Abstract
AIM: To analyze reliability among endoscopists in diagnosing portal hypertensive gastropathy (PHG) and to determine which criteria from the most utilized classifications are the most suitable.
METHODS: From January to July 2009, in an academic quaternary referral center at Santa Casa of São Paulo Endoscopy Service, Brazil, we performed this single-center prospective study. In this period, we included 100 patients, including 50 sequential patients who had portal hypertension of various etiologies; who were previously diagnosed based on clinical, laboratory and imaging exams; and who presented with esophageal varices. In addition, our study included 50 sequential patients who had dyspeptic symptoms and were referred for upper digestive endoscopy without portal hypertension. All subjects underwent upper digestive endoscopy, and the images of the exam were digitally recorded. Five endoscopists with more than 15 years of experience answered an electronic questionnaire, which included endoscopic criteria from the 3 most commonly used Portal Hypertensive Gastropathy classifications (McCormack, NIEC and Baveno) and the presence of elevated or flat antral erosive gastritis. All five endoscopists were blinded to the patients’ clinical information, and all images of varices were deliberately excluded for the analysis.
RESULTS: The three most common etiologies of portal hypertension were schistosomiasis (36%), alcoholic cirrhosis (20%) and viral cirrhosis (14%). Of the 50 patients with portal hypertension, 84% were Child A, 12% were Child B, 4% were Child C, 64% exhibited previous variceal bleeding and 66% were previously endoscopic treated. The endoscopic parameters, presence or absence of mosaic-like pattern, red point lesions and cherry-red spots were associated with high inter-observer reliability and high specificity for diagnosing Portal Hypertensive Gastropathy. Sensitivity, specificity and reliability for the diagnosis of PHG (%) were as follows: mosaic-like pattern (100; 92.21; High); fine pink speckling (56; 76.62; Unsatisfactory); superficial reddening (69.57; 66.23; Unsatisfactory); red-point lesions (47.83; 90.91; High); cherry-red spots (39.13; 96.10; High); isolated red marks (43.48; 88.31; High); and confluent red marks (21.74; 100; Unsatisfactory). Antral elevated erosive gastritis exhibited high reliability and high specificity with respect to the presence of portal hypertension (92%) and the diagnosis of portal hypertensive gastropathy (88.31%).
CONCLUSION: The most suitable endoscopic criteria for the diagnosis of PHG were mosaic-like pattern, red-point lesions and cherry-red spots with no subdivisions, which were associated with a high rate of inter-observer reliability.
Keywords: Endoscopy, Cirrhosis, Portal hypertension, Portal hypertensive gastropathy, Stomach
Core tip: This article proposes a simplified approach for the diagnosis of portal hypertensive gastropathy, considering the presence or the absence of mosaic-like pattern, red point lesions and cherry-red spots, without subdivisions, as those criteria exhibit high agreement among observers and high specificity. This simplified approach is useful for future research on the natural history of this disease and its related factors, thus helping to clarify some of the current controversies due to the lack of homogeneity on the diagnostic criteria of portal hypertensive gastropathy.
INTRODUCTION
Portal hypertensive gastropathy (PHG) is characterized by an alteration in gastric mucosa that causes digestive hemorrhage in patients with portal hypertension (PH) of any etiology. Its incidence varies in the medical literature from 4% to 80% owing to a lack of consensus on endoscopic criteria for diagnosis[1-6].
PHG is histologically characterized by the dilation and tortuosity of the sub mucosal vessels, the thinning of the vascular wall and the increased areas of gastric mucosa occupied by vessels[7-11]. These alterations stem from hemodynamic modifications caused by portal hypertension syndrome and are not related to inflammatory infiltration[7-18].
McCormack et al[7] first described PHG in 1985 and proposed the first classification, attributing a risk of bleeding of 38% to 62% for severe forms and 3.5% to 31% for mild forms of PHG. Although simplified, this classification is problematic for grading intermediate endoscopic findings.
In 1994, the New Italian Endoscopy Club (NIEC) proposed an alternative classification including a moderate aspect of PHG for grading intermediate endoscopic findings[19].
Shortly after, in 1996, the Baveno Consensus, a scoring system for the most relevant aspects of PHG (the Baveno Score System), was developed and attributed a higher risk of bleeding in patients with the severe form of PHG and an odds ratio of 2.56[20].
The medical literature is not in agreement regarding the best classification and endoscopic criteria for diagnosing PHG, nor is there a consensus on its therapeutic management[21-28].
The purpose of this study was to analyze reliability among endoscopists in diagnosing PHG and to determine which endoscopic criteria, from the most utilized classifications (McCormack, NIEC and Baveno), are most suitable for diagnosing PHG.
MATERIALS AND METHODS
In a prospective study, a total of 100 patients were selected from those undergoing upper digestive endoscopy between January and July 2009 at the Endoscopy Service - Santa Casa School of Medical Sciences (Santa Casa de São Paulo Medical School), São Paulo, Brazil. This study was approved by the local Research Ethics Committee, and patients were included only after signing informed consent forms.
Fifty sequential patients with portal hypertension of various etiologies previously diagnosed based on clinical, laboratory and imaging exams who presented with esophageal varices were selected (Table 1). All patients with clinical or endoscopic signs of upper hemorrhage were included in this study. A control group was formed, consisting of 50 sequential patients with dyspeptic symptoms referred for upper digestive endoscopy without portal hypertension or a previous history of hepatopathy or congestive cardiopathy, abdominal ultrasounds disclosing normal liver and spleen, and a portal vein caliber of less than 12 mm.
Table 1.
Group with portal hypertension and esophageal varices n (%)
| Character | n = 50 |
| Mean age (52.7 yr) | |
| Sex | |
| Male | 28 (56) |
| Female | 22 (44) |
| Etiology | |
| Alcohol | 10 (20) |
| Schistosomiasis | 18 (36) |
| Hepatitis B | 2 (4) |
| Hepatitis C | 5 (10) |
| Alcohol and schistosomiasis | 1 (2) |
| Alcohol, schistosomiasis and Hepatitis B | 1 (2) |
| Autoimmune hepatitis | 1 (2) |
| Portal vein thrombosis | 1 (2) |
| Non-alcoholic hepatic steatosis | 1 (2) |
| Budd-Chiari Syndrome | 1 (2) |
| Biliary cirrhosis | 1 (2) |
| Idiopathic or not yet identified | 8 (16) |
| Child-pugh classification | |
| A | 42 (84) |
| B | 6 (12) |
| C | 2 (4) |
| Previous digestive bleeding | 32 (64) |
| Previous endoscopic treatment | 33 (66) |
| Using propanolol | 25 (50) |
Exams were performed under sedation and digitally recorded. Six images were selected from recordings, consisting of two from the antrum, two from the gastric body and two from the gastric fundus (not showing varices). The images were then analyzed by five independent expert endoscopists with over 15 years of experience in our service. First, the examiners were familiarized with the standards used in this trial and subsequently evaluated the selected images of each patient while blinded to patients’ clinical information. The varices were deliberately excluded from the images that were presented in sequential order to each endoscopist. The endoscopists were also blinded to each other’s comments and evaluations.
An electronic questionnaire, which included endoscopic criteria from PHG classifications (McCormack, NIEC and Baveno) and recorded the presence or otherwise of elevated or flat antral erosive gastritis, was used to collect and collate the results (Figure 1). The results were independently analyzed to determine their relationship with PHG.
Figure 1.
Electronic questionnaire.
Figures 2-4 compare endoscopic aspects with their classifications.
Figure 2.
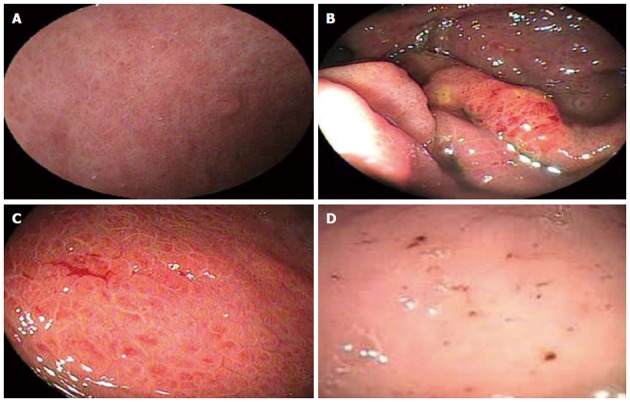
Endoscopic aspects of portal hypertensive gastropathy considered in the study and their corresponding classifications. A: Fine pink speckling - McCormack; B: Superficial reddening - McCormack; C: Diffuse hemorrhagic lesion - McCormack; D: Black brown spots - New Italian Endoscopy Club.
Figure 4.
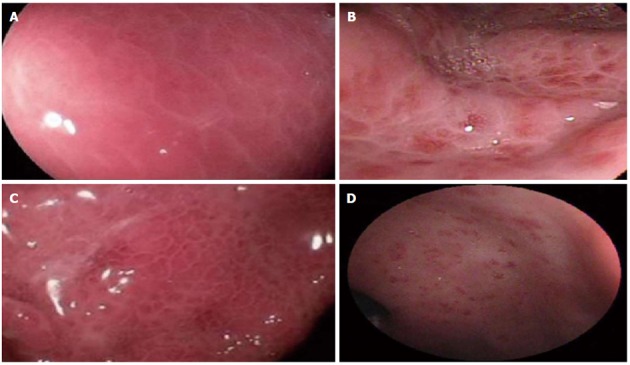
Endoscopic aspects of portal hypertensive gastropathy considered in the study and their corresponding classifications. A: Corresponds to “cherry-red spots” with different nomenclature in each classification as follows: discrete red spots - McCormack; cherry-red spots - New Italian Endoscopy Club (NIEC); B: Red-point lesions - NIEC; C: Isolated red marks - Baveno; D: Confluent red marks - Baveno.
Figure 3.
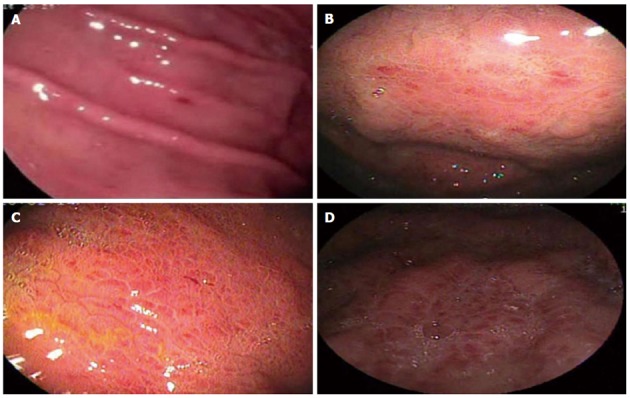
Endoscopic aspects of portal hypertensive gastropathy considered in the study and their corresponding classifications. A: Corresponds to “mosaic-like pattern” with different nomenclature in each classification as follows: mosaic-like pattern - McCormack; mild mosaic-like pattern - New Italian Endoscopy Club (NIEC); mild mosaic-like pattern - Baveno; B: Corresponds to “mosaic-like pattern” with different nomenclature in each classification as follows: red center mosaic-like pattern - McCormack; moderate mosaic-like pattern - NIEC; C: Corresponds to “mosaic-like pattern” with different nomenclature in each classification as follows: mosaic-like pattern - McCormack; severe mosaic-like pattern - NIEC; severe mosaic-like pattern - Baveno; D: GAVE - Baveno.
Due to inconsistencies in the medical literature on the role of histological analysis of standard endoscopic biopsies for diagnosing PHG, we decided not to perform biopsies in this study[29-31]. Due to the absence of an established gold standard for diagnosing PHG, the statistical analysis was performed in two stages. The first stage verified the correlation between each endoscopic criterion and the presence of PH, with the group of 50 patients without PH serving as a control. The second stage determined the correlation between each endoscopic criterion and the diagnosis of PHG. The establishment of a relationship between the endoscopic criterion and the presence of PH was a prerequisite for the potential correlation between the same criterion and PHG. If any criterion demonstrated an apparent relationship with PHG but not with PH, then it was deemed logically false.
The Statistical Package for Social Sciences version 17.0 was utilized for statistical analysis, adopting a 5% level of significance on Fisher’s Exact Test. Cronbach’s alpha was used to determine reliability among the five endoscopists, with values between 0 and 0.60 considered Unsatisfactory, values between 0.60 to 0.69 as Satisfactory, and values between 0.70 to 1.00 as a High degree of reliability.
RESULTS
For criteria from the McCormack classification (Table 2), the mosaic-like pattern was associated with high reliability, specificity (90%) and positive predictive value (82.76%) for the presence of PH, as well as sensitivity and negative predictive values of 100% for the diagnosis of PHG. Fine pink speckling and superficial reddening both exhibited unsatisfactory reliability, as well as low specificity (86%) and high false positive values (7%) for the presence of PH. In addition, these criteria exhibited low specificity (76.62%) and high false positive values (18%) for the diagnosis of PHG.
Table 2.
Analysis of the criteria from the McCormack classification for the presence of portal hypertension and portal hypertensive gastropathy
| Fine pink speckling | Superficial reddening | Mosaic-like pattern | Cherry-red spots | Diffuse hemorrhagic lesion | ||||||
| Diagnosis | PH | PHG | PH | PHG | PH | PHG | PH | PHG | PH | PHG |
| Sensitivity | 48.00% | 56.52% | 64.00% | 69.57% | 48.00% | 100.00% | 22.00% | 39.13% | 8.00% | 17.39% |
| Specificity | 86.00% | 76.62% | 80.00% | 66.23% | 90.00% | 92.21% | 98.00% | 96.10% | 100.00% | 100.00% |
| PPV | 77.42% | 41.94% | 76.19% | 38.10% | 82.76% | 79.31% | 91.67% | 75.00% | 100.00% | 100.00% |
| NPV | 62.32% | 85.51% | 68.97% | 87.93% | 63.38% | 100.00% | 55.68% | 84.09% | 52.08% | 80.21% |
| Accuracy | 67.00% | 72.00% | 72.00% | 67.00% | 69.00% | 94.00% | 60.00% | 83.00% | 54.00% | 81.00% |
| False negative | 26.00% | 10.00% | 18.00% | 7.00% | 26.00% | 0.00% | 39.00% | 14.00% | 46.00% | 19.00% |
| False positive | 7.00% | 18.00% | 10.00% | 26.00% | 5.00% | 6.00% | 1.00% | 3.00% | 0.00% | 0.00% |
| Significance | P < 0.001 | P =0.03 | P < 0.001 | P = 0.002 | P < 0.001 | P < 0.001 | P = 0.002 | P < 0.001 | P = 0.059 | P = 0.002 |
| Reliability (α) | 0.4301 | 0.4301 | 0.5321 | 0.5321 | 0.7992 | 0.7992 | 0.7532 | 0.7532 | 0.5741 | 0.5741 |
Unsatisfactory;
High. PPV: Positive predictive value; NPV: Negative predictive value; PH: Portal hypertension; PHG: Portal hypertensive gastropathy.
On the NIEC classification (Table 3), pink and red mosaic-like patterns were associated with unsatisfactory reliability, but red center mosaic-like patterns exhibited high reliability, thus defining PHG as moderate.
Table 3.
Analysis of the criteria from the New Italian Endoscopy Club classification for the presence of portal hypertension and portal hypertensive gastropathy
| Pink | Red center | Red | Red-point | Cherry-red | Black-brown | |||||||
| mosaic-like pattern | mosaic-like pattern | mosaic-like pattern | lesions | spots | spots | |||||||
| Diagnosis | PH | PHG | PH | PHG | PH | PHG | PH | PHG | PH | PHG | PH | PHG |
| Sensitivity | 26.00% | 52.17% | 16.00% | 34.78% | 6.00% | 13.04% | 28.00% | 47.83% | 22.00% | 39.13% | 2.00% | 4.35% |
| Specificity | 90.00% | 92.21% | 100.00% | 100.00% | 100.00% | 100.00% | 92.00% | 90.91% | 98.00% | 96.10% | 98.00% | 98.70% |
| PPV | 72.22% | 66.67% | 100.00% | 100.00% | 100.00% | 100.00% | 77.78% | 61.11% | 91.67% | 75.00% | 50.00% | 50.00% |
| NPV | 54.88% | 86.59% | 54.35% | 83.70% | 51.55% | 79.38% | 56.10% | 85.37% | 55.68% | 84.09% | 50.00% | 77.55% |
| Accuracy | 58.00% | 83.00% | 58.00% | 85.00% | 53.00% | 80.00% | 60.00% | 81.00% | 60.00% | 83.00% | 50.00% | 77.00% |
| False negative | 37.00% | 11.00% | 42.00% | 15.00% | 47.00% | 20.00% | 36.00% | 12.00% | 39.00% | 14.00% | 49.00% | 22.00% |
| False positive | 5.00% | 6.00% | 0.00% | 0.00% | 0.00% | 0.00% | 4.00% | 7.00% | 1.00% | 3.00% | 1.00% | 1.00% |
| Significance | P = 0.033 | P < 0.001 | P = 0.003 | P < 0.001 | P = 0.121 | P = 0.011 | P = 0.009 | P < 0.001 | P = 0.002 | P < 0.001 | P = 0.753 | P = 0.358 |
| Reliability (α) | 0.5691 | 0.5691 | 0.7272 | 0.7272 | 0.0792 | 0.0792 | 0.7522 | 0.7522 | 0.7532 | 0.7532 | 0.4081 | 0.4081 |
Unsatisfactory;
High. PPV: Positive predictive value; NPV: Negative predictive value; PH: Portal hypertension; PHG: Portal hypertensive gastropathy.
For criteria from the Baveno classification (Table 4), only red marks demonstrated high reliability and specificity (92%) for the presence of PH and high reliability for the diagnosis of PHG.
Table 4.
Analysis of the criteria from the Baveno classification for the presence of portal hypertension and portal hypertensive gastropathy
| Pink mosaic-like pattern | Red mosaic-like pattern | Isolated red marks | Confluent red marks | GAVE | ||||||
| Diagnosis | PH | PHG | PH | PHG | PH | PHG | PH | PHG | PH | PHG |
| Sensitivity | 26.00% | 52.17% | 6.00% | 13.04% | 30.00% | 43.48% | 10.00% | 21.74% | 8.00% | 4.35% |
| Specificity | 90.00% | 92.21% | 100.00% | 100.00% | 92.00% | 88.31% | 100.00% | 100.00% | 100.00% | 96.10% |
| PPV | 72.22% | 66.67% | 100.00% | 100.00% | 78.95% | 52.63% | 100.00% | 100.00% | 100.00% | 25.00% |
| NPV | 54.88% | 86.59% | 51.55% | 79.38% | 56.79% | 83.95% | 52.63% | 81.05% | 52.08% | 77.08% |
| Accuracy | 58.00% | 83.00% | 53.00% | 80.00% | 61.00% | 78.00% | 55.00% | 82.00% | 54.00% | 75.00% |
| False negative | 37.00% | 11.00% | 47.00% | 20.00% | 35.00% | 13.00% | 45.00% | 18.00% | 46.00% | 22.00% |
| False positive | 5.00% | 6.00% | 0.00% | 0.00% | 4.00% | 9.00% | 0.00% | 0.00% | 0.00% | 3.00% |
| Significance | P = 0.033 | P < 0.001 | P = 0.121 | P = 0.011 | P = 0.005 | P = 0.0014 | P = 0.028 | P < 0.001 | P = 0.059 | P = 0.429 |
| Reliability (α) | 0.5691 | 0.5691 | 0.0791 | 0.0791 | 0.7532 | 0.7532 | 0.5581 | 0.5581 | 0.5141 | 0.5141 |
Unsatisfactory;
High. PPV: Positive predictive value; NPV: Negative predictive value; PH: Portal hypertension; PHG: Portal hypertensive gastropathy.
Table 5 depicts the results of the statistical analysis of antral erosive gastritis and its variations, flat and elevated, in relation to the presence of portal hypertension and the diagnosis of portal hypertensive gastropathy. Antral elevated erosive gastritis exhibited high reliability and high specificity with respect to the presence of PH (92%) and the diagnosis of PHG (88.31%).
Table 5.
Analysis of antral erosive gastritis in the presence of portal hypertension and portal hypertensive gastropathy
| Antral erosive gastritis | Antral elevated erosive gastritis | Antral flat erosive gastritis | ||||
| Diagnosis | PH | PHG | PH | PHG | PH | PHG |
| Sensitivity | 34.00% | 39.13% | 26.00% | 34.78% | 8.00% | 4.35% |
| Specificity | 84.00% | 79.22% | 92.00% | 88.31% | 92.00% | 90.91% |
| PPV | 68.00% | 36.00% | 76.47% | 47.06% | 50.00% | 12.50% |
| NPV | 56.00% | 81.33% | 55.42% | 81.93% | 50.00% | 76.09% |
| Accuracy | 59.00% | 70.00% | 59.00% | 76.00% | 50.00% | 71.00% |
| False negative | 33.00% | 14.00% | 37.00% | 15.00% | 46.00% | 22.00% |
| False positive | 8.00% | 16.00% | 4.00% | 9.00% | 4.00% | 7.00% |
| Significance | P = 0.032 | P = 0.046 | P = 0.016 | P = 0.012 | P = 0.643 | P = 0.297 |
| Reliability (α) | 0.8402 | 0.8402 | 0.8622 | 0.8622 | 0.6411 | 0.6411 |
Satisfactory;
High. PPV: Positive predictive value; NPV: Negative predictive value; PH: Portal hypertension; PHG: Portal hypertensive gastropathy.
DISCUSSION
The analyzed classifications (McCormack, NIEC and BAVENO) comprise several common endoscopic aspects, albeit aspects that are occasionally analyzed from different perspectives, thereby affecting the level of agreement among the observers. Others classifications have been published, including pre- and post-treatment evaluations, but these classifications have been reported without exclusive diagnostic aspects[21-22].
The presence of any mosaic-like pattern, defined as polygonal areas with whitish reticular borders, is utilized in all three classifications studied. The McCormack Classification considers only its presence or absence but not variations in its inner polygonal area. In the present study, the mosaic-like pattern was associated with high reliability, specificity and positive predictive value for the presence of PH, as well as sensitivity and negative predictive values of 100% for the diagnosis of PHG, where its absence almost excluded this diagnosis. This finding corroborates the results of the study by Stewart et al[24] in which, out of the 100 patients diagnosed with PHG, 96 exhibited mosaic-like patterns.
Based on the NIEC classification, the mosaic-like pattern is subdivided into three and classified according to the color of the inner polygonal area as either pink, red center or red. In the present study, pink and red mosaic-like patterns were associated with unsatisfactory reliability, whereas a red center mosaic-like pattern had high reliability, defining PHG as moderate. Nevertheless, the red center may also be considered a red-point lesion or a cherry-red spot, characterizing the PHG as severe, thereby rendering this stratification of the pattern ambiguous and the NIEC classification inconsistent.
The Baveno Score System subdivides the mosaic-like pattern into two aspects: mild, corresponding to a pink mosaic-like pattern, and severe, which corresponds to a red mosaic-like pattern, which as mentioned above, was found to exhibit Unsatisfactory reliability and thus low agreement among observers. Although Stewart et al[24] also demonstrated agreement among observers analyzing the presence or absence of the mosaic-like pattern, with a Kappa Index of greater than 0.75, concordance decreased when this aspect was subdivided according to variation in the inner polygonal area.
The present results demonstrated that fine pink speckling and superficial reddening from the McCormack classification were associated with unsatisfactory reliability and, thus, the low agreement among observers. These criteria also exhibited low specificity and high false positive values for the presence of PH, as well as low specificity and high false positive values for the diagnosis of PHG. This result indicated that fine pink speckling and superficial reddening also occurred in the group without PH, possibly corresponding to enanthematous mucosal alterations unrelated to portal hypertension. McCormack et al[7], in his original article, emphasized that with the exception of the cherry-red spots, the endoscopic aspects he described for the diagnosis now called PHG were indistinguishable from gastritis.
PHG is classified according to its tendency to bleed. Nonetheless, diffuse hemorrhagic lesions and black brown spots (old mucosal hemorrhage) are utilized in the McCormack and NIEC classifications. This use reveals incoherence because these aspects are, concomitantly, both a cause (PHG) and a consequence (hemorrhage). Additionally, these parameters exhibited unsatisfactory reliability in the present study due to low inter-observer agreement. Occasionally, these tenuous hemorrhages may exhibit discrete clinical manifestations[2,18]. In our study, patients with suspected digestive bleeding were excluded. The exclusion of these cases may partly explain the low statistical significance of these criteria.
Mucosal delimited red alterations are utilized in all three classifications. Red-point lesions are employed in the NIEC classification whereas cherry-red spots are used in both the McCormack (called discrete red spots) and NIEC classification. The Baveno score system groups red-point lesions and cherry-red spots together under red marks. We found that the presence or absence of red alterations was associated with high reliability due to high agreement among the endoscopists, high specificity and high positive predictive value in relation to the presence of PH and the diagnosis of PHG (Tables 2-4), thus demonstrating that these endoscopic aspects are related to PH and PHG.
The Baveno score system splits these parameters by grouping the alterations as either isolated or confluent. Nevertheless, there is no definition of the confluence criterion, thus leading to subjective interpretation and unsatisfactory reliability in the present study. Stewart et al[24] studying patients with PHG, demonstrated high inter-observer agreement in relation to the presence or absence of red marks and a kappa index of greater than 0.75, indicating desirable agreement. However, this level became unsatisfactory when used with the confluence criterion, splitting the red marks of the endoscopic aspect into isolated and confluent categories.
Although the current study demonstrated that GAVE (Baveno score system) exhibited 100% specificity and 0% false positive results, suggesting a strong association with portal hypertension and PHG, this relationship failed to reach statistical significance. Some authors claim that PHG and GAVE are distinct entities with no correlations between them[32-34].
Analysis of antral erosive gastritis and its variations, flat and elevated, revealed that antral elevated erosive gastritis exhibited high reliability and high specificity with relation to the presence of PH and the diagnosis of PHG, thereby suggesting an association with PHG. Assef et al[35] observed a 37.5% rate of antral elevated erosive gastritis in patients with PHG. Using multivariate analysis, Auroux et al[36] demonstrated that 31.2% of patients with portal hypertension had gastric erosions related to PHG and not to the presence of Helicobacter pylori, alcohol abuse, Child classification or the severity of esophageal varices. Further studies including histological analyses are warranted to confirm this association.
All endoscopic parameters analyzed exhibited low accuracy for the presence of PH (Tables 2-4). This low accuracy is due to the low negative predictive values of each separate parameter. Therefore, it is important to analyze all of the endoscopic parameters in conjunction with PH.
Regarding criteria for the diagnosis of PHG, the mosaic-like pattern, pink mosaic-like pattern, mosaic-like pattern with red center, cherry-red spots and red-point lesions showed accuracies of 94%, 83%, 85%, 83% and 81%, respectively. Of these criteria, only the mosaic like-pattern offered high sensitivity (100%). As explained earlier, the subdivision of the mosaic-like pattern leads to low inter-observer agreement, whereas the mosaic-like pattern with red center is an incoherent subdivision, at the same time representing a mosaic-like pattern and a red point lesion or cherry red spot.
The unsatisfactory reliability and low inter-observer agreement of the analyzed classifications corroborate the findings reported in other studies. Yoo et al[25] analyzed McCormack and NIEC classifications and observed low kappa indices of 0.52 and 0.44, respectively, indicating low inter-observer agreement given that a desirable Kappa index is greater than 0.75. Stewart et al[24] analyzing the Baveno classification, found an unsatisfactory rate of agreement when mosaic-like patterns and red marks were subdivided.
It is clear that all three investigated classifications have inadequate endoscopic parameters. Nevertheless, analyzing binary criteria such as the presence or the absence of the mosaic-like pattern, red-point lesions and cherry-red spots, the diagnosis of PHG yields high inter-observer agreement and high specificity. This approach can prove useful for future research on the natural history of this disease and related factors, thus helping to clarify some of the current controversies, including studies with histologic findings and comparisons with the endoscopic criteria of the classifications that we have already begun.
In conclusion, the most suitable endoscopic criteria for the diagnosis of portal hypertensive gastropathy were mosaic-like pattern, red-point lesions and cherry-red spots (without subdivisions), all of which were associated with a high rate of inter-observer reliability.
COMMENTS
Background
Portal hypertensive gastropathy (PHG) is an alteration of gastric mucosa causing occult and sometimes massive digestive hemorrhage in patients with portal hypertension of any etiology.
Research frontiers
PHG remains an endoscopic diagnosis, and there are many endoscopic classifications. No histologic correspondence was proven, leading to an individual observer opinion in diagnosis and grading, with a low level of reliability among endoscopists.
Innovations and breakthroughs
The most used classifications of PHG comprise several common endoscopic aspects, albeit aspects that are sometimes analyzed from different perspectives, thereby affecting the level of agreement among observers and leading to no consensus on endoscopic diagnosis and grading.
Applications
By separating the most suitable endoscopic criteria for the diagnosis of PHG, authors found that mosaic-like pattern, red-point lesions and cherry-red spots (without subdivisions) were associated with high inter-observer reliability and should be used to simplify and standardize the PHG diagnosis and severity.
Peer review
PHG is frequently observed on an upper gastrointestinal endoscopy in patients of portal hypertension. However, there are no objective criteria to diagnose PHG, and there is no consensus on the best classification and endoscopic criteria in the medical literature. The authors have attempted to analyze the data regarding reliability of various endoscopic morphological features among different endoscopists based on the criteria of McCormack, New Italian Endoscopy Club and Baveno. The authors concluded that most suitable endoscopic criteria for the diagnosis of PHG are mosaic-like pattern, red point lesions and cherry red spot.
Footnotes
Supported by CAPES-MEC-Brazil-Grant master’s thesis
P- Reviewers Abbas Z, Fouad YM, Reddy DN, Hashimoto N S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Sarin SK, Sreenivas DV, Lahoti D, Saraya A. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;102:994–999. doi: 10.1016/0016-5085(92)90188-5. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, Montalbano L, Traina M, Pisa R, Menozzi M, Spanò C, Pagliaro L. Natural history of congestive gastropathy in cirrhosis. The Liver Study Group of V. Cervello Hospital. Gastroenterology. 1990;99:1558–1564. doi: 10.1016/0016-5085(90)90458-d. [DOI] [PubMed] [Google Scholar]
- 3.Calès P, Oberti F, Bernard-Chabert B, Payen JL. Evaluation of Baveno recommendations for grading esophageal varices. J Hepatol. 2003;39:657–659. doi: 10.1016/s0168-8278(03)00404-5. [DOI] [PubMed] [Google Scholar]
- 4.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645–663. doi: 10.1016/s1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 5.Sarin SK, Agarwal SR. Gastric varices and portal hypertensive gastropathy. Clin Liver Dis. 2001;5:727–67, x. doi: 10.1016/s1089-3261(05)70190-2. [DOI] [PubMed] [Google Scholar]
- 6.Thuluvath PJ, Yoo HY. Portal Hypertensive gastropathy. Am J Gastroenterol. 2002;97:2973–2978. doi: 10.1111/j.1572-0241.2002.07094.x. [DOI] [PubMed] [Google Scholar]
- 7.McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy. Gut. 1985;26:1226–1232. doi: 10.1136/gut.26.11.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albillos A, Colombato LA, Enriquez R, Ng OC, Sikuler E, Groszmann RJ. Sequence of morphological and hemodynamic changes of gastric microvessels in portal hypertension. Gastroenterology. 1992;102:2066–2070. doi: 10.1016/0016-5085(92)90333-t. [DOI] [PubMed] [Google Scholar]
- 9.Panés J, Bordas JM, Piqué JM, Bosch J, García-Pagán JC, Feu F, Casadevall M, Terés J, Rodés J. Increased gastric mucosal perfusion in cirrhotic patients with portal hypertensive gastropathy. Gastroenterology. 1992;103:1875–1882. doi: 10.1016/0016-5085(92)91447-c. [DOI] [PubMed] [Google Scholar]
- 10.Kotzampassi K, Eleftheriadis E, Aletras H. Gastric mucosal blood flow in portal hypertension patients--a laser Doppler flowmetry study. Hepatogastroenterology. 1992;39:39–42. [PubMed] [Google Scholar]
- 11.Ohta M, Yamaguchi S, Gotoh N, Tomikawa M. Pathogenesis of portal hypertensive gastropathy: a clinical and experimental review. Surgery. 2002;131:S165–S170. doi: 10.1067/msy.2002.119499. [DOI] [PubMed] [Google Scholar]
- 12.Dong L, Zhang ZN, Fang P, Ma SY. Portal hypertensive gastropathy and its interrelated factors. Hepatobiliary Pancreat Dis Int. 2003;2:226–229. [PubMed] [Google Scholar]
- 13.Iwao T, Toyonaga A, Ikegami M, Sumino M, Oho K, Shigemori H, Sakaki M, Nakayama M, Tanikawa K, Iwao J. Portal vein hemodynamics in cirrhotic patients with portal hypertensive gastropathy: an echo-Doppler study. Hepatogastroenterology. 1994;41:230–234. [PubMed] [Google Scholar]
- 14.Mezawa S, Homma H, Ohta H, Masuko E, Doi T, Miyanishi K, Takada K, Kukitsu T, Sato T, Niitsu Y. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol. 2001;96:1155–1159. doi: 10.1111/j.1572-0241.2001.03694.x. [DOI] [PubMed] [Google Scholar]
- 15.Bellis L, Nicodemo S, Galossi A, Guarisco R, Spilabotti L, Durola L, Dell’Unto O, Puoti C. Hepatic venous pressure gradient does not correlate with the presence and the severity of portal hypertensive gastropathy in patients with liver cirrhosis. J Gastrointestin Liver Dis. 2007;16:273–277. [PubMed] [Google Scholar]
- 16.Abraldes JG, Angermayr B, Bosch J. The management of portal hypertension. Clin Liver Dis. 2005;9:685–713, vii. doi: 10.1016/j.cld.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Sarin SK, Shahi HM, Jain M, Jain AK, Issar SK, Murthy NS. The natural history of portal hypertensive gastropathy: influence of variceal eradication. Am J Gastroenterol. 2000;95:2888–2893. doi: 10.1111/j.1572-0241.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- 18.Primignani M, Carpinelli L, Preatoni P, Battaglia G, Carta A, Prada A, Cestari R, Angeli P, Gatta A, Rossi A, et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. The New Italian Endoscopic Club for the study and treatment of esophageal varices (NIEC) Gastroenterology. 2000;119:181–187. doi: 10.1053/gast.2000.8555. [DOI] [PubMed] [Google Scholar]
- 19.Spina GP, Arcidiacono R, Bosch J, Pagliaro L, Burroughs AK, Santambrogio R, Rossi A. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. J Hepatol. 1994;21:461–467. doi: 10.1016/s0168-8278(05)80329-0. [DOI] [PubMed] [Google Scholar]
- 20.Sarin SK. Diagnostic issue: Portal hypertensive gastropathy and gastric varices. In: DeFranchis R, editor. Portal hypertension II. Proceeding of the second Baveno intenational consensus workshop on definitions, methodology and therapeutic strategies. Oxford: Blackwell Science; 1996. pp. 30–55. [Google Scholar]
- 21.Hashizume M, Sugimachi K. Classification of gastric lesions associated with portal hypertension. J Gastroenterol Hepatol. 1995;10:339–343. doi: 10.1111/j.1440-1746.1995.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanoue K, Hashizume M, Wada H, Ohta M, Kitano S, Sugimachi K. Effects of endoscopic injection sclerotherapy on portal hypertensive gastropathy: a prospective study. Gastrointest Endosc. 1992;38:582–585. doi: 10.1016/s0016-5107(92)70522-7. [DOI] [PubMed] [Google Scholar]
- 23.de Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol. 2000;33:846–852. doi: 10.1016/s0168-8278(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 24.Stewart CA, Sanyal AJ. Grading portal gastropathy: validation of a gastropathy scoring system. Am J Gastroenterol. 2003;98:1758–1765. doi: 10.1111/j.1572-0241.2003.07595.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoo HY, Eustace JA, Verma S, Zhang L, Harris M, Kantsevoy S, Lee LA, Kalloo AN, Ravich WJ, Thuluvath PJ. Accuracy and reliability of the endoscopic classification of portal hypertensive gastropathy. Gastrointest Endosc. 2002;56:675–680. doi: 10.1067/mge.2002.128539. [DOI] [PubMed] [Google Scholar]
- 26.Chiu YC, Lu LS, Wu KL, Tam W, Hu ML, Tai WC, Chiu KW, Chuah SK. Comparison of argon plasma coagulation in management of upper gastrointestinal angiodysplasia and gastric antral vascular ectasia hemorrhage. BMC Gastroenterol. 2012;12:67. doi: 10.1186/1471-230X-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prachayakul V, Aswakul P, Leelakusolvong S. Massive gastric antral vascular ectasia successfully treated by endoscopic band ligation as the initial therapy. World J Gastrointest Endosc. 2013;5:135–137. doi: 10.4253/wjge.v5.i3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5:6–13. doi: 10.4253/wjge.v5.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra SP, Dwivedi M, Misra V, Agarwal SK, Gupta R, Gupta SC, Mital VP. Endoscopic and histologic appearance of the gastric mucosa in patients with portal hypertension. Gastrointest Endosc. 1990;36:575–579. doi: 10.1016/s0016-5107(90)71167-4. [DOI] [PubMed] [Google Scholar]
- 30.Piñero R, Olavarría R, Urquiola G, Yaraure M, Ramón Poleo J. [Portal hypertensive gastropathy: histologic and endoscopic correlation] G E N. 1994;48:7–9. [PubMed] [Google Scholar]
- 31.Chaves DM, Sakai P, Mucenic M, Iriya K, Iriya Y, Ishioka S. Comparative study of portal hypertensive gastropathy in schistosomiasis and hepatic cirrhosis. Endoscopy. 2002;34:199–202. doi: 10.1055/s-2002-20291. [DOI] [PubMed] [Google Scholar]
- 32.Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138–144. doi: 10.1016/0016-5085(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 33.Spahr L, Villeneuve JP, Dufresne MP, Tassé D, Bui B, Willems B, Fenyves D, Pomier-Layrargues G. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739–742. doi: 10.1136/gut.44.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ripoll C, Garcia-Tsao G. Management of gastropathy and gastric vascular ectasia in portal hypertension. Clin Liver Dis. 2010;14:281–295. doi: 10.1016/j.cld.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assef MS, Valentino W, Nakamura MAC, Colaiacovo R, Rossini LG. The Study of Magnifying Endoscopy for the Diagnosis of Portal Hypertensive Gastropathy. Gastrointest Endosc. 2010;71:AB379. [Google Scholar]
- 36.Auroux J, Lamarque D, Roudot-Thoraval F, Deforges L, Chaumette MT, Richardet JP, Delchier JC. Gastroduodenal ulcer and erosions are related to portal hypertensive gastropathy and recent alcohol intake in cirrhotic patients. Dig Dis Sci. 2003;48:1118–1123. doi: 10.1023/a:1023772930681. [DOI] [PubMed] [Google Scholar]


