from locations 3 and 4 have highest peak (dominant period) at 6-8.5 s. This was typical of all spectra-celiacs tended to have longer dominant periods, perhaps due to an increase in motility at areas of injury. The threshold values for vote-casting to classify the data were again midpoints between means for celiacs and controls from prior data[19].
Figure 4.
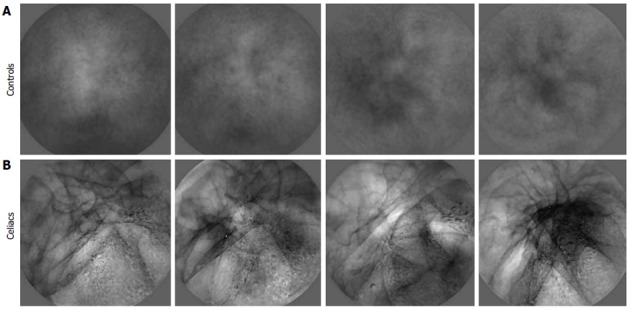
Examples of basis images derived from celiac vs control videoclip series. A: Control; B: Celiacs. The celiac basis has more heterogeneous structure. There are dark lines running through the celiac basis images as well as black and white blotches. In contrast, the control basis images are mostly uniform, with only diffuse features being evident.
Figure 5.
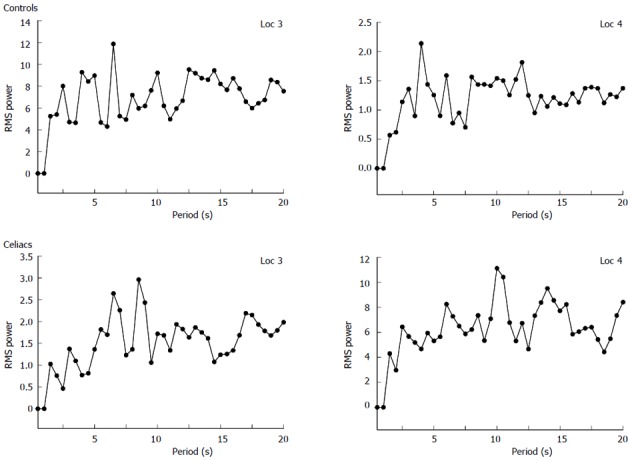
Examples of frequency spectra for celiac vs control. The dominant period (DP) is the highest peak in the physiologic range, taken as 1-20 s per oscillation. The graphs are shown for locations 3 and 4 in the small intestine. In the control patient the DP is 6 and 5 s for location 3 and 4, respectively. In the celiac patient the DP is 9 and 10 s for location 3 and 4, respectively. Thus the DP is higher for the celiac patient.
Overall a total of 24 automata from the five measurement methods outlined above were incorporated as functional nodes into the computational network. Each automaton was given one vote, and all automata were then polled to decide whether the sequences of images being analyzed was acquired from a patient with active celiac disease or not. Thus there were 24 vote-casting nodes, each having equal weight.
Statistical analysis
Votes for celiac and for control were tallied at all four intestinal levels for both celiac and control video clips. Summary results in terms of the number of votes cast were also expressed as mean ± SD, and the statistical significance based on the unpaired t-test was determined for each small intestinal level (SigmaPlot 2004, ver. 9.01, Systat Software, Chicago, IL, United States). The sensitivity, specificity, and accuracy of the method was then determined. The sensitivity was defined as the number of videoclip image sequences determined to be celiac out of the total number of actual celiac video clips that were classified. The sensitivity was defined as the number of video clips determined to be controls out of the total number of actual control videoclip sequences that were classified. The accuracy was defined as the total number of correct classifications out of the total number of video clips classified. Video clips that were not classified by the automata-based polling protocol were not included in the sensitivity, specificity, and accuracy statistical calculations. The statistical calculations were done separately for each of the small intestinal locations 1-4, and also for all four locations combined.
RESULTS
Automata-based voting and prediction are summarized in Table 1 for celiac and control patients. Votes are shown for locations 1 to 4, and by patient. More votes in the celiac as compared with the non-celiac column are predictive of a celiac patient, while more votes for non-celiac is predictive of a control patient. A tie indicates that no prediction was made. For most locations and patients, prediction by the automata-based polling protocol was correct. All predictions were correct for Marsh type IIIC celiac patients. However for the two celiac patients with Marsh type II pathology, prediction was incorrect at two locations, there was no prediction at location 4, and at only one location out of four was the prediction correct. For two of the Marsh type IIIA celiac patients, and for the patient lacking biopsy, no prediction was also made at location 4, suggesting that images acquired from this location (ileum) are more difficult to evaluate, or that there is a lesser degree of villous atrophy, as compared with more proximal small intestinal locations. The results for control patients are shown in Table 1. In 4/7 control patients, predictions at all four levels were correct, indicating no celiac disease. In 3/7 control patients, prediction at three of the four locations was correct.
Table 1.
Number of votes cast by automata, celiac patient data
| Mar | C1 | N1 | Vote | C2 | N2 | Vote | C3 | N3 | Vote | C4 | N4 | Vote |
| Celiac patients | ||||||||||||
| IIIC | 13 | 11 | C | 13 | 11 | C | 20 | 4 | C | 18 | 6 | C |
| IIIC | 13 | 11 | C | 16 | 6 | C | 17 | 7 | C | 16 | 8 | C |
| IIIC | 13 | 11 | C | 21 | 3 | C | 13 | 11 | C | 15 | 9 | C |
| IIIA | 17 | 7 | C | 16 | 8 | C | 19 | 5 | C | 14 | 10 | C |
| IIIA | 16 | 8 | C | 16 | 8 | C | 14 | 10 | C | 12 | 12 | - |
| ND | 16 | 8 | C | 17 | 7 | C | 16 | 9 | C | 12 | 12 | - |
| IIIA | 14 | 10 | C | 15 | 9 | C | 9 | 15 | N | 12 | 12 | - |
| II | 10 | 14 | N | 14 | 10 | C | 9 | 15 | N | 12 | 12 | - |
| II | 19 | 5 | C | 10 | 14 | N | 6 | 18 | N | 12 | 12 | - |
| Mean ± SD | 14.56 ± 2.70 | 9.44 ± 2.70 | P = 0.022 | 15.33 ± 3.00 | 8.44 ± 3.13 | P = 0.009 | 13.67 ± 4.85 | 10.44 ± 4.80 | P = 0.345 | 13.67 ± 2.24 | 10.33 ± 2.24 | P = 0.056 |
| Control patients | ||||||||||||
| 0 | 9 | 15 | N | 11 | 13 | N | 5 | 19 | N | 8 | 16 | N |
| 0 | 9 | 15 | N | 8 | 16 | N | 6 | 18 | N | 8 | 16 | N |
| 0 | 10 | 14 | N | 12 | 13 | N | 9 | 15 | N | 9 | 15 | N |
| 0 | 11 | 13 | N | 10 | 14 | N | 4 | 20 | N | 11 | 13 | N |
| 0 | 16 | 8 | C | 10 | 14 | N | 9 | 15 | N | 6 | 18 | N |
| 0 | 16 | 8 | C | 11 | 13 | N | 10 | 14 | N | 9 | 15 | N |
| 0 | 10 | 14 | N | 10 | 14 | N | 10 | 14 | N | 15 | 9 | C |
| Mean ± SD | 11.57 ± 3.10 | 12.43 ± 3.10 | P = 0.727 | 10.29 ± 1.25 | 13.86 ± 1.07 | P = 0.006 | 7.57 ± 2.51 | 16.43 ± 2.51 | P = 0.003 | 9.43 ± 2.88 | 14.57 ± 2.88 | P = 0.056 |
Automata vote tallies for celiac (C) and non-celiac controls (N). The number of votes for each are shown for small intestinal levels 1, 2, 3, and 4. Vote = the overall vote for celiac, non-celiac, or tie (-) based on the tally for each. The vote tallies for celiac patients with villous atrophy and control patients lacking villous atrophy confirmed by intestinal biopsy. The Marsh score based on the intestinal biopsy is given (Mar) except in one patient who was a hemophiliac it was not determined (ND). Means and standard deviations are provided in the lower two rows, with significances based on the paired t-test shown.
The mean and standard deviations for number of votes cast by automata are noted in the last row of each portion of the table. The significance of the difference based upon the paired t-test is also provided. At intestinal levels 1 and 2, there is significance for all but level 1 of control patients. At intestinal levels 3 and 4, there is significance only at level 1 of control patients. Thus there is a tendency for greater significance in the automated classification procedure at more proximate levels of the small intestine as compared to distal levels. When the data of Table 1 was pooled from all levels, for actual celiacs there was a mean of 14.31 ± 3.28 celiac votes cast, vs 9.67 ± 3.31 control votes cast (P < 0.001). When the data of Table 1 was pooled from all levels, for actual controls there was a mean of 9.71 ± 2.81 celiac votes cast vs 14.32 ± 2.79 control votes cast (P < 0.001).
The sensitivity, specificity, and accuracy of the automata-based polling protocol described in this study are shown in Table 2. At top are the results for sensitivity. Provided are the values for each method type alone and also based on location, as well as the value for data pooled from all locations. The method of first transforming the data into a series of basis vectors had the highest sensitivity at 93.8% (Table 2), while textural measurement without transformation being the second most sensitive method, at 77.8%. The other methods had approximately the same sensitivity. Values for specificity are also shown in Table 2. For data pooled from all locations, the subband method is the most specific at 76.0%. The transformation into basis vectors is least specific at 27.8%. The accuracy of the methods are also shown in Table 2. The transformation into basis vectors method is the most accurate at 70.8% for pooled data. The texture, subband, and volume methods have approximately equal accuracy overall.
Table 2.
Statistics of automata polling to correctly classify videocapsule data
| Sensitivity | 1st | 2nd | 3rd | 4th | All locations |
| Sensitivity | |||||
| Texture | 88.9 | 88.9 | 55.6 | 77.8 | 77.8 |
| Subband | 55.6 | 50.0 | 42.9 | 83.3 | 56.7 |
| Motility | 42.9 | 100.0 | 50.0 | 37.5 | 84.2 |
| Volume | 44.4 | 77.8 | 55.6 | 44.4 | 55.6 |
| Basis | 100.0 | 100.0 | 75.0 | 100.0 | 93.3 |
| Specificity | |||||
| Texture | 0.0 | 42.9 | 71.4 | 71.4 | 46.4 |
| Subband | 80.0 | 83.3 | 71.4 | 71.4 | 76.0 |
| Motility | 100.0 | 25.0 | 83.3 | 60.0 | 64.7 |
| Volume | 71.4 | 85.7 | 85.7 | 71.4 | 78.6 |
| Basis | 0.0 | 33.3 | 100.0 | 40.0 | 27.8 |
| Accuracy | |||||
| Texture | 50.0 | 68.8 | 62.5 | 75.0 | 64.1 |
| Subband | 64.3 | 64.3 | 57.1 | 76.9 | 65.4 |
| Motility | 55.6 | 70.0 | 64.3 | 46.2 | 58.7 |
| Volume | 56.3 | 81.3 | 68.8 | 56.3 | 64.1 |
| Basis | 50.0 | 80.0 | 81.8 | 76.9 | 70.8 |
| Overall | |||||
| Sensitivity | 88.9 | 88.9 | 66.7 | 100.0 | 83.9 |
| Specificity | 85.7 | 100.0 | 100.0 | 85.7 | 92.9 |
| Accuracy | 87.5 | 93.8 | 81.3 | 90.9 | 88.1 |
The sensitivity, specificity, and accuracy of the method at locations, and for all locations combined, separated by the measurement type (texture, subband coding, motility, volumetric analysis, and reconstruction using basis vectors.
In Table 2 at the bottom is shown the overall sensitivity, specificity, and accuracy of the automata-based polling protocol. Similar efficacy is evident at each small intestinal location. For pooled values from all locations, the overall sensitivity of vote-casting was 83.9%, the specificity was 92.9%, and the accuracy was 88.1%.
DISCUSSION
Videocapsule data was acquired from the small intestine of celiac patients with biopsy-proven active disease, and from control patients without mucosal lesions. Using an automata-based polling protocol to classify celiac vs control video clips, the overall specificity and accuracy were 92.9% and 88.1%, with the sensitivity being 83.9%. The method of transformation to basis vectors had the best overall sensitivity and accuracy for prediction, although the specificity using this method was reduced. Several tie votes were cast at location 4 (ileum) suggesting that videocapsule images acquired from this region are less differentiable as being active celiac or control data. Prediction using the automata-based polling protocol is an improved technique because votes are polled from many independent automata. Although extraneous features and random and phasic noise may degrade quantitative comparisons of videocapsule data, the methodology has been shown to be relatively robust to these external influences[22,23].
As compared to prior analyses[15-19], the automata-based polling protocol tended to improve prediction. Although the methods as introduced previously tended to be quite predictive of celiac and control patients, they made use of nonlinear discriminant functions which were specifically tailored to the data at hand. This previous methodology was computationally intensive and was developed for a specific data set. The texture method, the subband method, and the motility method by themselves all make use of manually-derived three-dimensional classifiers and nonlinear discriminant functions[15-17]. In contrast, the automata-based polling protocol described in this study does not use three-dimensional classifiers nor complex nonlinear discriminant functions. Rather, each measurement method is used independently for calculation. The threshold for determining whether the measurement is likely to have been from data acquired from an active celiac or a control patient was based on a predetermined threshold level from data analyzed previously. Pooled voting from all measurements was used for prediction, making the method robust to outliers in the individual measurements.
Other measurement methods may be useful to incorporate into the automata polling procedure for improved efficacy. These include texture-based methods[24-28]. Yet equally important will be the need for advances in videocapsule technology[29-32] and image resolution[33-36].
Limitations
The number of video clips analyzed was relatively small, and validation should be done in a prospective double-blinded study with larger data set. Our results suggest in part that classification of celiacs vs controls can be used for dynamic estimates of wall motility. Inclusion of a control group with severe intestinal motility disorders would be helpful for validation. The number of automata used for classification was 24. Classification accuracy may increase with a larger computational network, the subject of future study. The technique presented in the study presumed that camera angle and distance to the mucosal surface is uniform, and that coverage of the surface area of the small intestinal lumen is relatively constant during transit of the videocapsule. However, continual variation in these parameters actually occurs. These variations may act as random and phasic noise to reduce accuracy in quantitatively comparing celiac vs control videoclip images. Yet, these quantitative methods have been shown to be relatively robust to additive random and phasic noise[22]. Removal of extraneous image features prior to analysis[23] can potentially improve efficacy further. Capsule motion may also be erratic, further limiting analysis. The study was also performed with only one type of capsule endoscope and it is unclear if these results would be different with the use of a different capsule endoscopy system. Since there was no gold standard of biopsy specimen for levels 3 and 4 analysis, villous atrophy may have been absent from these regions in celiac patients, which would result in classification error. The findings were determined with a relatively small patient population, and should be confirmed in a larger study.
In conclusion, video clips from four small intestinal locations in the duodenum, jejunum, and ileum can be used to differentiate data acquired from active celiacs from controls. Several methods that were introduced previously, namely texture-based analysis, use of subbands, syntactic analysis of volumetric properties, estimation of motility, and use of transformed basis vectors to extract salient information, were all found to be useful for prediction. The system was implemented as an automata-based polling protocol, with pooling of the votes cast from a network of 24 automata. The findings of this study suggest that the technique may be useful for discerning images of celiac patients with villous atrophy from images of control patients lacking atrophy, though this must be confirmed with a larger data set that includes different Marsh grades of intestinal damage. The sensitivity of the method was less accurate due to the fact that Marsh type II celiac patients were not as readily discerned by the quantitative analysis.
COMMENTS
Background
In celiac disease patients and in other patients with gastrointestinal malady there may be atrophy of the small intestinal villi. Changes in the villi and other abnormalities of the intestinal mucosa may be detectable by quantitative analysis of videocapsule images.
Research frontiers
Quantitative biomedical image processing is becoming an important means to assist gastroenterologists during their evaluation of videocapsule images for the detection of gastrointestinal abnormalities.
Innovations and breakthroughs
In this study an automated, unbiased method was developed to quantify changes in videocapsule images of the small intestine. The method was found useful to distinguish images of celiac disease patients with biopsy-proven villous atrophy, vs control patients lacking villous atrophy.
Applications
The method is potentially useful as a real-time analysis tool during videocapsule image acquisition and playback at the clinical analysis console. The degree of abnormality can be posted on-screen with each image in the set of patient data.
Terminology
The method determines the degree of abnormality in videocapsule imagery by polling of specialized measurement automata. Each automaton is an independent measurement that is calculated without user intervention. By referring to threshold values from prior analysis, each automaton casts a vote as to whether their particular measurement value is indicative of abnormality, and the votes are tallied, or pooled. Classification as to whether villous atrophy is present or not at each small intestinal level is determined by which of the two classes garners the greater number of votes.
Peer review
This study outlines an approach for analysis of videocapsule endoscopy data to assess patients with celiac disease. In particular, the benefit of automated quantitative analysis is investigated. It is concluded from the findings that the method is useful in the detection of villous atrophy, especially in proximal locations of the small intestine.
Footnotes
Supported by (In part) a grant from the Celiac Sprue Association Peer Review Research Grant Program
P- Reviewers Day AS, Gassler N S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Lee SK, Green PH. Endoscopy in celiac disease. Curr Opin Gastroenterol. 2005;21:589–594. doi: 10.1097/01.mog.0000174218.00333.19. [DOI] [PubMed] [Google Scholar]
- 2.Green PH, Rubin M. Capsule endoscopy in celiac disease. Gastrointest Endosc. 2005;62:797–799. doi: 10.1016/j.gie.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Green PH, Rubin M. Capsule endoscopy in celiac disease: diagnosis and management. Gastrointest Endosc Clin N Am. 2006;16:307–316. doi: 10.1016/j.giec.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Gonda TA, Khan SU, Cheng J, Lewis SK, Rubin M, Green PH. Association of intussusception and celiac disease in adults. Dig Dis Sci. 2010;55:2899–2903. doi: 10.1007/s10620-009-1086-8. [DOI] [PubMed] [Google Scholar]
- 5.Tennyson CA, Green PH. The role of capsule endoscopy in patients with nonresponsive celiac disease. Gastrointest Endosc. 2011;74:1323–1324. doi: 10.1016/j.gie.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Ersoy O, Akin E, Ugras S, Buyukasik S, Selvi E, Güney G. Capsule endoscopy findings in celiac disease. Dig Dis Sci. 2009;54:825–829. doi: 10.1007/s10620-008-0402-z. [DOI] [PubMed] [Google Scholar]
- 7.Collin P, Rondonotti E, Lundin KE, Spada C, Keuchel M, Kaukinen K, DE Franchis R, Jacobs MA, Villa F, Mulder CJ. Video capsule endoscopy in celiac disease: current clinical practice. J Dig Dis. 2012;13:94–99. doi: 10.1111/j.1751-2980.2011.00561.x. [DOI] [PubMed] [Google Scholar]
- 8.Rokkas T, Niv Y. The role of video capsule endoscopy in the diagnosis of celiac disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:303–308. doi: 10.1097/MEG.0b013e32834fa914. [DOI] [PubMed] [Google Scholar]
- 9.Van Weyenberg SJ, Bouman K, Jacobs MA, Halloran BP, Van der Peet DL, Mulder CJ, Van Kuijk C, Van Waesberghe JH. Comparison of MR enteroclysis with video capsule endoscopy in the investigation of small-intestinal disease. Abdom Imaging. 2013;38:42–51. doi: 10.1007/s00261-012-9892-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green PH. Celiac disease: how many biopsies for diagnosis. Gastrointest Endosc. 2008;67:1088–1090. doi: 10.1016/j.gie.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 11.Ciaccio EJ, Bhagat G, Tennyson CA, Lewis SK, Hernandez L, Green PH. Quantitative assessment of endoscopic images for degree of villous atrophy in celiac disease. Dig Dis Sci. 2011;56:805–811. doi: 10.1007/s10620-010-1371-6. [DOI] [PubMed] [Google Scholar]
- 12.Naiyer AJ, Hernandez L, Ciaccio EJ, Papadakis K, Manavalan JS, Bhagat G, Green PH. Comparison of commercially available serologic kits for the detection of celiac disease. J Clin Gastroenterol. 2009;43:225–232. doi: 10.1097/MCG.0b013e31816200e5. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez S, Gupta A, Cheng J, Tennyson C, Lewis SK, Bhagat G, Green PH. Prospective study of the role of duodenal bulb biopsies in the diagnosis of celiac disease. Gastrointest Endosc. 2010;72:758–765. doi: 10.1016/j.gie.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 14.Lebwohl B, Kapel RC, Neugut AI, Green PH, Genta RM. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–109. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciaccio EJ, Tennyson CA, Lewis SK, Krishnareddy S, Bhagat G, Green PH. Distinguishing patients with celiac disease by quantitative analysis of videocapsule endoscopy images. Comput Methods Programs Biomed. 2010;100:39–48. doi: 10.1016/j.cmpb.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Classification of videocapsule endoscopy image patterns: comparative analysis between patients with celiac disease and normal individuals. Biomed Eng Online. 2010;9:44. doi: 10.1186/1475-925X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Quantitative estimates of motility from videocapsule endoscopy are useful to discern celiac patients from controls. Dig Dis Sci. 2012;57:2936–2943. doi: 10.1007/s10620-012-2225-1. [DOI] [PubMed] [Google Scholar]
- 18.Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Use of shape-from-shading to estimate three-dimensional architecture in the small intestinal lumen of celiac and control patients. Comput Methods Programs Biomed. 2013:Epub ahead of print. doi: 10.1016/j.cmpb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Transformation of videocapsule images to detect small bowel mucosal differences in celiac versus control patients. Comput Methods Programs Biomed. 2012;108:28–37. doi: 10.1016/j.cmpb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Nicopolitidis P, Papadimitriou GI, Pomportsis AS. Learning automata-based polling protocols for wireless LANs. IEEE Trans Commun. 2003;51:453–463. [Google Scholar]
- 21.Metzger YC, Adler SN, Shitrit AB, Koslowsky B, Bjarnason I. Comparison of a new PillCam™ SB2 video capsule versus the standard PillCam™ SB for detection of small bowel disease. Reports in Medical Imaging. 2009;2:7–11. [Google Scholar]
- 22.Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Robust spectral analysis of videocapsule images acquired from celiac disease patients. Biomed Eng Online. 2011;10:78. doi: 10.1186/1475-925X-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashar MK, Kitasaka T, Suenaga Y, Mekada Y, Mori K. Automatic detection of informative frames from wireless capsule endoscopy images. Med Image Anal. 2010;14:449–470. doi: 10.1016/j.media.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Vécsei A, Amann G, Hegenbart S, Liedlgruber M, Uhl A. Automated Marsh-like classification of celiac disease in children using local texture operators. Comput Biol Med. 2011;41:313–325. doi: 10.1016/j.compbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Hegenbart S, Uhl A, Vécsei A. Systematic assessment of performance prediction techniques in medical image classification: a case study on celiac disease. Inf Process Med Imaging. 2011;22:498–509. doi: 10.1007/978-3-642-22092-0_41. [DOI] [PubMed] [Google Scholar]
- 26.Hämmerle-Uhl J, Höller Y, Uhl A, Vécsei A. Endoscope distortion correction does not (easily) improve mucosa-based classification of celiac disease. Med Image Comput Comput Assist Interv. 2012;15:574–581. doi: 10.1007/978-3-642-33454-2_71. [DOI] [PubMed] [Google Scholar]
- 27.Hegenbart S, Maimone S, Uhl A, Vécsei A. Customised frequency pre-filtering in a local binary pattern-based classification of gastrointestinal images. Medical Content-Based Retrieval for Clinical Decision Support. Lect Notes Comput Sc. 2013;7723:99–109. [Google Scholar]
- 28.Hegenbart S, Uhl A, Vécsei A, Wimmer G. Scale invariant texture descriptors for classifying celiac disease. Med Image Anal. 2013;17:458–474. doi: 10.1016/j.media.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulder CJ, van Weyenberg SJ, Jacobs MA. Celiac disease is not yet mainstream in endoscopy. Endoscopy. 2010;42:218–219. doi: 10.1055/s-0029-1243903. [DOI] [PubMed] [Google Scholar]
- 30.Venkatesh K, Abou-Taleb A, Cohen M, Evans C, Thomas S, Oliver P, Taylor C, Thomson M. Role of confocal endomicroscopy in the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr. 2010;51:274–279. doi: 10.1097/MPG.0b013e3181d1b02c. [DOI] [PubMed] [Google Scholar]
- 31.Harris LA, Park JY, Voltaggio L, Lam-Himlin D. Celiac disease: clinical, endoscopic, and histopathologic review. Gastrointest Endosc. 2012;76:625–640. doi: 10.1016/j.gie.2012.04.473. [DOI] [PubMed] [Google Scholar]
- 32.Chang MS, Rubin M, Lewis SK, Green PH. Diagnosing celiac disease by video capsule endoscopy (VCE) when esophagogastroduodenoscopy (EGD) and biopsy is unable to provide a diagnosis: a case series. BMC Gastroenterol. 2012;12:90. doi: 10.1186/1471-230X-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culliford A, Daly J, Diamond B, Rubin M, Green PH. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55–61. doi: 10.1016/s0016-5107(05)01566-x. [DOI] [PubMed] [Google Scholar]
- 34.Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–S78. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Hopper AD, Sidhu R, Hurlstone DP, McAlindon ME, Sanders DS. Capsule endoscopy: an alternative to duodenal biopsy for the recognition of villous atrophy in coeliac disease. Dig Liver Dis. 2007;39:140–145. doi: 10.1016/j.dld.2006.07.017. [DOI] [PubMed] [Google Scholar]


