Abstract
OBJECTIVE
In this study, we seek to investigate the effects of simvastatin on proliferation, migration and apoptosis in human U251 and U87 glioma cells and the underlying molecular mechanism.
METHODS
We used colony formation assay to test the cell proliferation, in vitro scratch assay to examine the cell migration, and caspase-3 activity assay, annexin V staining and cytochrome C release to evaluate the cell apoptosis. Lipid raft fractions were isolated from glioma cells. Total cholesterol content assay was used to test the change of cholesterol level in lipid raft fractions. Immunocytochemistry staining was performed to detect the changes of lipid rafts in cell membrane. Western blotting analysis was performed to examine the signal transduction both in cells and in lipid raft fractions.
RESULTS
Simvastatin inhibited proliferation and migration of U251 and U87 cells dose-dependently. Simvastatin induced an increase of caspase-3 activity, annexin V staining, and downregulated the PI3K/Akt pathway. Simvastatin also decreased cholesterol content in lipid raft fractions, suppressed caveolin-1 expression in the lipid rafts and induced Fas translocation into lipid rafts, suggesting that simvastatin may inhibit pro-survival PI3K/Akt pathway and trigger caspase-3-dependent apoptotic cell death through the modulation of lipid rafts.
CONCLUSION
These results suggest that modulation of lipid rafts, Fas translocation and PI3K/Akt/caspase-3 pathway are involved in the antitumor effect of simvastatin and it may have a potential role in cancer prevention and treatment.
Keywords: apoptosis, glioma, lipid rafts, PI3K/Akt pathway, simvastatin
Gliomas account for approximately 70% of the new cases of malignant primary brain tumors that are diagnosed in adults in the United States each year (34). Gliomas are resistant to conventional therapies including radiation and chemotherapy. Despite the current advances in aggressive combination therapies, the median survival of patients with malignant glioma has not improved (17). To prolong this survival, novel therapeutic approaches are needed. Gliomas, along with other malignant brain tumors, have shown very high rates of cholesterol synthesis and increased activities of hydroxy-methyl-glutaryl-coenzyme A (HMG-CoA) reductase (14). Statins are HMG-CoA reductase inhibitors that are widely used and well tolerated drugs for treating hypercholesterolemia, coronary heart disease and stroke (11). Therefore, targeting lipid metabolism with statins may offer a promising approach to the treatment of glioma (28).
There are emerging interests in the use of statins as anticancer agents because of their anti-proliferative, pro-apoptotic and anti-invasive properties (8). Mevalonic acid is a product of HMG-CoA reductase and an essential precursor of isoprenoid compounds including farnesyl isoprenoid, cholesterol, dolichol, and ubiquinone (13). Results from several studies have implied that by limiting mevalonate availability statins may influence not only the cholesterol levels but also the expression of several proteins, including Ras/Raf/MAP kinases and RhoGTPases (15, 36). Simvastatin, a lipophilic statin which crosses the blood-brain barrier, inhibits the synthesis of mevalonic acid by suppressing activity of HMG-CoA reductase. By inhibiting protein isoprenylation, simvastatin exerts important cellular effects, including a reduction of cell proliferation and an induction of apoptosis (24). Thus, simvastatin may have significant antitumor activity, which is further supported by experimental (33) and clinical (27) studies.
In the current study, we tested the effects of simvastatin in the suppression of cell growth and induction of apoptosis in U251 and U87 cells, and investigated its underlying signaling mechanisms. Our results showed that simvastatin reduced cellular proliferation and migration and induced apoptotic cell death in U251 and U87 cells in a dose- and time-dependent manner. These effects of simvastatin were associated with a decrease of Akt phosphorylation, an increase of caspase-3 activation, a depletion of cholesterol content, a modification of lipid rafts in cell membrane, and a translocation of Fas into lipid rafts. These results suggest modulation of lipid rafts, Fas translocation and PI3K/Akt/caspase-3 pathway is involved in the antitumor effects of simvastatin.
Materials and Methods
Materials
Ten mg of simvastatin (0.024 mmol) was dissolved in 0.2 ml of 100% ethanol and then diluted to a volume of 1 ml with DSMO. The stock solution of simvastatin (24 mM) was further diluted to appropriate concentrations with cell culture medium immediately before use. Control experiments contain DMSO only. A specific PI3K inhibitor LY294002 [2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one] and a selective caspase-3 inhibitor DEVD [Z-DEVD-FMK, Z-Asp-(OH3)-Glu(OH3)-Val-Asp(OH3)-CH2F] were obtained from Calbiochem (La Jolla, CA). Antibodies against phospho-Akt (ser473), Akt and cleaved (active) caspase-3 (Asp-175) were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against actin (I-19) and pro-caspase-3 (E8) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody against caveolin-1 (Marker for lipid rafts) was purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
Human U251 and U87 glioma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 units/ml of penicillin and 100 μg/ml of streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified incubator containing 5% CO2 and 95% air. A U251-GFP clone stably expressing green fluorescent protein (GFP) was cultured under the same conditions and used for a colony formation assay, in vitro scratch assays and caveolin-1 immunostaining.
Isolation of Lipid Rafts
Lipid rafts were prepared using a Sigma Caveolea/Rafts Isolation Kit. All procedures were carried out on ice. After the cells were collected, 1 ml of lysis buffer containing 1% Triton X-100 was added and incubated on ice for 30 minutes. The density gradient was made of 5 layers of OptiPrep with the following different concentrations: 35%, 30%, 25%, 20%, and 0%. The lower layer (35% OptiPrep) contained the cell lysate. The density gradient was centrifuged at 200,000 × g (70.1 Ti rotor, Beckman Coulter, Fullerton, CA) for 4 h at 4 °C. One-ml fractions were carefully collected from top to bottom of the ultracentrifuge tube and transferred to a microcentrifuge tube. The protein concentration of each fraction was determined by a BCA (bicinchoninic acid) protein assay kit (Pierce, Rockford, IL) Total cholesterol of each fraction was determined using a Wako CII Total Cholesterol assay kit (Wako Chemicals, Inc., Richmond, VA). Lipid raft-containing fractions were tracked by the enrichment of the cholesterol-binding protein caveolin-1.
Colony Formation Assay
Two ml of 0.5% Low Melt agarose (Bio-Rad, Hercules, CA) in DMEM supplemented with 10% FBS was poured into each well of 6-well plates and cooled down at 4°C for 1 hour. Two ml of 0.35% agarose in DMEM+10% FBS containing 5000 U251-GFP cells were then poured onto the bottom agarose layer and left at room temperature for 30 minutes. Finally, 2 ml of DMEM+10% FBS containing 0, 1, 5, or 10 μM of simvastatin was added on the top of the second agarose layer. Plates were incubated at 37°C in a humidified incubator with 5% CO2. Medium was changed twice a week and fresh simvastatin was added. After 19 days of incubation, colonies were stained with 0.5 ml of 0.005% Crystal Violet for 2 hours at room temperature and counted in ten randomly selected fields from each well using a light microscope. The average number of colonies per field was calculated.
In vitro Scratch Assay
U251-GFP and U87 cells were seeded in two 4-well chambers until confluent. In vitro scratch assay was performed using a sterile 200-μl pipette tip to scratch several straight lines on the cell monolayer. Immediately after scratching, one 4-well chamber was washed with 1X PBS and fixed with 4% paraformaldehyde for 20 minutes and used as a baseline control. Cells in the other 4-well chamber were treated with 0, 1, 5, or 10 μM of simvastatin for 24 hours. Cells were then fixed and images were captured by a fluorescence microscope. The gap distance was measured and the average was calculated for each treatment condition.
Caspase-3 Activity Assay
Cells were collected from different experimental groups and cell lysates were prepared. Protein concentration was determined by using a BCA protein assay kit (Pierce). Equal amounts of lysates were used for caspase-3 activity assay measured at a wavelength of 405 nm using the detection of chromophore p-nitroaniline (pNA) after its cleavage by caspase-3 from the labeled caspase-3 specific substrate, DEVD-pNA (Calbiochem). The data are presented as pmoles of pNA per μg of cell lysate per hour of incubation.
Isolation of Mitochondrial and Cytosolic Fractions
Cells were collected from different experimental groups and mitochondrial and cytosolic fractions were isolated using a Mitochondrial/Cytosol Fractionation kit (Biovision, Mountain View, CA).
Western Blot Analysis
After various treatments, cells were collected and washed once with 1X phosphate-buffered saline (PBS) and lysed in lysis buffer on ice for 30 minutes and briefly sonicated. Protein concentrations were determined. Equal amounts of cell lysate were subjected to SDS-polyacrylamide electrophoresis on Novex tris-glycine pre-cast gels (Invitrogen) and separated proteins were then electrotransferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA). After exposure to various antibodies, specific proteins were detected using the SuperSignal West Pico chemiluminescence substrate system (Pierce Rockford, IL). The band intensity was analyzed using Scion image software (Scion, Frederick, MD).
Confocal Microscopy
U251-GFP cells were plated in 4-well chamber slides. After various treatments, cells were incubated with anti-Caveolin-1 antibody followed by a cy-3 conjugated secondary antibody and analyzed with a Bio-Rad MRC 1024 laser scan confocal microscope (Hemel, Hempstead, UK) for visualization of membrane rafts.
Annexin V-FITC/propidium iodide (PI) staining
Annexin V-FITC/propidium iodide staining was used to evaluate the number of cells undergoing apoptosis. After being treated with 0, 1, 5, or 10 μM of simvastatin for 48 hours, the cells growing on 4-well chamber slides were rinsed with ice cold PBS once, then stained by using an annexin V-FITC/propidium iodide kit (BioVision) (3). Each slide was mounted with Fluoromount G (EMS, PA). The stained cells were observed under a fluorescence microscope (Nikon Fluophot). Images were acquired and processed on the fluorescence microscope with an X40 objective lens. Image analysis and merging of images was done with Adobe PhotoShop CS software (Adobe System). A blind counting methodology was employed to determine the percentage of annexin V-positive cell number versus total cell number.
Statistical Analysis
All data were presented as means ± standard deviation (SD) of three independent experiments. Data were analyzed by a one-way analysis of variance (ANOVA) followed by post hoc Student–Newman–Keuls (SNK) tests. Differences were determined to be significant with P < 0.05.
Results
Simvastatin suppresses human U251 and U87 glioma cell proliferation
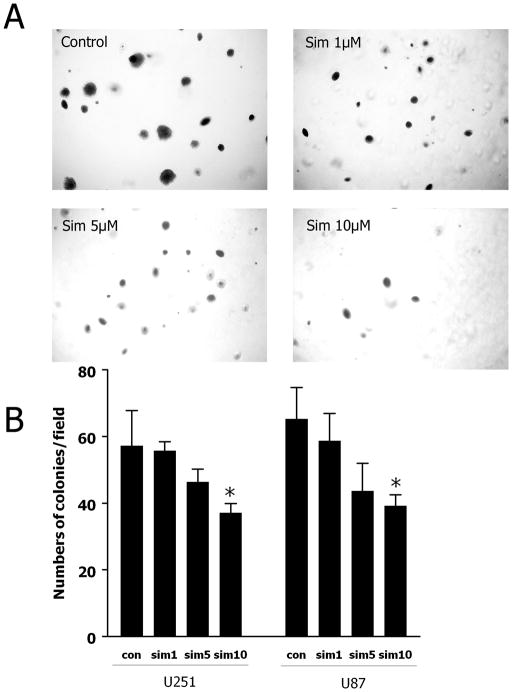
Colony formation assay was performed to examine the effect of simvastatin on cell proliferation (Fig. 1). In the control group, there was an average of 57/65 (U251/U87) colonies per field; simvastatin at 1 or 5 μM slightly reduced the number of colonies (i.e., an average of 55/58 and 46/43 colonies, respectively, P > 0.05). However, 10 μM of simvastatin significantly reduced the number to 37/39 colonies per field (P < 0.05) representing a 35% reduction of colony numbers, as compared to control (Fig. 1B). In addition, the size of the colonies after simvastatin treatment was much smaller as compared to control (Fig. 1A). These data suggest that simvastatin may suppress the proliferation of glioma cell in vitro, especially at higher concentrations. These effects may be due to retarded growth rather than to abrogate colony formation.
Fig. 1. Simvastatin decreases the number of colonies formed in soft agar.
U251-GFP and U87 cells (5000 cells/well) were plated in triplicate in 6-well plates with soft agar in the presence of 0, 1, 5, or 10 μM simvastatin for 19 days. Colonies were stained with 0.005% crystal violet for 2 hours. (A) Representative images of colonies after treatment with different dose of simvastatin. (B) The number of colonies was counted from 10 random fields under the light microscope and averages were calculated. Data are the mean ± SD. *P < 0.05 vs. control.
Simvastatin inhibits glioma cell migration
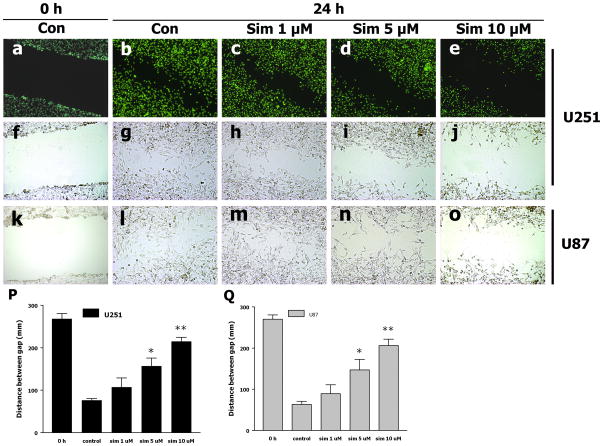
The in vitro scratch assay showed that cell migration was clearly inhibited by simvastatin in a dose-dependent manner, as examined by both light and fluorescent microscopy (Fig. 2A). The average gap distance of baseline control was 268 μm. After 24 hours of incubation without any treatment, the gap distance was reduced to 76 μm (Fig. 2B), suggesting an increase of cell migration. Simvastatin treatment at 1 μM did not notably change the gap distance as compared to control at 24 hours; however, 5 and 10 μM of simvastatin treatment significantly prevented the decrease of gap distances, which were 157 and 215 μm, respectively. These results suggest that simvastatin inhibits U251 and U87 glioma cell migration, especially at higher concentrations.
Fig. 2. Simvastatin suppresses U251 and U87 cell migration.
(A) a–o: representative images of each group taken by fluorescent microscope; f–j: representative images captured by phase contrast microscope. (B) The gap distance was measured with Photoshop and the average of gap distance was shown by the bar graph. *P < 0.05, **P < 0.01 vs. Con; n = 3. Scale bar = 100 μm
Simvastatin induces apoptotic cell death of glioma cells in a dose- and time-dependent manner
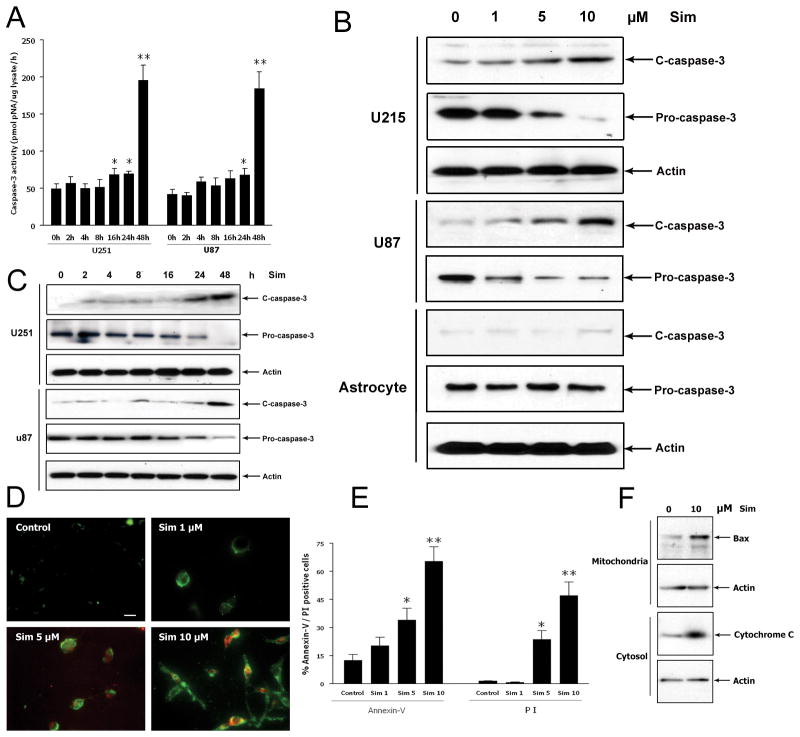
To determine the dose-dependent effect of simvastatin, U251 and U87 cells were treated with 0, 1, 5, or 10 μM of simvastatin for 48 hours. These doses were selected based on previous published studies on the effect of simvastatin in cancer cells (12). Western blot analysis showed that 10 μM of simvastatin induced an elevation of cleaved caspase-3 along with a reduction of pro-caspase-3 expression (Fig. 3B). As previous studies suggested that simvastatin induces apoptosis in prostate cancer cells in a time-dependent manner (37), the apoptotic effects of 10 μM of simvastatin was examined after 0, 2, 4, 8, 16, 24 and 48 hours of treatment. Caspase-3 activity assay showed that activity of caspase-3 appeared at 16 hours and reached the maximum at 48 hours (Fig. 3A). Western blot analysis showed a similar pattern where cleaved (active) caspase-3 expression was increased at 24 hours and peaked at 48 hours (Fig. 3C). These data suggest that simvastatin induces apoptosis in U251 cells as early as after 24 hours of treatment but the effect becomes notable after 48 hours. Annexin V is an anticoagulant protein that preferentially binds to negatively charged phospholipids and is used to identify cells in the early stages of apoptosis. Propidium iodide enters cells with membranes disrupted by necrosis or in the late stages of apoptosis. Therefore, conjugated annexin V/FTTC staining (green fluorescence) and propidium iodide (red fluorescence) were used to identify apoptosis and cell death by fluorescent microscopy (Fig. 3D). After different treatment for 48 hours, few cells in the control group were stained, whereas a majority of 10 μM simvastatin-treated cells displayed green and red staining (Fig. 3D). Quantitative analysis showed that the percentage of annexin V-positive and PI-positive cells in the 10 μM of simvastatin-treated group was significantly higher than that of control group (65.4% vs. 12.6% and 46.2% vs. 1.5%, respectively) (Fig. 3E). In addition, 10 μM of simvastatin induced Bax translocation into the mitochondria and enhanced cytochrome C release from the mitochondria (Fig. 3F). These data further confirm the pro-apoptotic effect of simvastatin.
Fig. 3. The pro-apoptotic effect of simvastatin is time and dose dependent.
(A) Caspase-3 activity assay after simvastatin treatment for different time points. (B) Western Blot analysis of caspase-3 expression after simvastatin treatment for different doses. Representative Western blot and protein band density analysis show that 10 μM of simvastatin induces cleavage of caspase-3. (C) Western Blot analysis of caspase-3 expression after simvastatin treatment for different time points. (D) Cell apoptosis was assessed by staining with annexin V-FITC (Green)/PI (Red). (E) Bar graph shows percentage of annexin V positive cells and PI positive cells after treatment with different doses of simvastatin. Scale bar = 25 μm. (F) Cells were treated with DMSO or 10 μM of simvastatin for 48 hours. Cytosolic and mitochondrial fractions were isolated, and Western blot analysis was performed using antibodies against Bax or cytochrome C. Data are expressed as means ± SD. n = 3. *P < 0.05 and **P < 0.01 vs. control. Scale bar = 10 μm.
Simvastatin suppresses Akt phosphorylation in a dose- and time-dependent manner
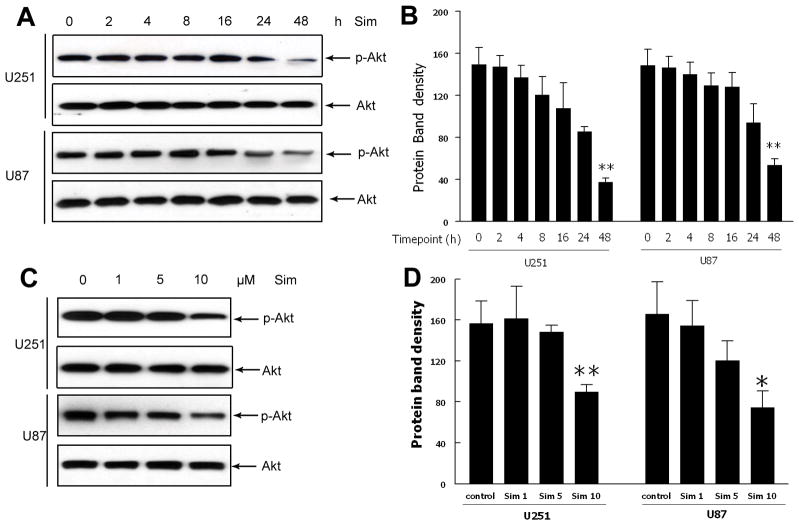
To evaluate the effect of simvastatin on phosphorylation of Akt, cells were treated with simvastatin at different time points and at different doses. Western blot analysis showed that simvastatin suppressed phospho-Akt after 24 and 48 hours of treatment, and 10 μM of simvastatin significantly decreased the Akt phosphorylation (Fig. 4).
Fig. 4. Effects of simvastatin treatment on Akt phosphorylation at different time point and dosage.
(A,B) Western blot analysis of phosphorylation of Akt at different time point after simvastatin treatment. (C,D) Western blot analysis of phosphorylation of Akt after simvastatin treatment of different dose. Data are the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01 vs. control.
Simvastatin-induced apoptosis in human U251 glioma cell is associated with the PI3K/Akt/caspase-3 pathway
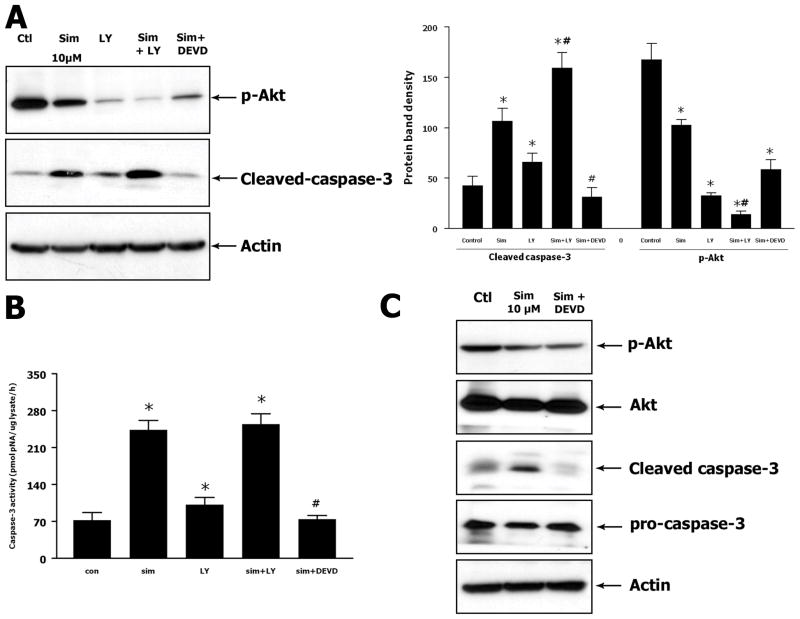
To investigate the mechanisms underlying the pro-apoptotic effect of simvastatin in U251 cells, Western blot analysis and caspase-3 assay were performed to measure the changes in signaling proteins related to cell apoptosis. As the PI3K/Akt/caspase-3 pathway was previously demonstrated to play a central role in regulating cell apoptosis, this signaling pathway was selected for further investigation. PI3K inhibitor LY294002 and caspase-3 inhibitor DEVD were used to inhibit the respective signaling proteins. Western blot analysis showed that simvastatin inhibited the Akt phosphorylation as compared to the control group. LY294002 itself greatly reduced the Akt phosphorylation. Combination of simvastatin and LY294002 further reduced the phosphorylation of Akt to a very low level (Fig. 5A). Pretreatment with DEVD prevented simvastatin-induced increase of active caspase-3 expression (Fig. 5C). Caspase-3 activity assay showed a similar pattern in activation of caspase-3 as compared to the results from Western blot analysis. Simvastatin, as well as LY294002, induced a significant elevation of caspase-3 activity. Combination of simvastatin and LY294002 further enhanced the caspase-3 activity. Pretreatment with DEVD greatly decreased the caspase-3 activity as compared to the simvastatin-treated group (Fig. 5B). These data suggest that simvastatin induces apoptosis through PI3K/Akt/caspase-3 signaling pathway. Blocking the PI3K/Akt pathway enhances the pro-apoptotic effect of simvastatin while inhibiting caspase-3 attenuates its effect.
Fig. 5. Simvastatin suppresses phosphorylation of Akt and induces activation of caspase-3.
(A) Representative Western blot and densitometric analysis on expression of p-Akt and cleaved caspase-3. Cells were treated with 10 μM of simvastatin alone for 48 hours or combination of simvastatin with LY294002 or DEVD. (B) Caspase-3 activity assay for cells treated with simvastatin and combination of simvastatin with LY294002 or DEVD. (C) Western blot shows that DEVD attenuates the simvastatin induced cleaved caspase-3 but did not affect simvastatin suppressed p-Akt. Data are the mean ± SD. n = 3. *P < 0.05 vs. control. #P < 0.05 vs. simvastatin-treated group.
Lipid raft modification and Fas relocation in cell membrane following treatment of simvastatin in human U251 glioma cells
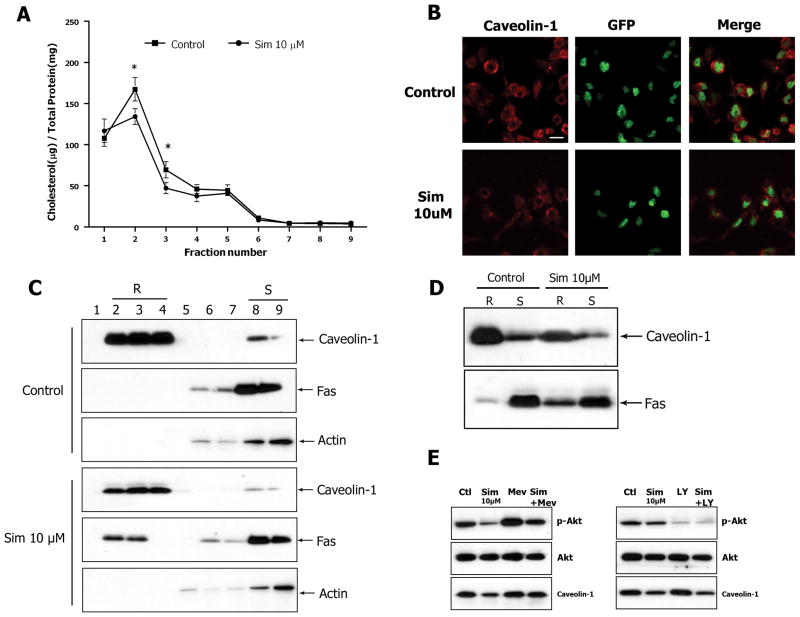
As previously reported data show that statins change the composition of lipid rafts on cell membranes, which offer a platform for many crucial signal transduction events that modulate cell growth and apoptosis (2), we examined changes in the lipid rafts after simvastatin treatment. U251 cells were treated with 0 or 10 μM of simvastatin for 48 hours and subjected to density gradient fractionation. Total cholesterol assay showed that simvastatin significantly reduced the cholesterol content in raft fractions (fraction 2 and 3) (Fig. 6A). Fractions were also subjected to SDS-PAGE and immunoblotted with antibodies for caveolin-1 (a marker for lipid rafts), Fas, and Actin. Fractions 2–4 were identified as raft fractions (R) and fractions 8–9 were soluble protein fractions (S) (Fig. 6C). Fluorescent immunostaining showed that simvastatin decreased the fluorescent signal of caveolin-1 on cell membrane as compared to control (Fig. 6B). Western blot analysis confirmed the downregulation of caveolin-1 levels in raft fractions after treatment with 10 μM of simvastatin (Fig. 6D). Furthermore, Fas expression was increased in the raft fractions in simvastatin-treated cells as compared to control (Fig. 6C and D), suggesting the recruitment of Fas to lipid rafts after simvastatin treatment. Mevalonate blocked the effect of simvastatin on lipid rafts thereby affecting the phosphorylation of Akt. However, blockage of the PI3K/Akt pathway with LY294002 did not affect the level of caveolin-1 in lipid raft fractions, suggesting that simvastatin-induced downregulation of phospho-Akt was partly due to changes in lipid rafts (Fig. 6E). Fraction number 3 was used to measure the caveolin-1 change after simvastatin treatment. These data suggest that simvastatin decreases cholesterol levels and disrupts the integrity of lipid rafts in U251 glioma cell membranes, promoting a Fas translocation into lipid rafts which is associated with cell apoptosis through the inhibition of the PI3K/Akt pathway.
Fig. 6. Simvastatin decreases cholesterol content, affects the integrity of lipid rafts, and promotes Fas translocation into lipid rafts in cell membrane.
(A) Total cholesterol assay was performed to test the cholesterol content in lipid raft fractions after density gradient fractionation. (B) U251-GFP cells were treated with DMSO or 10 μM of simvastatin. Immunostaining was performed with anti-Caveolin-1 followed by Cy-3 and examined with confocal microscope. Representative images from three independent experiments. (C) The lipid raft fractions (R) and soluble protein fractions (S) were identified by blot with anti-Caveolin-1, and anti-Actin. Ten μg of protein was loaded per lane for immunoblotting. (D) Fas translocation in membrane was detected by blot with anti-Fas using equal amounts of protein for each lane. (E) Mevalonate was used to block the effect of simvastatin on lipid rafts and LY294002 was used to block the effect of simvastatin on PI3K/Akt pathway. Fraction number 3 was used to measure the change caveolin-1 expression. Data are representative of at least three independent experiments. Scale bar = 10 μm.
Discussion
Our data demonstrate that simvastatin, a drug utilized as a plasma cholesterol-lowering agent, inhibits cell proliferation and migration and induces apoptosis in human U251 and U87 glioma cells in a dose- and time-dependent manner. Apoptosis induced by simvastatin is related to the downregulation of PI3K/Akt pathway. Simvastatin reduces cholesterol content in the cell membrane and disrupts the integrity of lipid rafts, leading to a Fas translocation into the lipid rafts.
Statins have been shown to synchronize tumor cells by blocking the transition of G1-S phase in the cell cycle, thereby exerting its anti-proliferative effect (16). This effect can be reversed by the addition of mevalonate. In primary cultures of human glioblastoma cells, inhibition of Ras farnesylation by statins is associated with reduction of proliferation (5). In C6 glioma cells treated with lovastatin, free geranylgeraniol overcomes the arrest of cell proliferation (9). These findings suggest that the mevalonate pathway is essential for progression of cell cycle in glioma cells and plays a key role in the simvastatin-induced inhibition of proliferation (33). Statins have also been shown to inhibit cell signaling proteins that are associated with the migration properties of tumor cells, such as RhoA, NF-κB, and matrix metalloproteinase-9 (10). Statins also induce disorganization of the actin cytoskeleton and disappearance of focal adherence site (8). These findings suggest that simvastatin may inhibit glioma cell proliferation and migration through the reduction of mevalonate and interference with related signaling pathways.
Simvastatin induces apoptosis in various cancer cells including human myeloma cells (6), breast cancer (1), leukemia cells (19), prostate cancer (37), C6 glioma cells (18), and U87 malignant glioblastoma cells (12). Based on the previous studies using simvastatin as an antitumor agent, we chose three doses (1, 5, and 10 μM) and several time points (0, 2, 4, 8, 16, 24, and 48 hours) to examine the effect of simvastatin in U251 and U87 cells. Caspase-3 activity assay and Western blot analysis show that phospho-Akt expression was decreased and active caspase-3 was increased maximally at 48 hours. Dose response studies by Annexin V staining and Western blot analysis demonstrate that 10 μM is more effective than other two doses. This result is consistent with other studies reporting treatment of human glioma cell lines GaMg and U-87 Mg with simvastatin in vitro (12). However, some studies reported that the maximum effective dose of simvastatin treatment for primary cultured human glioma cells was 1 μM (33). Our effective dose is much higher than that, possibly due to specific cell types and because we use simvastatin prodrug without pretreatment to activate it and we culture glioma cells with 10% FBS which provides some exogenous cholesterol.
Statin-induced apoptosis is reported to be mediated through the depletion of geranylgeranylated proteins (36). We investigated the changes in cell signaling after simvastatin treatment. Akt/protein kinase B (PKB) is a serine/threonine kinase that is a critical regulator for cell survival and proliferation, especially in human malignant cancer. Our results show that a high dose of simvastatin suppresses Akt phosphorylation, induces Bax translocation into the mitochondria and cytochrome C release from the mitochondria, and increases caspase-3 activation, leading to apoptosis. Blockade of PI3K with LY294002 enhances simvastatin-induced suppression of p-Akt as well as simvastatin-induced elevation of active caspase-3. Blockade of caspase-3 with DEVD abrogates the simvastatin-induced activation of caspase-3. However, our data suggest that DEVD does not affect the phosphorylation of Akt after simvastatin treatment. These results indicate that simvastatin-induced apoptosis is closely related to modulation of the PI3K/Akt/caspase-3 pathway. Statins have been found to inhibit Akt phosphorylation and induce apoptosis in tumor cells (35). Treating tumor cells with a combination of statins and the PI3K inhibitor LY294002 induced an additive effect on cell apoptosis. This additive effect confirms that statins act by inhibiting the PI3K/Akt pathway (29). However, this does not exclude the participation of other pathways in the apoptotic effect of simvastatin. A previous study has suggested the involvement of c-jun Nterminal kinase (JNK)-dependent cell death pathway in the simvastatin treatment of glioma (18). Our data are not consistent with the report of Koyuturk et al., who did not observe Akt downregulation with simvastatin treatment in C6 glioma cells (18). This inconsistency may be due to two reasons. One is the time point difference; they examined a time point of 0–24 hours, while we find the inhibitory effects of simvastatin shown mainly at 48 hours. The other is the phosphorylation residue difference; we tested residue ser473 instead of Thr308.
To further elucidate the mechanism of the pro-apoptotic effect of simvastatin, we examined the lipid rafts following simvastatin treatment. Lipid rafts are cholesterol-enriched microdomains in the plasma membrane. They act as molecular platforms that spatially organize membrane receptor molecules and are involved in the transduction of various signaling pathways (32). A recent study suggested that rafts are implicated in Akt activation (26). Cancer cells contain increased levels of rafts compared to their normal counterparts, suggesting the potential therapeutic value of using raft-modulating agents as anti-cancer drugs (4). Depletion of cholesterol from the plasma membrane causes disruption of rafts and renders them nonfunctional, indicating that cholesterol is crucial for maintaining lipid raft structure and function (30). Our data show that simvastatin decreases cholesterol content and attenuates the expression of caveolin-1 (the lipid raft marker) in raft fractions, resulting in a rearrangement of the lipid rafts. In some studies the cholesterol depletion drug methyl-cyclodextrin has been observed to alter Akt activity following caveolar disruption (20, 31). Caveolin-1 is also reported to be important for fine-tuning signals through the PI-3K/Akt pathway (22). Simvastatin-induced decrease of cholesterol and attenuation of caveolin-1 in the lipid raft disrupt the integrity of the raft; thereby damaging the membrane platforms for signaling molecules and down-regulating the Akt pathway that regulates various cellular functions, including cell survival, leading to apoptosis in an Akt/caspase-3-dependent pathway in glioma cells. These data are consistent with recent studies reporting that cholesterol depletion using methyl-β cyclodextrin causes tumor cell apoptosis, which involves decreased raft levels, Akt inactivation, and caspase-3 activation (21).
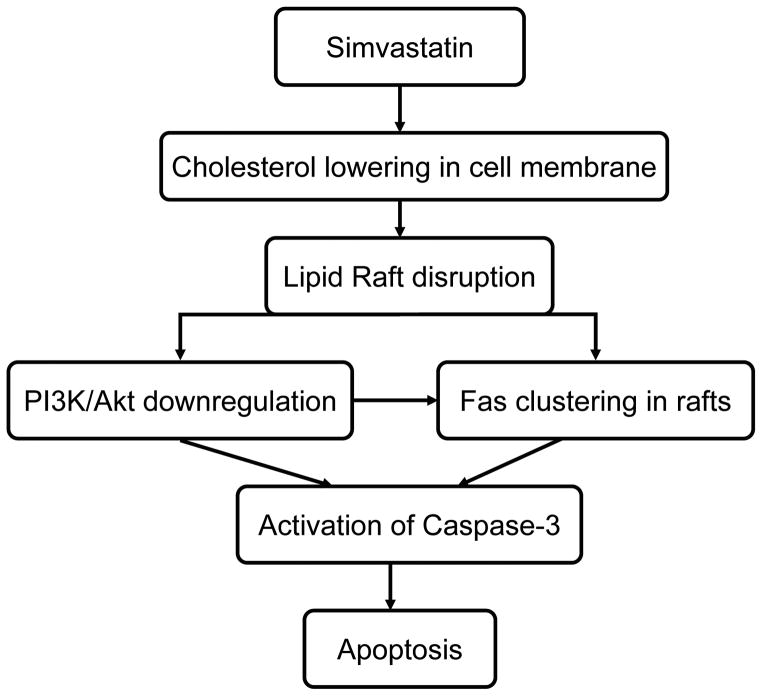
Our results show that simvastatin also promotes Fas translocation into lipid raft fractions. Fas-induced apoptosis has been proposed through caspase-8 and caspase-3-dependent pathways (25). However, recent evidence suggests that the Fas death receptor can also be FasL-independently activated through translocation of Fas into lipid rafts. The clustering of Fas in membrane rafts generates high local concentrations of death receptors that provide scaffolds for the coupling adaptor and effector proteins involved in Fas-mediated apoptosis. Therefore, lipid rafts play a key role where a potent death signal is initiated and become new promising anticancer targets (23). Gliomas express varied levels of Fas, yet constitutively high levels of Fas ligand (7). A recent study suggests that inhibition of the PI3K/Akt signal triggers a Fas-mediated apoptotic signal independently of the Fas/FasL interaction, and blockade of the PI3K activity by LY294002 leads to the clustering of Fas within the lipid rafts (3). Our findings suggest that simvastatin downregulates PI3K/Akt to sensitize tumor cells to apoptosis. This effect may be lead to the redistribution of Fas in cell membrane (Fig. 7).
Fig. 7.
Proposed antitumor mechanism of simvastatin in glioma cells.
Taken together, these studies demonstrate that simvastatin reduces cholesterol content in cell membrane of glioma cells, disrupts integrity of lipid rafts, downregulates PI3K/Akt signal pathway, and results in caspase-3-dependent apoptosis. Our results indicate that simvastatin-induced disruption of lipid raft integrity is closely involved in interfering with pro-survival pathways and triggering of apoptotic cell death in glioma cells. Further elucidation of the nature of this effect could ultimately augment the efficacy of simvastatin therapy.
Acknowledgments
Grant information/Acknowledgement
This work was supported by National Institutes of Health (NIH) grants R01NS052280-01A1. We thank Susan Macphee-Gray for her editorial assistance.
Financial Support: This work was supported by National Institutes of Health (NIH) grants R01NS052280-01A1.
References
- 1.Aberg M, Wickstrom M, Siegbahn A. Simvastatin induces apoptosis in human breast cancer cells in a NFkappaB-dependent manner and abolishes the anti-apoptotic signaling of TF/FVIIa and TF/FVIIa/FXa. Thromb Res. 2008;122:191–202. doi: 10.1016/j.thromres.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Bang B, Gniadecki R, Gajkowska B. Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp Dermatol. 2005;14:266–272. doi: 10.1111/j.0906-6705.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Beneteau M, Pizon M, Chaigne-Delalande B, Daburon S, Moreau P, De Giorgi F, Ichas F, Rebillard A, Dimanche-Boitrel MT, Taupin JL, Moreau JF, Legembre P. Localization of Fas/CD95 into the lipid rafts on down-modulation of the phosphatidylinositol 3-kinase signaling pathway. Mol Cancer Res. 2008;6:604–613. doi: 10.1158/1541-7786.MCR-07-0331. [DOI] [PubMed] [Google Scholar]
- 4.Bionda C, Athias A, Poncet D, Alphonse G, Guezguez A, Gambert P, Rodriguez-Lafrasse C, Ardail D. Differential regulation of cell death in head and neck cell carcinoma through alteration of cholesterol levels in lipid rafts microdomains. Biochem Pharmacol. 2008;75:761–772. doi: 10.1016/j.bcp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Bouterfa HL, Sattelmeyer V, Czub S, Vordermark D, Roosen K, Tonn JC. Inhibition of Ras farnesylation by lovastatin leads to downregulation of proliferation and migration in primary cultured human glioblastoma cells. Anticancer Res. 2000;20:2761–2771. [PubMed] [Google Scholar]
- 6.Cafforio P, Dammacco F, Gernone A, Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005;26:883–891. doi: 10.1093/carcin/bgi036. [DOI] [PubMed] [Google Scholar]
- 7.Cerrato JA, Khan T, Koul D, Lang FF, Conrad CA, Yung WK, Liu TJ. Differential activation of the Fas/CD95 pathway by Ad-p53 in human gliomas. Int J Oncol. 2004;24:409–417. [PubMed] [Google Scholar]
- 8.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- 9.Crick DC, Andres DA, Danesi R, Macchia M, Waechter CJ. Geranylgeraniol overcomes the block of cell proliferation by lovastatin in C6 glioma cells. J Neurochem. 1998;70:2397–2405. doi: 10.1046/j.1471-4159.1998.70062397.x. [DOI] [PubMed] [Google Scholar]
- 10.Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, Soria J, Soria C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22:1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 11.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 12.Gliemroth J, Zulewski H, Arnold H, Terzis AJ. Migration, proliferation, and invasion of human glioma cells following treatment with simvastatin. Neurosurg Rev. 2003;26:117–124. doi: 10.1007/s10143-003-0258-9. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 14.Grieb P, Ryba MS, Jagielski J, Gackowski W, Paczkowski P, Chrapusta SJ. Serum cholesterol in cerebral malignancies. J Neurooncol. 1999;41:175–180. doi: 10.1023/a:1006131418126. [DOI] [PubMed] [Google Scholar]
- 15.Jackson SM, Ericsson J, Edwards PA. Signaling molecules derived from the cholesterol biosynthetic pathway. Subcell Biochem. 1997;28:1–21. doi: 10.1007/978-1-4615-5901-6_1. [DOI] [PubMed] [Google Scholar]
- 16.Keyomarsi K, Sandoval L, Band V, Pardee AB. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 1991;51:3602–3609. [PubMed] [Google Scholar]
- 17.Kondo Y, Hollingsworth EF, Kondo S. Molecular targeting for malignant gliomas (Review) Int J Oncol. 2004;24:1101–1109. [PubMed] [Google Scholar]
- 18.Koyuturk M, Ersoz M, Altiok N. Simvastatin induces proliferation inhibition and apoptosis in C6 glioma cells via c-jun N-terminal kinase. Neurosci Lett. 2004;370:212–217. doi: 10.1016/j.neulet.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101:3628–3634. doi: 10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–1118. doi: 10.2353/ajpath.2006.050959. quiz 1404–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews LC, Taggart MJ, Westwood M. Modulation of caveolin-1 expression can effect signalling through the PI-3K/Akt pathway and cellular proliferation in response to IGF-I. Endocrinology. 2008 doi: 10.1210/en.2007-1211. [DOI] [PubMed] [Google Scholar]
- 23.Mollinedo F, Gajate C. Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist Updat. 2006;9:51–73. doi: 10.1016/j.drup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Newman A, Clutterbuck RD, Powles RL, Millar JL. Selective inhibition of primary acute myeloid leukaemia cell growth by simvastatin. Leukemia. 1994;8:2023–2029. [PubMed] [Google Scholar]
- 25.Nijhawan D, Honarpour N, Wang X. Apoptosis in neural development and disease. Annu Rev Neurosci. 2000;23:73–87. doi: 10.1146/annurev.neuro.23.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase Calpha in endothelial cells. Cell Signal. 2004;16:951–957. doi: 10.1016/j.cellsig.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen TR, Berg K, Cook TJ, Faergeman O, Haghfelt T, Kjekshus J, Miettinen T, Musliner TA, Olsson AG, Pyorala K, Thorgeirsson G, Tobert JA, Wedel H, Wilhelmsen L. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch Intern Med. 1996;156:2085–2092. [PubMed] [Google Scholar]
- 28.Prasanna P, Thibault A, Liu L, Samid D. Lipid metabolism as a target for brain cancer therapy: synergistic activity of lovastatin and sodium phenylacetate against human glioma cells. J Neurochem. 1996;66:710–716. doi: 10.1046/j.1471-4159.1996.66020710.x. [DOI] [PubMed] [Google Scholar]
- 29.Roudier E, Mistafa O, Stenius U. Statins induce mammalian target of rapamycin (mTOR)-mediated inhibition of Akt signaling and sensitize p53-deficient cells to cytostatic drugs. Mol Cancer Ther. 2006;5:2706–2715. doi: 10.1158/1535-7163.MCT-06-0352. [DOI] [PubMed] [Google Scholar]
- 30.Scheel-Toellner D, Wang K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun. 2002;297:876–879. doi: 10.1016/s0006-291x(02)02311-2. [DOI] [PubMed] [Google Scholar]
- 31.Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res. 2005;96:635–642. doi: 10.1161/01.RES.0000160610.61306.0f. [DOI] [PubMed] [Google Scholar]
- 32.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 33.Soma MR, Pagliarini P, Butti G, Paoletti R, Paoletti P, Fumagalli R. Simvastatin, an inhibitor of cholesterol biosynthesis, shows a synergistic effect with N,N′-bis(2-chloroethyl)-N-nitrosourea and beta-interferon on human glioma cells. Cancer Res. 1992;52:4348–4355. [PubMed] [Google Scholar]
- 34.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 35.Woodard J, Sassano A, Hay N, Platanias LC. Statin-dependent suppression of the Akt/mammalian target of rapamycin signaling cascade and programmed cell death 4 up-regulation in renal cell carcinoma. Clin Cancer Res. 2008;14:4640–4649. doi: 10.1158/1078-0432.CCR-07-5232. [DOI] [PubMed] [Google Scholar]
- 36.Xia Z, Tan MM, Wong WW, Dimitroulakos J, Minden MD, Penn LZ. Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells. Leukemia. 2001;15:1398–1407. doi: 10.1038/sj.leu.2402196. [DOI] [PubMed] [Google Scholar]
- 37.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]