Abstract
Background
Patient self-care support via Interactive Voice Response (IVR) can improve disease management. However little is known about the factors affecting program engagement.
Methods
We compiled data on IVR program engagement for 1,173 patients with: heart failure, depression, diabetes, and cancer who were followed for 28,962 person-weeks. Patients in programs for diabetes or depression (N=727) had the option of participating along with an informal caregiver who received electronic feedback based on the patient’s IVR assessments. Analyses focused on factors associated with completing weekly IVR calls.
Results
Patients were on average 61 years old, 37% had at most a high school education, and 48% reported incomes < $30,000. Among patients given the option of participating with an informal caregiver, 65% chose to do so. Patients completed 83% of attempted IVR assessments, with rates higher for heart failure (90%) and cancer programs (90%) than for the diabetes (81%) or depression programs (71%) (p<0.001). Among patients in diabetes or depression programs, those opting to have feedback provided to an informal caregiver were more likely to complete assessments (adjusted odds ratio: 1.36; 95% CI: 1.06, 1.75). Older patients had higher call completion rates, even among patients > 75 years of age. Missed clinic appointments, prior hospitalizations, depression program participation, and poorer mental health were associated with lower completion rates.
Conclusions
Patients with a variety of chronic conditions will complete IVR self-care support calls regularly. Risk factors for missed IVR calls overlap with those for missed appointments. Involvement of informal caregivers may significantly increase engagement.
Keywords: mobile health, telemedicine, chronic-disease management, caregiver support
Introduction
Activities competing for clinicians’ attention during visits with chronically-ill patients far exceed available time,1,2 and patients needing support for self-management are often the ones most likely to miss their appointments.3,4 Although telephone follow-up improves disease management,5–8 clinician-delivered telephone care has shown limited cost savings.9–11 Automated technologies for monitoring and education via mobile phones, i.e., mobile health or “mHealth,” may fill the gap between what patients need and what clinicians can deliver sustainably.
Most mHealth studies have focused on text messaging or SmartPhones. Text messaging interventions can have positive effects on disease self-management,12–15 but are challenging for individuals with limited vision, dexterity, or literacy. Also, text messaging is less interactive than clinician counseling, limiting the depth of information exchanged. Smartphones allow more complex information exchanges,16–19 but may be difficult for patients with limited computer literacy or incomes.
“Interactive Voice Response” or IVR allows patients to communicate with clinicians asynchronously using a mobile or landline telephone. Patients using IVR hear recorded messages and respond to queries using their touch-tone keypad or voice-recognition. Based on their responses, patients receive tailored, recorded health information. Clinicians or family caregivers can receive automated updates and recommendations based on patients’ responses. IVR monitoring can provide reliable and valid information about patients,20–27 and IVR-supported nursing care can improve self-care and health outcomes.28–34 IVR calls may be useful in developing countries, where mobile phones are common.34–36
Despite the potential benefits of IVR-supported self-management, concerns remain that patients may be unable to use IVR or perceive it as an effort to limit access to clinicians via “robo-calls.” Most studies describe IVR use in small samples from clinical trials, and few reports include information regarding trends in IVR call completion or patient determinants of engagement. Moreover, no studies have examined the extent to which informal caregivers impact patients’ use of mHealth services such as IVR, despite the importance of caregivers in promoting self-management.37
Here we describe the experience of 1,173 chronically-ill patients enrolled in four IVR monitoring and self-management support programs. We report patients’ IVR assessment completion rates, trends in completion, and characteristics associated with persistent participation. Patients with diabetes or depression participating in clinical demonstration programs had the option of participating with an informal caregiver who received automated feedback based on patients’ IVR-reported information. Because caregiver support is associated with improved disease management,37,38 we used these data to test the hypothesis that caregiver feedback increases patients’ engagement in mobile health communication.
Methods
Study Design
We analyzed pre-existing data from four IVR chronic disease self-management support programs serving English-speaking patients with heart failure, depression, diabetes, or cancer. The study was approved by university and Department of Veterans Affairs (VA) Human Subjects Committees.
Eligibility Criteria
In each program, patients were initially identified from electronic medical records. Patients were ineligible if they: had diagnoses of cognitive impairment, schizophrenia, or bipolar disorder; had a limited life expectancy (e.g., end-stage renal disease); were not receiving the majority of their primary care in the recruitment site; or were unable to respond to IVR calls using a touch-tone telephone.
Characteristics Common to All Programs
Patients presented with each program were told that its goal is to help the patient stay as healthy as possible by providing support for their disease self-management. Enrollers explained that the IVR calls could: help to remind the patient about what to do to manage their self-care effectively, provide feedback to the patient’s clinicians, and update a family member or friend so that they can better support the patient’s efforts to stay well.
IVR systems were programmed to automatically attempt to contact patients a maximum of three different times each week that the patient indicated were convenient. For each calling time, up to three attempts were made (i.e., a maximum of nine attempts per week). Patients were given a toll-free number that they could use to contact program staff during business hours and request an immediate IVR call. At the beginning of each IVR call, patients’ identities were verified using their self-reported birth year. Call content for each program was developed by specialists in the clinical area, health behavior change, and IVR program design. Calls used tree-structured algorithms to present recorded queries and tailored information. Calls lasted 5–20 minutes depending on the problems reported. Clinician experts and participating primary care sites defined criteria for faxing alerts to clinicians based on patients’ responses. With the exception of the automatic transfer to staff at a national suicide hotline described below (for depression program patients), clinicians evaluated fax alerts and contacted patients for follow-up using their own staff, clinical protocols, and judgment.
Involvement of Informal Caregivers or “CarePartners”
Some patients enrolled with a family member or friend who we refer to as their “CarePartner.” Based on patients’ IVR assessments, CarePartners received automated emails with feedback about the patient’s status and how they could support the patient’s self-management. Feedback regarding urgent patient reports was sent via IVR, and CarePartners had the option to call a toll-free number to receive their weekly update. For patients in the heart failure and cancer programs, caregiver participation was required, and enrollees were randomized to receive CarePartner feedback, while control-group caregivers did not. Analyses testing the impact of CarePartner feedback in those trials will be reported elsewhere. For the diabetes and depression programs, CarePartner participation was optional. In all programs, patients received identical IVR messages, regardless of CarePartner involvement.
Program-Specific Features
Heart Failure
The heart failure CarePartner program is a randomized trial among patients managed at the Cleveland VA Medical Center. IVR calls monitored patients’ weight, dyspnea, salt and fluid intake, and medication adherence; and patients were provided immediate feedback to improve self-management. Participants received weekly IVR calls for 12 months and were required to enroll with an informal caregiver. Only caregivers randomized to the intervention arm received weekly emails about the patient’s status with suggestions for how they could support self-management. Fax alerts were generated automatically to the clinical team in the event that patients reported frequent or worsening shortness of breath, acute weight gain, medication adherence problems, or overconsumption of salt and fluids. Patients were enrolled between June 2009 and December 2011, and 57% of eligible patients chose to participate. Ninety-four percent of patients remained enrolled throughout the program.
Depression
The depression CarePartner program is an ongoing demonstration project that includes patients from 12 Michigan primary care practices. IVR calls monitor patients’ depression symptoms using the PHQ-939 and provide advice to improve medication adherence and prompt appropriate clinical follow-up. For patients reporting suicidal ideation: an alert is automatically faxed to the clinical team, patients are instructed to contact their provider or 911, and patients are given the option to connect immediately to a suicide hotline. Faxes also are generated when patients experience a significant increase in their PHQ-9 scores or medication adherence problems. Patients included in this report were enrolled between March 2010 and January 2012, during which 95% of eligible patients chose to participate. Patients participated for 3–4 months, and 85% of patients remained enrolled throughout the program.
Diabetes
The diabetes CarePartner program is a demonstration program offered in a Midwestern VA health services network (i.e., VISN 11). IVR calls monitor patients’ symptoms, blood glucose readings, medication adherence, and blood pressure. Based on information reported, patients receive tailored suggestions for managing blood sugar and improving medication adherence. Faxes to the clinical team are generated when patients report medication adherence problems, high or low blood sugar, or high or low blood pressure. Patients included in this report were recruited between March 2010 and January 2012, during which 86% of eligible patients chose to participate. Patients received IVR calls for 12 weeks and 95% remained enrolled throughout that time.
Cancer
The cancer CarePartner program is a randomized trial conducted in four VA oncology clinics nationwide40 for patients with solid tumors within 10 weeks of initiating chemotherapy. Patients were excluded if they had treatment involving bone marrow transplantation, were in hospice, or did not have a CarePartner. IVR calls monitor and provide self-care support for managing eight prevalent chemotherapy symptoms. Feedback alerts are generated to the clinical team when patients report medium or high severity symptoms. Data for the current study were collected from patients enrolled between October 2010 and January 2012, during which 38% of eligible patients chose to participate. Patients received weekly IVR calls for 10 weeks, and 95% of patients remained enrolled throughout that time. Only CarePartners randomized to the intervention group received email alerts and access to a website with tailored decisional support.
Data Analysis
Each IVR system captured data on the calling process, including the date and time of each call attempt. We created a dataset including one record representing each patient-week of follow-up, with an indicator for whether or not the patient completed that week’s assessment. Follow-up time excluded periods when calls were suspended at the patient’s request, e.g., because of hospitalization or travel. Additional patient-level information was compiled from screening interviews and baseline surveys, including sociodemographic characteristics and health status (measured by the SF-1241 and reports of hospitalizations within the year prior to enrollment). As part of their baseline survey, patients reported whether they had missed one or more outpatient visits in the prior year.
In initial analyses, we examined variation in patient characteristics across programs using chi-square, t-tests, and one-way ANOVA. We used multivariate logistic regression models to identify predictors of whether a weekly IVR assessment was completed. We used the cluster command in Stata (version 11.2) to compute model-free coefficient standard errors that took into account the clustering of person-weeks by patient. Potential patient-level predictors included patient demographics, measures of vulnerability (prior missed appointments, prior hospitalizations, and SF-12 mental and physical functioning scores), and program type. SF-12 scores were standardized before entering into multivariable models. Models also included a variable for the number of weeks elapsed between patient enrollment and the call week. Models included terms representing the main effects of heart failure, diabetes, and cancer program participation (relative to depression) as well as interactions between each program and time since enrollment. Parameters with p-values > 0.40 were dropped from the final model.
Based on model coefficients, we computed predicted probabilities of call completion separately by program. For those computations, other patient characteristics were set to the distribution average, and plots of the predicted probability of completing a weekly assessment by time were constructed using the prgen command in Stata. We constructed plots predicting call completion over 52 weeks for patients in the heart failure program, and over 12 weeks for patients in programs for depression, diabetes, and cancer. We used a similar multivariate logistic regression model and cubic splines to graphically display variation in call completion rates across patient ages.
For the 727 patients in the depression and diabetes programs who were given the option of participating with or without a CarePartner, we used bivariate analyses and a multivariate model to identify the patient characteristics associated with that choice. We then re-examined the predictors of call completion, using a logistic regression model as above, including an indicator for whether the patient participated with a CarePartner.
Results
Patient Characteristics
Of the 1,173 patients whose data are represented in these analyses, 394 (34%) participated in the heart failure program, 442 (38%) in the depression program, 285 (24%) in the diabetes program, and 52 (4%) in the cancer program. Patients were a mean of 61 years of age (SD=13.4), with 22% of all participants over age 70. Most patients were white (78%) and male (70%). A total of 37% of patients had a high school education or less, and 48% had annual household incomes of $30,000 or less. At baseline, 41% of patients rated their physical health as fair or poor, and 40% had been hospitalized in the year prior to enrollment. Variation in patient characteristics across programs is presented in Table 1.
Table 1.
Participant Characteristics
| Program | |||||
|---|---|---|---|---|---|
| Total | Heart Failure |
Depression | Diabetes | Cancer | |
| No of patients | 1,173 | 394 | 442 | 285 | 52 |
| Mean Age (SD) | 60.9(13.4) | 68.0(10.7) | 51.5(12.6) | 65.4(9.9) | 61.2(6.9) |
| White (%) | 77.6 | 76.4 | 90.3 | 57.5 | 88.5 |
| Male (%) | 70.2 | 99.0 | 24.9 | 96.8 | 90.4 |
| Married (%) | 79.9 | 60.9 | 87.1 | 93.3 | 90.4 |
| ≤ High school (%) | 37.0 | 49.5 | 20.6 | 46.7 | 34.6 |
| Income ≤ $30K (%) | 48.3 | 71.3 | 38.3 | 28.4 | 59.6 |
| VA (%) | 65.3 | 100.0 | 7.9 | 100.0 | 100.0 |
| Mean MCS (SD) | 44.(13.7) | 47.5(12.0) | 36.5(13.1) | 50.1(11.8) | 50.1(10.5) |
| Mean PCS (SD) | 34.5(13.0) | 30.1(10.3) | 40.2(13.5) | 32.5(12.3) | 29.1(11.8) |
| Missed Visits(a) | 41.6 | 44.2 | 37.3 | 30.5 | 43.7 |
| Prior Admission(b) | 39.8 | 57.1 | 25.6 | 27.0 | 100.0 |
Notes: Patients varied significantly across programs on all characteristics (p< .001). VA=Department of Veterans Affairs Healthcare System. MCS=SF-12 Mental Composite Summary Score; higher scores indicate better functioning. PCS=SF-12 Physical Composite Summary Score; higher scores indicate better functioning. (a)One or more missed outpatient visits in the year prior to enrollment. (b)One or more hospitalizations in the year prior to enrollment.
Call Completion
Patients received IVR calls for a total of 28,962 person-weeks. The median number of weeks participants received calls was 16 (interquartile range: 11–34). The length of follow-up varied by program design, with heart failure participants having the longest participation (Table 2). Overall, patients completed assessments in 24,053 of the weeks (83%) during which a call was attempted. As shown in Table 2, call completion rates were higher for patients enrolled in the heart failure (90%) and cancer programs (90%) than for those in the diabetes (81%) or depression programs (71% p<0.001). Based on the multivariate logistic model (Table 3), older age was significantly associated with greater odds of completing a weekly IVR call (adjusted odds ratio [AOR]: 1.13 per 10 year increase in age; 95% CI: 1.03, 1.23). Call completion rates increased over time among patients in the heart failure program (p<0.001), but decreased over time among patients in the depression program (p<0.001). Overall, lower mental health summary scores at enrollment, one or more missed clinic appointments in the prior year, prior hospitalization, and increasing weeks of program participation were significantly associated with lower odds of completing a weekly call. Odds of call completion were not associated with patients’ race, marital status, educational attainment, or income.
Table 2.
Call Completion
| Program | |||||
|---|---|---|---|---|---|
| Total | Heart Failure |
Depression | Diabetes | Cancer | |
| N (patients) | 1,173 | 394 | 442 | 285 | 52 |
| N (call weeks) | 28,962 | 15,519 | 7,815 | 5,166 | 462 |
| Median Follow-up (Q-Q) | 16(11,34) | 50(32,52) | 15(13,21) | 11(7,12) | 10(10,10) |
|
N of Completed Assessments |
24,053 | 13,907 | 5,544 | 4,188 | 414 |
| % of Assessments Completed |
83.1 | 89.6 | 70.9 | 81.1 | 89.6 |
Notes: Q-Q = interquartile range. Programs were similar in the frequency of patient calls. See Methods for information about the content of each program’s calls and the patient-reported information triggering fax alerts to clinical teams.
Table 3.
Predictors of Call Completion from Multivariable Logistic Regression Models
| All Patients | Optional CP Patients(f) | |||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p-value | AOR | 95% CI | p-value | |
| White | 1.23 | 0.96, 1.56 | 0.09 | 1.08 | 0.81, 1.42 | 0.53 |
| Age (10 years) | 1.13 | 1.03, 1.23 | 0.009 | 1.20 | 1.08, 1.32 | 0.001 |
| Female | 0.72 | 0.54, 0.96 | 0.02 | 0.77 | 0.57, 1.03 | 0.08 |
| CHF | ||||||
| Main Effect(a) | 1.28 | 0.87, 1.88 | 0.21 | --- | --- | --- |
| Change per call week | 1.04 | 1.02, 1.06 | <0.001 | --- | --- | --- |
| Depression | ||||||
| Main Effect | ref | --- | --- | ref | --- | --- |
| Change per call week | 0.96 | 0.95, 0.98 | <0.001 | 0.96 | 0.95, 0.98 | <0.001 |
| Diabetes | ||||||
| Main Effect(a) | 1.17 | 0.78, 1.75 | 0.44 | 1.11 | 0.72, 1.70 | 0.63 |
| Change per call week | 1.01 | 0.99, 1.04 | 0.34 | 1.02 | 0.99, 1.04 | 0.25 |
| Cancer | ||||||
| Main Effect(a) | 2.70 | 1.24, 5.89 | 0.01 | --- | --- | --- |
| Change per call week | 0.93 | 0.85, 1.03 | 0.18 | --- | --- | --- |
| Mental Functioning(b) | 1.13 | 1.02, 1.25 | 0.02 | 1.06 | 0.94, 1.21 | 0.34 |
| Physical Functioning(c) | 0.95 | 0.86, 1.04 | 0.25 | 0.99 | 0.87, 1.11 | 0.80 |
| Missed Visits(d) | 0.75 | 0.67, 0.85 | <0.001 | 0.79 | 0.69, 0.90 | <0.001 |
| CarePartner | --- | --- | --- | 1.36 | 1.06, 1.75 | 0.02 |
Notes: Marital status, educational attainment, and income had no association with call completion (all p>0.40) in the full model and were dropped from the final model. AOR = adjusted odds ratio; CI = confidence interval. (a) Referent: depression program patients. (b) SF-12 Mental Composite Summary Score; higher scores indicate better functioning. AOR represents the adjusted change in the odds of completion for one standard deviation increase in standardized scores. (c) SF-12 Physical Composite Summary Score; higher scores indicate better functioning. AOR represents the adjusted change in the odds of completion for one standard deviation increase in standardized scores. (d) One or more missed outpatient visits in the year prior to enrollment. (e) One or more hospitalizations in the year prior to enrollment. (f) Model fit based on the subset of 727 patients enrolled in the depression or diabetes programs who had the option of enrolling alone or with an informal caregiver or “CarePartner.”
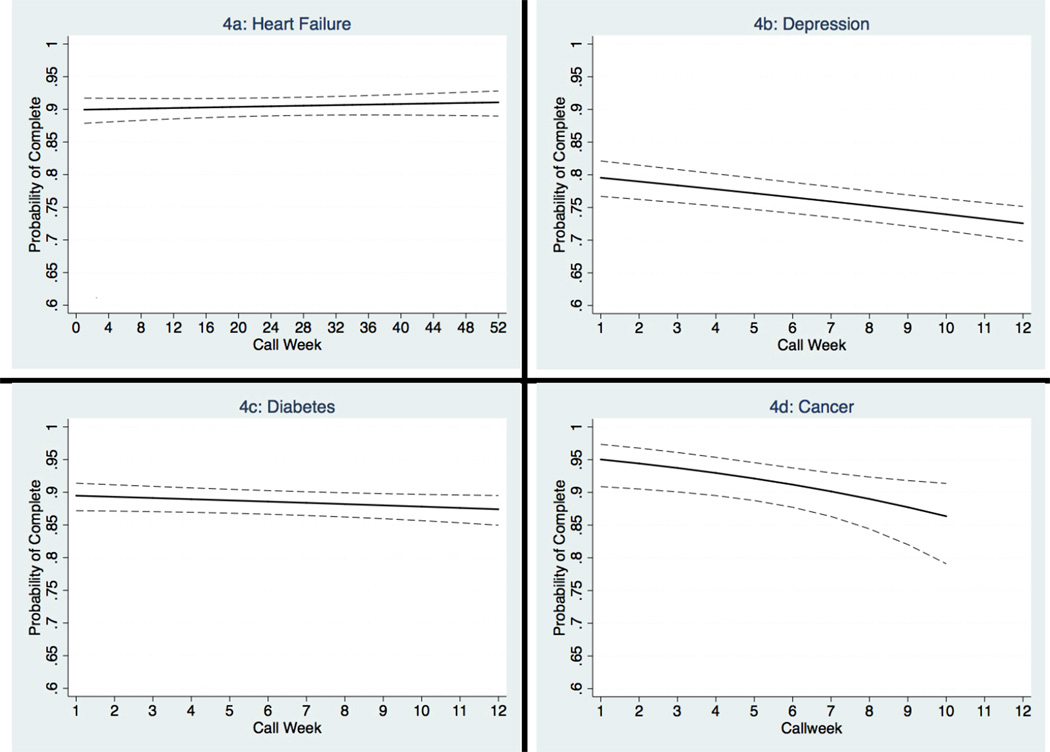
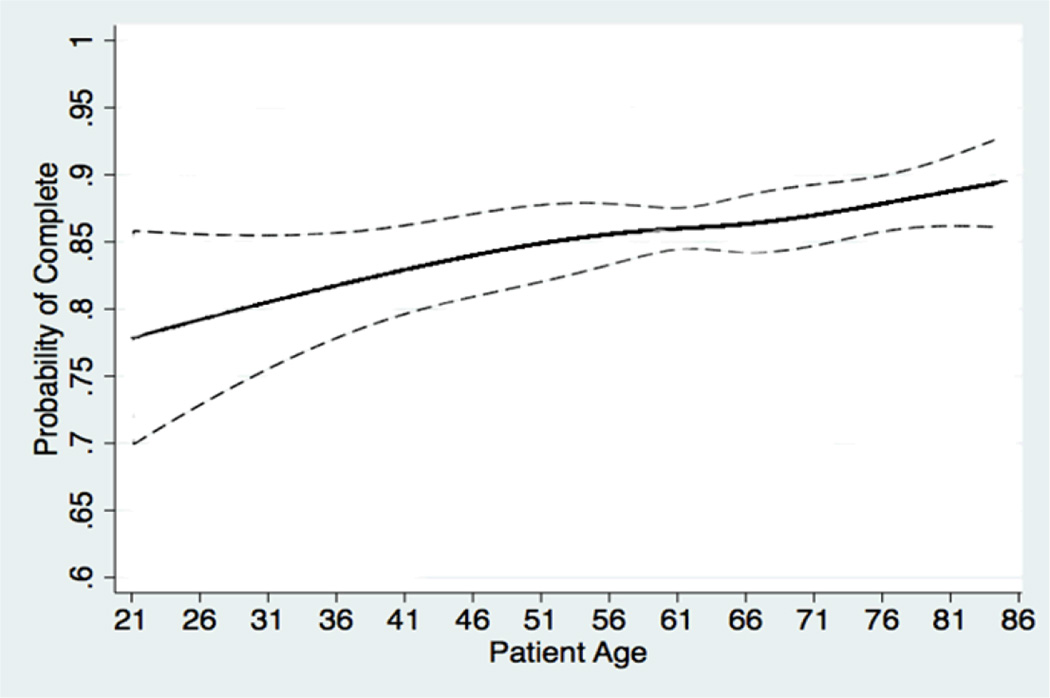
For patients in the heart failure program, calls were consistently answered for a year of follow-up with roughly 90% of patients completing calls in any given week (Figure 1). Call completion rates were consistent over three months among patients with diabetes, but decreased over this time period among patients with depression or cancer. Call completion rates increased monotonically with patient age, with the highest completion rates among patients older than 75 (Figure 2).
Figure 1. Probability of Call Completion Over Time by Program.
Dashed lines represent 95% confidence bands. Predicted probabilities were generated from multivariable logistic regression models using the predictors shown in the first column of Table 3.
Figure 2. Probability of Call Completion by Patient Age.
Dashed lines represent 95% confidence bands. Predicted probabilities were generated from a multivariable logistic regression model using the predictors shown in the first column of Table 3. Age was modeled using cubic splines with four knots in order to detect non-linear age effects on the probability of call completion.
CarePartner Selection and Impact on Call Completion
Of the 727 patients with diabetes or depression given the option to participate with an informal caregiver, 65% chose to do so. Bivariate and multivariate analyses identified no significant sociodemographic or clinical predictors of choosing to enroll with a CarePartner. Participation with a CarePartner was associated with increased odds of completing an IVR assessment (AOR 1.36, 95% CI: 1.06, 1.74) compared to participation alone (Table 3). We identified no interaction between having a CarePartner and patients’ marital status, i.e., CarePartner participation was associated with an increased odds of completing IVR assessments whether or not the patient was married.
Discussion
This study is noteworthy because it includes over a thousand patients with a variety of chronic conditions, in both clinical demonstration programs and randomized trials. As such, the experience reported here is likely to represent what can be expected when IVR disease management programs are translated into primary care and specialty practice. Our data suggest that many concerns regarding IVR self-management support services are unfounded:
Concern #1: Patients will not answer IVR calls. We found that IVR call completion rates were generally high.
Concern #2: Socioeconomically vulnerable patients will be less likely to use IVR. IVR call completion did not vary by race, education, marital status, or income. This suggests that IVR may be one means for eliminating disparities in access to health information supporting disease self-management.
Concern #3: Older patients will not use IVR. While some might assume that older patients who often have poorer computer literacy42 would avoid using IVR, we found that the odds of call completion increased significantly with age, even among patients greater than 75 years old. This suggests that IVR may be an appropriate strategy for increasing monitoring and self-care support for frail elders, particularly those who have difficulty attending face-to-face clinic appointments.
Concern #4: Patients with mental illness will not participate in IVR self-management support programs. Depression program patients completed 10–20% fewer assessments than patients in other programs, however their call completion rates were still reasonably high (71%). Both depression program participation per se and lower baseline SF-12 mental health functioning scores were associated with less frequent call completion. Patients with mental illness may benefit from additional support (e.g. a CarePartner) to maintain adherence to IVR calls. In the current study, only depression program patients were recruited from non-VA healthcare systems. VA has made an enormous investment in patient centered care and service integration, and VA patients tend to be highly satisfied with their healthcare. As a result, it may be that differences between VA and non-VA services, rather than between patients with and without depression are responsible for some of the variation in call completion rates we observed. Similarly, the gender effect suggested in Table 3 may also represent a “VA effect.”
Concern #5: IVR participation will wane significantly over time. While we did see some decline over time, patients with heart failure followed for up to a year completed 90% of their weekly calls. As in a previous report,43 call completion rates decreased among patients with depression and cancer. It is unclear whether patients tired of the IVR interactions or felt they had learned a sufficient amount about their self-care. However, user fatigue is likely, and future applications should seek to address fatigue using intelligent algorithms that adapt to patients’ changing needs.44
Despite the favorable findings, IVR cannot fully address the barriers to health service engagement among some of the most high risk patients. Patients who had missed a prior clinic visit, had been hospitalized, and had lower mental health functioning at enrollment missed their IVR calls at higher rates. The optimal “dose” of IVR intervention remains unknown, and it could be that these vulnerable patients benefit disproportionately, despite a lower exposure to the service. Studies of IVR self-management support among socioeconomically vulnerable patients in Honduras and Mexico suggest that patients can benefit from IVR self-management support, despite call completion rates of less than 60%.34,45 Ultimately, usability testing and qualitative research on specific programs for specific populations will be important to ensure that these services meet diverse patients’ needs.
Although the current study’s compilation of a large sample of patients from multiple IVR programs is a strength, it also implies important limitations in our ability to examine the predictors of call completion separately within each program. For example, because we limited the multivariate analyses of completion rates to information collected uniformly across programs, we could not examine the potentially important role of disease-specific quality-of-life measures (e.g., the Minnesota Living with Heart Failure Questionnaire46) or disease-specific physiologic information (e.g., cancer type or diabetes patients’ glycemic control). In particular, the predictors of decreased (or increased) call completion over time may vary across programs, although those three-way interactions (e.g., program-by-time-by baseline SF-12 scores) could not be examined exhaustively in the current study.
To our knowledge, this is one of the only patient-focused health IT applications that includes feedback to an informal caregiver. The current study suggests that most patients will enroll in IVR self-management support programs with a caregiver if given the option. This is consistent with other research showing that most chronically-ill adults have significant family member or friend involvement in their health care.47 Patients who elected CarePartner involvement were more likely to complete their IVR calls, and this increase was independent of the patient’s marital status. That CarePartner selection was not associated with other identified patient characteristics suggests that its relationship with program engagement represents a direct effect of caregiver feedback. Nevertheless, it remains possible that unmeasured confounders uncorrelated with patient demographics explain the relationship between patients’ participation with a CarePartner and tendency to respond to more of their IVR calls. Future studies should investigate patient perceptions of the benefits of including caregivers in mHealth monitoring, and future mHealth chronic illness interventions using technologies other than IVR should evaluate the effect of caregiver participation on patient engagement.
It is important to remember that patient responsiveness to IVR interventions is largely determined by specific program characteristics, such as call frequency, length, complexity, feedback to others, and the targeted health problem. Thus, these results should be taken as a starting point for understanding the expected engagement of patients using IVR. It is unknown how these findings will generalize to other populations, particularly patients with cognitive and other mental health limitations. However, much of the research on IVR patient monitoring has come from psychiatry,48–50 and the current study suggests that patients with depression will use IVR self-management support. Future research should explore IVR use by community-dwelling patients with mild dementia, schizophrenia, and active substance abuse. Finally, the current study did not have available information about important psychosocial factors, such as health literacy or self-efficacy, which could determine patients’ willingness to complete IVR calls and their ability to utilize the information.
In summary, we found that in a large sample of patients participating in four IVR disease management programs, proactive IVR assessment and behavior change calls were a viable way to gather patient data and provide self-management education between visits. Call completion rates decreased over time among patients with depression or cancer, but remained consistent among patients with diabetes and heart failure patients receiving calls for a year. When given the option, many chronically ill patients choose to have feedback about their health and self-care challenges delivered to an informal caregiver. Caregiver feedback can increase call completion rates, and interventions including an emphasis on caregiver involvement should be explored further. Future studies should continue evaluating the impact of IVR self-management support systems on patients’ treatment outcomes.
Acknowledgements
John Piette is a VA Senior Research Career Scientist, and Ann Marie Rosland is a VA Career Development Awardee. The current study was supported by the Department of Veterans Affairs Health Services Research and Development Program (Cancer CarePartners was supported by VA IIR 08-309-2 and the Heartfailure CarePartners study was supported by VA IIR-07-185). Other financial support came from the University of Michigan Health System Faculty Group Practice, Blue Cross and Blue Shield of Michigan, and grant number P30DK092926 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Contributor Information
John D. Piette, Email: jpiette@umich.edu.
Ann-Marie Rosland, Email: arosland@umich.edu.
Nicolle Stec Marinec, Email: stecn@umich.edu.
Dana Striplin, Email: Dana.striplin@va.gov.
Steven J. Bernstein, Email: sbernste@umich.edu.
Maria J. Silveira, Email: mariajs@umich.edu.
References
- 1.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konrad TR, Link CL, Shackelton RJ, et al. It's about time: Physicians' perceptions of time constraints in primary care medical practice in three national healthcare systems. Medical Care. 2010;48:95–100. doi: 10.1097/MLR.0b013e3181c12e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker MM, Moffet HH, Schillinger D, et al. Ethnic differences in appointment-keeping and implications for the patient-centered medical home: Findings from the diabetes study of northern California (DISTANCE) Health Services Research. doi: 10.1111/j.1475-6773.2011.01337.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karter AJ, Parker MM, Moffet HH, et al. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Medical Care. 2004;42(2):110–115. doi: 10.1097/01.mlr.0000109023.64650.73. [DOI] [PubMed] [Google Scholar]
- 5.Abudagga A, Resnick HE, Alwan M. Impact of blood pressure telemonitoring on hypertension outcomes: A literature review. Telemedicine and e-Health. 2010;16(7):830–838. doi: 10.1089/tmj.2010.0015. [DOI] [PubMed] [Google Scholar]
- 6.Piette JD, Richardson C, Himle J, et al. A randomized trial of telephone counseling plus walking for depressed diabetes patients. Medical Care. 2011;49:641–648. doi: 10.1097/MLR.0b013e318215d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 8.Clark RA, Inglis SC, McAlister FA, Cleland JGF, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. British Medical Journal. 2007;334:942–950. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 10.Bott DM, Kapp MC, Johnson LB, Magno LM. Disease management for chronically ill beneficiaries in traditional Medicare. Health Affairs. 2009;28(1):86–98. doi: 10.1377/hlthaff.28.1.86. [DOI] [PubMed] [Google Scholar]
- 11.Whitten PS, Mair FS, Haycox A, May CR, Williams TL, Hellmich S. Systematic review of cost-effectiveness studies of telemedicine interventions. British Medical Journal. 2002;324:1434–1437. doi: 10.1136/bmj.324.7351.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick K, Raab F, Adams MA, et al. A text message-based intervention for weight loss: randomized controlled trial. Journal of Medical Internet Research. 2009;11:e1. doi: 10.2196/jmir.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong AW, Watson AJ, Makredes M, Frangos JE, Kimball AB, Kvedar JC. Text-message reminders to improve sunscreen use: a randomized, controlled trial using electronic monitoring. Archives of Dermatology. 2009;145(11):1230. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 14.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiologic Reviews. 2010;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemedicine and e-Health. 2009;15(3):231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 16.Joundi RA, Brittain J-S, Jenkinson N, Green AL, Aziz T. Rapid tremor frequency assessment with the iPhone accelerometer. Parkinsonism and Related Disorders. 2011;17(4):288–290. doi: 10.1016/j.parkreldis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Kosaka R, Sankai Y, Takiya R, Jikuya T, Yamane T, Tsutsui T. Tsukuba remote monitoring system for continuous-flow artificial heart. Artificial Organs. 2003;27(10):897–906. doi: 10.1046/j.1525-1594.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 18.Savenstedt S, Zingmark K, Sandman PO. Video-phone communication with cognitively impaired elderly patients. Journal of Telemedicine and TeleCare. 2003;9(Suppl. 2):52–54. doi: 10.1258/135763303322596264. [DOI] [PubMed] [Google Scholar]
- 19.Abroms LC, Padmanabhan N, Thaweethai L, Phillips T. iPhone apps for smoking cessation. American Journal of Preventive Medicine. 2011;40(3):279–285. doi: 10.1016/j.amepre.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodey BB, Rosen CS, Brodey IS, Sheetz BM, Steinfeld RR, Gastfriend DR. Validation of the Addiction Severity Index (ASI) for internet and automated telephone self-report administration. J Subst Abuse Treat. 2004 Jun;26(4):253–259. doi: 10.1016/j.jsat.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Toll BA, Cooney NL, McKee SA, O'Malley SS. Do daily interactive voice response reports of smoking behavior correspond with retrospective reports? Psychol Addict Behav. 2005 Sep;19(3):291–295. doi: 10.1037/0893-164X.19.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobak KA, Taylor LV, dottle SL, et al. A computer-administered telephone interview to identify mental disorders. JAMA. 1997;278:905–910. [PubMed] [Google Scholar]
- 23.Midanik LT, Greenfield TK. Interactive voice response versus computer-assisted telephone interviewing (CATI) surveys and sensitive questions: the 2005 National Alcohol Survey. J Stud Alcohol Drugs. 2008 Jul;69(4):580–588. doi: 10.15288/jsad.2008.69.580. [DOI] [PubMed] [Google Scholar]
- 24.Schroder KE, Johnson CJ, Wiebe JS. Interactive voice response technology applied to sexual behavior self-reports: a comparison of three methods. AIDS and Behavior. 2007 Mar;11(2):313–323. doi: 10.1007/s10461-006-9145-z. [DOI] [PubMed] [Google Scholar]
- 25.Balas EA, Jaffrey F, Kuperman GJ, et al. Electronic communication with patients. Evaluation of distance medicine technology. Journal of the American Medical Association. 1997 Jul 9;278(2):152–159. [PubMed] [Google Scholar]
- 26.Corkrey R, Parkinson L. Interactive Voice Response: review of studies 1989–2000. Behavioral Research Methods and Instrumental Computing. 2002;34(3):342–353. doi: 10.3758/bf03195462. [DOI] [PubMed] [Google Scholar]
- 27.Perrine MW, Mundt JC, Searles JS, Lester LS. Validation of daily self-reported alcohol consumption using interactive voice response (IVR) technology. J Stud Alcohol. 1995;56(5):487–490. doi: 10.15288/jsa.1995.56.487. [DOI] [PubMed] [Google Scholar]
- 28.Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000;108(1):20–27. doi: 10.1016/s0002-9343(99)00298-3. [DOI] [PubMed] [Google Scholar]
- 29.Piette JD, Weinberger M, Kraemer FB, McPhee SJ. Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs health care system: a randomized controlled trial. Diabetes Care. 2001;24(2):202–208. doi: 10.2337/diacare.24.2.202. [DOI] [PubMed] [Google Scholar]
- 30.Bender BG, Apter A, Bogen DK, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. J Am Board Fam Med. 2010 Mar-Apr;23(2):159–165. doi: 10.3122/jabfm.2010.02.090112. [DOI] [PubMed] [Google Scholar]
- 31.Reid RD, Pipe AL, Quinlan B, Oda J. Interactive voice response telephony to promote smoking cessation in patients with heart disease: a pilot study. Patient Educ Couns. 2007 Jun;66(3):319–326. doi: 10.1016/j.pec.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney DF, Tarlow BJ, Jones RN. Effects of an automated telephone support system on caregiver burden and anxiety: findings from the REACH for TLC intervention study. Gerontologist. 2003;43(4):556–567. doi: 10.1093/geront/43.4.556. [DOI] [PubMed] [Google Scholar]
- 33.Aharonovich E, Hatzenbuehler ML, Johnston B, et al. A low-cost, sustainable intervention for drinking reduction in the HIV primary care setting. AIDS Care. 2006 Aug;18(6):561–568. doi: 10.1080/09540120500264134. [DOI] [PubMed] [Google Scholar]
- 34.Piette JD, Datwani H, Gaudioso S, et al. Hypertension management using mobile technology and home blood pressure monitoring: results of a randomized trial in two low/middle income countries. Telemedicine and e-Health. doi: 10.1089/tmj.2011.0271. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piette JD, Mendoza-Avelares M, Milton EC, et al. Access to mobile communication technology and willingness to participate in automated telemedicine calls among chronically-ill patients in Honduras. Telemedicine and e-Health. 2010;16(10):1030–1041. doi: 10.1089/tmj.2010.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piette JD, Lun KC, Moura LA, et al. Impacts of eHealth on outcomes of care in low and middle-income countries: Where do we go from here? WHO Bulletin. 2012;90(5):366–372. doi: 10.2471/BLT.11.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosland AM, Piette JD, Choi H, Heisler M. Family and friend participation in primary care visits of patients with diabetes or heart failure: patient and physician determinants and experiences. Medical Care. 2011;49(1):37–45. doi: 10.1097/MLR.0b013e3181f37d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff JL, Roter DL. Family presence in routine medical visits: A meta-analytic review. Social Science and Medicine. 2011;72:823–831. doi: 10.1016/j.socscimed.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silveira MJ, Given CW, Cease KB, et al. Cancer Carepartners: Improving patients' symptom management by engaging informal caregivers. BMC Palliat Care. 2011;10:21. doi: 10.1186/1472-684X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Pub Health Med. 1997;19(2):179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 42.Rideout V, Neuman T, Kitchman M, Brodie M. Kaiser Family Foundation: e-Health and the Elderly: How Seniors Use the Internet for Health Information. 2005 http://www.kff.org/entmedia/upload/e-Health-and-the-Elderly-How-Seniors-Use-the-Internet-for-Health-Information-Key-Findings-From-a-National-Survey-of-Older-Americans-Survey-Report.pdf.
- 43.Piette JD, McPhee SJ, Weinberger M, Mah CA, Kraemer FB. Use of automated telephone disease management calls in an ethnically diverse sample of low-income patients with diabetes. Diabetes Care. 1999;22(8):1302–1309. doi: 10.2337/diacare.22.8.1302. [DOI] [PubMed] [Google Scholar]
- 44.Singh S, Litman DJ, Kearns M, Walker M. Optimizing dialogue management with reinforcement learning: experiments wtih the NJFun System. Journal of Artificial Intelligence Research. 2000:1–28. [Google Scholar]
- 45.Piette JD, Mendoza-Avelares MO, Ganser M, Mohamed M, Marinec N, Krishnan S. A preliminary study of a cloud-computing model for chronic illness self-care support in an underdeveloped country. American Journal of Preventive Medicine. 2011;40(6):629–632. doi: 10.1016/j.amepre.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure Questionnaire: reliability, validity during a randomized, double-blind, placebo-controlled trial of pimobendan pimobendan multicenter research group. Am Heart J. 1992;124(4):1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 47.Rosland AM, Heisler M, Choi H, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illness. 2010;6(1):22–33. doi: 10.1177/1742395309354608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helzer JE, Rose GL, Badger GJ, et al. Using interactive voice response to enhance brief alcohol intervention in primary care settings. J Stud Alcohol Drugs. 2008 Mar;69(2):251–258. doi: 10.15288/jsad.2008.69.251. [DOI] [PubMed] [Google Scholar]
- 49.Mundt JC, Kinoshita LM, Hsu S, Yesavage JA, Greist JH. Telephonic remote evaluation of neuropsychological deficits (TREND): longitudinal monitoring of elderly community-dwelling volunteers using touch-tone telephones. Alzheimer Dis Assoc Disord. 2007 Jul-Sep;21(3):218–224. doi: 10.1097/WAD.0b013e31811ff2c9. [DOI] [PubMed] [Google Scholar]
- 50.Turvey CL, Willyard D, Hickman DH, Klein DM, Kukoyi O. Telehealth screen for depression in a chronic illness care management program. Telemedicine and e-Health. 2007 Feb;13(1):51–56. doi: 10.1089/tmj.2006.0036. [DOI] [PubMed] [Google Scholar]