Abstract
The 95% ethanol extract of Astragalus has been demonstrated to have potent activity as an immunological adjuvant when administered with vaccines of various types. We endeavor here to identify the components of this extract that are responsible for this adjuvant activity. Mice were immunized with KLH conjugated to cancer carbohydrate antigens globo H and GD3 and cancer peptide antigen MUC1 combined with different Astragalus fractions or with commercially available Astragalus saponins and flavonoids. The antibody responses against cancer antigens and KLH were quantitated in ELISA assays, and toxicity was calculated by weight loss. Astragalosides II and IV were the most active components, but the toxicity of these two differed dramatically. Astragaloside II was the most toxic Astragalus component with 5–10% weight loss at a dose of 500 µg while astragaloside IV showed no weight loss at all at this dose, suggesting that astragaloside IV might be utilized as an immunological adjuvant in future studies. Several flavonoids also had significant adjuvant activity. However, when the activities of these known immunologically active components of Astragalus (and of endotoxin) are calculated based on the extent of their presence in the 95% ethanol extract, they provide only a small proportion of the immunological activity. This raises the possibility that additional uniquely active components of Astragalus may contribute to adjuvant activity, or that the adjuvant activity of Astragalus is greater than the activity of the sum of its parts.
Keywords: Astragalus membranaceus, Leguminosae, botanicals, conjugate vaccine, cancer vaccine, astragalosides, saponin
Introduction
Many widely used botanicals are claimed to have immunostimulant effects, but clear evidence that they are able to augment immunological responses against defined antigens is lacking. Screening botanicals for immunomodulatory activity after oral ingestion is difficult due to unknowns such as bioavailability (depending in part on issues such as formulation and concomitant food intake), the amount of active ingredient in the botanical selected, optimal dose, and appropriate assay. As a prelude to testing popular botanical medicines as immune modulators after oral ingestion, we have chosen to test their immunological activity as immunological adjuvants, mixed with antigens and injected subcutaneously, using resulting antibody titers as a read-out. We hypothesized that this would permit us to identify active botanicals and botanical fractions and to identify the active ingredient(s). Once identified, these ingredients could then be administered orally in bioavailability and immunogenicity studies.
When the breast cancer carbohydrate antigen globo H [1] or glycoprotein antigen MUC1, or neuroectodermal cancer ganglioside GD3 are conjugated to KLH, mixed with an immunological adjuvant and administered subcutaneously (s.c.), the magnitude of the antibody responses against globo H, MUC1, and GD3 and against KLH depend largely on the potency of the adjuvant [2]. This mix of carbohydrate autoantigens, peptide xenoantigen, and protein xenoantigen, weak, moderate, and strong immunogens, reflects the range of antigens targeted in vaccines against cancer and infectious diseases, and the range of antigens encountered in life. We have previously used this s.c. immunization model in mice to screen the immunomodulatory activity of a wide range of immunological adjuvants [2] and seven widely used botanicals selected based on prevalence of use and reports describing immunomodulatory activity [3]. The seven botanicals selected for that initial study were Astragalus [4], Coriolus [5,6], Echinacea [6,7], H-48 (a formula consisting of ten herbs) [8,9], maitake [7, 10], β-glucan of yeast origin [11], and turmeric [7, 12]. We found that only the 95% ethanolic extract of Astragalus (but not the water extract), Coriolus extracts, and yeast β-glucan had potent adjuvant activity. We focus here on identifying the active components in the 95% ethanolic extract of Astragalus.
Yang and colleagues have tested saponins extracted from a variety of botanical sources for hemolytic effect (a surrogate for toxicity) and have constructed vaccines containing ovalbumin as an antigen to measure their potency as immunological adjuvants when mixed with ovalbumin and injected subcutaneously [21]. They have identified the saponins from Astragalus as having a particularly high activity/toxicity ratio. While these reports are suggestive (they are the reason we included Astragalus in our initial screen), they do not guide us in the identification of the Astragalus components most responsible for the potent adjuvant activity identified in these or our previous study. Based on the Yang study, clearly the saponin fraction of Astragalus has adjuvant activity. Our goal here was to determine whether all of the Astragalus adjuvant activity resides in the saponin fraction and if so, which of the multiple saponins in this fraction are most active. Since activity of most adjuvants is dose related and the dose that can be administered is determined by local and systemic toxicity, our study includes toxicity determinations as well.
Materials and Methods
Botanicals (see Table 1)
Table 1.
Botanicals tested with vaccines: source, dose, and active ingredients.
| Botanical extract | Source | Lot # | Purity by HPLC |
Dose | Probable active ingredients |
|---|---|---|---|---|---|
| Astragalus (A. membranaceus) water crude extract | ICM | 500 µg | No activity | ||
| Astragalus (A. membranaceus) 50% ethanol crude extract | ICM | 500 µg | Saponin | ||
| Astragalus (A. membranaceus) 95% ethanol crude extract | ICM | 0.5–2 mg | Saponin | ||
| Astragalus 95% ethanol crude extract – water subfraction F1 | ICM | 2 mg | Saponin | ||
| Astragalus 95% ethanol crude extract – 30% ethanolic subfraction F2 | ICM | 2 mg | Saponin | ||
| Astragalus 95% ethanol crude extract – 95% ethanolic subfraction F3 | ICM | 100 µg – 2 µg | Saponin | ||
| Standards | Source | Lot # | Purity | Dose | Probable active Ingredients |
| Calycosin-7-O-β-D-glucoside | Weilie Xiao | 98% | 20–100 µg | Flavonoid | |
| Astragalosides I | Chromadex | 111195-(607) | 99.0% | 20–500 µg | Saponin |
| Astragalosides II | Chromadex | 11196-(607) | 99.5% | 4–500 µg | Saponin |
| Astragalosides IV | Chromadex | 11197-(203) | 99.9% | 20–500 µg | Saponin |
| Calycosin | Chromadex | 3071-(109) | 93.1% | 20–100 µg | Flavonoid |
| Formononetin | Chromadex | 6190-(971) | 91.0% | 20–500 µg | Flavonoid |
| Isoquercitrin | Chromadex | 9505-(311) | 91.2% | 500 µg | Flavonoid |
| Isorhamnetin-3-b-glucoside | Chromadex | 9535-(509) | 500 µg | Flavonoid | |
| Ononide ononin | Chromadex | 15371-(206) | 99.9% | 500 µg | Flavonoid |
| Yeast β-glucan | Biotec Pharmacon | 100 µg–2mg β-glucan | β-1,3 backbone β-1,6 side chains | ||
| Positive controls | |||||
| OPT-821 Quillaja saponaria, fraction 21 | Optimer Pharmaceuticals | >99% | 20 µg | Saponin |
The root of Astragalus membranaceus (Fisch.) Bge. (purchased from an herbal store in Mainland China) was provided by the Institute of Chinese Medicine (ICM), Chinese University of Hong Kong. A voucher specimen (no. HK 40399) of the Astragalus membranaceus studied here was deposited at the Herbarium of Agriculture, Fisheries and Conservation Department in Hong Kong. For the preparation of Astragalus extracts, the raw herb was cut into small pieces and refluxed with 95% ethanol for an hour twice. The supernatants obtained were combined and dried using a rotary evaporator to give the 95% ethanolic extract. Three subfractions of the 95% ethanolic extract of Astragalus were further prepared by passing through a D101 resin column and successively eluted with water, 30% ethanol, and 95% ethanol to give the corresponding water, 30%, and 95% ethanolic subfractions. Yeast β-glucan (SBG) was provided by Biotec Pharmacon and was > 95% pure.
Analysis of botanical composition
HPLC PDA and LC-MS technologies were used for the quantitative analysis. Standards isoquercitrin, isorhamnetin-3-O-glucoside, ononin, and calycosin were purchased from ChromaDex. Standards astragalosides I, II, and IV, and formononetin were obtained from Zhongxin Innova Laboratories. The purities of the standards are listed in Table 1. Standard calycosin-7-O-β-D-glucoside was isolated from 95% EtOH Astragalus extract using the following procedures. Dried Radix Astragali samples (5.0 g) were extracted with 95% aqueous ethanol at room temperature (3 × 100 mL). After the EtOH was removed in vacuo, the residue (1.52 g) was separated over reversed-phase C18 eluting with MeOH-H2O (1:4, 2:3, 1:1, 3:1, and 0:1) to give five fractions (I–V). Fraction II (250.0 mg) was further separated by Sephadex LH-20 eluting with methanol to give four subfractions (A–D). Calycosin-7-O-β-D-glucoside (1) (10.2 mg) was obtained from subfraction B by recrystallization. Its structure was determined by 1D and 2D NMR spectra, and the purity was more than 98% determined by HPLC-PDA technology.
Standard calycosin-7-O-β-D-glucoside was isolated from 95% EtOH Astragalus extract using reversed-phase chromatography as previously described [13]. Its structure was determined by 1D and 2D NMR spectra, and the purity was more than 98% determined by HPLC-PDA technology.
HPLC grade acetonitrile was from J. T. Baker; HPLC grade methanol was from E. Merck; distilled water was further purified by Milli-Q system (Millipore). Polyvinylidene difluoride (PVDF) syringe filters with a pore size of 0.45 µm were procured from National Scientific Co.
Stock solutions (1 mg/mL) for the nine standards were prepared by dissolving individual standards in HPLC grade MeOH. Working standard solutions containing each of the nine compounds were prepared by diluting the stock solutions with methanol to a series of proper concentrations. The solutions were brought to room temperature, and an aliquot of 10 µL was injected into HPLC or LC-MS for analysis. Calibration curves were obtained by plotting the peak area vs. the concentration of the standard.
Analytes including 95% ethanol crude extract, water subfraction F1, 30% ethanolic subfraction F2, and 95% ethanolic subfraction F3 were dissolved in HPLC grade MeOH and prepared into 20 mg/mL, which were filtered through a 0.45 µm syringe filters before injection. 10 µL of each sample was injected to HPLC and LC-MS for quantitative analysis of flavonoids and astragalosides, respectively. The mobile phase, both flavonoids and astragalosides, consisted of water (A) and MeCN (B) with a flow rate of 1mL/min. The mobile phase composition began with 0% B, followed by a linear increase to 10% B in 5min, 10–55% B in 30 min, 55–100% B in 10min. The column for separation is Phenomenex Hydro C18 column (4.6 × 250 mm, 5 mm) at 25°C. The peaks for the nine standards were baseline separated. For the six flavonoids, separations were carried out on a Waters 2695 HPLC equipped with a PDA dectector. For astragalosides I, II, and IV, LC-MS was performed on a Thermo Finnigan LCQ mass spectrometer in the positive mode with a Waters 2690 separations module. The instrument was equipped with an atmospheric pressure chemical ionization (APCI) source and controlled by Xcalibur software. The discharge current was set to 5 µA. The vaporizer and capillary temperatures were set to 450 and 200°C, respectively. Nitrogen was used as the sheath gas and auxiliary gas at flow rates of 80 and 10 units, respectively. A mass range of 200–2000 amu was scanned.
Vaccine production
Globo H hexasaccharide (molecular weight 1055 Da) was synthesized and conjugated to KLH (8 × 106 Da) essentially as previously described [12]. Globo H/KLH molar ratios in the conjugate ranging between 500/1 and 800/1were used in these studies. The globo H-KLH used here was purchased from Optimer Pharmaceuticals who synthesized it under contract. It was provided as globo H ceramide for use as a target in ELISA assays and as globo H-KLH conjugate for vaccine production. GD3was extracted from bovine buttermilk, purchased from Matreya, Inc. and conjugated to KLH as previously described [14,15]. The GD3/KLH molar ratio in the conjugate was 950/1. MUC1 peptide containing 1 ½ tandem repeats (32 amino acids) was conjugated to KLH using the hetero-bifunctional linker MBS as previously described [16]. KLH for vaccine production and serological target was purchased from Sigma. OPT-821 (> 95% pure QS-21) at a dose of 20 µg and provided by Optimer Pharmaceuticals or yeast β-glucan [3] at a dose of 250 µg were used as positive controls. The Astragalus fractions or purified components were used at doses between 4 µg and 2mg in individual experiments as indicated.
Vaccine administration
Six-week-old female C57Bl/6 mice were obtained from the Jackson Laboratory. Groups of 5 mice were immunized s.c. three times at 1-week intervals with globo H-KLH containing 3–5 µg of globo H, GD3-KLH containing 5 µg GD3, or MUC1-KLH containing 1 µg MUC1 mixed with either Astragalus fractions or purified components in 0.1 mL saline, 20 µg OPT-821 as a positive control. The Memorial Sloan-Kettering Cancer Center’s (MSKCC) Animal Care and Use Program operates in compliance with all applicable federal, state, and local regulations. Specifically, the program complies with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and has an assurance, # A33110-01, on file with the Office of Laboratory Animal Welfare. MSKCC is a registered research institution with the United States Department of Agriculture (registrant # 21-R-0072) and is fully accredited by the Association for the Accreditation of Laboratory Animal Care International.
Serological assays
Mice were bled from the retro-orbital sinus under general anesthesia seven days after the third immunization for ELISA, and sera was frozen for future testing.
ELISA: Enzyme linked immunosorbent assays were performed as described previously [14,15]. The target antigens are globo H ceramide, GD3, MUC1 peptide, or KLH. To determine the titers of antibodies, ELISA plates were coated with antigen, generally at 0.1 µg/well. Serially diluted sera in 1% HSA in PBS were added to wells of the coated plate and incubated for 1 hour at room temperature. Goat anti-mouse IgM or IgG conjugated with alkaline phosphatase (Southern Biotechnology) serve as second antibodies. The antibody titer is defined as the highest serum dilution showing an absorbance of 0.1 or greater over that of normal sera. A response is considered positive by ELISA if the titer of reactivity increased from undetectable pretreatment to at least 1:40 after vaccination, or in case of detectable pretreatment, by 8-fold.
Endotoxin assay
Endotoxin levels in the botanical samples were tested at Charles River Laboratories, using their Endosafe® PTS™ system.
Statistical analysis
For each experimental run, results in serological assays for treated mice were compared to the no-adjuvant controls using the Mann-Whitney test or Student’s t-test.
Results
The adjuvant activity of Astragalus fractions was determined. After vaccination with globo H-KLH plus or minus crude Astragalus extracts, we found that the entire potency of Astragalus as immunological adjuvant with globo H-KLH was present in the 95% ethanol crude extract portion as indicated in Table 2. This strongly suggested that activity was concentrated in the saponin or possibly flavonoid components. Significant augmentation of anti-KLH antibody titers using the 95% ethanol crude extract of Astragalus was confirmed in the 500 µg-2000 µg dose range in 9 of 11 subsequent experiments, though augmentation of antibody titers against globo H or GD3 was not seen. This 95% ethanol crude extract of Astragalus was further fractionated into three subfractions (eluted by water, 30% ethanol, or 95% ethanol), and the individual fractions tested. Each of these 3 fractions significantly augmented antibody titers against KLH in 2 of 3 experiments but in no case were antibody titers higher than with the same dose of the initial 95% ethanol “crude” Astragalus extract. The saponin (astragaloside) and flavonoid content of these fractions was quantified using HPLC and available known astragaloside and flavonoid positive controls, as summarized in Table 3. While neither could be detected in subfraction F1, subfraction F3 contained the astragalosides and most of the flavonoids, and subfraction F2 contained a high concentration of the flavonoid calycosin-7-O-β-D-glucoside.
Table 2.
ELISA antibody titers after vaccination with globo H-KLH plus or minus crude Astragalus extracts.
| Globo H-KLH vaccine plus |
Globo H IgM | KLH IgG |
|---|---|---|
| No adjuvant | 800(3), 1600, 3200 | 400, 800(2), 1600, 3200 |
| Water crude extract 500 µg | 400, 800, 1600(2), 3200 | 0, 400, 800(2), 3200 |
| 50% ethanol crude extract 500 µg | 400(2), 800(2), 1600 | 1600, 3200(2), 6400, 12800 |
| 95%ethanol crude extract 500 µg | 800, 1600(2), 3200, 6400 | 25600(3), 51200(2) |
| Yeast β-glucan 250 µg | 800, 1600(2), 3200, 12800 | 102400, 819200(2), 1638400(2) |
Reciprocal antibody titer. Number in parentheses is the number of mice with that titer, if more than 1.
Groups in bold are significantly (p ≤ 0.05) higher than the no adjuvant control group
Table 3.
Quantity of astragalosides and flavonoids in dried Astragalus root extracts.
| Qi, 2006 [26] Yu, 2007 [27] | ||||||
|---|---|---|---|---|---|---|
| Astragalus | 95% Ethanol crude extract (µg/mg) |
Water subfraction F1 (µg/mg) |
30% Ethanolic subfraction F2 |
95% Ethanolic subfraction F3 |
||
| Astragalosides | ||||||
| I | 0.61 | 1.15 | 1.86 ± 0.06* | ND** | ND | 21.15 ± 0.36* |
| II | 0.24 | 0.14 | 0.63 ± 0.06 | ND | ND | 7.27 ± 0.23 |
| Iv | 0.14 | 0.042 | 0.44 ± 0.03 | ND | ND | 3.76 ± 0.02 |
| Flavonoids | ||||||
| Calycosin | 0.09 | 0.03 | 1.92 ± 0.05 | ND | ND | 23.11 ± 0.14 |
| Calycosin-7-O-β-D-glucoside | 0.35 | 0.43 | 1.34 ± 0.02 | ND | 16.41 ± 0.07* | 9.03 ± 0.26 |
| Formononetin | 0.059 | 0.022 | 1.59 ± 0.05 | ND | ND | 12.03 ± 0.05 |
| Isoquercitrin | 0.051 | ND | ND | ND | ND | ND |
| Isorhamnetin-3-O-β-glucoside | 0.13 | 0.042 | ND | ND | ND | ND |
| Ononin | 0.092 | 0.101 | 1.14 ± 0.01 | ND | ND | 6.69 ± 0.17 |
Standard deviation of triplicates.
ND: not detectable < 0.005 µg/mg
The commercially available purified saponin components of Astragalus are astragaloside I, astragaloside II, and astragaloside IV. Seven experiments were conducted with these astragalosides. Table 4 demonstrates that all 3 astragalosides are able to augment the antibody response against both MUC1 and KLH when injected with MUC1-KLH. Overall, astragaloside I significantly augmented KLH antibody titers in 2 of 4 experiments and astragaloside IV in 3 of 5 experiments. In most cases, significant reactivity was limited to the 500 µg dose level. Astragaloside II demonstrated significant elevations in titers against KLH in 6 of 6 experiments, 4 of 6 experiments, and 3 of 6 experiments at the 500 µg, 100 µg, and 20 µg dose levels, respectively. In 2 of 6 experiments, the anti-KLH titers induced with astragaloside II at the 500 µg dose level approached the titers of (were not significantly lower than) those obtained with 20 µg of OPT-821. Despite the high anti-KLH antibody titers induced and the consistent induction of anti-GD3 titers between 1/80 and 1/320 when 20 µg of OPT-821 was used as adjuvant with GD3-KLH, in none of the experiments were astragalosides able to induce detectable antibodies against GD3.
Table 4.
Median ELISA antibody titers after vaccination with MUC1-KLH plus or minus Astragalus extract and astragalosides.
| GD3-KLH vaccine plus: | MUC1 IgG | KLH IgG |
|---|---|---|
| Pretreatment | < 40(5) | < 100(5) |
| No adjuvant | < 40(3), 40(2), 80(2), 320, 640, 1280 | 100(2), 400(6), 1600, 6400 |
| 95% Ethanol crude extract 2mg | 640(3),1280(2) | 1600(5) |
| 95% Ethanol crude extract 500 µg | 40, 320, 640(2), 1280 | 400(3), 1600(2) |
| Astragaloside I 500 µg | 160, 1280 2560(2), 5120 | 400,1600(2) 6400(2) |
| Astragaloside I 100 µg | 320(4),5120 | 400(2), 1600 (2), 6400 |
| Astragaloside II 100 µg | 1280(3) 2560, 5120 | 6400, 25600(3) 102400 |
| Astragaloside II 20 µg | 320, 640 1280(2), 5120 | 400, 1600(4) |
| Astragaloside IV 100 µg | 1280, 2560(2) 5120(2) | 1600(2), 6400(3) |
| Astragaloside IV 20 µg | 40, 80, 320(2), 1280 | 400,1600(2) 6400(2) |
| OPT-821 20 µg | 640, 1280(2) 2560, 5120 | 102, 400(5) |
Reciprocal antibody titer of each mouse (number of mice at that titer).
Groups in bold are significantly (p ≤ 0.05) higher than the 10 no adjuvant control mice
The commercially available purified flavonoid components of Astragalus are isoquercitrin, isorhamnetin-3-b-glucoside, ononin, calycosin, and formononetin. The adjuvant activities of these were tested in a series of experiments with MUC1-KLH as the antigen. MUC1 was highly immunogenic, as immunogenic as KLH, and a more sensitive indicator of adjuvant activity. The results of one experiment are summarized in Table 5. Astragalosides II and IV at a dose of 100 µg, and Astragalus 95% EtOH extract at a dose of 500 µg or 2 mg were the most active, though the flavonoids were also active in this experiment. Overall, ononin and isorhamnetin-3-O-glucoside were tested in a single experiment at a dose of 500 µg and failed to significantly elevate antibody titers against KLH while formononetin and isoquercitrin were positive at a dose of 500 µg against KLH in 1 of 2 experiments and at a dose of 100 µg induced antibodies against MUC1, but not KLH, in 1 experiment.
Table 5.
Median ELISA antibody titers after vaccination with MUC1-KLH plus Astragalus extract, subfractions, astragalosides, flavonoids, or a mixture of astragalosides and flavonoids.
| MUC1-KLH vaccine plus |
MUC1 IgG |
Mann- Whitney |
KLH IgG |
Mann- Whitney |
Endotoxin EU/dose |
|---|---|---|---|---|---|
| Calycosin 20 µg | 320 | 0.214 | 400 | 0.608 | |
| Calycosin 100 µg | 640 | 0.048 | 1600 | 0.075 | < 0.06 EU |
| Calycosin-7-O-β-D-glucoside20 µg | 160 | 0.901 | 400 | 0.295 | |
| Calycosin-7-O-β-D-glucoside 100 µg | 160 | 0.901 | 400 | 0.366 | |
| Formononetin 20 µg | 320 | 0.138 | 400 | 0.366 | |
| Formononetin 100 µg | 1280 | 0.016 | 1600 | 0.179 | < 0.06 EU |
| Astragaloside I 100 µg | 320 | 0.069 | 1600 | 0.142 | |
| Astragaloside I 500 µg | 2560 | 0.009 | 1600 | 0.058 | |
| Astragaloside II20 µg | 1280 | 0.016 | 1600 | 0.075 | |
| Astragaloside II 100 µg | 1280 | 0.004 | 25600 | 0.002 | 0.018 EU |
| Astragaloside IV 20 µg | 320 | 0.268 | 1600 | 0.143 | |
| Astragaloside IV 100 µg | 2560 | 0.003 | 6400 | 0.011 | < 0.005 EU |
| Astragalus 95 % EtOH extract crude 500 µg | 640 | 0.122 | 400 | 0.366 | < 0.009 EU |
| Astragalus 95% EtOH extract crude 2 mg | 640 | 0.026 | 1600 | 0.031 | 0.035 EU |
| Astragalus 95 % EtOH subfraction F1 100 µg | 640 | 0.048 | 400 | 0.366 | |
| Astragalus 95% EtOH subfraction F1 500 µg | 320 | 0.048 | 400 | 0.366 | |
| Astragalus 95 % EtOH subfraction F2 100 µg | 640 | 0.019 | 1600 | 0.075 | |
| Astragalus 95 % EtOH subfraction F2 500 µg | 2560 | 0.012 | 6400 | 0.011 | |
| Astragalus 95 % EtOH subfraction F3 100 µg | 800 | 0.122 | 1600 | 0.432 | |
| Astragalus 95 % EtOH subfraction F3 500 µg | 640 | 0.031 | 1600 | 0.020 | |
| Mixture 20 µg | 640 | 0.036 | 400 | 0.661 | |
| Mixture 100 µg | 1280 | 0.026 | 1600 | 0.015 | |
| OPT-821 20 µg | 1280 | 0.007 | 102400 | 0.002 | < 0.005 EU |
| Endotoxin 2EU | 2560 | 0.004 | 6400 | 0.007 | |
| No adjuvant (10 mice) | 60 | 400 |
Median antibody titer of 5 mice.
Mixture is Calycosin, Calycosin-7-O-β-D-glucoside, formononetin, astragaloside I, astragaloside II, and astragaloside IV in weight of 3, 2, 3, 3, 1, and 1
To address the possibility that endotoxin contamination of the various extracts was responsible for some or all reactivity, several of the relevant extracts were sent to Charles River Laboratories for determination of endotoxin levels. All levels were less than 0.04 EU per dose (see Table 5). When the 2 EU dose of endotoxin demonstrated significant adjuvant activity (Table 5), we performed an additional experiment to gage the impact of lower doses of endotoxin. Doses of 0.5 EU or lower had no significant adjuvant activity (Table 6).
Table 6.
Median ELISA antibody titers after vaccination with MUC1-KLH plus or minus Astragalus extract, astragaloside IV, OPT-821, and endotoxin.
| MUC1-KLH vaccine plus: | KLH IgG | Mann-Whitney |
|---|---|---|
| Astragalus 95% EtOH crude extract 2 mg | 25600 | 0.011 |
| Astragaloside IV100 µg | 25600 | 0.050 |
| OPT-821 20 µg | 102400 | < 0.001 |
| Endotoxin 0.5 EU | 6400 | 0.259 |
| Endotoxin 0.1 EU | 6400 | 0.125 |
| Endotoxin 0.02 EU | 6400 | 0.178 |
| No adjuvant (10 mice) | 6400 |
Median antibody titer of 5 mice
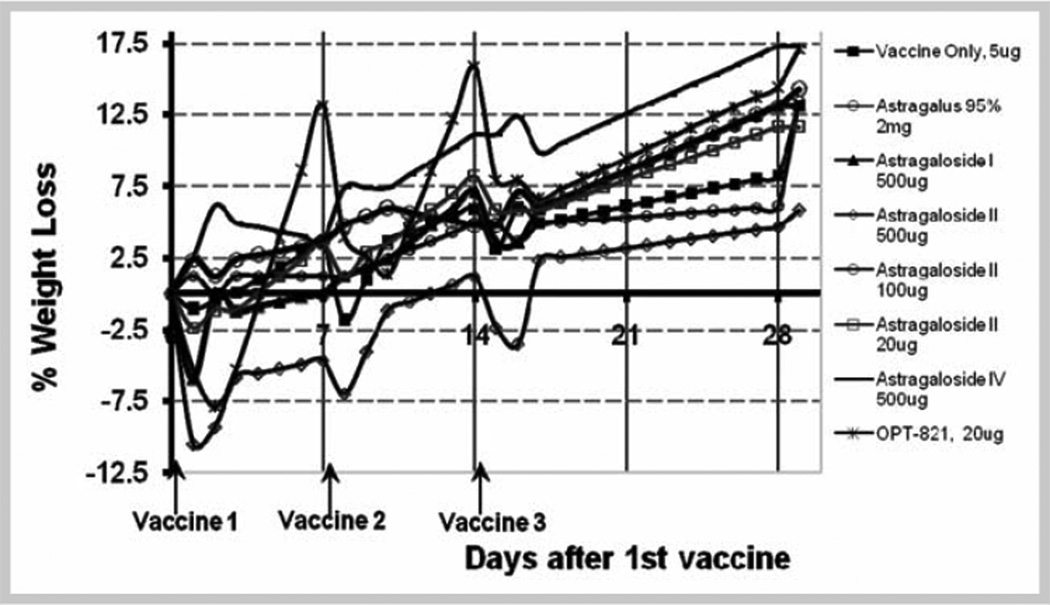
Weight loss after immunizations was used as a way to gauge toxicity. Significant weight loss (between 5% and 10% of body weight) was consistently seen with the 500 µg dose of astragaloside II in the 4 experiments where this was evaluated (see Fig. 1). The same applies to OPT-821 at the 20 µg dose level, but not to any of the other extracts tested in the experiments described here. This is an indication that astragaloside II and OPT-821 doses cannot be further escalated to gain immunogenicity. Doses of the other preparations could be further escalated safely, but doses here were limited by expense (all of the commercially available astragalosides and flavonoids) or volume (Astragalus extracts).
Fig. 1.
Toxicity study in C57BL/6J female mice injected s.c. with different doses of astragaloside compared to 20 µg OPT 821.
Compared to the quantities of astragalosides and flavonoids described by Qi and Yu in whole Astragalus root preparations, the ethanol extraction procedure that we utilized did purify the astragalosides by several fold and most of the flavonoids by 4–30 fold [26,27]. Consistent adjuvant activity was demonstrated in our experiments with the 500 µg and 2 mg doses of this 95% EtOH extract of Astragalus. At the 1000 µg dose level, there are less than 2 µg of any of the major individual astragalosides or flavonoids known to be in Astragalus (see Table 3 under 95% EtOH extract). A mixture of the 6 astragalosides and flavonoids most highly expressed in the 95% EtOH extract of astragalus was tested at the 20 and 100 µg dose levels. Significantly increased antibody titers resulted against MUC1 at the lower mixture dose and against MUC1 and KLH at the 100 µg dose level (Table 5). The 100 µg dose level contained 23 µg of calycosin, formononetin, and astragaloside I, 15 µg of calycosin-7-O-β-D-glucoside, and 8 µg of astragaloside II and astragaloside IV. None of these individual doses resulted in demonstrable adjuvant activity, so certainly the 20 µg mixture dose and probably the 100 µg dose demonstrated more than additive reactivity.
Discussion
We have identified saponins and in particular the saponin fraction QS-21 and the semisynthetic saponin mix GPI-0100 as uniquely potent immunological adjuvants when mixed with conjugate vaccines containing glycolipids or peptides chemically conjugated to keyhole limpet hemocyanin (KLH) [2,17]. The maximal doses of QS-21 used in mice (20 µg) and patients (100 µg) were selected based on toxicity, acceptable weight loss in mice, and acceptable local erythema/induration and systemic flu-like symptoms in patients [18]. The quest continues for immunological adjuvants with more potent adjuvant activity and more limited local and systemic toxicities. While most studies have been focused on Quillaja saponaria saponins, there are many additional botanicals expressing other saponins. A possible case in point is Astragalus membranaceus which we have demonstrated previously [3] and demonstrate here to have significant adjuvant activity in the 95% ethanolic subfraction.
A. membranaceus is a well-known traditional Chinese medicinal plant used widely for a variety of indications. The applicable part of Astragalus is the root. Astragalus root preparations contain a variety of constituents thought to be immunologically active including isoflavonoids and triterpene saponins (primarily multiple astragalosides), as well as a variety of other components including polysaccharides, multiple trace minerals, amino acids, and coumarins [19–23]. We have attempted to identify the components with greatest adjuvant activity in our model by further fractionating the 95% ethanolic Astragalus fraction into 3 fractions containing saponins, flavonoids, and the remainder, and by testing the commercially available components which include the 3 major saponins and most of the major flavonoids. We demonstrate here that the 95% ethanolic subfraction of A. membranaceus (the saponin rich fraction) augments antibody responses not only against strong xenoantigens such as KLH but also against moderately immunogenic peptides such as MUC1 and weak glycolipid autoantigens such as globo H by 4- to 20-fold in different experiments. At least 15 separate saponins have been identified among A. membranaceus saponins and their chemical structures defined [21]. Four of these (astragalosides I–IV), the most heavily expressed, are commercially available. While purifying the other 11 individual saponins from extracted saponins for use as adjuvants would be difficult, the recent description of the total chemical synthesis of QS-21 [24] raises the possibility that these remaining individual A. membranaceus saponins could be synthesized. The described low hemolytic activity of these saponins and their potent adjuvant activity suggest that this might be a fruitful approach to identification of new adjuvants. It is expected that even among this family of saponins, some would have greater or lesser toxicity and that this might be distinct from their adjuvant activity.
We have now conducted a quantitative analysis of the saponins and flavonoids present in the 95% ethanol fraction. Most of the major peaks have been identified and quantitated previously and are available commercially. Consequently, we were able to explore the immunological adjuvant potency of these components in the context of antibody response against our KLH conjugate vaccines. We confirmed the safety and adjuvant potency of astragalosides II and IV which are clearly the most immunologically active of the various components present in the 95% ethanol extract of Astragalus but also identified a variety of flavonoids with significant adjuvant activity. Each of these components were well tolerated at immunologically active doses with only astragaloside II at a dose of 500 µg inducing significant weight loss after each immunization.
None of the flavonoids demonstrated detectable immunological activity in our model at doses of 100 µg or lower, and even astragalosides II and IV, the most active Astragalus components, demonstrated only low level activity at a dose of 20 µg and no detectable activity at a dose of 4 µg. The 500 µg dose of 95% ethanolic fraction of Astragalus contains less than 1.1 µg of astragalosides II and IV combined and less than 10 µg of the major flavonoids combined. When the eight active Astragalus components were mixed together at the ratios detected in the 95% ethanolic extract and administered at different doses, activity was seen only at the 100 µg total dose but not at the 20 µg dose. This represents 10- fold the amount of this mixture in 500 µg of the 95% EtOH extract of Astragalus. These results suggest that the immunologic activity demonstrated by the 95% ethanolic extract of Astragalus is not determined by any one or two components but by the entire mix including components not yet detected or at least not identified here.
The contribution of even low levels of contaminating endotoxin is always a concern when studying adjuvant activity. The low levels of endotoxin (0.035 and 0.018 EU) present in the Astragalus 95% EtOH and astragaloside II extracts, but not in astragaloside IV, were sufficient to contribute to adjuvant activity. While these levels of endotoxin were not high enough to explain all or even most of the adjuvant activity seen with these extracts, they may have been responsible for a small amount of the activity. Given the low levels of the known adjuvant active components in the 95% ethanol Astragalus extract, it may be that there are additional active components yet to be defined. The recent report from Liu et al. [25] demonstrating that part of the immunological activity of Radix Astragali and Radix Hedysari resides in their polysaccharides is consistent with this possibility. It is also possible that the components are synergistic and more active than the sum of the immunologic activities of the component parts (including endotoxin), although this is less likely since the mixture of known components was not uniquely active. The consequences of these findings are that there will be no easy short cuts to determining batch-to-batch immunologic consistency for different batches of the same Astragalus preparations or batches obtained from different sources. It also suggests that studies aimed at determining the relevant bioavailability from an immunologic point of view of Astragalus after oral ingestion will be exceedingly difficult given the many individual components which contribute to activity. Finally, they suggest that astragaloside IV has a uniquely favorable adjuvant potency/toxicity ratio and might be an excellent immunological adjuvant for further study if it could be obtained more economically.
Acknowledgments
This project was supported by Grant Number 1 P50 AT002779-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, or the National Institute of Health.
The authors would like to thank Professor Cao Hui (National Engineering Research Centre for Modernization of Traditional Chinese Medicine, Guangdong, China) for the morphological identification of the root of Astragalus membranaceus.
Abbreviations
- BSA
bovine serum albumin
- HSA
human serum albumin
- KLH
keyhole limpet hemocyanin
References
- 1.Ragupathi G, Park TK, Zhang S, Kim IJ, Graeber K, Adluri S, Lloyd KO, Danishefsky SJ, Livingston PO. Immunization of mice with the synthetic hexasaccharide Globo H results in antibodies against human cancer cells. Angew Chem Int Ed Engl. 1997;36:125–128. [Google Scholar]
- 2.Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000;19:530–537. doi: 10.1016/s0264-410x(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 3.Ragupathi G, Yeung KS, Leung PC, Lee M, Lau CB, Vickers A, Hood C, Deng G, Cheung NK, Cassileth B, Livingston P. Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine. 2008;26:4860–4865. doi: 10.1016/j.vaccine.2008.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 5. Astragalus membranaceus. Monograph. Altern Med Rev. 2003;8:72–77. [No authors listed]. [PubMed] [Google Scholar]
- 6.Chu KK, Ho SS, Chow AH. Coriolus versicolor: a medicinal mushroom with promising immunotherapeutic values. J Clin Pharmacol. 2002;42:976–984. [PubMed] [Google Scholar]
- 7.Spellman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 2006;11:128–150. [PubMed] [Google Scholar]
- 8.Kamiyama H, Takano S, Ishikawa E, Tsuboi K, Matsumura A. Anti-angiogenic and immunomodulatory effect of the herbal medicine “Juzen-taiho-to” on malignant glioma. Biol Pharm Bull. 2005;28:2111–2116. doi: 10.1248/bpb.28.2111. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Sakurai MH, Kiyohara H, Yamada H. Orally administered decoction of Kampo (Japanese herbal) medicine, “Juzen-Taiho-To” modulates cytokine secretion and induces NKT cells in mouse liver. Immunopharmacology. 2000;46:149–161. doi: 10.1016/s0162-3109(99)00166-6. [DOI] [PubMed] [Google Scholar]
- 10.Kodama N, Komuta K, Nanba H. Can maitake MD-fraction aid cancer patients? Altern Med Rev. 2002;7:236–239. [PubMed] [Google Scholar]
- 11.Smith JESR. Immunomodulatory activities of mushroom glucans and polysaccharide-protein complexes in animals and humans, Chapter 6. London. Cancer Res UK. 2002:106. [Google Scholar]
- 12.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Xiao W, Wang Q, Unachukwu U, Lau CB, Jiang B, Hong F, et al. Chemical and genetic assessment of commercial Radix Astragali (Astragalus spp.) by ion trap LC-MS and nuclear ribosomal DNA sequence analyses. J Agric Food Chem. doi: 10.1021/jf1028174. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragupathi G, Cappello S, Yi SS, Canter D, Spassova M, Bornmann WG, Danishefsky SJ, Livingston PO. Comparison of antibody titers after immunization with monovalent or tetravalent KLH conjugate vaccines. Vaccine. 2002;20:1030–1038. doi: 10.1016/s0264-410x(01)00451-0. [DOI] [PubMed] [Google Scholar]
- 15.Ragupathi G, Meyers M, Adluri S, Howard L, Musselli C, Livingston PO. Induction of antibodies against GD3 ganglioside in melanoma patients by vaccination with GD3-lactone-KLH conjugate plus immunological adjuvant QS-21. Int J Cancer. 2000;85:659–666. doi: 10.1002/(sici)1097-0215(20000301)85:5<659::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, Moynahan M, Houghton A, Norton L, Livingston PO. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000;6:1693–1701. [PubMed] [Google Scholar]
- 17.Kim SK, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston PO. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine. 1999;18:597–603. doi: 10.1016/s0264-410x(99)00316-3. [DOI] [PubMed] [Google Scholar]
- 18.Livingston PO, Adluri S, Helling F, Yao TJ, Kensil CR, Newman MJ, Marciani D. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994;12:1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 19.Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. II. Reversal of cyclophosphamide-induced immune suppression by administration of fractionated Astragalus membranaceus in vivo . J Clin Lab Immunol. 1988;25:125–129. [PubMed] [Google Scholar]
- 20.Sun HX, Qin F, Ye YP. Relationship between haemolytic and adjuvant activity and structure of protopanaxadiol-type saponins from the roots of Panax notoginseng . Vaccine. 2005;23:5533–5542. doi: 10.1016/j.vaccine.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 21.Yang ZG, Sun HX, Fang WH. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice. Vaccine. 2005;23:5196–5203. doi: 10.1016/j.vaccine.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Leung AY, Foster S. 2nd. New York, NY: John Wiley & Sons; 1996. Encyclopedia of common natural ingredients used in food, drugs and cosmetics. [Google Scholar]
- 23.Upton RSC. Astragalus root: Analytical, quality control, and therapeutic monograph. Scotts Valley: American Herbal Pharmacopoeia. 1999:1–25. [Google Scholar]
- 24.Kim YJ, Wang P, Navarro-Villalobos M, Rohde BD, Derryberry J, Gin DY. Synthetic studies of complex immunostimulants from Quillaja saponaria: synthesis of the potent clinical immunoadjuvant QS-21Aapi. J Am Chem Soc. 2006;128:11906–11915. doi: 10.1021/ja062364i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Hu X, Yang Q, Yu Z, Zhao Z, Yi T, Chen H. Comparison of the immunoregulatory function of different constituents in radix astragali and radix hedysari. J Biomed Biotechnol 2010. 2010:479426. doi: 10.1155/2010/479426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi LW, Yu QT, Li P, Li SL, Wang YX, Sheng LH, Yi L. Quality evaluation of Radix Astragali through a simultaneous determination of six major active isoflavonoids and four main saponins by high-performance liquid chromatography coupled with diode array and evaporative light scattering detectors. J Chromatogr A. 2006;1134:162–169. doi: 10.1016/j.chroma.2006.08.085. [DOI] [PubMed] [Google Scholar]
- 27.Yu QT, Qi LW, Li P, Yi L, Zhao J, Bi Z. Determination of seventeen main flavonoids and saponins in the medicinal plant Huang-qi (Radix astragali) by HPLC-DAD-ELSD. J Sep Sci. 2007;30:1292–1299. doi: 10.1002/jssc.200600422. [DOI] [PubMed] [Google Scholar]