Abstract
Mu opioid receptors are densely expressed in the patch compartment of striatum and contribute to methamphetamine-induced patch-enhanced gene expression and stereotypy. In order to further elucidate the role of mu opioid receptor activation in these phenomena, we examined whether activation of mu opioid receptors would enhance methamphetamine-induced stereotypy and prodynorphin, c-fos, arc and zif/268 expression in the patch and/or matrix compartments of striatum, as well as the impact of mu opioid receptor activation on the relationship between patch-enhanced gene expression and stereotypy. Male Sprague-Dawley rats were intrastriatally infused with D-Ala(2)-N-Me-Phe(4),Gly(5)-ol]enkephalin (DAMGO; 1 μg/μl), treated with methamphetamine (0.5 mg/kg) and sacrificed at 45 minutes or 2 hours later. DAMGO augmented methamphetamine-induced zif/268 mRNA expression in the patch and matrix compartments, while prodynorphin expression was increased in the dorsolateral patch compartment. DAMGO pretreatment did not affect methamphetamine-induced arc and c-fos expression. DAMGO enhanced methamphetamine-induced stereotypy and resulted in greater patch versus matrix expression of prodynorphin in the dorsolateral striatum, leading to a negative correlation between the two. These findings indicate that mu opioid receptors contribute to methamphetamine-induced stereotypy, but can differentially influence the genomic responses to methamphetamine. These data also suggest that prodynorphin may offset the overstimulation of striatal neurons by methamphetamine.
Keywords: psychostimulant, opioid, immediate early gene, caudate putamen, behavior
1. Introduction
Psychostimulants modify basal ganglia function and induce significant changes in behavior as a result of alterations in neuropeptide and immediate early gene expression in the striatum (Hanson et al., 1987, Moratalla et al., 1992, Wang and McGinty, 1995, Wang et al., 1995, Harlan and Garcia, 1998, Canales and Graybiel, 2000, Tan et al., 2000, Adams et al., 2003, Gonzalez-Nicolini et al., 2003, Horner and Keefe, 2006, Horner et al., 2010). For example, treatment with methamphetamine induces a higher degree of dynorphin, c-fos, arc and zif/268 mRNA expression in the patch (striosome) compartment relative to the surrounding matrix compartment of rostral striatum, resulting in a patch-enhanced pattern of gene expression (Wang et al., 1995, Adams et al., 2003, Horner and Keefe, 2006, Horner et al., 2010). The immediate early genes zif/268 and c-fos code for transcription factors that act on downstream target genes, including those encoding neuropeptides in the striatum, whereas arc mRNA is trafficked to activated synapses (Milbrandt, 1987, Cole et al., 1995, Lyford et al., 1995, Steward et al., 1998, Steward and Worley, 2001). On the other hand, dynorphin could serve as a negative feedback mechanism to regulate striatal neuron function, possibly in response to overstimulation of striatal neurons by psychostimulants (Steiner and Gerfen, 1998, Horner et al., 2010). Thus, activation of arc, c-fos and/or zif/268 may be an initial step in a chain of transcriptional events that impact long-term plasticity in neurons and along with dynorphin, could ultimately influence the behavioral responses to treatment with methamphetamine.
It is thought that psychostimulant-induced stereotypy may be related to the induction of patch-enhanced gene expression in the rostral striatum (Canales and Graybiel, 2000, Graybiel and Canales, 2000, Graybiel et al., 2000, Canales, 2005). The neurons of the patch compartment receive inputs from limbic-related areas, such as prelimbic cortex and based upon its connections with periallocortical regions, possess circuitry that limbic in nature, whereas neurons in the matrix compartment receive inputs from sensorimotor and association cortices, and due to its connections with neocortex, possesses a circuitry that is less limbic in nature (Gerfen, 1984, Bolam et al., 1988, Ragsdale and Graybiel, 1988, Gerfen, 1989, 1992b, Wang and Pickel, 1998). It has been suggested that enhanced activity of patch-based, limbic-associated circuits, relative to the matrix-based, motor-associated circuits may be related to inflexible, internally driven behaviors, such as stereotypy (Canales and Graybiel, 2000, Graybiel and Canales, 2000, Canales, 2005). Yet, the exact nature of the relationship between enhanced activation of the patch compartment relative to the matrix compartment and stereotypic behavior following psychostimulant treatment is not completely understood, as previous studies have shown positive, negative, or no correlation between patch-enhanced activity and psychostimulant-induced stereotypy (Canales and Graybiel, 2000, Saka et al., 2002, Glickstein and Schmauss, 2004, Horner et al., 2010).
However, despite the disparate findings regarding the precise relationship between patch-enhanced activity and psychostimulant-induced stereotypy, several lines of evidence point to a role for the activation of mu opioid receptors in psychostimulant-induced patch-enhanced gene expression, as well as stereotypic behavior. First, mu opioid receptors are expressed in high density by the neurons of the patch compartment, and may be located extrasynaptically on dendrites where they are co-localized with tyrosine hydroxylase-containing afferents, or on dendritic spines, where they receive asymmetric inputs from prefrontal corticostriatal afferents (Pert et al., 1976, Herkenham and Pert, 1981, Tempel and Zukin, 1987, Wang et al., 1996, Wang and Pickel, 1998). Thus, mu opioid receptors are anatomically positioned to influence gene expression within the neurons of the patch compartment both directly and indirectly through modulation of post-synaptic responses to corticostriatal and nigrostriatal activation (Wang et al., 1997, Wang and Pickel, 1998). Second, blockade of mu opioid receptors attenuates psychostimulant-induced dynorphin expression in the patch compartment of rostral striatum, and prevents patch-enhanced expression of dynorphin in the dorsolateral striatum by methamphetamine as a result of a decrease in the ratio of patch-to-matrix mRNA expression in this region (Horner and Keefe, 2006, Horner et al., 2010). Finally, blockade of striatal mu opioid receptors can reduce methamphetamine-induced stereotypic behavior, while pretreatment with the mu opioid receptor agonist morphine has been shown to enhance amphetamine-induced stereotypy (Woo et al., 1985, Horner et al., 2010).
Together, these data indicate that striatal mu opioid receptor activation contributes to methamphetamine-induced gene expression and behavior, but also suggest that methamphetamine-induced stereotypic behavior and/or gene expression could be intensified if striatal mu opioid receptors are activated during treatment with a low dose of methamphetamine that induces relatively low levels stereotypic behavior and gene expression. However, whether activation of striatal mu opioid receptors in combination with a low dose of methamphetamine results in an increase in dynorphin or immediate early gene mRNA expression in the patch relative to the matrix compartment or increased stereotypy is presently unknown. Thus, the purpose of the present study was to determine whether activation of striatal mu opioid receptors followed by treatment with a low dose of methamphetamine results in relatively higher levels of dynorphin and immediate early gene mRNA expression in the patch versus matrix compartments striatum, and whether mu opioid receptor activation increases the severity of stereotypic behavior. In addition, we examined whether activation of mu opioid receptors altered the correlation between the ratio of mRNA expression in the patch versus matrix compartments and stereotypy.
2. Methods
2.1. Animals and Surgery
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA), weighing 250–350g were used in all experiments. Rats were housed in groups of four in plastic cages in a temperature-controlled room. Rats were on a 14:10 h light/dark cycle and had free access to food and water. All animal care and experimental manipulations were approved by the Institutional Animal Care and Use Committee of Mercer University School of Medicine and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The minimum possible number of animals (based on power analyses) was used for our experiments and steps were taken to minimize any suffering that might occur during our procedures.
Five to seven days prior to the experiment, rats were implanted bilaterally in the rostral striatum (coordinates relative to bregma: AP +1.68 to +2.16 mm, ML ±2.6 mm, DV −3.5 mm) with 26-gauge guide cannulae (Plastics One, Roanoke, VA, USA) that were 3.5 mm in length that were kept patent with 31-gauge obturators that were also 3.5 mm in length, as previously described (Horner et al., 2010). The day before the experiment the obturators were removed and replaced with dummy cannulae that extended 2.0 mm beyond the guide cannulae, in order to minimize acute tissue damage and spurious immediate early gene expression during the experiment (Keefe and Gerfen, 1995, Adams et al., 2000, Horner et al., 2010). The day of the experiment, 31-gauge injection cannulae that extended 1.5 mm beyond the guide were inserted into the guide cannulae and a 1-μl volume of buffered artificial cerebrospinal fluid (144 mM NaCl; 2.68 mM KCl; 1.6 mM CaCl2; 2.6 mM MgCl2; 0.4 mM KH2PO4, pH, 7.2) or the mu opioid receptor agonist, D-Ala(2)-N-Me-Phe(4),Gly(5)-ol]enkephalin (DAMGO; 1 μg/μl, Woo et al., 1985) was administered bilaterally at a rate of 0.1 μl/minute to the freely moving animal. After each infusion, the injection cannulae were left in place for 5 minutes in order to minimize fluid back flow through the cannulae. Only animals whose cannulae were in the rostral striatum were included in subsequent analyses.
2.2. Experimental Design and Behavior
Twenty-four hours prior to the experiment, animals were habituated to plexiglass activity chambers (Frankel et al., 2007, Horner et al., 2010) by placing them in the chambers for 60 minutes, giving them sham injections and returning them to the chambers for either 45 minutes or 2 hours. The next day, animals were placed in the chambers for 60 minutes, after which time they were bilaterally infused with either artificial cerebrospinal fluid or 1 μg/μl DAMGO, as described above. The animals were then injected with methamphetamine (0.5 mg/kg, s.c., a dose determined by dose-response pilot studies performed in our laboratory) or saline and returned to the activity chambers for 45 minutes or 2h, during which time the behavior was digitally recorded for post-hoc analyses. During the post-hoc analyses, each animal was observed for 1 minute every 5 minutes for the entire 45 minute or 2 hour observation period after the injection of methamphetamine or saline by an observer blind to the experimental conditions (Horner et al., 2010). Stereotypy was rated on a scale of 1–10, with 10 representing the highest degree of the response, and scores were generated as previously described (Canales and Graybiel, 2000, Horner et al., 2010), by averaging the scores from four behavioral dimensions: repetitiveness/flexibility, frequency, duration and spatial distribution of the motor response.
2.3. In Situ Hybridization Histochemistry
In situ hybridization histochemistry for detection of changes in mRNA expression was performed as previously described (Horner et al., 2010). Forty-five minutes (for immediate early gene expression; (Horner and Keefe, 2006) or 2 hours (for prodynorphin expression; (Wang et al., 1995) after treatment with methamphetamine or saline, rats were sacrificed by exposure to CO2 for 1 minute followed by decapitation. The brains were rapidly harvested, quick-frozen in isopentane on dry ice and stored at −80°C until they were cut into 12-μm sections on a cryostat (Minotome Plus, Triangle Biomedical Sciences, Durham, N.C., USA). Sections were thaw-mounted onto Superfrost slides (VWR, Westchester, PA, USA) and stored at −20°C. Slides from all animals were then post-fixed in 4% paraformaldehyde/0.9% NaCl, acetylated in fresh 0.25% acetic anhydride in 0.1 M triethanolamine/0.9% NaCl (pH 8.0), dehydrated in alcohol, delipidated in chloroform and gradually re-hydrated in a descending series of alcohol concentrations. Slides were air-dried and stored at −20°C.
Oligonucleotide probes (GeneDetect, Bradenton, FL, USA) complementary to bases 762–809 of prodynorphin (Civelli et al., 1985), 1227–1274 of c-fos (Curran et al., 1987), 377–424 of arc (Lyford et al., 1995) or 355–399 of zif/268 (Milbrandt, 1987) mRNA were end-labeled with [33P]-dATP (Perkin Elmer NEN, Wellesley, MA, USA). Each probe was diluted in hybridization buffer (0.6 M sodium chloride, 80 mM Tris, 4 mM EDTA, 0.1% w/v sodium pyrophosphate, 10% w/v dextran, 0.2% w/v lauryl sulfate, 0.5 mg/ml heparin, 50% formamide) and 90 μl of the probe in hybridization buffer was applied to each slide and covered with glass coverslips. Slides were hybridized overnight in humid chambers at 37°C, followed by four washes in 1X saline-sodium citrate (SSC; 0.15 M NaCl, 0.015 M sodium citrate, pH 7.2) at room temperature and then three washes in 2X SSC with 50% (v/v) formamide at 42°C. Slides were washed twice in 1X SSC at room temperature, dipped in deionized H2O and air-dried. All labeled slides were apposed to X-ray film (Kodak Biomax MR film, Kodak Company, Rochester, NY, USA) for approximately 30 days.
2.4. Mu Opioid Receptor Immunohistochemistry
In order to anatomically distinguish the patch and matrix compartments of striatum, immunohistochemistry for mu opioid receptors was performed on serial, 12-μm sections through the striatum that were adjacent to those used for in situ hybridization, as previously described (Horner et al., 2005, Horner and Keefe, 2006, Horner et al., 2010). Briefly, sections were post-fixed in 4% paraformaldehyde/0.9% NaCl, rinsed three times in 0.1 M phosphate-buffered saline (PBS) and blocked with 10% bovine serum albumin (BSA)/0.3% Triton X-100 (TX)/0.1 M PBS for 2 h followed by overnight incubation at 4°C with a polyclonal antibody for the mu opioid receptor (Immunostar, Hudson, WI, USA), diluted in 1:1000 in 0.3% TX/0.1M PBS/5% BSA. The slides were then washed several times in PBS and incubated for 2 h at room temperature in biotinylated goat anti-rabbit IgG antiserum (Vector Laboratories, Burlingame, CA, USA) diluted 1:200 in 0.1M PBS/5% BSA. Slides were then washed three times in PBS, incubated 1 h in ABC solution (Elite ABC Kit, Vector Laboratories) and washed three more times in PBS. Bound antibody was detected using a 3′,3-diaminobenzidine/Ni+ solution (Vector Laboratories). Slides were washed with deionized H2O, dehydrated in a series of alcohols and coverslipped out of xylene.
2.5. Film Analysis
Film autoradiograms were analyzed using the image analysis program ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij), as previously described (Adams et al., 2003, Horner et al., 2005, Horner and Keefe, 2006, Horner et al., 2010). Briefly, images from 5 to 8 animals in each treatment group were analyzed for each mRNA and one section per animal was analyzed for each region of interest examined. Measurements were made according to the coordinates of Paxinos and Watson (Paxinos and Watson, 2005) in the left hemisphere of the rostral striatum (approximately +1.7 mm anterior to bregma). The average gray value of the white matter overlying the structure being measured was subtracted from the average gray value of the region of interest to correct for background labeling.
In order to distinguish the patch and matrix compartments of the striatum, sections adjacent to those used for in situ hybridization for prodynorphin, c-fos, arc or zif/268 mRNA were processed for mu opioid receptor immunohistochemistry, as described above. Measurements were made in the patch and matrix of the rostral striatum, as previously described (Adams et al., 2003, Horner et al., 2005, Horner and Keefe, 2006, Horner et al., 2010), and encompassed four sub-regions: dorsolateral, dorsomedial, ventrolateral and ventromedial striatum (Adams et al., 2001, Horner et al., 2010). Immunohistochemically labeled sections were captured at the same magnification as the in situ hybridization-labeled sections. Patches of mu opioid receptor immunoreactivity were outlined using the ImageJ software and superimposed over corresponding areas on the in situ hybridization-labeled striatal sections and analyzed as described above. Areas where mu opioid receptor immunoreactivity was absent were analyzed as a measure of mRNA expression in the matrix compartment of striatum (Horner et al., 2005, Horner and Keefe, 2006, Horner et al., 2010). A ratio of patch-to-matrix mRNA expression (Canales and Graybiel, 2000, Horner et al., 2009, Horner et al., 2010) was calculated for each mRNA, at each survival time, in each of the four sub-regions of the rostral striatum and was accomplished by dividing the average gray value of the patch by the average gray value of the matrix, for each animal in the study.
2.6. Statistical Analysis
The effects of mu opioid receptor activation on methamphetamine-induced prodynorphin, c-fos, arc and zif/268 mRNA expression in the rostral striatum was analyzed using a two-way (pretreatment x acute treatment) analysis of variance. A separate analysis was performed for the patch and matrix compartments in each of the four sub-regions of the rostral striatum. Behavioral rating data was represented as the area under the curve and was also analyzed using a two-way (pretreatment x acute treatment) analysis of variance. Post-hoc analysis of significant effects was accomplished using individual Bonferroni (Dunn) t-tests. The alpha level for all analyses was set at 0.05. In the case of a significant overall main effect of treatment, the alpha level was not corrected, as only one comparison was made in the post-hoc analysis (saline versus methamphetamine). For the post-hoc analysis of significant interaction terms, four comparisons were made (vehicle/saline versus vehicle/methamphetamine; vehicle/saline versus DAMGO/saline; vehicle/methamphetamine versus DAMGO/methamphetamine; DAMGO/saline versus DAMGO/methamphetamine), thus requiring a P-value=0.0125 (0.05/4) for statistical significance. Correlations between the ratio of patch-to-matrix gene expression in each of the four sub-regions of the rostral striatum for each mRNA and the cumulative stereotypy score for the either the entire 45 minute or 2 hour observation period, were calculated according to the Spearman method. Statistical significance was set at P<0.05.
2.7. Drugs
(±)Methamphetamine hydrochloride was a generous gift from the National Institute on Drug Abuse (Bethesda, MD, USA). Ketamine hydrochloride and xylazine hydrochloride were obtained from Sigma Aldrich (St. Louis, MO, USA). Methamphetamine, ketamine and xylazine doses were calculated as the free base and dissolved in normal saline. All drugs were given in a volume of 1 ml/kg. DAMGO was obtained from Sigma Aldrich (St. Louis, MO, USA) and dissolved in artificial cerebrospinal fluid.
3. Results
3.1. Effects of DAMGO pretreatment on arc mRNA expression in the patch and matrix compartments of striatum, 45 minutes after treatment with methamphetamine
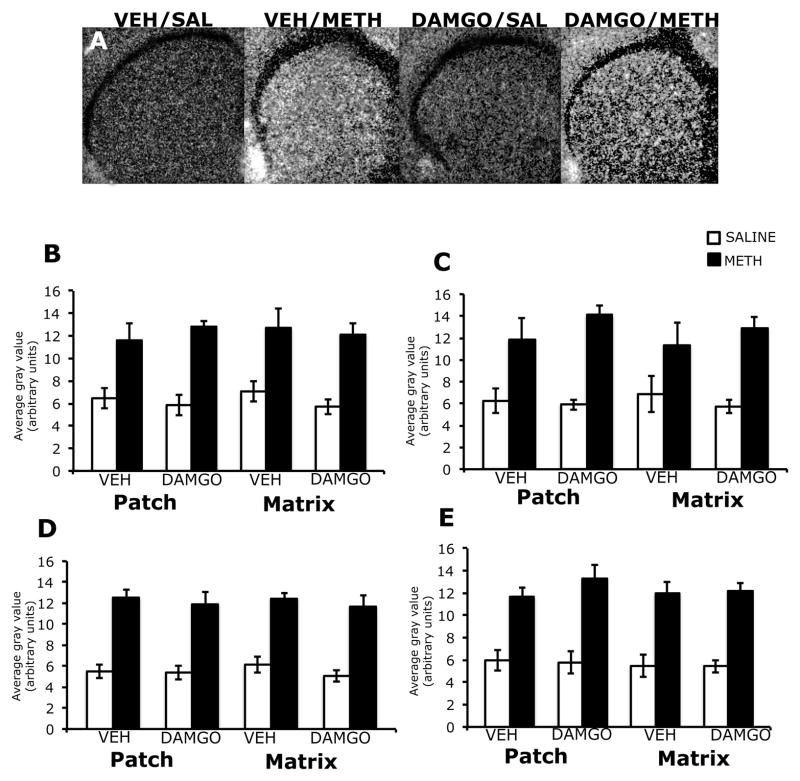
Expression of arc mRNA was homogeneous in appearance in the striatum of both vehicle and DAMGO-pretreated animals, 45 minutes after treatment with methamphetamine (Figure 1A). Two-way analysis of variance of the effects of mu opioid receptor activation on methamphetamine-induced arc mRNA expression revealed a significant overall effect of methamphetamine treatment, without significant effects of DAMGO pretreatment or a pretreatment x treatment interaction in both the patch and matrix compartments of all four sub-regions of striatum examined. Post-hoc analyses found that compared to saline-treated controls, methamphetamine significantly increased the arc mRNA signal in the patch compartment of dorsolateral (t=5.84; P<0.0001), dorsomedial (t=5.20; P<0.0001), ventrolateral (t=8.52; P<0.0001) and ventromedial (t=8.04; P<0.0001) striatal sub-regions, as well as the matrix compartment of dorsolateral (t=5.04; P<0.0001), dorsolateral (t=3.95; P=0.0008), ventrolateral (t=8.92; P<0.0001) and ventromedial (t=8.68; P<0.0001) striatum (Figure 1B–E). DAMGO pretreatment did not significantly alter basal arc mRNA expression in the patch or matrix of any sub-region of striatum examined.
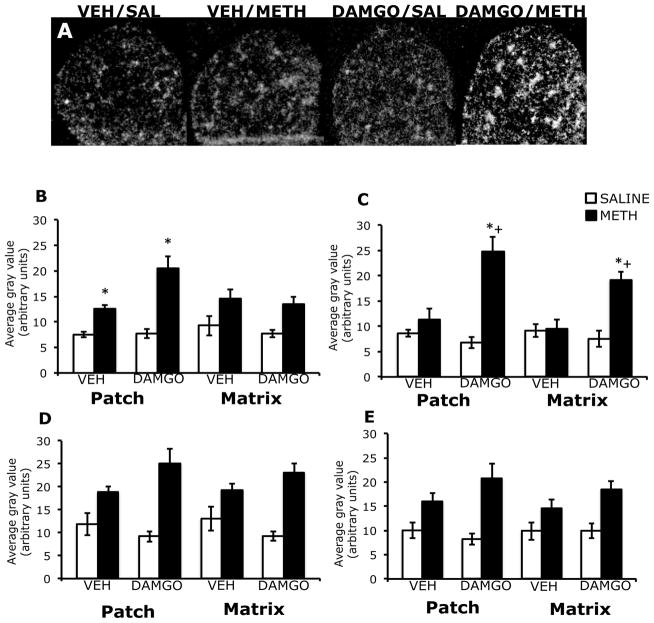
Figure 1.
Effects of DAMGO pretreatment on methamphetamine-induced arc mRNA expression in the rostral striatum, 45 minutes post-treatment. In situ hybridization films (A) showing arc mRNA expression in the rostral striatum. Note the homogenous pattern of arc mRNA expression in methamphetamine-treated animals, and the similar levels of arc mRNA expression between vehicle- and DAMGO-pretreated methamphetamine-treated animals. Quantitative analysis of arc mRNA expression in the patch and matrix compartments of dorsolateral (B), dorsomedial (C), ventrolateral (D) and ventromedial (E) striatum, from rats intrastriatally infused with vehicle or DAMGO (1 μg/μl) 15 minutes prior to treatment with methamphetamine (0.5 mg/kg). Quantitative values are average gray values (arbitrary units, ±SEM, n=5–8 animals). There was a significant overall main effect of methamphetamine treatment in the patch and matrix compartments of all four sub-regions of striatum.
3.2. Effects of DAMGO pretreatment on zif/268 mRNA expression in the patch and matrix compartments of striatum, 45 minutes after treatment with methamphetamine
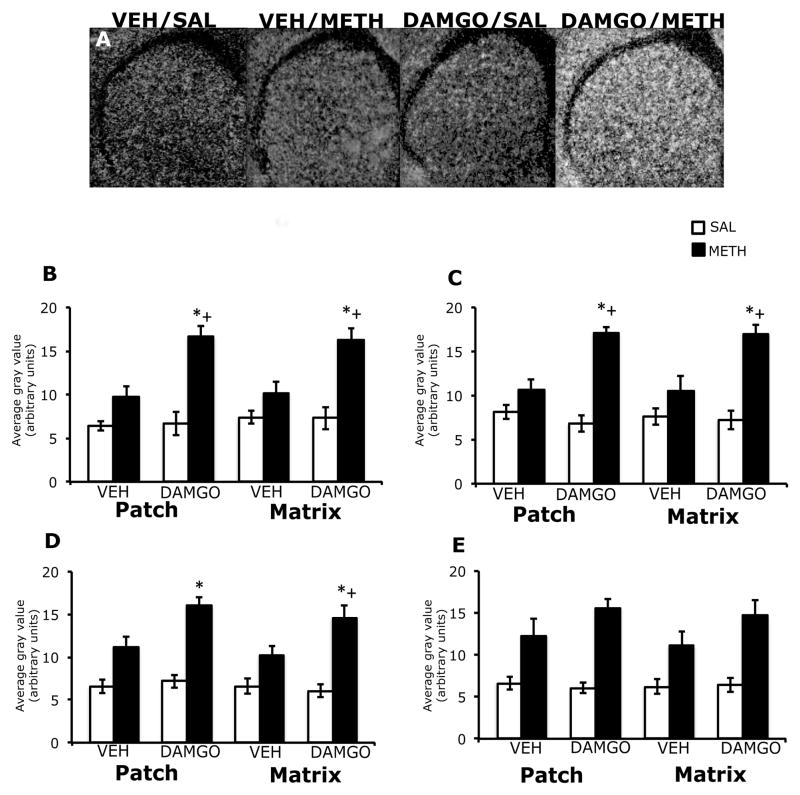
In the striatum of vehicle-pretreated animals, 45 minutes after treatment with methamphetamine, zif/268 mRNA expression was diffuse in appearance, while in DAMGO pre-treated animals this effect appeared to be accentuated (Figure 2A). Two-way analysis of variance of the effects of striatal mu opioid receptor activation on methamphetamine-induced zif/268 mRNA expression revealed a significant effect of DAMGO pretreatment, a significant effect of methamphetamine treatment and a significant pretreatment x treatment interaction in both the patch and matrix compartments of dorsolateral, dorsomedial, and ventrolateral striatum (Figure 2B–E). Post-hoc analyses revealed that methamphetamine treatment alone did not significantly increase zif/268 mRNA expression in the patch compartment of dorsolateral (t=2.7; P=0.02), dorsomedial (t=1.8; P=0.10), ventrolateral (t=2.8; P=0.02) striatum of vehicle-pretreated animals. However, in DAMGO-pretreated animals, methamphetamine treatment significantly increased zif/268 mRNA in the patch compartment of dorsolateral (t=5.3; P=0.0005), dorsomedial (t=8.6; P<0.0001) and ventrolateral (t=7.2; P<0.0001) striatum. In addition, the effect of methamphetamine treatment on zif/268 mRNA expression in the patch compartment was significantly greater in DAMGO-pretreated animals than vehicle-pretreated animals in the dorsolateral (t=3.9; P=0.005) and dorsomedial (t=4.9; P=0.001), but not ventrolateral (t=3.0; P=0.02) striatum. Post-hoc analyses also revealed that methamphetamine treatment alone did not significantly increase zif/268 mRNA expression in the matrix compartment of dorsolateral (t=2.1; P=0.07), dorsomedial (t=1.7; P=0.13) or ventrolateral (t=2.7; P=0.02) striatum in vehicle-pretreated animals, but did significantly increase zif/268 mRNA expression in the matrix compartment of dorsolateral (t=4.9; P=0.001), dorsomedial (t=6.7; P<0.0001) and ventrolateral (t=8.4; P<0.0001) striatum in DAMGO-pretreated animals. In addition, the effect of methamphetamine treatment on zif/268 mRNA expression in the matrix compartment was significantly greater in DAMGO-pretreated animals than vehicle-pretreated animals in the dorsolateral (t=3.4; P=0.01), dorsomedial (t=3.3; P=0.01) and ventrolateral (t=3.6; P=0.007) striatum. In the both the patch and matrix compartments of ventromedial striatum, two-way analysis of variance revealed an overall significant main effect of treatment, but not a significant effect of pretreatment or a pretreatment-treatment interaction (Figure 2E). Post-hoc analysis showed that methamphetamine significantly increased zif/268 mRNA expression in both the patch (t=7.9; P<0.0001) and matrix (t=6.0; P<0.0001) compartments of this striatal sub-region. DAMGO pretreatment did not significantly alter basal zif/268 mRNA expression in the patch or matrix of any sub-region of striatum examined.
Figure 2.
Effects of DAMGO pretreatment on methamphetamine-induced zif/268 mRNA expression in the rostral striatum, 45 minutes post-treatment. In situ hybridization films (A) showing zif/268 mRNA expression in the rostral striatum. Note the enhanced zif/268 mRNA expression, as well as the homogenous pattern of expression in DAMGO-pretreated, methamphetamine-treated animals. Quantitative analysis of zif/268 mRNA expression in the patch and matrix compartments of dorsolateral (B), dorsomedial (C), ventrolateral (D) and ventromedial (E) striatum, from rats intrastriatally infused with vehicle or DAMGO (1 μg/μl) 15 minutes prior to treatment with methamphetamine (0.5 mg/kg). Quantitative values are average gray values (arbitrary units, ±SEM, n=5–8 animals). *Significantly different from vehicle-pretreated control group, P<0.05; +Significantly different from vehicle-pretreated methamphetamine-treated group, P<0.05. There was a significant overall main effect of methamphetamine treatment in the patch and matrix compartments of ventromedial striatum.
3.3. Effects of DAMGO pretreatment on c-fos mRNA expression in the patch and matrix compartments of striatum, 45 minutes after treatment with methamphetamine
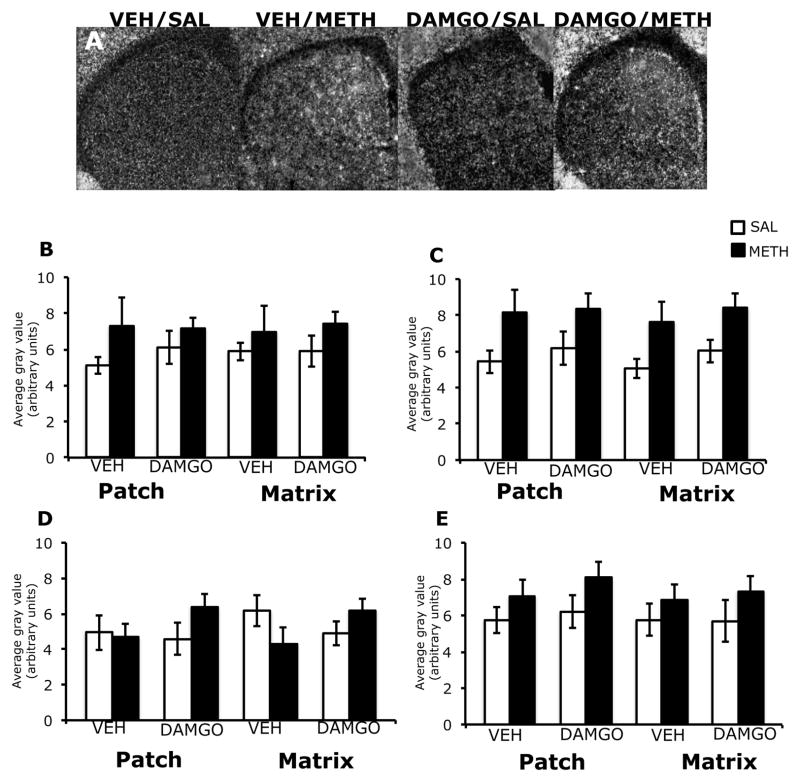
Expression of c-fos mRNA appeared to be increased medially in the striatum of both vehicle and DAMGO-pretreated animals, 45 minutes after treatment with methamphetamine (Figure 3A). Two-way analysis of variance of the effects of mu opioid receptor activation on methamphetamine-induced c-fos mRNA expression revealed a significant overall effect of methamphetamine treatment, without a significant effect of DAMGO pretreatment or a pretreatment-treatment interaction for both the patch and matrix compartments of dorsomedial striatum (Figure 3C). Post-hoc analyses found that c-fos mRNA expression was significantly increased in the patch (t=2.7; P=0.01) and matrix (t=3.2; P=0.005) compartments within this sub-region following methamphetamine treatment. There was not a significant effect of pretreatment, treatment or pretreatment x treatment interaction in the patch or matrix compartment of any other sub-region examined, nor did DAMGO pretreatment significantly alter basal c-fos mRNA expression (Figure 3B, D, E).
Figure 3.
Effects of DAMGO pretreatment on methamphetamine-induced c-fos mRNA expression in the rostral striatum, 45 minutes post-treatment. In situ hybridization films (A) showing c-fos mRNA expression in the rostral striatum. Notice the slight increase in c-fos mRNA expression in the dorsomedial aspects of striatum in methamphetamine-treated animals, and the similar levels of c-fos mRNA expression between vehicle- and DAMGO-pretreated methamphetamine-treated animals. Quantitative analysis of c-fos mRNA expression in the patch and matrix compartments of dorsolateral (B), dorsomedial (C), ventrolateral (D) and ventromedial (E) striatum, from rats intrastriatally infused with vehicle or DAMGO (1 μg/μl) 15 minutes prior to treatment with methamphetamine (0.5 mg/kg). Quantitative values are average gray values (arbitrary units, ±SEM, n=5–8 animals). There was a significant overall main effect of methamphetamine treatment in the patch and matrix compartments all four sub-regions of striatum.
3.4. Effects of DAMGO pretreatment on arc mRNA expression in the patch and matrix compartments of striatum, 2 hours after treatment with methamphetamine
Two-way analysis of the effects of striatal mu opioid receptor activation on methamphetamine-induced arc mRNA expression revealed that 2 hours following treatment, there was not a significant effect of DAMGO pretreatment, methamphetamine treatment or significant pretreatment x treatment interaction in either patch or matrix compartment of any sub-region of striatum examined, nor did DAMGO pretreatment significantly alter basal arc mRNA expression at this time point (data not shown).
3.5. Effects of DAMGO pretreatment on zif/268 mRNA expression in the patch and matrix compartments of striatum, 2 hours after treatment with methamphetamine
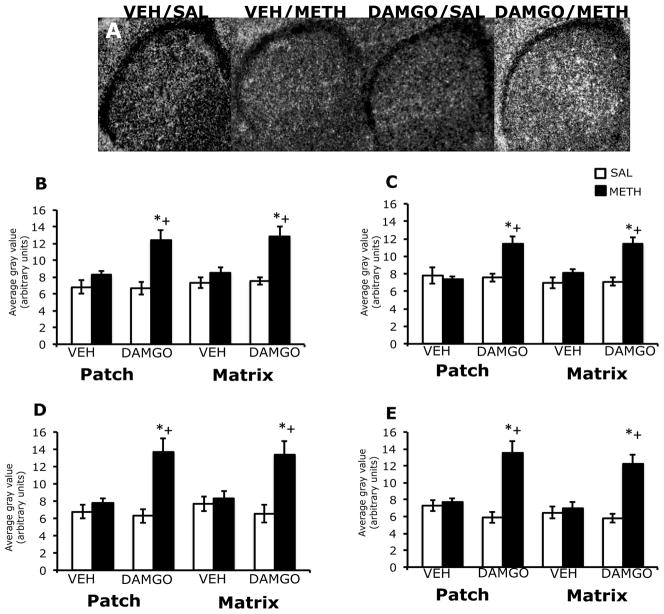
In the striatum of vehicle pre-treated animals, 2 hours after treatment with methamphetamine, zif/268 mRNA expression was diffuse in appearance, while in DAMGO pre-treated animals, this effect appeared to be enhanced (Figure 4A), similar to what was observed 45 minutes post-methamphetamine treatment. Two-way analysis of variance of the effects of striatal mu opioid receptor activation on methamphetamine-induced zif/268 mRNA expression revealed a significant effect of DAMGO pretreatment, a significant effect of methamphetamine treatment, and a significant pretreatment x treatment interaction in both the patch and matrix compartments of all four sub-regions of striatum (Figure 4B–E). Post-hoc analyses revealed that methamphetamine treatment alone did not significantly increase zif/268 mRNA expression in the dorsolateral (t=1.7; P=0.12), dorsomedial (t=0.53; P=0.60), ventrolateral (t=1.5; P=0.16) or ventromedial (t=0.53; P=0.63) patch compartment of vehicle-pretreated animals. However, in the patch compartment of DAMGO-pretreated, methamphetamine-treated animals, zif/268 mRNA expression was significantly increased in all four striatal sub-regions (dorsolateral, t=3.4; P=0.004; dorsomedial, t=3.7; P=0.005; ventrolateral, t=3.9; P=0.003; ventromedial, t=4.8; P=0.0009). In addition, the effect of methamphetamine treatment on zif/268 mRNA expression in the patch compartment was significantly greater in DAMGO-pretreated animals than vehicle-pretreated animals in the dorsolateral (t=3.6; P=0.004), dorsomedial (t=4.3; P=0.001), ventrolateral (t=4.1; P=0.002) and ventromedial (t=4.6; P=0.0006) striatum. Post-hoc analyses revealed that in the matrix compartment, methamphetamine treatment alone did not significantly increase zif/268 mRNA expression in the dorsolateral (t=1.6; P=0.16), dorsomedial (t=1.6; P=0.13), ventrolateral (t=1.0; P=0.36) or ventromedial (t=0.85; P=0.42) sub-regions of vehicle-pretreated animals, but in DAMGO pre-treated, methamphetamine-treated animals, zif/268 mRNA expression was significantly increased in all four sub-regions of striatum (dorsolateral, t=7.5; P=0.004; dorsomedial, t=4.9; P=0.0009; ventromedial, t=4.8; P=0.001; ventrolateral, t=3.6; P=0.007). In addition, the effect of methamphetamine treatment on zif/268 mRNA expression in the matrix compartment was significantly greater in DAMGO-pretreated animals than vehicle-pretreated animals in the dorsolateral (t=3.7; P=0.004), dorsomedial (t=4.3; P=0.001), ventrolateral (t=3.6; P=0.007) and ventromedial (t=4.7; P=0.0005) striatum. DAMGO pretreatment did not significantly alter basal zif/268 mRNA expression in the patch or matrix of any sub-region of striatum examined at this time point.
Figure 4.
Effects of DAMGO pretreatment on methamphetamine-induced zif/268 mRNA expression in the rostral striatum, 2 hours post-treatment. In situ hybridization films (A) showing zif/268 mRNA expression in the rostral striatum. Note the enhanced zif/268 mRNA expression in DAMGO-pretreated, methamphetamine-treated animals. Quantitative analysis of zif/268 mRNA expression in the patch and matrix compartments of dorsolateral (B), dorsomedial (C), ventrolateral (D) and ventromedial (E) striatum, from rats intrastriatally infused with vehicle or DAMGO (1 μg/μl) 15 minutes prior to treatment with methamphetamine (0.5 mg/kg). Quantitative values are average gray values (arbitrary units, ±SEM, n=5–8 animals). *Significantly different from vehicle-pretreated control group, P<0.05; +Significantly different from vehicle-pretreated methamphetamine-treated group, P<0.05.
3.6. Effects of DAMGO pretreatment on prodynorphin mRNA expression in the patch and matrix compartments of striatum, 2 hours after treatment with methamphetamine
In the striatum of vehicle pre-treated animals, 2 hours after treatment with a low dose of methamphetamine, prodynorphin mRNA expression was slightly increased in the patch and matrix compartments of striatum, while in DAMGO pre-treated animals, prodynorphin expression in the patch compartment appeared to be enhanced (Figure 5A). Two-way analysis of variance of the effects of striatal mu opioid receptor activation on methamphetamine-induced prodynorphin mRNA expression in the patch compartment revealed a significant effect of DAMGO pretreatment, a significant effect of methamphetamine treatment, and a significant pretreatment x treatment interaction in the dorsolateral and dorsomedial sub-regions of striatum (Figure 5B, C). Post-hoc analyses revealed that methamphetamine treatment significantly increased prodynorphin mRNA expression in dorsolateral (t=8.1; P<0.0001), but not dorsomedial (t=1.2; P=0.27) patch compartment of vehicle-pretreated animals, while in DAMGO-pretreated animals, methamphetamine also significantly increased prodynorphin mRNA expression in the patch compartment of both dorsolateral (t=4.5; P=0.001) and dorsomedial (t=5.8; P=0.0004) striatum. Furthermore, the effect of methamphetamine treatment on prodynorphin mRNA expression in the patch compartment was significantly greater in DAMGO-pretreated animals than vehicle-pretreated animals in the dorsomedial striatum (t=3.7; P=0.006), and trended towards significance in dorsolateral striatum (t=2.9; P=0.016). In the ventral aspects of striatum, two-way analysis of variance revealed a lack effect of DAMGO pretreatment on prodynorphin mRNA expression in the patch compartment, and no interaction between DAMGO pretreatment and methamphetamine treatment within either the ventrolateral or ventromedial sub-regions of striatum (Figure 5D, E). However, there was a significant main effect of treatment for the patch compartment in the ventral aspects of striatum, with methamphetamine treatment significantly increasing prodynorphin RNA expression within the patch compartment of ventrolateral (t=4.5; P=0.0002) and ventromedial (t=4.1; P=0.0006) striatum.
Figure 5.
Effects of DAMGO pretreatment on methamphetamine-induced prodynorphin mRNA expression in the rostral striatum, 2 hours post-treatment. In situ hybridization films (A) showing prodynorphin mRNA expression in the rostral striatum. Notice the slight increase in prodynorphin expression in vehicle-pretreated, methamphetamine treated animals, and how the intensity of the prodynorphin mRNA expression is increased, particularly within the patches of DAMGO-pretreated, methamphetamine-treated animals. Quantitative analysis of prodynorphin mRNA expression in the patch and matrix compartments of dorsolateral (B), dorsomedial (C), ventrolateral (D) and ventromedial (E) striatum, from rats intrastriatally infused with vehicle or DAMGO (1 μg/μl) 15 minutes prior to treatment with methamphetamine (0.5 mg/kg). Quantitative values are average gray values (arbitrary units, ±SEM, n=5–8 animals). *Significantly different from vehicle-pretreated control group, P<0.05; +Significantly different from vehicle-pretreated methamphetamine-treated group, P<0.05. There was a significant overall main effect of methamphetamine treatment in the patch and matrix compartments of ventromedial striatum and ventrolateral striatum.
Two-way analysis of variance of the effects of striatal mu opioid receptor activation on methamphetamine-induced prodynorphin mRNA expression in the matrix compartment of dorsomedial striatum revealed a significant effect of DAMGO pretreatment, methamphetamine treatment and a significant pretreatment x treatment interaction (Figure 5C). Additional post-hoc analysis revealed that methamphetamine treatment did not significantly increase prodynorphin mRNA expression in the dorsomedial matrix compartment of vehicle-pretreated animals (t=0.15; P=0.88), but significantly increased prodynorphin mRNA expression in the dorsomedial matrix compartment of DAMGO-pretreated animals (t=4.8; P=0.001), with a significantly greater effect of methamphetamine treatment on prodynorphin mRNA expression in DAMGO-pretreated animals than vehicle-pretreated animals (t=3.7; P=0.006). Two-way analysis of variance also revealed a lack of effect of DAMGO pretreatment or a significant interaction between DAMGO pretreatment and methamphetamine treatment in the dorsolateral, ventrolateral and ventromedial matrix compartments (Figure 5B, D, E). However, there was a significant main effect of methamphetamine treatment in the matrix compartment of all three of these sub-regions, with methamphetamine treatment significantly increasing matrical prodynorphin mRNA expression in the dorsolateral (t=3.9; P=0.001), ventrolateral (t=5.2; P<0.0001) and ventromedial (t=4.0; P=0.0006) striatum. DAMGO pretreatment did not significantly alter basal prodynorphin mRNA expression in the patch or matrix of any sub-region of striatum examined.
3.7. Effects of striatal mu opioid receptor activation on methamphetamine-induced stereotypy
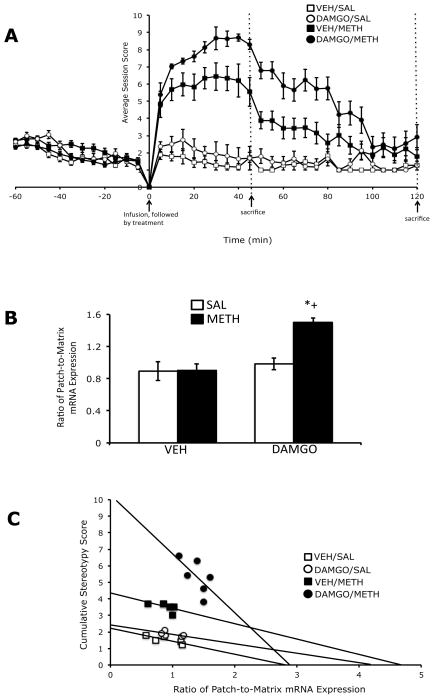
Acute treatment with a low dose methamphetamine resulted in a mild to moderate level of stereotypy, which peaked during the first 45 minutes following treatment, and tapered off by 2 hours post-treatment (Figure 6A). Pretreatment with DAMGO resulted in an increase stereotypic behavior in methamphetamine-treated animals, that also peaked at approximately 45 minutes post-methamphetamine treatment, followed by a gradual tapering-off by 2 hours post-treatment (Figure 6A). Two-way analysis of variance of the area under the curve values for stereotypy at the 45 minute and 2 hour sacrifice time points revealed that at both time points there were significant effects of DAMGO pretreatment, methamphetamine treatment and a significant pre-treatment x treatment interaction. Post-hoc analyses found that methamphetamine treatment significantly increased stereotypic behavior in vehicle-pretreated animals, at 45 minutes (t=7.5; P<0.0001) and 2 hours (t=6.4, P<0.0001) post-methamphetamine treatment, and in DAMGO-pretreated animals, at 45 minutes (t=30; P<0.0001) and 2 hours (t=6.2; P<0.0001) post-methamphetamine treatment. In addition, the degree of stereotypy induced by methamphetamine treatment was significantly greater in DAMGO-pretreated versus vehicle-pretreated animals, at both 45 minutes (t=6.9; P<0.0001) and 2 hours (t=3.7, P=0.001) post-methamphetamine treatment.
Figure 6.
Effects of intrastriatal infusion of DAMGO (1 μg/μl) and methamphetamine treatment (0.5 mg/kg) on stereotyped behavior (A). Values are expressed as the mean ±SEM. The two time points were combined into single graph for conciseness. Methamphetamine treatment significantly increased stereotypy at 45 minutes and 2 hours post-treatment, and was enhanced by pretreatment with DAMGO at both time points. The AUC values are as follows: at 45 minutes post-treatment, Vehicle/Saline=61; Vehicle/METH=203; DAMGO/Saline=72; DAMGO/METH=334 and 2 hours post-treatment, Vehicle/Saline=156; Vehicle/METH=392; DAMGO/Saline=187; DAMGO/METH=610. Patch-enhanced gene expression in the dorsolateral striatum (B). Acute METH treatment did not significantly increase the ratio of patch-to-matrix prodynorphin mRNA expression in the dorsolateral striatum; however, pretreatment with DAMGO resulted in a significant increase in the ratio of patch-to-matrix prodynorphin mRNA expression in this region. Correlation between cumulative stereotypy scores and the ratio of patch-to-matrix prodynorphin mRNA expression in the dorsolateral striatum (C). There was a significant negative correlation between the cumulative stereotypy scores and the ratio of patch-to-matrix prodynoprhin mRNA expression in DAMGO-pretreated, methamphetamine-treated animals. *Significantly different from vehicle-pretreated control group, P<0.05; +Significantly different from vehicle-pretreated methamphetamine-treated group, P<0.05.
3.8. Effects of DAMGO pretreatment and methamphetamine treatment on the ratio of patch-to-matrix mRNA expression and correlation with stereotypy
Two-way analysis of variance of the ratio of patch-to-matrix prodynorphin mRNA expression, 2 hours after treatment with a low dose of methamphetamine revealed that in the dorsolateral striatum, there were significant effects of DAMGO pretreatment, methamphetamine treatment and a significant pretreatment x treatment interaction. Post-hoc analysis revealed that methamphetamine significantly increased the ratio of patch-to-matrix prodynorphin mRNA expression in the dorsolateral striatum of DAMGO-pretreated (t=4.8, P=0.0005), but not vehicle-pretreated (t=0.04; P=0.97) animals, as the effects of methamphetamine treatment on the ratio patch-to-matrix prodynorphin mRNA expression was significantly greater in DAMGO-pretreated versus vehicle-pretreated animals (t=5.5; P=0.0001; Figure 7B). In order to examine the relationship between DAMGO pretreatment, the relative expression of prodynorphin in the patch versus matrix compartments and stereotyped behavior in methamphetamine-treated animals, we determined if there was a correlation between the ratio of patch-to-matrix prodynorphin mRNA expression, for each treatment group, within each of the four sub-regions of striatum and the cumulative stereotypy scores for the entire 2 hour behavioral session. At 2 hours post-methamphetamine treatment, in the dorsolateral sub-region of striatum, there was a significant negative correlation between cumulative stereotypy scores and the ratio of patch-to-matrix prodynorphin mRNA expression in the dorsolateral striatum for DAMGO- (rs=−0.80; P=0.03) pretreated, methamphetamine-treated animals, but not for vehicle-pretreated methamphetamine-treated animals (rs=-0.89; P=0.08), vehicle-pretreated saline-treated animals (rs=−0.90; P=0.10) or DAMGO-pretreated, saline-treated animals (rs=−0.72; P=0.23; Figure 6C). There was not a significant correlation between the ratio of patch-to-matrix prodynorphin mRNA expression and the cumulative stereotypy scores for the 2-hour sacrifice time point for any other sub-region of striatum examined (data not shown).
Two-way analysis of variance of the patch-to-matrix ratio of zif/268 mRNA expression revealed that 45 minutes after methamphetamine treatment, the pattern of zif/268 mRNA expression was patch-enhanced, but only in the dorsolateral striatum, as the ratio of patch-to-matrix zif/268 mRNA expression was significantly greater in methamphetamine-treated versus saline-treated animals in this region (t=2.7; P=0.014). However, there was not a significant effect of DAMGO pretreatment or a pretreatment x treatment interaction, nor was there a significant correlation between patch-enhanced zif/268 mRNA expression and cumulative stereotypy scores for the 45 minute sacrifice time point (data not shown). Arc and c-fos mRNA expression, 45 minutes following methamphetamine treatment and zif/268 and arc mRNA expression, 2 hours following methamphetamine treatment were not patch-enhanced in any sub-region of striatum, nor was there a significant effect of DAMGO pretreatment or a significant pretreatment x treatment interaction. In addition, there was not a significant correlation between the ratio of patch-to-matrix mRNA expression for c-fos or arc and the cumulative stereotypy scores at the 45 minute sacrifice time point or between the ratio of patch-to-matrix mRNA expression for arc or zif/268 and cumulative stereotypy scores at the 2 hour sacrifice time point for any treatment group, in any striatal sub-region examined (data not shown).
4. Discussion
The goal of the current study was to determine whether activation of mu opioid receptors in the striatum would result in enhanced gene expression and stereotypic behavior when combined with a low dose of methamphetamine. Activation of striatal mu opioid receptors prior to treatment with a low dose of methamphetamine augmented zif/268 mRNA expression in both the patch and matrix compartments of all four sub-regions of striatum, whereas methamphetamine-induced c-fos and arc mRNA expression in the patch and matrix compartments were unaltered by striatal mu opioid receptor activation in any sub-region of striatum. In addition, methamphetamine-induced prodynorphin expression was enhanced in the patch, but not matrix compartment of dorsolateral striatum by striatal mu opioid receptor activation and resulted in an increase the ratio of patch-to-matrix expression of prodynorphin mRNA in this region. Finally, mu opioid receptor activation significantly increased methamphetamine-induced stereotypy and induced a negative correlation between the ratio of patch-to-matrix prodynorphin mRNA expression in the dorsolateral striatum and the intensity of the stereotypic behavior. The current study provides additional evidence that striatal mu opioid receptor activation can differentially modulate methamphetamine-induced gene expression, as well as contribute to methamphetamine-induced stereotypy. These data also lend support to the notion that patch-enhanced dynorphin expression may serve as a homeostatic response to psychostimulant-induced overstimulation of the striatum.
4.1 Striatal mu opioid receptor activation and methamphetamine-induced immediate early gene mRNA expression
Activation of mu opioid receptors augmented methamphetamine-induced zif/268 mRNA expression, with little effect on the expression of arc or c-fos. These data are similar to previous work from our laboratory where mu opioid receptor blockade attenuated methamphetamine-induced zif/268 mRNA expression, but had no effect on methamphetamine-induced c-fos mRNA expression (Horner and Keefe, 2006, Horner et al., 2010). The differential regulation of methamphetamine-induced zif/268 expression by mu opioid receptors could be the result of mu-mediated differences in calcium signaling and/or extracellular signal-regulated kinase (ERK) activity. Increases in nuclear calcium, which occur as a result of increased calcium influx at proximal synapses, may stimulate cyclic AMP response element (CRE)-mediated gene transcription, while increases in cytoplasmic calcium, which occur as a result of calcium influx at distal synapses, may stimulate serum response element (SRE)-mediated transcription (Ginty, 1997, Hardingham et al., 1997). In addition, ERK-mediated Elk-1 activation (which targets SRE) takes place in the cytoplasm, while ERK-mediated CREB activation (which targets CRE) takes place in the nucleus (Sgambato et al., 1998). Interestingly, zif/268 has four putative SREs and one or two CREs, c-fos has one SRE and three functional CREs and arc has a synaptic activity responsive element (SARE) that is comprised of SRE, CRE and myocyte enhancer factor-2 (MEF-2) binding sites (Christy et al., 1988, Sassone-Corsi et al., 1988, Kawashima et al., 2009). Activation of mu opioid receptors, which are found on the distal dendrites of medium spiny neurons in the striatum, has been shown to increase intracellular calcium, as well as ERK activity (Smith and Bolam, 1990, Wang and Pickel, 1998, Wang et al., 2000, Macey et al., 2006). Therefore, it is possible that activation of mu opioid receptors at distal synapses lead to increased calcium and Elk-1 activity in the cytoplasm, resulting in enhanced SRE-mediated zif/268 transcription, while leaving nuclear calcium and CREB levels unaltered and thus CRE-mediated c-fos transcription unaffected. Since the SARE requires binding at both the SRE and CRE sites in order to initiate arc transcription, a lack of CREB (and possibly MEF-2) activity may have prevented an enhancement of arc expression by mu opioid receptor activation.
4.2. Striatal mu opioid receptor activation and methamphetamine-induced gene expression in the matrix compartment
Interestingly, the selective enhancement of zif/268 expression by mu opioid receptor activation was also apparent in matrix compartment, despite the relative paucity of mu opioid receptors in this sub-region, as compared to the patch compartment (Pert et al., 1976, Herkenham and Pert, 1981, Tempel and Zukin, 1987), but is in line with previous work from our laboratory where blockade of mu opioid receptors resulted in a diminution of zif/268 mRNA expression in the matrix compartment (Horner and Keefe, 2006, Horner et al., 2010). The mu opioid receptor-mediated changes in zif/268 expression that originate within the patch compartment could be communicated to the matrix as a result of local interactions between the patch and matrix compartments. A portion of medium spiny neurons have dendritic arborizations that cross from one compartment into the other; thus dendrites from a portion of matrix neurons may cross over into the patch where they are influenced by DAMGO-induced changes in the patch compartment (Bolam et al., 1988, Walker et al., 1993). In addition, cholinergic interneurons have dendritic fields that extend across both compartments, and target the medium spiny neurons of the matrix with widespread axon collaterals (Bolam et al., 1988, Kawaguchi, 1992, Tepper and Bolam, 2004). However, if local circuit mechanisms were responsible for changes in the matrix compartment, then it is likely that the strongest effects of DAMGO infusion would be seen primarily around the site of injection. Alternatively, circuit-based mechanisms may be responsible for mu opioid receptor-mediated enhancement of methamphetamine-induced gene expression in the matrix. For example, activation of mu opioid receptors could reduce the activity of GABAergic medium spiny neurons in the patch compartment that send projections to the dopaminergic neurons of the substantia nigra pars compacta, relieving these neurons from inhibition and resulting in enhanced striatal dopamine release and increased striatal output (Gerfen, 1984, Graybiel, 1990, Gonzalez-Nicolini et al., 2003, Pereira et al., 2006). This increase in striatal output could lead to increased cortical activity via disinhibition of thalamocortical pathways, which may then increase corticostriatal input to the matrix, leading to enhanced gene expression in this region (Gerfen and Wilson, 1996, Parthasarathy and Graybiel, 1997, Sgambato et al., 1997). Interestingly, there appears to be enhanced cortical gene expression in DAMGO-pretreated, methamphetamine-treated animals where zif/268 mRNA expression was enhanced in the matrix (see Figures 2A, 4A). However, it is important to note that this scenario does not explain how mu opioid receptor activation can simultaneously induce a patch-enhanced pattern of gene expression when combined with methamphetamine, or why this effect appears to be selective for certain genes. Clearly, additional studies are needed in order to further understand the potential contribution of mu opioid receptor activation to changes in circuit-level activity and the impact on methamphetamine-induced gene expression in the matrix compartment of striatum.
4.3. Striatal mu opioid receptor activation and methamphetamine-induced prodynorphin expression in the dorsolateral striatum
Treatment with a low dose of methamphetamine resulted in a homogenous pattern of prodynorphin mRNA expression in the dorsolateral striatum, as mRNA levels were significantly increased in the patch and matrix compartments, and to a similar degree. Activation of mu opioid receptors prior to treatment with methamphetamine significantly increased the level of prodynorphin expression in the patch, but not matrix compartment of dorsolateral striatum. Accordingly, striatal mu opioid receptor activation increased the ratio of patch-to-matrix prodynorphin mRNA expression in the dorsolateral striatum, which, if we use the negative correlation between patch-enhanced prodynorphin expression and the intensity of stereotypy as a predictor, should result in a decrease in stereotypical behavior. However, this was not the case, as striatal mu opioid receptor activation increased METH-induced stereotypical behavior. One possible explanation for this incongruity is that striatal mu opioid receptor activation results in alterations in the release other neurotransmitters in the striatum. As mentioned above, reduced output from the patch compartment, due to mu opioid receptor activation, may disinhibit dopamine neurons in the substantia nigra pars compacta, resulting in enhanced striatal dopamine release and an exacerbation of methamphetamine-induced stereotypy (Gerfen, 1984, Ujike et al., 1989, Graybiel, 1990, Capper-Loup et al., 2002, Gonzalez-Nicolini et al., 2003, Pereira et al., 2006, Lan et al., 2009). Increased striatal dopamine release may then trigger a patch-enhanced pattern of prodynorphin expression, particularly in the dorsolateral striatum, possibly as a homeostatic response to dampen the overstimulation of striatal neurons, although the mechanism by which this may take place is unclear. Nevertheless, the dorsolateral striatum is though to play a role in repetitive behaviors and habit formation (Canales and Graybiel, 2000; Yin and Knowlton, 2006), and increased dynorphin expression in this region may serve to eventually reduce methamphetamine-induced stereotypy. A role for dynorphin as a homeostatic modulator in the striatum is supported by the observations that striatal prodynorphin expression is usually not evident until the peak of methamphetamine-induced stereotypy has passed and that kappa opioid receptor agonists can reduce stereotypic behavior and inhibit striatal dopamine release (Walker et al., 1987, Heidbreder et al., 1993, Toyoshi et al., 1996, You et al., 1999, Meshul and McGinty, 2000, Ito et al., 2002, Margolis et al., 2003, Horner et al., 2010). However, it is important to note that psychostimulant-induced increases in c-fos and arc mRNA expression are dependent on dopamine D1 receptor activation (Moratalla et al., 1996, Yamagata et al., 2000), and thus should have been affected if methamphetamine-induced dopamine release was augmented by DAMGO. Clearly, additional studies are needed in order to address the role of mu opioid receptor activation in methamphetamine-induced neurotransmitter release in the striatum, as well as the specific impact of these potential changes on methamphetamine-induced gene expression and behavior.
4.4. Striatal mu opioid receptor activation and methamphetamine-induced prodynorphin mRNA expression in the dorsomedial striatum
It is also interesting to note that mu opioid receptor activation significantly enhanced prodynorphin mRNA expression in both the patch and matrix compartments of dorsomedial striatum. Superimposed upon the topography cortical inputs to the patch and matrix compartments, is a medial-to-lateral topography of cortical inputs to the striatum as a whole, such that the medial aspects of the frontal cortex (e.g., the prelimbic cortex) project to the patch and matrix compartments of medial striatum, while the lateral aspects of frontal cortex (e.g., motor cortex) project to the patch and matrix compartments of lateral striatum (Gerfen, 1989, 1992a). Thus, the medial striatum is also considered to be important for the transfer of limbic-related information through the basal ganglia. Of note is recent data that indicates psychostimulant-induced stereotypy may be the result of a functional imbalance between the medial prefrontal circuits that traverse the dorsomedial striatum and the sensorimotor circuits that traverse the dorsolateral striatum, as the psychostimulant-induced neurochemical and electrophysiological changes that occurred during stereotypy were observed in the medial prefrontal, but not sensorimotor circuits (Aliane et al., 2009). Thus, enhanced methamphetamine-induced prodynorphin expression in the dorsomedial striatum following DAMGO pretreatment could reflect an imbalance in the medial prefrontal versus sensorimotor circuits through the striatum, and indicates that mu opioid receptor activation contributes to this imbalance. Interestingly, the methamphetamine-induced c-fos expression observed in the current study was restricted to the dorsomedial striatum, which also supports a functional imbalance between medial prefrontal versus sensorimotor circuits during stereotypy. However, methamphetamine-induced c-fos expression in the dorsomedial striatum was unaltered by DAMGO pretreatment, which argues against a potential role for mu opioid receptor activation in the imbalance between medial prefrontal and sensorimotor circuits during stereotypy. Nevertheless, the relationship between methamphetamine-induced alterations in prodynophin and/or c-fos expression in the dorsomedial striatum and the functional imbalance between limbic and motor-based circuits through the basal ganglia and methamphetamine-induced stereotypy warrants further investigation.
5. Conclusions
Our findings demonstrate that striatal mu opioid receptor activation differentially contributes to methamphetamine-induced immediate early gene expression in the striatum. Furthermore, mu opioid receptor activation modulates zif/268 expression in both the patch and matrix compartments of the striatum, suggesting that striatal mu opioid receptors may differentially modulate of intracellular signaling cascades and that compartmental cross-talk and/or circuit-level changes may allow for modification of methamphetamine-induced gene expression in both the patch and matrix compartments. Our findings also provide further evidence for a role of mu opioid receptors in the expression of methamphetamine-induced stereotypy, as well as methamphetamine-induced patch-enhanced pattern of prodynorphin mRNA expression. In addition, the current study confirms that striatal mu opioid receptors contribute to the negative relationship between patch-enhanced pattern of prodynorphin mRNA expression in the dorsolateral striatum and the intensity of methamphetamine-induced stereotyped behavior. Together, these data indicate that the mu opioid receptor system contributes to the systemic changes in basal ganglia function and organismal behavior that occur as a result of methamphetamine treatment. The present data also lend support to the notion that patch-enhanced expression of prodynorphin of may be the response to methamphetamine-induced overstimulation of the striatum and stereotypy, rather than the source of methamphetamine-induced stereotypy.
Abbreviations
- DAMGO
D-Ala(2)-N-Me-Phe(4),Gly(5)-ol]enkephalin
- METH
methamphetamine
- SAL
saline
- VEH
vehicle
References
- Adams AC, Layer RT, McCabe RT, Keefe KA. Effects of conantokins on L-3,4-dihydroxyphenylalanine-induced behavior and immediate early gene expression. Eur J Pharmacol. 2000;404:303–313. doi: 10.1016/s0014-2999(00)00640-3. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hanson GR, Keefe KA. Differential effects of cocaine and methamphetamine on neurotensin/neuromedin N and preprotachykinin messenger RNA expression in unique regions of the striatum. Neuroscience. 2001;102:843–851. doi: 10.1016/s0306-4522(00)00530-3. [DOI] [PubMed] [Google Scholar]
- Adams DH, Hanson GR, Keefe KA. Distinct effects of methamphetamine and cocaine on preprodynorphin messenger RNA in rat striatal patch and matrix. J Neurochem. 2003;84:87–93. doi: 10.1046/j.1471-4159.2003.01507.x. [DOI] [PubMed] [Google Scholar]
- Aliane V, Perez S, Nieoullon A, Deniau JM, Kemel ML. Cocaine-induced stereotypy is linked to an imbalance between the medial prefrontal and sensorimotor circuits of the basal ganglia. Eur J Neurosci. 2009;30:1269–1279. doi: 10.1111/j.1460-9568.2009.06907.x. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM. Cellular substrate of the histochemically defined striosome and matrix system of the caudate nucleus: a combined golgi and immunohistochemical study. Neuroscience. 1988;24:853–875. doi: 10.1016/0306-4522(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Stimulant-induced adaptations in neostriatal matrix and striosome systems: transiting from instrumental responding to habitual behavior in drug addiction. Neurobiol Learn Mem. 2005;83:93–103. doi: 10.1016/j.nlm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM. Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci. 2002;22:6218–6227. doi: 10.1523/JNEUROSCI.22-14-06218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy BA, Lau LF, Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with “zinc finger” sequences. Proc Natl Acad Sci U S A. 1988;85:7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat dynorphin gene. Proc Natl Acad Sci USA. 1985;82:4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Gordon MB, Rubinko KL, Sambucetti LC. Isolation and characterization of the c-fos (rat) cDNA and analysis of posttranslational modification in vitro. Oncogene. 1987;2:79–84. [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Danaceau JP, Hanson GR. Mechanism of an exaggerated locomotor response to a low-dose challenge of methamphetamine. Pharmacol Biochem Behav. 2007;86:511–515. doi: 10.1016/j.pbb.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends in neurosciences. 1992a;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. J Neural Transm Suppl. 1992b;36:43–59. doi: 10.1007/978-3-7091-9211-5_4. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. Integrated systems of the CNS. Amsterdam: Elsevier Sciences; 1996. [Google Scholar]
- Ginty DD. Calcium regulation of gene expression: isn’t that spatial? Neuron. 1997;18:183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Glickstein SB, Schmauss C. Focused motor stereotypies do not require enhanced activation of neurons in striosomes. J Comp Neurol. 2004;469:227–238. doi: 10.1002/cne.11000. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini MV, Berglind W, Cole KS, Keogh CL, McGinty JF. Local mu and delta opioid receptors regulate amphetamine-induced behavior and neuropeptide mRNA in the striatum. Neuroscience. 2003;121:387–398. doi: 10.1016/s0306-4522(03)00488-3. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv Neurol. 2000;85:123–131. [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–S77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Methamphetamine-induced changes in the striatal-nigral dynorphin system: role of D-1 and D-2 receptors. Eur J Pharmacol. 1987;144:245–246. doi: 10.1016/0014-2999(87)90527-9. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Harlan RE, Garcia MM. Drugs of abuse and immediate-early genes in the forebrain. Mol Neurobiol. 1998;16:221–267. doi: 10.1007/BF02741385. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Pert CB. Mosaic distibution of opiate receptors, parafascilular projections and acetylcholinesterase in rat striatum. Nature. 1981;291:415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynoprhin mRNA expression in the rat striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Horner KA, Keefe KA. Regulation of psychostimulant-induced preprodynorphin, c-fos and zif/268 messenger RNA expression in the rat dorsal striatum by mu opioid receptor blockade. Eur J Pharmacol. 2006;532:61–73. doi: 10.1016/j.ejphar.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Horner KA, Noble ES, Gilbert YE. Methamphetamine-induced stereotypy correlates negatively with patch-enhanced prodynorphin and arc mRNA expression in the rat caudate putamen: the role of mu opioid receptor activation. Pharmacol Biochem Behav. 2010;95:410–421. doi: 10.1016/j.pbb.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Noble ES, Lauterbach EC. Differential regulation of prodynophin, c-fos, and serotonin transporter mRNA following withdrawal from a chronic, escalating dose regimen of D-amphetamine. Synapse. 2009;63:257–268. doi: 10.1002/syn.20606. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H. Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc Natl Acad Sci U S A. 2009;106:316–321. doi: 10.1073/pnas.0806518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe KA, Gerfen CR. D1-D2 dopamine receptor synergy in striatum: effects of intrastriatal infusions of dopamine agonists and antagonists on immediate early gene expression. Neuroscience. 1995;66:903–913. doi: 10.1016/0306-4522(95)00024-d. [DOI] [PubMed] [Google Scholar]
- Lan KC, Chang AC, Liu SH, Ho IK, Lin-Shiau SY. Enhancing effects of morphine on methamphetamine-induced reinforcing behavior and its association with dopamine release and metabolism in mice. J Neurochem. 2009;109:382–392. doi: 10.1111/j.1471-4159.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratalla R, Vallejo M, Elibol B, Graybiel AM. D1-class dopamine receptors influence cocaine-induced persistent expression of Fos-related proteins in striatum. Neuroreport. 1996;8:1–5. doi: 10.1097/00001756-199612200-00001. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:2477–2491. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Pereira FC, Lourenco E, Milhazes N, Morgadinho T, Ribeiro CF, Ali SF, Macedo TR. Methamphetamine, morphine, and their combination: acute changes in striatal dopaminergic transmission evaluated by microdialysis in awake rats. Ann N Y Acad Sci. 2006;1074:160–173. doi: 10.1196/annals.1369.016. [DOI] [PubMed] [Google Scholar]
- Pert CB, Kuhar M, Snyder SH. Opiate receptor: autoradiographic localization in the rat brain. Proc Natl Acad Sci USA. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale CW, Jr, Graybiel AM. Fibers from the basolateral amygdala selectively innervate the striosomes in the caudate nucleus of the cat. J Comp Neurol. 1988;269:506–522. doi: 10.1002/cne.902690404. [DOI] [PubMed] [Google Scholar]
- Saka E, Iadarola M, Fitzgerald DJ, Graybiel AM. Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc Natl Acad Sci U S A. 2002;99:9004–9009. doi: 10.1073/pnas.132212499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988;2:1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Abo V, Rogard M, Besson MJ, Deniau JM. Effect of electrical stimulation of the cerebral cortex on the expression of the Fos protein in the basal ganglia. Neuroscience. 1997;81:93–112. doi: 10.1016/s0306-4522(97)00179-6. [DOI] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford G, Worley PF, Graybiel AM. The activity-regulated cytoskeletal-associated protein Arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta and kappa opioid receptors of the rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci USA. 1987;43:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Toyoshi T, Ukai M, Kameyama T. Opioid receptor agonists selective for mu and kappa receptors attenuate methamphetamine-induced behavioral sensitization in the mouse. Biol Pharm Bull. 1996;19:369–374. doi: 10.1248/bpb.19.369. [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology (Berl) 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- Walker JM, Thompson LA, Frascella J, Friederich MW. Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol. 1987;134:53–59. doi: 10.1016/0014-2999(87)90130-0. [DOI] [PubMed] [Google Scholar]
- Walker RH, Arbuthnott GW, Baughman RW, Graybiel AM. Dendritic domains of medium spiny neurons in the primate striatum: relationship to striosomal borders. J Comp Neurol. 1993;337:614–628. doi: 10.1002/cne.903370407. [DOI] [PubMed] [Google Scholar]
- Wang D, Tolbert LM, Carlson KW, Sadee W. Nuclear Ca2+/calmodulin translocation activated by mu-opioid (OP3) receptor. J Neurochem. 2000;74:1418–1425. doi: 10.1046/j.1471-4159.2000.0741418.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J Comp Neurol. 1996;375:659–674. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu-opioid receptors in dendritic targets of dopaminergic terminals in the rat caudate-putamen nucleus. Neuroscience. 1997;81:757–771. doi: 10.1016/s0306-4522(97)00253-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dendritic spines containing mu-opioid receptors in rat striatal patches receive asymmetric synapses from prefrontal corticostriatal afferents. J Comp Neurol. 1998;396:223–237. [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alterations in zif/268 and preprodynorphin mRNA exprsesion induced by amphetamine and methamphetamine in rat forebrain. J Pharmacol Exp Ther. 1995;273:909–917. [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in the rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- Woo SK, Hitzemann RJ, Loh HH. Specific opioid-amphetamine interactions in the caudate putamen. Psychopharmacology (Berl) 1985;85:371–376. doi: 10.1007/BF00428204. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Suzuki K, Sugiura H, Kawashima N, Okuyama S. Activation of an effector immediate-early gene arc by methamphetamine. Ann N Y Acad Sci. 2000;914:22–32. doi: 10.1111/j.1749-6632.2000.tb05180.x. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]