Abstract
Neuropeptide Y (NPY) and galanin have both been implicated in the regulation of body weight, yet mice bearing deletions of either of these molecules have unremarkable metabolic phenotypes. To investigate whether galanin and NPY might compensate for one another, we produced mutants lacking both neuropeptides (GAL−/−/NPY−/−). We found that male GAL−/−/NPY−/− mice ate significantly more and were much heavier (30%) than wild-type (WT) controls. GAL−/−/NPY−/− mice responded to a high-fat diet by gaining more weight than WT mice gain, and they were unable to regulate their weight normally after a change in diet. GAL−/−/NPY−/− mice had elevated levels of leptin, insulin, and glucose, and they lost more weight than WT mice during chronic leptin treatment. Galanin mRNA was increased in the hypothalamus of NPY−/− mice, providing evidence of compensatory regulation in single mutants. The disruption of energy balance observed in GAL−/−/NPY−/− double knockouts is not found in the phenotype of single knockouts of either molecule. The unexpected obesity phenotype may result from the dysregulation of the leptin and insulin systems that normally keep body weight within the homeostatic range.
The control of feeding and metabolism in mammals is an exquisitely controlled physiological process, and neuropeptides that regulate energy balance circuits are potential targets for the treatment of obesity. In the brain, neuropeptide Y (NPY) and galanin are both thought to be important regulators of body weight (22). NPY is a potent orexigenic factor (51), and its expression is robustly upregulated during fasting (11). Repeated administration of NPY leads to obesity in rodents (5), perhaps by interaction with other regulatory molecules, such as leptin and insulin (39). Several NPY receptors, including Y1, Y2, Y4, and Y5, have been implicated as transducers of NPY's effects on feeding and body weight (25, 28, 32, 36, 44).
Galanin is also an orexigenic molecule (10), although its effects in this regard are neither as robust nor as long lived as those of NPY (50). The actions of galanin on energy balance may be related more to either dietary preference or the initiation of hunger responses (1, 45). Galanin has many other roles in neuroendocrine regulation, including modulating the release of growth hormone and the gonadotropins (14, 34, 42). Galanin's role in metabolic homeostasis is unclear, but its robust regulation of (and by) pancreatic hormones implies an important functional role for this neuropeptide (27, 54). Galanin is expressed in various hypothalamic nuclei, including the arcuate (Arc), dorsomedial (DMN), paraventricular, and preoptic areas, which are nodal points in the regulation of energetics and reproduction (8, 30).
Considerable evidence suggests roles for both galanin and NPY in the neuroendocrine regulation of energy metabolism and reproductive physiology; however, phenotypic analysis of single deletions of these molecules and their receptors in mice has been incongruous and confusing. For example, despite compelling pharmacological evidence that NPY plays a critical role in the regulation of appetite and body weight, the targeted deletion of NPY in knockout mice (NPY−/−) produces animals that have only mild metabolic and neuroendocrine phenotypes (3, 12, 13, 47, 48). On the other hand, targeted deletion of several NPY receptor subtypes produce animals with unequivocal disturbances in body weight and hormonal regulation (25, 28, 32, 36, 44). Mice with a genetic deletion of galanin (GAL−/−) also defend their body weight normally under basal conditions, and they are, like NPY−/− mice, reproductively competent (55). GAL−/− mice do have nociceptive and memory deficits (23, 33), and they are more susceptible to seizures (29), suggesting unique roles for galanin in these processes.
Galanin and NPY may complement the physiological actions of one another. The two neuropeptides are often regulated in a similar manner following metabolic perturbations (41, 49), and neurons expressing galanin and NPY make synaptic contact with each other (20). In addition, galanin and NPY both dampen sympathetic nervous system function (7). If NPY and galanin complement each other, it seems plausible that in the congenital absence of one of these molecules, the other might take over some functions of the missing molecule. To test the hypothesis that galanin and NPY have overlapping functions in the energy balance circuitry, we generated double knockout mice (GAL−/−/NPY−/−), wherein both neuropeptides were genetically deleted. We report that distinct metabolic phenotypes become manifest in GAL−/−/NPY−/− mice which are not evident in single knockouts of either neuropeptide.
MATERIALS AND METHODS
Experimental animals.
All animals used in these studies were housed in the animal care facilities under the auspices of the Department of Comparative Medicine at the University of Washington. The University of Washington Animal Care Committee approved all procedures, in accordance with the National Institutes of Health (NIH) Guide to Care and Use of Laboratory Animals. All animals were kept on a constant light cycle (12:12, with lights off at 1800 h).
Breeding.
Seven NPY−/− founder mice (129/C57BL/6 mixed strain, kindly donated by Richard Palmiter, University of Washington) were mated with seven GAL−/− mice (129OLA strain, our colony) to produce NPY+/−/GAL+/− compound heterozygotes. These heterozygotes were mated together to produce mice with null mutations in both NPY and GAL (NPY−/−/GAL−/−) and WT littermates. Other genotypes resulting from these matings were not used experimentally.
Genotyping.
Tail DNA was extracted by standard phenol-chloroform methods. Southern blots were performed as previously described (13, 55) to confirm genotypes. Additionally, PCR was employed with the following primer sets: GAL common, 5′TTGGCTGGGTCTGAGACTGT3′; GAL WT, 5′GAGGACTAGAGCCTGACAAG3′; GAL−/−, 5′TTGAATGGAAGATTGGAGCTA3′; NPY common, 5′GAGCGGCAGTGGCTCCAG3′; NPY WT, 5′CACTGGCGTCTGGGAGCC3′; NPY−/−, 5′GCAACTGTTGGGAAGGGCG3′. PCRs were performed for 30 cycles as follows: 94°C for 1 min, 62°C for 1 min, 75°C for 2 min, with a final 5-min extension at 75°C.
Body weights, food intake, temperature, and length.
Food used for all studies except the high-fat diet study was Picolab Rodent Diet 20 (protein, 23.5%; fat, 11.9%; carbohydrate, 64.5%) (Animal Specialties, Hubbard, Ore.). Food consumption was measured in home cages or after animals were single-housed for a minimum of 7 days to acclimatize. For circadian feeding studies, mice were placed in clean cages and food was weighed 10 times during a 24-h period, beginning at 1800 h. At study completion, cages were examined for residual spillage. Body temperatures were obtained with a rectal probe in hand-restrained animals in age-matched young adult male mice. Body length was measured under isoflurane anesthesia, from the tip of the nose to the anus in age-matched young adult male mice.
High-fat diet.
Male NPY−/−/GAL−/− and WT mice were weaned onto a high-fat diet (Diet no. D12331; 58% fat, 25% carbohydrate; Research Diets, Inc., New Brunswick, N.J.) on day 21. Animals were group housed due to the length of the study, and total cage food intakes and individual body weights were recorded weekly. At 21 weeks, mice were switched to a low-fat high-carbohydrate diet (11% fat, 73% carbohydrate; Research Diets no. D12328) for another 11 weeks.
Hormone measurements.
Blood was collected either by orbital eye bleed or by cardiac puncture at sacrifice. Isoflurane anesthesia was used for all collection procedures. All hormones were measured in duplicate volumes of serum. Leptin (50 μl) concentrations were measured with a Linco radioimmunoassay kit (Linco, St. Louis, Mo.), and assay sensitivity was 0.1 ng/ml. Insulin and glucagon (100 μl each) were measured in the Diabetes Research Center at the University of Washington. Assay sensitivities were 2.5 μU/ml for insulin and 20 pg/ml for glucagon. Glucose measurements were taken with a hand-held glucometer (Glucometer Elite; Bayer, Elkhart, Ind.). Serum prolactin (25 μl) was measured with reagents from NIH. Sensitivity was 0.12 ng/tube, and intra-assay coefficient of variation (CV) was 14.4%. Corticosterone (25 μl) was measured with a double antibody kit (ICN, Inc., Costa Mesa, Calif.). Sensitivity was 0.5 μg/dl, and intra-assay CV was 4.12%. Follicle-stimulating hormone (FSH) (50 μl) was measured with reagents from NIH. Sensitivity was 0.05 ng/tube, and intra-assay CV was 6.85%. Thyroid hormone (T4) (25 μl) was measured with a Coat-a-Count kit (DPC, Inc., Los Angeles, Calif.). Sensitivity was 1.0 μg/dl, and intra-assay CV was 5.30%. Insulin-like growth factor 1 (IGF-1) was measured in the laboratory of Gloria Tannenbaum (McGill University, Montreal, Quebec, Canada). Testosterone levels (25 μl) were assayed with a Delphia fluoroimmunoassay kit (EG & G Wallac, Turku, Finland). The sensitivity of the assay was 5 pg/100 μl. Luteinizing hormone (LH) concentrations were assayed with reagents from NIH. The standard used was rLH-CSU-S1. The sensitivity of the assay was 0.05 ng/ml.
Activity measurements.
Locomotor activity was measured by placing mice individually in 40- by 20- by 20-cm Plexiglas cages fitted with infrared beams (San Diego Instruments, San Diego, Calif.). Activity levels were recorded as total beam breaks in a 24-h block, starting at 0900 h.
Leptin treatments.
Zymogenetics Inc. (Seattle, Wash.) kindly supplied recombinant full-length human leptin. GAL−/−/NPY−/− mice and aged-matched WT mice were treated intraperitoneally with either leptin (100 μg/mouse in 500 μl of saline with 50 mM boric acid) or saline (500 μl with 50 mM boric acid) for 18 days. Injections were given 1 h before lights out. To weight match mutants with WT as closely as possible, we used young adult mice (12 weeks old) that were not yet markedly obese. Body weight and food intake were monitored daily. At the end of the study, mice were sacrificed under isoflurane anesthesia, and white fat pads were removed and weighed.
In situ hybridization.
Tissue sections from GAL−/− mice, NPY −/− mice, and their respective WT controls were sectioned and processed as previously described (8). The plasmid vector pGemT-Easy, containing a 493-bp cDNA corresponding to the entire coding region of preprogalanin, was kindly provided by James Hyde of the University of Kentucky. The plasmid vector pBLNPY-1, containing a 511-bp rat NPY cDNA (kindly provided by Steven Sabol), was subcloned into the plasmid pBSKS. In situ hybridization reactions were performed as previously described (8). Slides were dipped in NTB-2 emulsion (Kodak, Rochester, N.Y.), exposed for 5 days (galanin) or 3 days (NPY), and then counterstained with cresyl violet. Slides were analyzed for the presence of silver grains clustered over individual cell bodies, using an automated image analysis system (Don Clifton, University of Washington). The high density of galanin-containing cells in the DMN prevented accurate cell counting, so total grain counts for this area were analyzed with the MCID image analysis system (Imaging Research, St. Catherines, Ontario, Canada).
Statistical analysis.
All data are presented as means ± standard errors of the means (SEM). Significant differences were determined by factorial analysis of variance, followed by post hoc tests to identify differences between individual groups. For developmental body weights, gene expression, circadian feeding, and high-fat diet studies, significant differences were determined by repeated-measures analysis of variance, followed by post hoc tests. Differences were considered significant when P values were <0.05.
RESULTS
General phenotype of GAL−/−/NPY−/− mice.
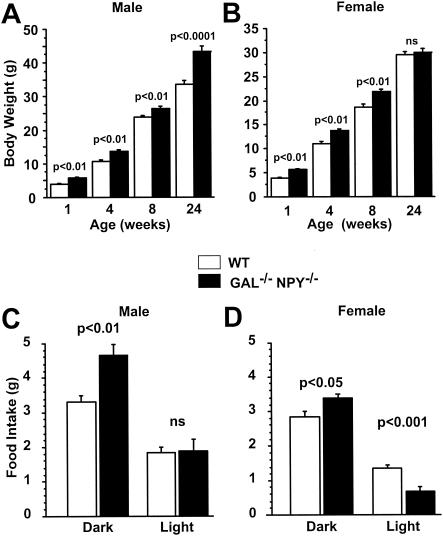
Breeding together compound heterozygote offspring from seven founder pairs of NPY−/− female and GAL−/− male mice produced animals with null mutations in genes for both NPY and galanin. Fertility and parturition were normal in GAL−/−/NPY−/− females, and mothers reared their offspring adequately. Litter sizes were not different between WT and GAL−/−/NPY−/− mice. GAL−/− mice do not lactate normally on the 129OLA background (55), but when crossed into the mixed 129/C57BL/6 background this deficit was overcome, and GAL−/−/NPY−/− mice nursed normally. Both male and female GAL−/−/NPY−/− mice had normal reproductive organ weights (Table 1). Body weights and food intakes were monitored throughout development and as adults. Both male and female GAL−/−/NPY−/− mice were significantly heavier than their WT counterparts by the age of 1 week (Fig. 1A and B). By 24 weeks of age, Male GAL−/−/NPY−/− mice were obese, being 30% heavier than WT mice (weight for GAL−/−/NPY−/− mice, 43.4 g ± 1.4 g; WT weight, 33.7 g ± 1.0 g; P < 0.0001), while the weights of female GAL−/−/NPY−/− mice were normal (compared to WT controls). The obesity of male GAL−/−/NPY−/− mice could be partially attributed to increased food intake, which was significantly greater than in WT controls at all time points measured, beginning at 8 weeks of age (Table 1). Activity levels, body length, and rectal temperatures were normal in GAL−/−/NPY−/− mice (Table 1).
TABLE 1.
Hormones and physiological parameters in WT and GAL−/−/NPY−/− micea
| Parameter | Value for groupb
|
|
|---|---|---|
| WT | GAL−/−/NPY−/− | |
| Male | ||
| Body length (cm) | 10.2 ± 0.2 | 10.5 ± 0.1 |
| Activity (24-h beam breaks) | 2714 ± 328 | 2788 ± 338 |
| 8-wk food intake (g/24 h) | 2.81 ± 0.1 | 3.50 ± 0.2*** |
| 16-wk food intake (g/24 h) | 5.19 ± 0.2 | 6.56 ± 0.4** |
| Rectal temp (°C) | 36.5 ± 0.1 | 36.5 ± 0.1 |
| Body mass index (g/cm2) | 0.27 ± 0.01 | 0.30 ± 0.01* |
| Prolactin level (ng/ml) | 24.2 ± 2.3 | 22.9 ± 3.9 |
| Corticosterone level (ng/ml) | 18.7 ± 1.3 | 15.5 ± 1.4 |
| T4 level (ng/ml) | 2.4 ± 0.1 | 2.1 ± 0.4 |
| Testosterone level (ng/ml) | 11.2 ± 1.6 | 6.00 ± 1.2* |
| LH level (ng/ml) | 9.4 ± 3.0 | 9.4 ± 2.8 |
| FSH level (ng/ml) | 16.3 ± 0.7 | 15.3 ± 0.7 |
| Glucagon level (pg/ml) | 53.5 ± 4.9 | 65.6 ± 5.3 |
| IGF-1 level (ng/ml) | 189 ± 6.4 | 188 ± 9.0 |
| Seminal vesicle wt (mg) | 295 ± 11 | 333 ± 41 |
| Testes wt (mg) | 214 ± 6 | 245 ± 16 |
| Female | ||
| Body length (cm) | 8.2 ± 0.1 | 8.1 ± 0.1 |
| Activity (24-h beam breaks) | 3281 ± 385 | 3613 ± 447 |
| 16-wk food intake (g/24 h) | 4.19 ± 0.2 | 4.04 ± 0.2 |
| Rectal temp (°C) | 36.3 ± 0.1 | 36.5 ± 0.1 |
| Body mass index (g/cm2) | 0.32 ± 0.01 | 0.32 ± 0.01 |
| Corticosterone level (ng/ml) | 48.0 ± 3.7 | 42.9 ± 3.7 |
| T4 level (ng/ml) | 3.4 ± 0.3 | 3.1 ± 0.3 |
| LH level (ng/ml) | 7.6 ± 2.3 | 8.4 ± 2.5 |
| FSH level (ng/ml) | 4.0 ± 1.4 | 5.2 ± 1.1 |
| Insulin, level (μU/ml) | 1.0 ± 0.4 | 1.4 ± 0.4 |
| Glucose level (mg/dl) | 75.3 ± 6.6 | 125 ± 11.7** |
| Glucagon level (pg/ml) | 77.5 ± 8.2 | 94.7 ± 22.3 |
| IGF-1 level (ng/ml) | 144 ± 11.5 | 175 ± 8.0* |
| Uterus wt (mg) | 124 ± 19 | 108 ± 24 |
| Ovary wt (mg) | 14 ± 1 | 14 ± 2 |
Data are expressed as mean values ± SEM (n = 6 to 12 per group).
*, P < 0.05 versus WT; **, P < 0.01 versus WT; ***, P < 0.001 versus WT.
FIG. 1.
Developmental body weights in male (A) and female (B) WT and GAL−/−/NPY−/− mice and light-phase and dark-phase food intake in male (C) and female (D) WT and GAL−/−/NPY−/− adult mice. White bars, WT; dark bars, GAL−/−/NPY−/−. Data are expressed as mean values ± SEM (n = 10 to 12 per group).
Circadian feeding patterns.
Since both NPY and galanin have been implicated in regulating the rhythmicity of neuroendocrine functions (56), we evaluated the possibility that deletion of both neuropeptides would perturb circadian feeding patterns. Food intake was measured 10 times during a 24-h period beginning at the commencement of the dark phase. Adult male GAL−/−/NPY−/− mice ate significantly more than male WT mice did during the 24-h period, while female GAL−/−/NPY−/− ate normal amounts of food. The increase in consumption by male GAL−/−/NPY−/− mice could be attributed entirely to elevated intake during the dark phase, since no differences between genotypes were noted in light-phase feeding (Fig. 1C). In contrast, although female GAL−/−/NPY−/− mice also had elevated food intake during the dark phase, they exhibited a compensatory decrease in light-phase feeding, resulting in no overall increase in their 24-h intake (Fig. 1D).
Response to a high-fat diet.
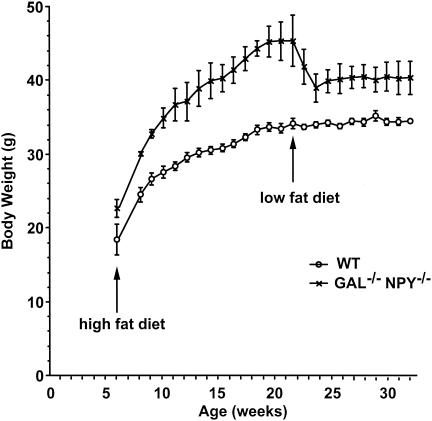
To determine whether GAL−/−/NPY−/− mice respond abnormally to a dietary challenge, we weaned male mutants and their WT controls onto a high-fat diet (58% kcal of fat and 26% kcal of carbohydrate) and then switched their food to high-carbohydrate chow (11% kcal of fat and 73% kcal of carbohydrate). WT mice gained weight normally during the high-fat diet regime, whereas GAL−/−/NPY−/− mice became obese during this period compared to WT mice (Fig. 2), demonstrating that the presence of these two peptides is not necessary for the preferential ingestion and storage of fat calories. However, when the diet was changed to high-carbohydrate chow, GAL−/−/NPY−/− mice lost significant amounts of weight in the following 2 weeks (−6.4 g ± 1.7 g; P < 0.05), whereas the weights of WT mice were unchanged by the switch in diet (−0.1 g ± 0.5 g; P = 0.30). The weights of GAL−/−/NPY−/− mice stabilized 3 weeks after the dietary change, and the animals' weight was recalibrated to a new, lowered set-point. Due to the length of this experiment, animals were group housed, so individual food intakes were not obtained. However, based on combined food intakes, GAL−/−/NPY−/− mice ate moderately more than WT mice at all time points, except during the 2 weeks immediately following the dietary switch (data not shown).
FIG. 2.
Developmental body weights of WT and GAL−/−/NPY−/− mice weaned onto a high-fat diet and then switched to a low-fat, high-carbohydrate diet at 21 weeks of age. Body weights were significantly different between genotypes at all ages. Data are expressed as mean values ± SEM (n = 5 per group).
Differences in circulating hormones.
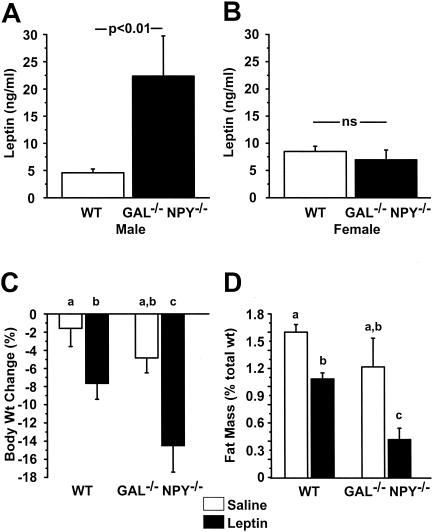
Measurement of several hormones revealed some notable differences as a function of genotype and sex. Serum levels of leptin were dramatically higher in male GAL−/−/NPY−/− mice than in male WT mice (+487%; P < 0.01), whereas in females there was no difference between genotypes (Fig. 3A and B). Circulating levels of testosterone were within the physiological range but lower in GAL−/−/NPY−/− males than in WT animals (Table 1). Curiously, levels of insulin-like growth factor 1 were higher in female GAL−/−/NPY−/− mice than in WT controls, while in males there was no difference between genotypes (Table 1).
FIG. 3.
Serum levels of leptin in male (A) and female (B) WT and GAL−/−/NPY−/− adult mice. (C) Change in body weight as a percentage of initial weight after 18 days of peripheral leptin or saline treatment in young adult male (12 weeks old) WT and GAL−/−/NPY−/− mice. (D) Adipose tissue weight as a percentage of total body weight following leptin treatment. Groups that do not share a common superscript are significantly different from each other (P < 0.05). Data are expressed as mean values ± SEM (n = 5 to 6 per group).
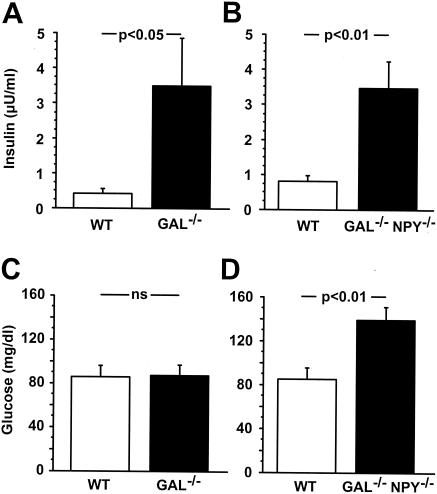
Metabolic indices have been characterized for NPY−/− mice but have not been examined for GAL−/− mice (12, 48). Therefore, we measured pancreatic hormones and glucose levels in both GAL−/− and GAL−/−/NPY−/− mice and found sex-specific differences in both mutant models compared to their respective WT controls. Levels of insulin were significantly elevated in male GAL−/− and GAL−/−/NPY−/− mice relative to their WT controls (Fig. 4A and B), whereas in females, levels of insulin were not different between genotypes (Table 1). Serum levels of glucagon were modestly higher in male and female GAL−/− and GAL−/−/NPY−/− mice than in WT controls (Table 1); however, these differences were statistically significant only between male GAL−/− mice and their WT controls (WT level, 53.5 pg/ml ± 4.9 pg/ml; GAL−/− level, 74.8 pg/ml ± 8.0 pg/ml; P < 0.05). Levels of glucose were markedly higher in both sexes of GAL−/−/NPY−/− animals than in their WT controls (Table 1 and Fig. 4D). In contrast, serum levels of glucose were not different between male GAL−/− mice and their WT controls (Fig. 4C). In female GAL−/− mice, glucose levels were modestly higher than those in the WT controls, while serum levels of insulin and glucagon were normal (data not shown).
FIG. 4.
Serum levels of insulin in WT and GAL−/− adult male mice (A) and WT and GAL−/−/NPY−/− adult male mice (B). Serum levels of glucose in WT and GAL−/− adult male mice (C) and WT and GAL−/−/NPY−/− adult male mice (D). Data are expressed as mean values ± SEM (n = 5 to 9 per group).
Response to chronic leptin treatments.
Because both NPY and galanin may oppose the actions of leptin in the brain, we compared the relative sensitivities of young preobese male GAL−/−/NPY−/− mice and WT mice to leptin by subjecting them to chronic intraperitoneal leptin infusions. There was a significant effect of treatment on food intake (P < 0.05), since leptin-treated mice ate less than saline-treated mice (data not shown). There was a significant effect of treatment (P < 0.01) and genotype (P < 0.05) on body weight, with leptin-treated GAL−/−/NPY−/− mice losing more weight than leptin-treated WT mice (P < 0.01) (Fig. 3C). To determine whether this weight loss was due to a decrease in adiposity, fat pads were weighed and calculated as a percentage of total body weight. There was a significant effect of treatment (P < 0.001) and genotype (P < 0.01) on fat pad percentage, with leptin-treated GAL−/−/NPY−/− mice having smaller fat pads than leptin-treated WT mice (P < 0.01) (Fig. 3D).
Upregulation of galanin mRNA in NPY−/− mice.
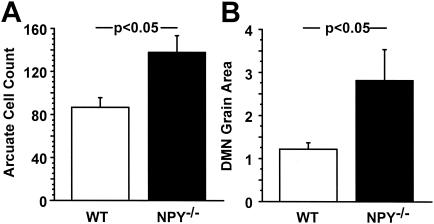
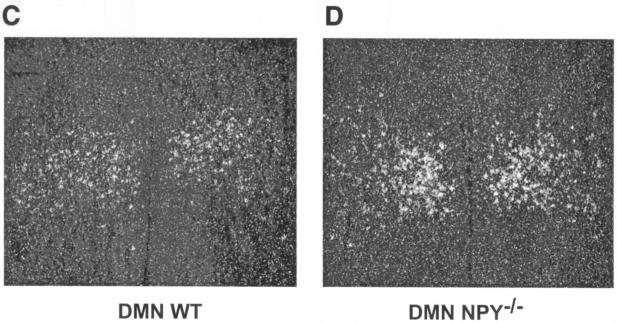
To test the hypothesis that compensatory alterations in galanin or NPY systems may exist when one of the molecules is absent, we used in situ hybridization to measure levels of galanin mRNA in the hypothalami of NPY−/− mice and levels of NPY mRNA in the hypothalami of GAL−/− mice. We found a significant upregulation of galanin mRNA in both the Arc and DMN of NPY−/− mice (Fig. 5). No differences between genotypes were noted in any other region of the brain, including other energy balance centers, such as the central nucleus of the amygdala (mean cell count for WT, 56 ± 8; for NPY−/−, 55 ± 6 [not significant]). Additionally, we found no evidence for differences between GAL−/− mice and their WT controls in the expression of NPY mRNA in the Arc or any other region of the brain (data not shown).
FIG. 5.
Galanin mRNA levels in WT and NPY−/− adult male mice. (A) Total mean cell count of galanin-positive cells in the arcuate nucleus. (B) Total relative grain area of galanin-positive cells in the dorsomedial nucleus. (C and D) Representative photomicrographs of galanin mRNA expression in the DMN of WT and NPY−/− mice, respectively. Data are expressed as mean values ± SEM (n = 5 to 6 per group).
DISCUSSION
GAL−/−/NPY−/− mice have unique energy balance phenotypes.
The targeted deletion of both galanin and NPY in mice resulted in a phenotype unique from that produced by single knockouts of either neuropeptide. Male GAL−/−/NPY−/− mice became hyperphagic and obese, with attendant hyperinsulinemia, hyperglycemia, and hyperleptinemia. However, female GAL−/−/NPY−/− mice were heavier than WT mice during development, but their food intake and body weights normalized in adulthood. In contrast, GAL−/− mice of both sexes are reported to have normal body weights and food intake but do exhibit moderate neuroendocrine disturbances (18, 55). NPY−/− mice have also been reported to have normal basal body weights and feeding behaviors (3, 12, 13, 48), although a recent study reports that NPY−/− mice backcrossed onto a C57BL/6 background are slightly obese and exhibit an impaired response to a 24-h fast (47). It is notable that although galanin receptor 1 knockouts have no body weight phenotype (21), mutations in several NPY receptors do show disturbances in body weight regulation (25, 28, 32, 36, 43, 44). Each NPY receptor mutation displays a unique constellation of energy balance phenotypes, which are all more severe than the phenotypes exhibited when the ligand itself is absent in NPY−/− mice. It is of interest that each NPY receptor mutant also leads to a sexually dimorphic phenotype, as was the case with the GAL−/− and GAL−/−/NPY−/− mutants in the present study. This may not be unexpected, considering that the expression of both galanin and NPY is sexually dimorphic in brain regions involved in energy homeostasis, such as the arcuate nucleus (38, 53).
The increase in 24-h food consumption in male GAL−/−/NPY−/− mice can be attributed entirely to an increase in dark-phase feeding, with no genotype differences noted in light-phase intake. In female GAL−/−/NPY−/− mice, the increase in dark-phase feeding was followed by a corresponding decrease in light-phase intake. This may reflect the females' unique ability to generate a compensatory adaptation of defending body weight by reducing overall intake which is absent in the male for reasons attributable to its hormonal milieu. Curiously, no alterations have been reported in dark-phase feeding in previous reports of male or female NPY−/− mice (3, 12, 47), and preliminary studies from our lab show no significant gender or genotype differences in circadian feeding patterns of GAL−/− mice (J.G. Hohmann, personal observation). NPY has certainly been implicated in the circadian timing of neuroendocrine rhythms, and both NPY and galanin show distinct diurnal patterns in the hypothalamus (56). Notably, galanin peptide levels show two distinct peaks (at dark and light onset) in the suprachiasmatic circadian pacemaker (2). Our results suggest that galanin and NPY may have overlapping roles in maintaining the appropriate rhythm of a rodent's drive to engage in food-seeking behaviors, which is revealed as dysfunction in the ingestive timing mechanisms when both molecules are absent.
Galanin has been linked to a preference for fat calories, and NPY has been implicated in the regulation of carbohydrate ingestion (6). When GAL−/−/NPY−/− mice were weaned onto a high-fat diet, they gained weight at a greater rate than WT mice fed the same diet, suggesting that the presence of galanin or NPY is not necessary for the preferential ingestion and storage of fat. However, when the diet was switched to a low-fat, high-carbohydrate diet, GAL−/−/NPY−/− mice responded differently than WT controls, with the GAL−/−/NPY−/− mice losing significantly more weight than WT mice before stabilizing. It is interesting that although body weights of the double mutants did recalibrate, it was at a lower set-point, and for the duration of the study (11 weeks post-diet change), their weights did not reach the levels achieved while on the high-fat diet. NPY−/− mice respond normally to dietary challenges (19), but GAL−/− mice have yet to be examined in this regard. Whether this phenotype in GAL−/−/NPY−/− mice is related to a deficit in carbohydrate metabolism or reflects an inability to efficiently react to a dietary challenge is unknown. However, based on the present initial experiments, a more finely grained analysis of macronutrient choice in GAL−/− and GAL−/−/NPY−/− mice may be informative.
Circulating levels of leptin and insulin are altered in GAL−/−/NPY−/− mice.
Differences in levels of several hormones were observed in GAL−/−/NPY−/− mice. Leptin levels were much higher in obese mutant males than in WT males. These elevated leptin levels could reflect a primary lesion or could simply be a weight-appropriate leptin response. Paradoxically, when preobese, young male GAL−/−/NPY−/− mice were treated chronically with leptin, they exhibited an enhanced response to the weight-loss and adipose-depleting effects of this hormone. However, no genotype differences were observed between mutant and WT mice in their adaptive feeding response to leptin, suggesting that the greater loss of body weight in GAL−/−/NPY−/− mice was primarily metabolic. Leptin stimulates the sympathetic nervous system, favoring activation of catabolic circuits, whereas both galanin and NPY reduce sympathetic tone (7). In addition, leptin receptors are expressed on galanin and NPY neurons in the hypothalamus (4, 17), and leptin treatment reduces mRNA levels for both these peptides (41). It would appear that in the dual absence of the anabolic molecules NPY and galanin the weight-reducing effects of leptin are enhanced, at least in nonobese animals.
Other metabolic molecules were also perturbed in GAL−/−/NPY−/− mice, including both insulin and glucose. We also report that insulin and glucagon were higher in GAL−/− mice than in WT mice, while previous studies report that metabolic factors are mostly normal in NPY−/− mice (12, 48). Collectively, these observations suggest that the perturbations in circulating levels of pancreatic hormones and glucose in GAL−/−/NPY−/− mice result primarily from the loss of galaninergic signaling. Galanin inhibits insulin secretion by acting directly in pancreatic islets (27), and in turn, insulin acts in the hypothalamus to reduce galaninergic tone (54). If galanin has an important role in maintaining insulin levels within the homeostatic range—as a function of nutritional state—then the loss of galanin may contribute to the derangement of metabolic hormone and glucose levels observed in GAL−/−/NPY−/− mice.
The question remains: why does the loss of both galanin and NPY lead to obesity phenotypes that are not evident in single knockouts of either neuropeptide? One possible explanation is that while galanin may have a predominant role in controlling insulin secretion, NPY may have an overlapping role in this function. It is worth noting that the deletion of the NPY-Y1 receptor in mice leads to hyperinsulinemia (25), and it has been proposed that NPY acts through the Y1 receptor subtype to inhibit insulin release (31). Collectively, the effect of lack of inhibition by galanin on insulin release combined with lack of NPY-Y1 activation in male GAL−/−/NPY−/− mice may produce a syndrome of severe hyperinsulinemia and hyperglycemia, which promotes the obese state. In turn, increased circulating insulin has been shown to stimulate leptin production, potentially leading to the leptin resistance indicative of mammalian obesity (40). The lack of an obesity phenotype in adult female GAL−/−/NPY−/− mice may result from the observation that leptin and insulin are normal in female mutants. There are clear differences between sexes in the processing of and sensitivity to leptin and insulin (9), and the inference from our studies is that galanin and/or NPY is more important as a regulator of these metabolic hormones in males than in females.
Another plausible explanation for the obesity phenotype of GAL−/−/NPY−/− mice could involve altered signaling by other orexigenic or anorectic molecules in the brain. Agouti-related peptide (AgRP), which is coexpressed with NPY in the hypothalamus, has been proposed as a potential compensatory molecule in the absence of NPY (16). Arguing against this hypothesis are the observations that AgRP knockout mice have normal body weights and feeding behaviors and that mice deficient in both AgRP and NPY are also completely normal in these phenotypic aspects (37). There is, however, evidence for deficits in the melanocortinergic system of NPY−/− mice, since these mutants are more sensitive to the effects of the melanocortin analogue MTII (52). In addition, NPY−/− mice have an abnormal corticotrophin-releasing hormone response to fasting (35); moreover, these mutants are more sensitive to the infusion of an orexigenic ghrelin receptor agonist (52). Thus, peptidergic signaling is perturbed in NPY−/− mutants, and it's conceivable that the added insult of removing galanin from the energy balance equation is sufficient to “tip the scales” towards dysregulation of body weight in GAL−/−/NPY−/− mice.
Lending credence to this argument is the finding that galanin mRNA was significantly upregulated in the hypothalami of NPY−/− mice, suggesting that galanin could functionally compensate for the loss of NPY. It is intriguing that the two regions where galanin mRNA was increased, the Arc and DMN, are also the primary sites of NPY synthesis in the hypothalamus. Levels of NPY mRNA increase markedly in the arcuate nucleus during fasting or food restriction (46), and the expression of NPY is robustly increased in the DMN in some rodent models of obesity (15, 24). Additionally, NPY is upregulated in the DMN during lactation, a period of high nutritional demand (26). Thus, if galanin becomes functionally more important in NPY−/− mice, it would make sense that increased galaninergic tone would be evident in the Arc and DMN.
It may seem paradoxical that the genetic deletion of two orexigenic neuropeptides that have both been widely implicated in the regulation of body weight and reproduction would result in animals that are obese. In mammals, the need to maintain adequate nutritional reserves that foster reproductive competency is critical for continuity of the species. Thus, it seems plausible, and perhaps expected, that parallel systems exist in the brains of mammals to ensure the maintenance of energetic sufficiency. What is apparently lost when molecules with overlapping functions—in this case galanin and NPY—are both absent is the ability to control the fine balance of metabolic hormones, such as insulin and leptin, that assist in keeping body weight within a narrow homeostatic range.
Acknowledgments
This work was supported by grants from the USPHS/NIH (R01 HD27142, R01 DK61517, AG05136, and the Specialized Cooperative Centers Program for Reproduction Research/U5412629) and the NSF (IBN-9720143/0110686).
We thank Tom Teal and Jennifer Holloman for their technical assistance, Stephanie Krasnow, Matt Cunningham, and Greg Fraley for comments on the manuscript, and Richard Palmiter for providing NPY−/− mice and advice on experimental design. We thank Brigitte Mann at Northwestern University, Evanston, Ill., Gloria Tannenbaum at McGill University, Montreal, Quebec, Canada, and William Bremner and the Diabetes Research Center at the University of Washington for performing hormone assays.
REFERENCES
- 1.Akabayashi, A., J. I. Koenig, Y. Watanabe, J. T. Alexander, and S. F. Leibowitz. 1994. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc. Natl. Acad. Sci. USA 91:10375-10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akabayashi, A., C. T. Zaia, J. I. Koenig, S. M. Gabriel, I. Silva, and S. F. Leibowitz. 1994. Diurnal rhythm of galanin-like immunoreactivity in the paraventricular and suprachiasmatic nuclei and other hypothalamic areas. Peptides 15:1437-1444. [DOI] [PubMed] [Google Scholar]
- 3.Bannon, A. W., J. Seda, M. Carmouche, J. M. Francis, M. H. Norman, B. Karbon, and M. L. McCaleb. 2000. Behavioral characterization of neuropeptide Y knockout mice. Brain Res. 868:79-87. [DOI] [PubMed] [Google Scholar]
- 4.Baskin, D. G., J. F. Breininger, and M. W. Schwartz. 1999. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48:828-833. [DOI] [PubMed] [Google Scholar]
- 5.Beck, B., A. Stricker-Krongrad, J. Nicolas, and C. Burlet. 1992. Chronic and continuous intracerebroventricular infusion of neuropeptide Y in Long-Evans rats mimics the feeding behavior of obese Zucker rats. Int. J. Obes. 16:295-302. [PubMed] [Google Scholar]
- 6.Bray, G. A. 1993. The nutrient balance hypothesis: peptides, sympathetic activity, and food intake. Ann. N. Y. Acad. Sci. 676:223-241. [DOI] [PubMed] [Google Scholar]
- 7.Bray, G. A. 2000. Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int. J. Obes. Relat. Metab. Disord. 24(Suppl. 2):S8-S17. [DOI] [PubMed] [Google Scholar]
- 8.Cheung, C. C., J. G. Hohmann, D. K. Clifton, and R. A. Steiner. 2001. Distribution of galanin messenger RNA-expressing cells in murine brain and their regulation by leptin in regions of the hypothalamus. Neuroscience 103:423-432. [DOI] [PubMed] [Google Scholar]
- 9.Clegg, D. J., C. A. Riedy, K. A. Smith, S. C. Benoit, and S. C. Woods. 2003. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 52:682-687. [DOI] [PubMed] [Google Scholar]
- 10.Crawley, J. N., M. C. Austin, S. M. Fiske, B. Martin, S. Consolo, M. Berthold, U. Langel, G. Fisone, and T. Bartfai. 1990. Activity of centrally administered galanin fragments on stimulation of feeding behavior and on galanin receptor binding in the rat hypothalamus. J. Neurosci. 10:3695-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dube, M. G., A. Sahu, P. S. Kalra, and S. P. Kalra. 1992. Neuropeptide Y release is elevated from the microdissected paraventricular nucleus of food-deprived rats: an in vitro study. Endocrinology 131:684-688. [DOI] [PubMed] [Google Scholar]
- 12.Erickson, J. C., R. S. Ahima, G. Hollopeter, J. S. Flier, and R. D. Palmiter. 1997. Endocrine function of neuropeptide Y knockout mice. Regul. Pept. 70:199-202. [DOI] [PubMed] [Google Scholar]
- 13.Erickson, J. C., K. E. Clegg, and R. D. Palmiter. 1996. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381:415-421. [DOI] [PubMed] [Google Scholar]
- 14.Finn, P. D., K. Y. Pau, H. G. Spies, M. J. Cunningham, D. K. Clifton, and R. A. Steiner. 2000. Galanin's functional significance in the regulation of the neuroendocrine reproductive axis of the monkey. Neuroendocrinology 71:16-26. [DOI] [PubMed] [Google Scholar]
- 15.Guan, X. M., H. Yu, and L. H. Van der Ploeg. 1998. Evidence of altered hypothalamic pro-opiomelanocortin/neuropeptide Y mRNA expression in tubby mice. Brain Res. Mol. Brain Res. 59:273-279. [DOI] [PubMed] [Google Scholar]
- 16.Hahn, T. M., J. F. Breininger, D. G. Baskin, and M. W. Schwartz. 1998. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat. Neurosci. 1:271-272. [DOI] [PubMed] [Google Scholar]
- 17.Hakansson, M. L., H. Brown, N. Ghilardi, R. C. Skoda, and B. Meister. 1998. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 18:559-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohmann, J. G., S. M. Krasnow, D. Teklemichael, D. Wynick, D. K. Clifton, and R. A. Steiner. 2003. Neuroendocrine profiles of galanin transgenic and knockout mice. Neuroendocrinology 77:354-366. [DOI] [PubMed] [Google Scholar]
- 19.Hollopeter, G., J. C. Erickson, and R. D. Palmiter. 1998. Role of neuropeptide Y in diet-, chemical- and genetic-induced obesity of mice. Int. J. Obes. Relat. Metab. Disord. 22:506-512. [DOI] [PubMed] [Google Scholar]
- 20.Horvath, T. L., F. Naftolin, C. Leranth, A. Sahu, and S. P. Kalra. 1996. Morphological and pharmacological evidence for neuropeptide Y-galanin interaction in the rat hypothalamus. Endocrinology 137:3069-3078. [DOI] [PubMed] [Google Scholar]
- 21.Jacoby, A. S., Y. J. Hort, G. Constantinescu, J. Shine, and T. P. Iismaa. 2002. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res. Mol. Brain Res. 107:195-200. [DOI] [PubMed] [Google Scholar]
- 22.Kalra, S. P., M. G. Dube, S. Pu, B. Xu, T. L. Horvath, and P. S. Kalra. 1999. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 20:68-100. [DOI] [PubMed] [Google Scholar]
- 23.Kerr, B. J., W. B. Cafferty, Y. K. Gupta, A. Bacon, D. Wynick, S. B. McMahon, and S. W. Thompson. 2000. Galanin knockout mice reveal nociceptive deficits following peripheral nerve injury. Eur. J. Neurosci. 12:793-802. [DOI] [PubMed] [Google Scholar]
- 24.Kesterson, R. A., D. Huszar, C. A. Lynch, R. B. Simerly, and R. D. Cone. 1997. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Mol. Endocrinol. 11:630-637. [DOI] [PubMed] [Google Scholar]
- 25.Kushi, A., H. Sasai, H. Koizumi, N. Takeda, M. Yokoyama, and M. Nakamura. 1998. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc. Natl. Acad. Sci. USA 95:15659-15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, C., P. Chen, and M. S. Smith. 1998. The acute suckling stimulus induces expression of neuropeptide Y (NPY) in cells in the dorsomedial hypothalamus and increases NPY expression in the arcuate nucleus. Endocrinology 139:1645-1652. [DOI] [PubMed] [Google Scholar]
- 27.Lindskog, S., and B. Ahren. 1992. Effects of galanin and norepinephrine on insulin secretion in the mouse. Pancreas 7:636-641. [DOI] [PubMed] [Google Scholar]
- 28.Marsh, D. J., G. Hollopeter, K. E. Kafer, and R. D. Palmiter. 1998. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat. Med. 4:718-721. [DOI] [PubMed] [Google Scholar]
- 29.Mazarati, A. M., J. G. Hohmann, A. Bacon, H. Liu, R. Sankar, R. A. Steiner, D. Wynick, and C. G. Wasterlain. 2000. Modulation of hippocampal excitability and seizures by galanin. J. Neurosci. 20:6276-6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melander, T., T. Hokfelt, and A. Rokaeus. 1986. Distribution of galaninlike immunoreactivity in the rat central nervous system. J. Comp. Neurol. 248:475-517. [DOI] [PubMed] [Google Scholar]
- 31.Morgan, D. G., R. N. Kulkarni, J. D. Hurley, Z. L. Wang, R. M. Wang, M. A. Ghatei, A. E. Karlsen, S. R. Bloom, and D. M. Smith. 1998. Inhibition of glucose stimulated insulin secretion by neuropeptide Y is mediated via the Y1 receptor and inhibition of adenylyl cyclase in RIN 5AH rat insulinoma cells. Diabetologia 41:1482-1491. [DOI] [PubMed] [Google Scholar]
- 32.Naveilhan, P., H. Hassani, J. M. Canals, A. J. Ekstrand, A. Larefalk, V. Chhajlani, E. Arenas, K. Gedda, L. Svensson, P. Thoren, and P. Ernfors. 1999. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat. Med. 5:1188-1193. [DOI] [PubMed] [Google Scholar]
- 33.O'Meara, G., U. Coumis, S. Y. Ma, J. Kehr, S. Mahoney, A. Bacon, S. J. Allen, F. Holmes, U. Kahl, F. U. Wang, I. R. Kearns, S. O. Ogren, D. Dawbarn, E. J. Mufson, C. Davies, G. Dawson, and D. Wynick. 2000. Galanin regulates the post-natal survival of a sub-set of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 97:11569-11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottlecz, A., W. K. Samson, and S. M. McCann. 1986. Galanin: evidence for a hypothalamic site of action to release growth hormone. Peptides 7:51-53. [DOI] [PubMed] [Google Scholar]
- 35.Palmiter, R. D., J. C. Erickson, G. Hollopeter, S. C. Baraban, and M. W. Schwartz. 1998. Life without neuropeptide Y. Recent Prog. Horm. Res. 53:163-199. [PubMed] [Google Scholar]
- 36.Pedrazzini, T., J. Seydoux, P. Kunstner, J. F. Aubert, E. Grouzmann, F. Beermann, and H. R. Brunner. 1998. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat. Med. 4:722-726. [DOI] [PubMed] [Google Scholar]
- 37.Qian, S., H. Chen, D. Weingarth, M. E. Trumbauer, D. E. Novi, X. Guan, H. Yu, Z. Shen, Y. Feng, E. Frazier, A. Chen, R. E. Camacho, L. P. Shearman, S. Gopal-Truter, D. J. MacNeil, L. H. Van der Ploeg, and D. J. Marsh. 2002. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol. Cell. Biol. 22:5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajendren, G., N. Levenkova, and M. J. Gibson. 2000. Galanin immunoreactivity in mouse basal forebrain: sex differences and discrete projections of galanin-containing cells beyond the blood-brain barrier. Neuroendocrinology 71:27-33. [DOI] [PubMed] [Google Scholar]
- 39.Raposinho, P. D., D. D. Pierroz, P. Broqua, R. B. White, T. Pedrazzini, and M. L. Aubert. 2001. Chronic administration of neuropeptide Y into the lateral ventricle of C57BL/6J male mice produces an obesity syndrome including hyperphagia, hyperleptinemia, insulin resistance, and hypogonadism. Mol. Cell Endocrinol. 185:195-204. [DOI] [PubMed] [Google Scholar]
- 40.Rohner-Jeanrenaud, F. 2000. Hormonal regulation of energy partitioning. Int. J. Obes. Relat. Metab. Disord. 24(Suppl. 2):S4-S7. [DOI] [PubMed] [Google Scholar]
- 41.Sahu, A. 1998. Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology 139:795-798. [DOI] [PubMed] [Google Scholar]
- 42.Sahu, A., W. R. Crowley, K. Tatomoto, A. Balasubramaniam, and S. P. Kalra. 1987. Effects of neuropeptide Y, NPY analogue (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides 8:921-926. [DOI] [PubMed] [Google Scholar]
- 43.Sainsbury, A., C. Schwarzer, M. Couzens, S. Fetissov, S. Furtinger, A. Jenkins, H. M. Cox, G. Sperk, T. Hokfelt, and H. Herzog. 2002. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. USA 99:8938-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sainsbury, A., C. Schwarzer, M. Couzens, A. Jenkins, S. R. Oakes, C. J. Ormandy, and H. Herzog. 2002. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 16:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schick, R. R., S. Samsami, J. P. Zimmermann, T. Eberl, C. Endres, V. Schusdziarra, and M. Classen. 1993. Effect of galanin on food intake in rats: involvement of lateral and ventromedial hypothalamic sites. Am. J. Physiol. 264:R355-R361. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz, M. W., A. J. Sipols, C. E. Grubin, and D. G. Baskin. 1993. Differential effect of fasting on hypothalamic expression of genes encoding neuropeptide Y, galanin, and glutamic acid decarboxylase. Brain Res. Bull. 31:361-367. [DOI] [PubMed] [Google Scholar]
- 47.Segal-Lieberman, G., D. J. Trombly, V. Juthani, X. Wang, and E. Maratos-Flier. 2003. NPY ablation in C57BL/6 mice leads to mild obesity and to an impaired refeeding response to fasting. Am. J. Physiol. 284:E1131-E1139. [DOI] [PubMed] [Google Scholar]
- 48.Sindelar, D. K., P. Mystkowski, D. J. Marsh, R. D. Palmiter, and M. W. Schwartz. 2002. Attenuation of diabetic hyperphagia in neuropeptide Y-deficient mice. Diabetes 51:778-783. [DOI] [PubMed] [Google Scholar]
- 49.Singh, S. N., P. Vats, R. Shyam, S. Suri, M. M. Kumria, S. Ranganathan, K. Sridharan, and W. Selvamurthy. 2001. Role of neuropeptide Y and galanin in high altitude induced anorexia in rats. Nutr. Neurosci. 4:323-331. [DOI] [PubMed] [Google Scholar]
- 50.Smith, B. K., D. A. York, and G. A. Bray. 1994. Chronic cerebroventricular galanin does not induce sustained hyperphagia or obesity. Peptides 15:1267-1272. [DOI] [PubMed] [Google Scholar]
- 51.Stanley, B. G., S. E. Kyrkouli, S. Lampert, and S. F. Leibowitz. 1986. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides 7:1189-1192. [DOI] [PubMed] [Google Scholar]
- 52.Tschop, M., M. A. Statnick, T. M. Suter, and M. L. Heiman. 2002. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 143:558-568. [DOI] [PubMed] [Google Scholar]
- 53.Urban, J. H., A. C. Bauer-Dantoin, and J. E. Levine. 1993. Neuropeptide Y gene expression in the arcuate nucleus: sexual dimorphism and modulation by testosterone. Endocrinology 132:139-145. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J., and K. L. Leibowitz. 1997. Central insulin inhibits hypothalamic galanin and neuropeptide Y gene expression and peptide release in intact rats. Brain Res. 777:231-236. [DOI] [PubMed] [Google Scholar]
- 55.Wynick, D., C. J. Small, A. Bacon, F. E. Holmes, M. Norman, C. J. Ormandy, E. Kilic, N. C. Kerr, M. Ghatei, F. Talamantes, S. R. Bloom, and V. Pachnis. 1998. Galanin regulates prolactin release and lactotroph proliferation. Proc. Natl. Acad. Sci. USA 95:12671-12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, B., P. S. Kalra, W. G. Farmerie, and S. P. Kalra. 1999. Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology 140:2868-2875. [DOI] [PubMed] [Google Scholar]