Abstract
Although insulin resistance and Type 2 diabetes are associated with upper body fat distribution, it is unknown whether insulin resistance predisposes to upper body fat gain or whether upper body fat gain causes insulin resistance. Our objective was to determine whether insulin sensitivity predicts abdominal (subcutaneous and/or visceral) fat gain in normal weight adults. Twenty-eight (15 men) lean (BMI = 22.1±2.5 kg/m2), healthy adults underwent ~8 weeks of overfeeding to gain ~4 kg fat. Body composition was assessed before and after overfeeding using DXA and abdominal CT to measure total and regional (visceral, abdominal, and lower body subcutaneous) fat gain. We assessed insulin sensitivity with an IV glucose tolerance test and the 24h insulin area-under-the-curve(AUC). We found a wide range of insulin sensitivity and a relatively narrow range of body fat distribution in this normal weight cohort. Participants gained 3.8±1.7 kg of body fat (4.6±2.2 kg body weight). The baseline 24h AUC of insulin concentration was positively correlated with percent body fat (r=0.43, p<0.05). The contribution of leg fat gain to total fat gain ranged from 29–79%, while the contributions of abdominal subcutaneous fat and visceral fat gain to total fat gain ranged from 17–69% and −5–22%, respectively. Baseline insulin sensitivity, whether measured by an IVGTT or the 24h AUC insulin Si, did not predict upper body subcutaneous or visceral fat gain in response to overfeeding. We conclude that reduced insulin sensitivity is not an obligate precursor to upper body fat gain.
Keywords: obesity, visceral fat, body composition, IVGTT, dual energy x-ray absorptiometry
Introduction
Insulin resistance and T2DM are associated with upper body obesity and excess visceral fat (1). Offspring of T2DM are insulin resistant as assessed by the euglycemic, hyperinsulinemic insulin clamp technique (2), suggesting a constitutional, perhaps genetic predisposition to T2DM. If normal weight insulin resistant offspring of T2DM become obese enough to develop T2DM, they would most likely develop central obesity. This raises the possibility that constitutional insulin resistance predisposes to central fat gain rather than central fat gain being the main cause of insulin resistance.
The purpose of this study was to determine whether insulin sensitivity is a predictor of upper body fat gain in normal weight individuals. We overfed healthy, young adults with varying degrees of insulin sensitivity for ~8 weeks to achieve ~4 kg of fat gain. We hypothesized that the most insulin resistant persons would gain the most abdominal subcutaneous and/or visceral fat whereas the most insulin sensitive subjects would preferentially gain leg fat. In addition, we studied the 24h hormone profiles (glucose, insulin, c-peptide, growth hormone, ghrelin, and catecholamines) before and after a period of overfeeding to assess any affect of fat gain on these parameters.
Subjects and Methods
This study was approved by the Mayo Clinic Institutional Review Board and informed, written consent was obtained from 28 volunteers (15 men, 13 women). The men who completed the study were all Caucasian. The women completing the study were Asian-American (1), African-American, (1) and Caucasian (11). Participants were recruited if their BMI was <26 kg/m2and provided they were taking no medications, with the exception of oral contraceptives. Study volunteers were recruited with the goal of obtaining a wide range of insulin sensitivity: some participants were physically active by self-report, others (n=3) had an immediate family history of type 2 diabetes, but were not diabetic themselves. Prior to participation in the study, a complete blood count and a chemistry panel were documented to be within normal limits.
Protocol
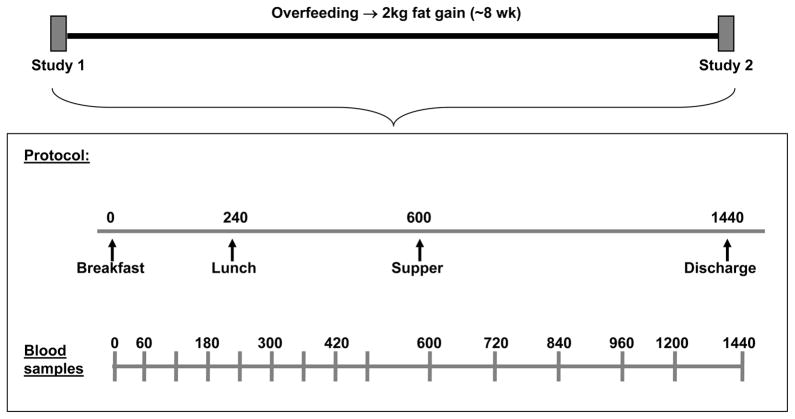
Weight maintenance meals were provided by the Mayo Clinic General Clinical Research Center (GCRC) metabolic kitchen for 10 days prior to the test meal to ensure constant macronutrient composition (50% carbohydrate, 35% fat, and 15% protein), as previously described (3). Participants consumed at least one meal/day at the GCRC and were allowed to take the remaining meals home. If weight changed by ±1 kg, the amount of food provided to participants was adjusted accordingly. The weight change over the maintenance period was 0.54 ±0.42 kg. Body composition was assessed by duplicate dual-energy x-ray absorptiometry (DXA) scans and computed tomography (CT), as described in detail below. During the baseline feeding interval oxygen consumption (VO2 peak) and maximum heart rate were determined by a graded exercise test performed on a Quinton (Seattle, WA) motor driven treadmill using a modified Bruce protocol (4).
Following the weight maintenance period, the volunteers were admitted to the GCRC at 1700 for their first inpatient study (Visit 1; Figure 1). They were given a meal at 1800 and remained in the GCRC for the next 2 days. After an overnight fast (Day 1), subjects were given a liquid meal at 0800 (Ensure Plus, Ross Laboratories) followed by weighed research meals of solid food at 1300 and 1800. Energy intake for visit 1 was based on the weight maintenance energy intake established during the 10 days prior to admittance. Blood samples were taken hourly until 1600 and then less frequently until 0800 the following morning (Day 2). These blood samples were assayed for glucose, insulin, c-peptide, growth hormone, ghrelin, and catecholamines. An overnight fasting blood sample was used to measure leptin. On day 2, after an overnight fast, the volunteers underwent a 4 hour IVGTT to assessment insulin sensitivity using the minimal model approach (5). Briefly, a baseline blood sample for the measurement of glucose and insulin was taken at time 0. Then, 50% dextrose (0.33 g/kg body weight) was given intravenously over two minutes. At 20 minutes, insulin (0.02 U/kg body weight) was given intravenously. Blood samples for the measurement of plasma glucose and insulin were taken at 2, 4, 8, 10, 18, 24, 32, 40, 60, 70, 120, and 180 minutes.
Figure 1.
Schematic of study protocol.
Upon completion of visit 1, participants entered the ad libitum overfeeding phase of the study, with a goal of achieving similar amounts of fat gain between subjects over ~8 weeks. Participants were instructed to eat until they were more full than usual. In addition, to promote more predictable weight gain they allowed to choose supplemental food from the GCRC metabolic kitchen -ice cream shakes (402 kcal,40% fat), king -sized Snickers bars (510 kcal, Mars Inc.), and Boost Plus (360 kcal/8 oz; Nestle Nutrition, Glendale, CA, USA). Body weight was measured at least 5 days/week to assess weight gain. The rate of fat gain was determined by a repeat DXA ~4 weeks into the overfeeding period. The amount of supplemental food provided to study participants was determined by their rate of weight and fat gain, and ranged from 1–4 supplements per day. The supplements varied slightly in macronutrient composition, but all were available to all subjects and a variety of them were eaten by all participants over the ~8 weeks of overfeeding. Additionally, macronutrient content of the supplements was not held constant because subjects were eating ad libitum from their own food during the weight gain phase of the study and therefore diet composition was not necessarily similar between individuals.
Once participants achieved a fat gain of 2–4 kg, they again received 10 days of controlled meals from the GCRC metabolic kitchen given in excess of weight maintenance intake. The increase in energy intake of each participant compared to the controlled feeding period prior to visit 1 was determined by the energy content of the supplements they had been consuming to gain weight. Daily weights were measured to insure that patients were either maintaining or slightly increasing their weight.
The second inpatient stay at the GCRC (Visit 2) was identical to visit 1 in all ways. The level of energy intake during visit 2 was the same as visit 1, except in 3 subjects who received the greater energy intake they had been receiving over the prior 10 days eating in the GCRC. Thus, we have the ability to directly compare 24 AUC insulin concentrations and hormone concentrations between the two visits for 25 of the 28 volunteers.
Assays and Methods
Total body fat, leg fat, and total fat-free mass (FFM) were measured in duplicate with dual-energy X-ray absorptiometry (DXA; DPX-IQ, Lunar Radiation, Madison, WI) at both visits; the DXA in the middle of the overfeeding period was a single scan. The average values of the two scans at each visit were used to assess body composition. Visceral fat was determined at both visits using three computed tomography (CT) images obtained at the levels of L2-3, L3-4, and L4-5(the average of the three slices used) in combination with duplicate DXA abdominal fat analysis (6).
Whole blood glucose concentrations were determined using the Beckman glucose analyzer (Beckman Instruments, Fullerton, CA, USA). Insulin and c-peptide concentrations were determined by chemiluminescent sandwich assays (Sanofi Diagnostics, Chaska, MN, USA). Leptin was measured by a double antibody radioimmunoassay kit (Linco Research, Inc. St. Louis, MO). Intra-assay CV’s were 6.1%, 7.7%, and 6.3% at 39.7, 21.6, and 3.8 ng/mL and inter-assay CV’s were 11%, and 13% at 20.4, and 3.0 ng/mL. C-peptide was measured by a direct, double antibody sequential radioimmunoassay (RIA; Linco Research, St. Charles MO) with inter-assay CV’s of 4.9 %, 4.3% and 8.0% at 0.43, 1.75 and 4.36 nmol/L. Growth hormone (hGH) was measured via a two-site immuno-enzymatic assay (Beckman Instruments, Chaska, MN) with inter-assay CV’s of 4.3% at 3.03 ng/mL, 5.0% at 7.23 ng/mL, and 4.8% at 13.62 ng/mL. Intra-assay CV’s were 3.5% at 2.50 ng/mL and 3.2% at 14.8 ng/mL. Active ghrelin was measured by competitive radioimuunoassay (RIA; Linco/Millipore Research, St. Charles, MO) with intra-assay CV’s of 7.3% and 7.8% at 77.5 and 538.3 pg/mL and inter-assay CV’s of7.1% and 11.9% at 42.6 and 333.9 pg/mL. Plasma catecholamine concentrations were measured by HPLC (7).
Calculations
The power calculations for this study were based on the primary hypothesis that there exists a positive relationship between insulin resistance and visceral fat gain. We estimated variations in regional fat gain using data from a previous overfeeding study (8). This study was designed to have 80% power to detect a correlation (r ≥ 0.50) between insulin sensitivity and regional fat gain. To estimate the range and precision of one of the insulin sensitivity measures we used published data (5) to anticipate a 20 fold range of Si values (from 2.0 to 40 – the units are 100 ml*min-1*kg-1 per mU/ml) with an average difference between a single measure and the mean of duplicate measures of 1.5 (5, 9). The reproducibility of measuring intra-abdominal fat area is ~1% in our hands (6). The power calculations were based upon an average fat gain of 2.0 kg. In order to detect a correlation of at least 0.50 between the change in visceral fat area and baseline Si we needed to include 30 volunteers. The observed average fat gain was ~3.5 kg and we observed a wider range of regional fat gain as a fraction of total fat gain than we assumed in our power calculations, which should improve the statistical power. However, only 25 subjects completed the protocol with complete data, which would reduce the statistical power.
Insulin sensitivity was determined using MINMOD Millennium (10). Insulin sensitivity was also assessed by determining the 24-hour insulin AUC (11)for the inpatient days. For the former, blood samples for the measurement of plasma insulin concentration were taken at 0, 60, 120, 180, 240, 300, 360, 420, 480, 600, 720, 840, 960, 1200, and 1440 minutes. Area-under-the-curve (AUC) was calculated using the trapezoidal method.
Because the total fat gain was not identical between individuals, the regional fat gain in response to overfeeding was expressed as a percent of the total change in fat mass.
Statistics
All data are presented as mean ± SD, unless stated otherwise, and were analyzed using SAS version 8.02 and SAS Enterprise Guide 4.2, and a p-value of <0.05 was taken as significant. Changes in anthropometric measures and hormone AUC from visit 1 to visit 2 were analyzed by a paired t -test. Regression analysis was performed to determine whether baseline insulin sensitivity, as measured by both 24-hour insulin AUC and Si from the IVGTT and HOMA IR, predicted regional upper body fat gain. General linear models were used to adjust for covariates such as age and sex, when appropriate. Spearman correlation coefficients were determined for the relationship between insulin sensitivity (Si and 24h AUC) and regional body fat and fitness.
Results
Subject characteristics
Table 1 provides the characteristics of the participants by sex. The range in baseline Si was 1.01 to 8.72 and the range of 24h insulin AUC was 10734 to 56859 μU/ml/24h. In general, men and women were similar, except, as would be expected, men were heavier and leaner than women. Also, as expected, women had more subcutaneous fat than men, both in the upper and lower body areas, and men had a greater amount of visceral fat than women.
Table 1.
Visit 1 subject characteristics by sex.
| Males N=15 | Females N=13 | P-value | |
|---|---|---|---|
| 24h insulin AUC | 20587 ± 11788 | 26157 ± 11972 | 0.2503 |
| Insulin Sensitivity (IVGTT) | 5.3 ± 2.3 | 3.7 ± 2.2 | 0.0812 |
| VO2max (ml/kgffm/min) | 50.1 ± 5.2 | 46.3 ± 7.4 | 0.1460 |
| Age (years) | 29 ± 8 | 28 ± 8 | 0.8539 |
| Weight (kg) | 76.3 ± 7.0 | 55.3 ± 6.7 | <0.0001 |
| BMI (kg/m2) | 23.5 ± 2.2 | 20.5 ± 1.7 | 0.0003 |
| % body fat | 16.1 ± 4.1 | 29.5 ± 6.3 | <0.0001 |
| UBSQ fat (kg) | 5.8 ± 1.9 | 7.9 ± 7.6 | 0.0130 |
| LBSQ fat (kg) | 5.2 ± 1.4 | 7.6 ± 1.7 | 0.0006 |
| Visceral fat (kg) | 1.3 ± 0.5 | 0.7 ± 0.2 | 0.0010 |
| Fasting insulin (μU/ml) | 4.3 ± 2.1 | 4.5 ± 1.4 | 0.7930 |
| Fasting glucose (mg/dL) | 91 ± 6 | 91 ± 5 | 0.8231 |
| Fasting c-peptide (nmol/L) | 0.32 ± 0.09 | 0.33 ± 0.10 | 0.8641 |
UBSQ/LBSQ = upper body subcutaneous and lower body subcutaneous
AUC = area under the curve; IVGTT = intravenous glucose tolerance test; FFM = fat free mass;
P-value for unequal variance by t-test.
Measures of insulin sensitivity
Because we used both the Si from the IVGTT and the 24h insulin AUC to assess insulin action we examined the relationships between these two indices and how they relate to body composition. The adjusted Spearman correlation coefficients for insulin sensitivity and regional body fat, total body fat percent, and VO2max are provided in Table 2. The baseline, unadjusted 24h insulin AUC was correlated with abdominal subcutaneous fat (r =0.39, p < 0.05) and percent body fat (r = 0.43, p < 0.05), but after adjusting for sex the relationships were no longer significant. Interestingly, even in this normal weight, healthy group of volunteers, the 24h insulin AUC correlated significantly with visceral fat mass after adjusting for sex. Baseline insulin sensitivity from the IVGTT (Si) was negatively correlated with abdominal subcutaneous fat (p < 0.005), lower body subcutaneous (p < 0.05) fat and percent body fat (p < 0.005), but, with the exception of abdominal subcutaneous fat (r = −0.45, p < 0.05), these relationships were no longer significant after adjusting for sex. Insulin sensitivity, as assessed by IVGTT, was significantly correlated with abdominal subcutaneous fat (p = 0.019) after adjusting for sex (Table 2). As might be expected, given the narrow range of body fat in our lean subjects, neither measure of insulin sensitivity was significantly correlated with percent body fat after adjusting for sex. Insulin secretion is responsive to the glycemic response to meals (12). To understand whether the differences in insulinemia were merely a function of different glucose concentrations we tested for associations between the 24-h glucose AUC and body composition variables – there were no significant relationships between any of the body fat parameters and plasma glucose responses.
Table 2.
Spearman correlations coefficients with insulin sensitivity at baseline.
| IVGTT Si | 24h insulin AUC | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||||
| r | p-value | r | p-value | r | p-value | r | p-value | |
| UBSQ fat(kg) | −0.58 | 0.0016 | −0.45 | 0.0191 | 0.39 | 0.0377 | 0.36 | 0.0573 |
| LBSQ fat (kg) | −0.43 | 0.0241 | −0.21 | 0.2866 | 0.22 | 0.2690 | 0.22 | 0.2593 |
| Visceral fat (kg) | 0.10 | 0.6206 | −0.04 | 0.8620 | 0.23 | 0.2491 | 0.38 | 0.0469 |
| % body fat | −0.54 | 0.0039 | −0.31 | 0.1210 | 0.43 | 0.0240 | 0.28 | 0.1494 |
| VO2max (ml/kgffm/min) | 0.08 | 0.7125 | 0.003 | 0.9686 | −0.31 | 0.1183 | −0.35 | 0.0769 |
Adjusted for sex.
UBSQ/LBSQ = upper body/lower body subcutaneous fat
Response to overfeeding
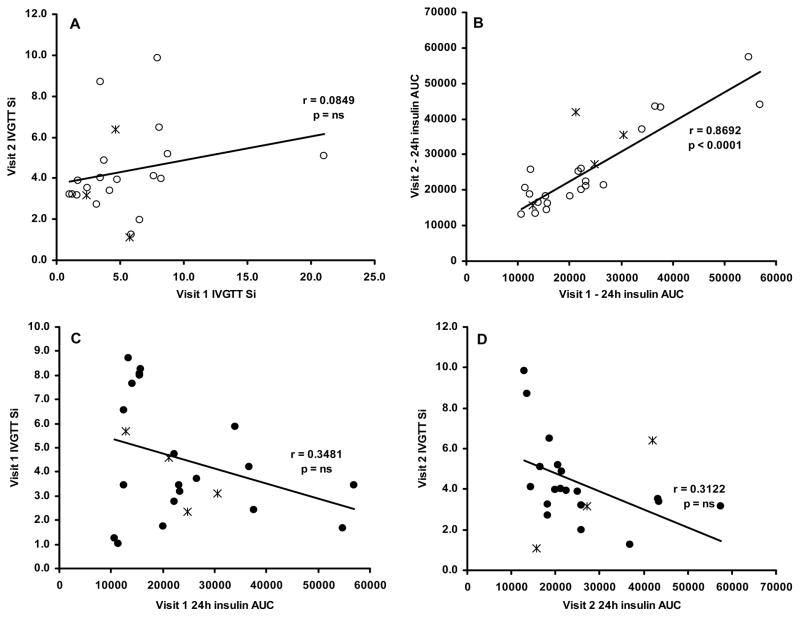
As measured by the 24h insulin AUC, insulin sensitivity decreased as a result of fat gain. Despite this, there was a significant correlation between the 24h insulin AUC measured at visits 1 and 2 (r = 0.87, CV = 23.0, p < 0.0001; Figure 2B). We did not find a significant correlation between Si measured at visit 1 and visit 2 (r = 0.08, CV = 52.5, p = 0.71; Figure 2A). SI measured by IVGTT and 24h insulin AUC at visit 1 (Figure 2C; r = 0.35, CV =52.1, p = 0.10) and visit 2 (Figure 2D; r = 0.31, CV = 49.0, p = 0.16) were not significantly correlated.
Figure 2.
The reproducibility between visits 1 and 2 of the IVGTT (A) and 24h insulin AUC (B). The correlation between insulin sensitivity as measured by IVGTT and 24h insulin AUC at visit 1 (C) and visit 2 (D). The “*” symbols represent women who were taking oral contraceptives.
Body composition changes
Total body weight increased 4.6 ±2.2 kg and percent body fat increased by 4.0 ±2.2% (Table 3). Regional fat mass increased significantly for all volunteers combined: 1.9 ±1.0 kg, 1.6 ±0.7 kg, and 0.4 ±0.3 kg for abdominal subcutaneous, lower body subcutaneous, and visceral fat, respectively (p < 0.0001) and men and women separately. The contribution of leg fat gain to total fat gain ranged from 29–79%, while the contributions of abdominal subcutaneous and visceral fat gain to total fat gain ranged from 17–69% and −5–22%, respectively.
Table 3.
Change in subject characteristics
| All (n=25) | P-value | |
|---|---|---|
| 24h insulin AUC | 2685 ± 6252 | 0.0421 |
| Insulin Sensitivity (IVGTT) | −0.08 ± 3.10 | 0.9058 |
| VO2max (ml/kgffm/min) | 0.3 ± 5.2 | 0.7659 |
| Weight (kg) | 4.7 ± 2.3 | <0.0001 |
| Fat free mass (kg) | 0.8 ± 1.3 | <0.0001 |
| Fat mass (kg) | 3.9 ± 1.8 | <0.0001 |
| Abd subcutaneous fat (kg) | 1.9 ± 1.0 | <0.0001 |
| Lower body subcutaneous fat (kg) | 1.6 ± 0.8 | <0.0001 |
| Visceral fat (kg) | 0.4 ± 0.3 | <0.0001 |
AUC = area-under-the-curve; IVGTT = intravenous glucose tolerance test;
p-value by paired t-test for visit 1 vs visit 2
Hormone responses
The hormone profiles, by sex, at visits 1 and 2 are provided in table 4. In all subjects combined, the only significant change in hormone levels from visit 1 to visit 2 was an increase in 24h insulin AUC (p < 0.05) and an increase in fasting plasma leptin (p < 0.0001). In males, there was also a significant decrease in growth hormone AUC from visit 1 to visit 2 (p =0.0021) but this was not seen in females. Catecholamines were measured from time 0 to 300 minutes. Norepinephrine and epinephrine AUC did not change with overfeeding.
Table 4.
Hormone values at visits 1 and 2.
| Males (n=13) | Females (n=12) | p-value* | |||
|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | ||
| Glucose AUC (mL/dL/day) | 151207 ± 15936 | 146130 ± 8433 | 149480 ± 9046 | 150585 ± 8985 | 0.3954 |
| Insulin AUC (uU/mL/day) | 21417 ± 12436 | 22505 ± 9041 | 25989 ± 12489 | 30310 ± 13798# | 0.0446 |
| C-peptide AUC (nmol/L/day) | 1190 ± 445 | 1225 ± 424 | 1277 ± 413 | 1403 ± 538 | 0.1664 |
| hGH AUC (ng/mL/day) | 2824 ± 1795 | 1449 ± 1283# | 3414 ± 2294 | 3731 ± 2610 | 0.2157 |
| Fasting Leptin (ng/mL) | 2.6 ± 1.7 | 5.0 ± 3.0# | 12.9 ± 4.9 | 21.9 ± 8.3# | <0.0001 |
| Ghrelin AUC (pg/mL/day)^ | 236423 ± 1219322 | 146130 ± 8433 | 210260 ± 96529 | 220291 ± 76834 | 0.3559 |
| Norepinephrine AUC (pg/mL/day) | 53767 ± 20500 | 48540 ± 12821 | 66303 ± 30198 | 61125 ± 29753 | 0.2940 |
| Epinephrine AUC (pg/mL/day) | 4786 ± 2133 | 4938 ± 3405 | 6112 ± 5155 | 4695 ± 2861 | 0.3026 |
AUC = area-under the curve
P-value by paired t-test for all subjects combined (visit 1 vs visit 2)
Acylated (active) ghrelin
p<0.05 visit 2 vs visit 1 by sex
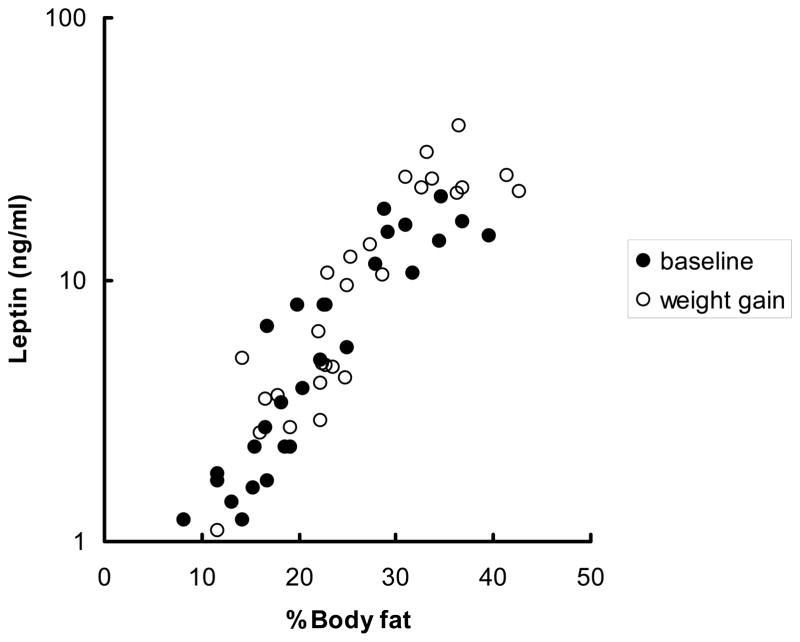
There was a significant, positive relationship (Figure 3; r = 0.91, p < 0.0001) between the log of fasting leptin and percent body fat; this relationship persisted following fat gain (visit 2).
Figure 3.
The relationship between the log of fasting leptin (y-axis) and percent body fat at baseline (r = 0.91, P <0.0001) and following weight gain (r = 0.90, P < 0.0001).
Insulin sensitivity and fat gain
The 24h insulin AUC increased by 2685 ± 6252 mU/ml/24h (Table 4; p < 0.05) across all subjects, but the insulin sensitivity by IVGTT did not change significantly (−0.08 ±3.10, p = 0.91). Across all subjects, there was a significant increase across all parameters of body composition. VO2max did not change with overfeeding.
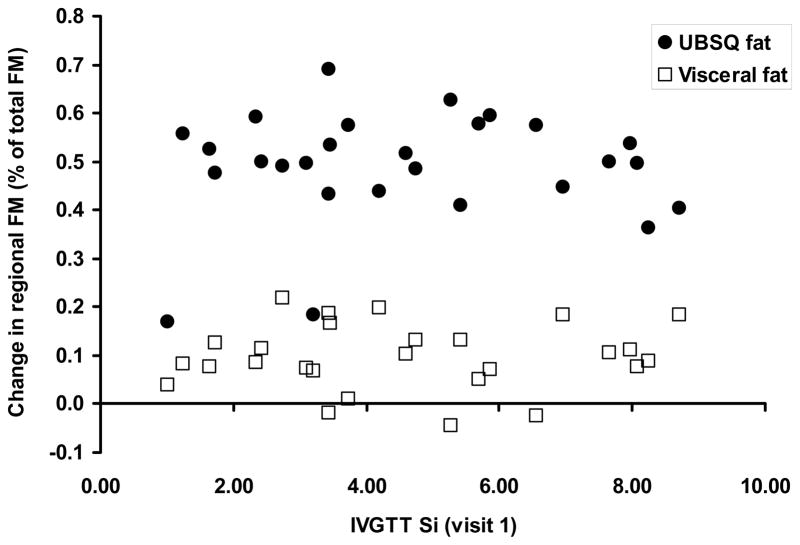
To further test whether baseline insulin sensitivity relates to abdominal body fat gain we performed simple linear regression with change in abdominal subcutaneous and visceral fat mass as a percent of total fat mass change as the independent variable and the visit 1 24h insulin AUC as the dependent variable. Additionally, HOMA IR also did not predict abdominal subcutaneous or visceral fat gain (as a percent of total fat gain) in a general linear model, even when adjusted for age and sex. Baseline 24h insulin AUC was not significantly related to abdominal subcutaneous fat(r = 0.21, CV = 23.2, p = 0.27) or visceral fat mass (r = 0.07, CV = 73.8, p = 0.71) change. Likewise, insulin sensitivity as assessed by the IVGTT (Si) did not predict abdominal subcutaneous fat (r = 0.07, CV = 24.1, p = 0.73) or visceral fat mass (r = 0.05, CV = 73.1, p = 0.80) change regardless of adjustment for body fat and sex (Figure 4).
Figure 4.
The relationship between insulin sensitivity (Si) as assessed by a 4-h IVGTT at visit 1 and the subsequent change in regional fat mass as a percent of total fat mass change. There was no significant relationship between baseline insulin sensitivity and either measure of upper body adiposity.
Discussion
The aim of this study was to test the hypothesis that whole body insulin resistance is a precursor, rather than strictly a consequence of an upper body fat distribution. To address this hypothesis we performed two measures of insulin sensitivity before and after an overfeeding protocol designed to result in an ~3.5 kg fat gain in healthy, normal weight men and women. If insulin resistance predisposes to visceral fat gain then those who were most insulin resistant at baseline should gain the greatest proportion of fat in the visceral depot. Instead, we found no relationship between baseline insulin sensitivity and visceral fat gain. We conclude that peripheral insulin resistance is not a necessary antecedent to upper body fat gain.
Of the methods for determining insulin sensitivity with respect to glucose metabolism, the euglycemic, hyperinsulinemic clamp is considered to be the gold standard (13). Glucose disposal under euglycemic, hyperinsulinemic conditions is almost exclusively muscle, however, and thus the traditional insulin clamp is primarily a measure of muscle insulin sensitivity with respect to glucose metabolism. Insulin is hugely important in regulating lipolysis, proteolysis and interacts in a complex fashion with blood glucose concentrations to promote glucose uptake. In this study we used the IVGTT, a widely accepted measure of insulin sensitivity and the 24 hour insulin AUC, a parameter know to be elevated in insulin resistant states such as obesity (11). The insulin AUC also reflects the availability of free fatty acids (14), which are increased with adipose tissue insulin resistance, and the insulin AUC is responsive to exercise in the expected direction (15). Our data suggest that these two measures of insulin sensitivity provide somewhat different and complementary indices of insulin’s ability to maintain metabolic homeostasis.
We reasoned that the insulinemia required for nutrient disposal over the postprandial and postabsorptive period would provide a good physiological profile of integrated insulin requirements/action. The 24 hr insulin AUC was reproducible, correlated well with body composition parameters known to be related to insulin sensitivity, and changed in the expected direction as a result of weight gain. The 24 insulin profile does not provide information regarding insulin secretion and glucose effectiveness that is gained using the IVGTT, however. In our group of non-obese men and women the 24 h insulin AUC was correlated with visceral fat mass whereas the Si from the IVGTT was better related to upper body subcutaneous fat (Table 2). This suggests that these two measures of insulin action may reflect somewhat different processes. In this regard it is not surprising that these two measures do not correlate well with one another in a population of only non-obese adults.
Insulin sensitivity is correlated with the amount of upper body subcutaneous (16) and visceral (17) adipose tissue when a wide range of lean and obese adults are studied. Likely because we enrolled only non-obese participants in our study, with a narrow range of visceral fat mass (0.42 – 2.68 kg), we could not detect this association between baseline insulin sensitivity and visceral fat when the data was not adjusted for sex. In fact, the range of fat distribution in our lean subjects at baseline was relatively small and this may have precluded us from detecting relationships that have previously been reported in groups that included both lean and obese individuals (18). That said, we had a wide range of insulin sensitivity, which indicates that, in these mostly Caucasian subjects, relative insulin resistance (decreased insulin sensitivity) can occur in the absence of exaggerated amounts of visceral fat.
The question of the role of insulin sensitivity in body fat distribution over time has not been addressed as frequently as general weight gain. To our knowledge, ours is the first study to directly address the role of insulin sensitivity in the distribution of regional body fat gain. Despite not seeing a relationship between fasting insulin and total weight gain, Gould et al (19) reported that in women with normal glucose tolerance, fasting plasma insulin was significantly associated with the percentage increase waist-to-hip ratio over a 4.4 year follow-up with mean total weight change of ~2.5 kg. This relationship was mainly confined to women >50 years, but could potentially indicate a role of insulin sensitivity in regional body fat distribution in this group. Our results suggest that in normal weight, premenopausal women and men of the same age, insulin sensitivity does not predict regional fat gain as measured by more sophisticated means.
The macronutrient content of excess energy intake has been reported to affect the metabolic response (20) – pure carbohydrate resulted in greater metabolic derangements and a greater increase in waist circumference than mixed fat/protein overfeeding. In our study, the food supplements provided to create weight gain were quite similar in fat/carbohydrate content, and thus likely did not contribute to inter-individual differences in regional fat gain.
There are limitations to our study that should be acknowledged. First, it may be that~4 kg fat gain is not enough to determine whether insulin sensitivity relates to regional differences in adiposity that develop over longer periods of time and with greater weight gain. Additionally, our volunteers were relatively homogenous, in that most of the participants were Caucasian, and thus generalizing our results to other ethnic groups may be inappropriate. Oral contraceptives, depending on type of medication, may decrease insulin action and could affect the response to overfeeding, however only 4 of our 12 females were on oral contraceptives and their results did not deviate from those not using oral contraceptives. Lastly, despite a relatively wide range of insulin sensitivity for lean, non-diabetic individuals, we only had 3 participants with a parental history of T2DM. Several studies suggest that it is not insulin resistance/sensitivity per se that results in future T2DM, but the family history, or genetic link, itself (2, 21, 22). We limited our study to lean individuals and few potential volunteers with a family history of T2DM qualified based on our entry criteria. Including a larger population of T2DM offspring in the normal weight category may be important in future studies. Offspring of T2DM respond differently to overfeeding (23), and, if constitutional factors other than insulin resistance result in upper body fat gain it will be necessary to compare equally insulin resistant persons with and without a family history of T2DM.
In conclusion, we found that insulin sensitivity is not predictive of abdominal subcutaneous/visceral fat gain in normal weight individuals. This suggests that insulin sensitivity is not directly causative in the development of an upper body fat distribution in normal weigh Caucasians. We might conclude from this that whatever environment or constitutional factors that drive upper body/visceral fat gain in the face of excess energy intake are not necessarily creating insulin resistance in the lean state. Future studies should include a larger population of offspring of T2DM and consider predictors in addition to pre-existing insulin resistance.
Acknowledgments
We thank the staff of the Mayo GCRC and Ms. Jessica Eastman for their assistance in performing these studies. Dr. Susanne Votruba – performing experiments, collecting data, analyzing and interpreting data, writing manuscript. Dr. Michael Jensen – principle investigator, designing experiments, analyzing and interpreting data, writing the manuscript. Supported by grants DK45343, DK50456 and R00585 from the U.S. Public Health Service and by the Mayo Foundation.
Footnotes
Disclosure summary: The authors have no conflicts to disclose.
Clinical Trial Registration Number: N/A
Disclosure Statement
No conflict of interest for Dr. Votruba or Dr. Jensen.
References
- 1.Gastaldelli A, Miyazaki Y, Pattiti M, et al. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab. 2002;87:5098–5103. doi: 10.1210/jc.2002-020696. [DOI] [PubMed] [Google Scholar]
- 2.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes. 1997;46:1001–1009. doi: 10.2337/diab.46.6.1001. [DOI] [PubMed] [Google Scholar]
- 3.Romanski SA, Nelson R, Jensen MD. Meal fatty acid uptake in adipose tissue: Gender effects in non-obese humans. Am J Physiol. 2000;279:E455–E462. doi: 10.1152/ajpendo.2000.279.2.E455. [DOI] [PubMed] [Google Scholar]
- 4.Doan AE, Peterson DR, Blackmon JR, Bruce RA. Myocardial ischemia after maximal exercise in healthy men. Am Heart J. 1965;69:11–25. doi: 10.1016/0002-8703(65)90211-5. [DOI] [PubMed] [Google Scholar]
- 5.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–278. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 7.Causon RC, Carruthers ME, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem. 1981;116:223–226. doi: 10.1016/0003-2697(81)90347-x. [DOI] [PubMed] [Google Scholar]
- 8.Levine JA, Eberhardt NL, Jensen MD. Role of non-exercise activity thermogenesis (NEAT) in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 9.Cobelli C. Models to interpret kinetic data in stable isotope tracer studies. Am J Physiol. 1987;253:E551. doi: 10.1152/ajpendo.1987.253.5.E551. [DOI] [PubMed] [Google Scholar]
- 10.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 11.Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frape DL, Williams NR, Scriven AJ, Palmer CR, O’Sullivan K, Fletcher RJ. Diurnal trends in responses of blood plasma concentrations of glucose, insulin, and C-peptide following high-and low -fat meals and their relation to fat metabolism in healthy middle-aged volunteers. Br J Nutr. 1997;77:523–535. doi: 10.1079/bjn19970054. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Ferrannini E, Hendler R, Wahren J, Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci. 1978;75:5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinari S, Bertuzzi A, Manco M, Mingrone G. NEFA-glucose comodulation model of β-cell insulin in 24-h multiple-meal test. Am J Physiol. 2007;2007:E1890–E1898. doi: 10.1152/ajpendo.00563.2006. [DOI] [PubMed] [Google Scholar]
- 15.Larsen JJS, Dela F, Madsbad S, Galbo H. The effect of intense exercise on postprandial glucose homeostasis in type II diabetes patients. Diabetologia. 1999;42:1282–1292. doi: 10.1007/s001250051440. [DOI] [PubMed] [Google Scholar]
- 16.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S12–S18. doi: 10.1016/j.amjmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. AmJ Physiol. 2000;278:E941–E948. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 19.Gould AJ, Williams DEM, Byrne CD, Hales CN, Wareham NJ. Prospective cohort study of the relationship of markers of insulin resistance and secretion with weight gain and changes in regional adiposity. Int J Obes Relat Metab Disord. 1999;23:1256–1261. doi: 10.1038/sj.ijo.0801060. [DOI] [PubMed] [Google Scholar]
- 20.Claesson AL, Holm GEA, Lindstrom T, Nystrom FH. Two weeks of overfeeding with candy, but not peanuts, increases insulin levels and body weight. Scand J Clin Lab Invest. 2009;69:598–605. doi: 10.1080/00365510902912754. [DOI] [PubMed] [Google Scholar]
- 21.Silver RJ, Mehta S, Soeldner JS, Marton BC, Warram JH, Goldfine AB. Acute insulin secretion as a predictor of weight gain in health humans. Obesity. 2006;14:67–72. doi: 10.1038/oby.2006.9. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson J, Smith U, Waagstein F, Wysocki M, Jansson P. Glucose turnover and adipose tissue lipolysis are insulin-resistant in healthy relatives of type 2 diabetes patients. Is cellular insulin resistance a secondary phenomenon? Diabetes. 1999;48:1572–1578. doi: 10.2337/diabetes.48.8.1572. [DOI] [PubMed] [Google Scholar]
- 23.Samocha-Bonet D, Campbell LV, Viardot A, et al. A family history of type 2 diabetes increases risk factors associated with overfeeding. Diabetologia. 2010;53:1700–1708. doi: 10.1007/s00125-010-1768-y. [DOI] [PubMed] [Google Scholar]