Abstract
Synthetic oleanane triterpenoids are novel agents which have shown strong antitumorigenic activity against a wide range of cancer types in vitro. The objective of the present study was to determine the anticancer activity of methyl-2-cyano-3, 12-dioxooleana-1, 9(11)-dien-28-oate (CDDO-Me) derived from CDDO, a synthetic analog of oleanolic acid, and its mechanism of action in killing of human ovarian cancer cells. CDDO-Me strongly inhibited the growth of ovarian cancer cells by inducing apoptosis characterized by increased annexin V binding, cleavage of poly (ADP-ribose) polymerase (PARP-1) and procaspases-3, -8 and -9. In addition, CDDO-Me induced mitochondrial depolarization. Western blot analysis showed inhibition of prosurvival (antiapoptotic) phospho-AKT (p-AKT), nuclear factor kappa B (NF-κB) (p65) and phospho- mammalian target of rapamycin (p-mTOR) signaling proteins in cells treated with CDDO-Me. Abrogation of AKT which regulates both NF-κB and mTOR increased the sensitivity of tumor cells to CDDO-Me. Thus, these data showing strong growth-inhibitory and apoptosis-inducing activity of CDDO-Me for ovarian cancer cells through the inhibition of AKT/NF-κB/mTOR signaling pathway provide basis for evaluation of CDDO-Me for ovarian cancer.
Keywords: Ovarian cancer; methyl-2-cyano-3; 12-dioxooleana-1,9(11)-dien-28-oate (CDDO-Me); apoptosis; AKT; NF-κB; mTOR
Carcinoma of the ovary is the most common gynecologic malignancy and the fifth most common cause of cancer-related death in women in the United States. According to the American Cancer Society, 21,880 new cases of ovarian cancer will have been diagnosed and 13,850 women will have died from the disease in the United States in 2010 (1). While surgical resection of tumors confined to the ovary can result in 5-year survival rate of approximately 90%, unfortunately most patients have widespread disease at the time of diagnosis (2). Carboplatin/paclitaxel is the current standard of care for first-line treatment of ovarian cancer; however, development of drug resistance limits the effectiveness of these chemotherapeutic agents, underscoring the dire need for developing new strategies and novel agents for the prevention and treatment of ovarian cancer (3, 4).
Novel agents that promote apoptosis in ovarian cancer cells could compliment chemotherapy and lead to tumor regression and improved prognosis. Triterpenoids are members of a larger family of structurally related compounds known as cyclosqualenoids that are widely distributed in nature. Oleanolic acid is a naturally occurring triterpenoid that has been used in traditional medicine as antibacterial, antifungal, anti cancer, and anti-inflammatory agent (5, 6). Recently, synthetic derivatives of oleanolic acid have been tested for anti-inflammatory and anticancer activity. 2-Cyano-3, 12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), an oleanane triterpenoid and its C-28 methyl ester derivative methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate (CDDO-Me) exhibit potent anti-inflammatory and antitumorigenic activity (7, 8).
Synthetic CDDOs inhibit the proliferation of diverse types of tumor cell lines, including glioblastoma, leukemia, osteosarcoma, breast, lung, prostate and pancreatic cancer cell lines (9–12). CDDOs induce apoptosis and inhibit (MAPK; ERK1/2), (NF-κB) and (PPARγ) signaling and induce phase 2 response through (NRF2) signaling (13–15). They also inhibit liver, lung and prostate cancer development in animal models (16–18). However, the response of ovarian cancer cells to CDDOs has not been adequately studied. In one study, CDDO-Me was shown to inhibit (IL-6) and (STAT3) phoshorylation in two ovarian cancer cell lines (19). In the present study, we evaluated the response and the mechanism of growth inhibition by CDDO-Me in four human ovarian cancer cell lines.
Materials and Methods
Reagents and antibodies
CDDO-Me was obtained from the National Cancer Institute, Bethesda, MD, USA, through the Rapid Access to Intervention Development Program. Anti-caspase-3, caspase-8, and caspase-9 antibodies were purchased from BD Pharmingen (San Diego, CA, USA). Anti-p-AKT (ser473) and anti-p-mTOR (ser2448) antibodies were from Cell Signaling Technology (Danvers, MA, USA). Anti-NF-κB (p65) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). CellTiter 96® AQueous One Solution Proliferation Assay System was from Promega (Madison, WI, USA).
Cell lines
Human ovarian cancer cell lines OVCAR-3, OVCAR-5 and SK-OV3 and ovarian endometrioid adenocarcinoma cell line MDAH-2774 were obtained from the American Type Tissue Collection (Rockville, MD, USA). Cells were maintained in tissue culture using fully supplemented cell line-specific tissue culture medium.
Measurement of cell viability (MTS assay)
Tumor cells (1×104) were seeded into each well of a 96-well plate in 100 μl of tissue culture medium. After 24 h incubation, cell were cultured at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air, cells were treated with CDDO-Me at concentrations of 0.625 to 10 μM for 72 h. Cell viability was then determined by the (MTS) assay using CellTiter 96® AQueous One Solution Proliferation Assay System. The percentage cytotoxicity was calculated from the loss of cell viability in cultures.
Annexin V-(FITC) binding
Tumor cells treated with CDDO-Me for 20 h were suspended in the binding buffer provided in the annexin V-FITC apoptosis detection kit II from BD Pharmingen (San Diego, CA, USA) and reacted with 5 μl of annexin V-FITC reagent plus 5 μl of propidium iodide (PI) for 30 min at room temperature in the dark. Stained cells were analyzed by flow cytometry.
Western blotting
Total cellular proteins were isolated by NP 40 cell lysis buffer from Invitrogen (Camarillo, CA, USA). Samples (50 μg) were boiled in an equal volume of sample buffer (20% glycerol, 4% SDS, 0.2% bromophenol blue, 125 mM Tris-HCl (pH 7.5), and 640 mM 2-mercaptoethanol) and separated on pre-casted Tris-glycine polyacrylamide gels (6–10%) using the XCell Surelock™ Mini-Cell, in Tris-Glycine SDS running buffer, all from Novex (Invitrogen, Carlsbad, CA,USA). Proteins resolved on the gels were transferred to nitrocellulose membranes. Membranes were blocked with 5% milk in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl with 0.05% Tween 20 (TPBS) and incubated with protein-specific antibody or anti-β-actin antibody (loading control) followed by (HRP)-conjugated secondary antibody from jakson Immunoresearch laboratories (West Grove, PA, USA). Immune complexes were visualized with enhanced chemiluminescence (ECL).
Mitochondrial depolarization assay
Alteration in mitochondrial potential by CDDO-Me was determined using mitochondrial potential sensor dye JC-1 (Molecular Probes, Invitrogen, San Diego, CA, USA). Briefly, 1×106 control (untreated) and CDDO-Me- treated cells in 1 ml culture medium were loaded with JC-1 (10 μg/ml) for 10 minutes at 22°C and analyzed by flow cytometry. In normal cells, dye is aggregated in mitochondria, fluoresces red, and is detected in the FL2 channel. In cells with altered mitochondrial potential, the dye fails to accumulate in the mitochondria, remains as monomers in the cytoplasm, fluoresces green, and is detected in the FL1 channel.
Transfection
For silencing of AKT, OVCAR-5 and MDAH-2774 cells were transfected with double-stranded siRNA of AKT using SignalSilence siRNA kits from Cell Signaling Technology (Beverly, MA,USA). Untransfected cells and those transfected with scrambled siRNA sequence served as controls. Briefly, 106 tumor cells were plated in each 60 mm Petri dish for 24 h and treated with 3 ml of transfection medium (serum-free) containing 20 μg LipofectAmine and 100 nM siRNA for 48 h. The inhibition of AKT was confirmed by Western blotting.
Statistical analysis
Most data are presented as means±S.D. and outcomes for treated and untreated cells were compared by Student’s t-test. Differences were considered significant at p<0.05.
Results
Growth-inhibitory effect of CDDO-Me on ovarian cancer cells
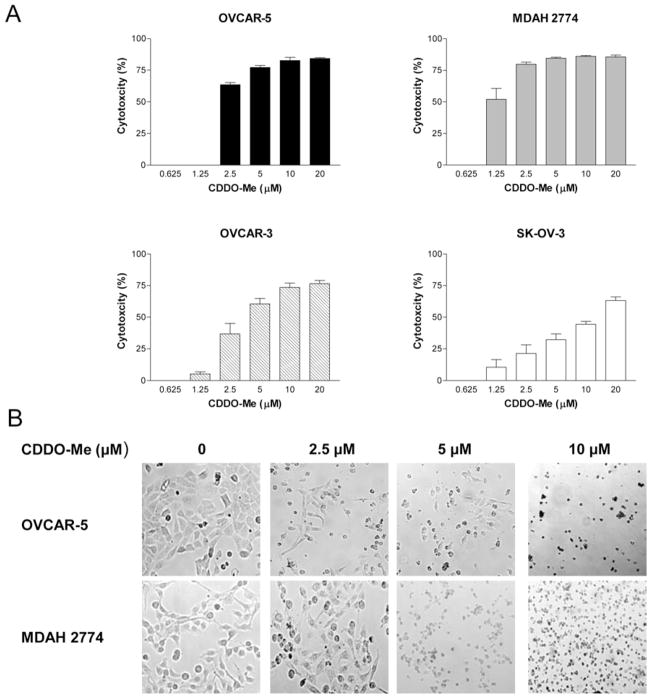
The effect of CDDO-Me on the proliferation of OVCAR-5, MDAH-2774, OVCAR-3 and SK-OV-3 ovarian cancer cells was measured by MTS assay. As shown in Figure 1A, CDDO-Me at concentrations of 2.5 to 20 μM significantly inhibited the growth of OVCAR-5 (63% to 83%), MDAH-2774 (79% to 86%) and OVCAR-3 (36% to 74%) compared to untreated cells (p<0.05). The growth of MDAH-2774 cells was also significantly inhibited at 1.25 μM (51%). None of the cell lines showed growth inhibition at 0.625 μM CDDO-Me. At all concentrations (0.625 to 20 μM), SK-OV-3 cells were less responsive to CDDO-Me compared to other cell lines.
Figure 1.
CDDO-Me inhibits the growth of ovarian cancer cells. A: Ovarian cancer cells (OVCAR-5, MDAH-2774, OVCAR-3 and SK-OV3) were treated with CDDO-Me at concentrations ranging from 0 to 20 μM for 72 h in triplicate in 96-well plates. Cell viability was measured by MTS assay using CellTiter AQueous Assay System. B: Morphological changes in cell cultures of OVCAR-5 and MDAH-2774 cells treated or not with CDDO-Me for 48 h as visualized by light microscopy. Similar results were obtained in three independent experiments. *p<0.05 compared to control cells (no CDDO-Me).
Microscopic examination of cell cultures of OVCAR-5 and MDAH-2774 showed partial rounding of cells at 2.5 μM to complete detachment and significant cell death (trypan blue dye exclusion) after treatment with CDDO-Me at 5 and 10 μM for 48 h (Figure 1B). Together, these data demonstrate that CDDO-Me potently inhibits the growth of ovarian cancer cells.
CDDO-Me induces apoptosis in ovarian cancer cells
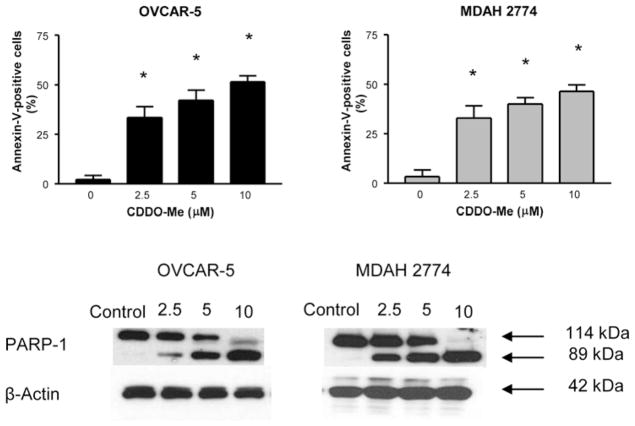
Whether CDDO-Me induces apoptosis in ovarian cancer cells was measured by the binding of annexin-V-FITC to OVCAR-5 and MDAH-2774 cells treated with CDDO-Me by flow cytometry. As shown in Figure 2, a small percentage of untreated OVCAR-5 and MDAH-2774 cells bound annexin V-FITC (<5%); however, the percentage of annexin V-FITC-binding OVCAR-5 cells increased from 33% to 52% and that of MDAH-2774 from 33% to 47% following treatment with CDDO-Me at 2.5 to 10 μM for 20 h (p<0.05, at all concentrations). In addition, CDDO-Me also induced the cleavage of native PARP-1 (110 kDa). These data demonstrate that CDDO-Me induces apoptosis in ovarian cancer cells.
Figure 2.
Treatment with CDDO-Me induces apoptosis in ovarian cancer cells. OVCAR-5 and MDAH-2774 cells were treated with CDDO-Me at 0 to 10 μM for 20 h. Cells were then incubated with 5 μl of annexin V-FITC and 5 μl propidium iodide for 30 min and the percentage of annexin V-FITC-binding cells was determined by flow cytometry (A). Cleavage of PARP-1 in cells treated with CDDO-Me was analyzed by immunoblotting (B). Each experiment was repeated at least three times. *p<0.05 compared to control cells (no CDDO-Me).
CDDO-Me activates procaspases in ovarian cancer cells
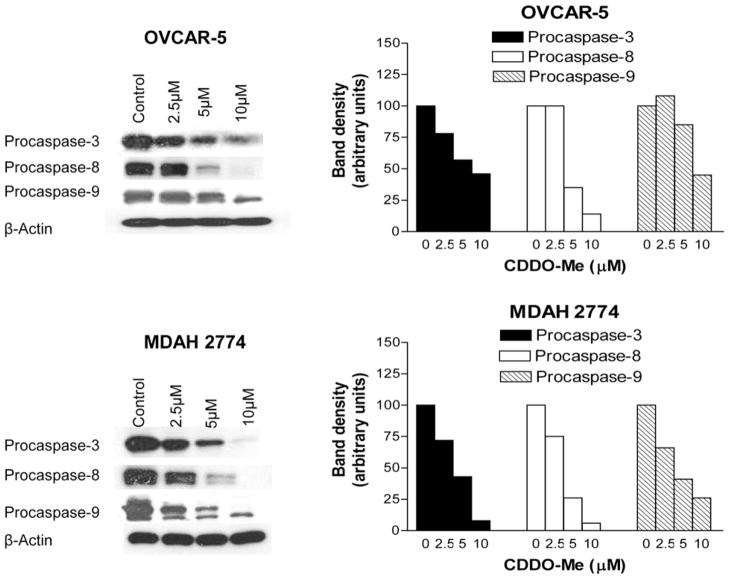
Further evidence that CDDO-Me induces apoptosis was obtained from the effect of CDDO-Me on the processing of procaspases-3, -8 and -9. Western blot analysis of OVCAR-5 and MDAH-2774 cells treated with CDDO-Me for 20 h showed almost complete processing of procaspases-3, -8 and -9 in both cell lines at 5.0 to 10 μM CDDO-Me (Figure 3). Some processing of these procaspases was also seen at 2.5 μM CDDO-Me, especially in MDAH-2774 cells.
Figure 3.
Treatment with CDDO-Me cleaves procaspases-3, -8 and -9. OVCAR-5 and MDAH-2774 cells were treated with CDDO-Me at concentrations of 0 to 10 μM for 20 h. Cellular lysates prepared from untreated (control) and treated cells were analyzed for processing of procaspase-3, -8 and -9 and levels of anti-β-actin (loading control) by Western blotting. Bar graphs represent dose-related changes in signal densities normalized to β-actin bands. Each experiment was repeated at least three times.
CDDO-Me induces mitochondrial depolarization in ovarian cancer cells
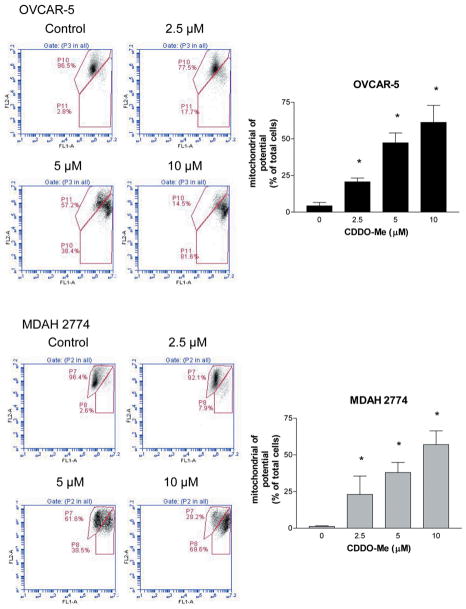
The cleavage of procaspase-9 suggested the involvement of mitochondria in the induction of apoptosis by CDDO-Me in ovarian cancer cells. To confirm this, we evaluated depolarization of mitochondria in cells treated with CDDO-Me from the fluorescent shift of cells loaded with JC-1 probe. As shown in Figure 4, the percentage of OVCAR-5 and MDAH-2774 cells emitting green fluorescence significantly increased (p<0.05) after treatment with CDDO-Me for 20 h (OVCAR-5, 4%, 21%, 47% and 61%; MDAH-2774, 1%, 23%, 38% and 57% at 0, 2.5, 5 and 10 μM CDDO-Me, respectively).
Figure 4.
CDDO-Me induces mitochondrial depolarization. OVCAR-5 and MDAH-2774 cells were treated with CDDO-Me at 0 to 10 μM for 20 h. Cells were loaded with mitochondrial potential sensor JC-1 (10 μg/ml) for 10 minutes at 22°C and analyzed by flow cytometry for cells fluorescing red (FL2 channel) or green (FL1 channel) (A). Bar graphs show the percentage of cells with loss of mitochondrial potential (B). Similar results were obtained in two separate experiments. *p<0.05 compared to control cells (no CDDO-Me).
Collectively, increase in annexin V-binding of cells, cleavage of PARP-1, processing of procaspases and mitochondrial depolarization demonstrated induction of apoptosis in ovarian cancer cells by CDDO-Me, both through the non-mitochondrial and mitochondrial pathways.
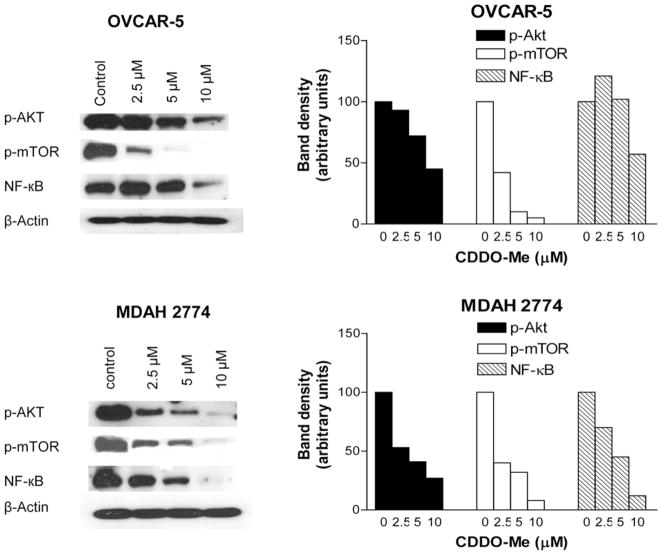
CDDO-Me inhibits prosurvival p-AKT, p-mTOR and NF-κB
AKT/NF-κB/mTOR is a major antiapoptotic signaling pathway that confers survival advantage and resistance to various forms of anticancer therapies. We investigated the effect of CDDO-Me on these signaling proteins in OVCAR-5 and MDAH-2774 cells treated with CDDO-Me for 20 h by immunoblotting. As shown in Figure 5, CDDO-Me markedly to completely reduced the levels of p-AKT, p-mTOR and NF-κB in both cell lines at concentrations of 5 to 10 μM. Measurable reduction in the levels of these proteins also occurred at 2.5 μM CDDO-Me.
Figure 5.
CDDO-Me inhibits prosurvival p-Akt, NF-κB and p-mTOR. A: OVCAR-5 and MDAH-2774 cells were treated with CDDO-Me at 0 to 10 μM for 20 h. After treatment, cell lysates and nuclear lysates were prepared and analyzed by Western blotting for p-AKT and p-mTOR (cell lysates), NF-κB (nuclear lysates) and β-actin (loading control). B: Bar graphs show dose-related changes in signal densities of target proteins.
AKT is a functionally relevant target of CDDO-Me
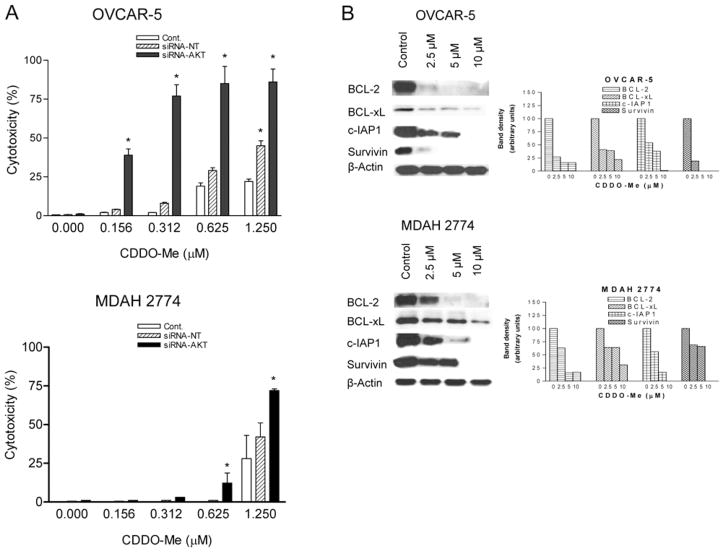
Since AKT plays a central role in survival and resistance of cancer cells to apoptosis by regulating the activation of NF-κB and m-TOR, we measured the response of OVCAR-5 and MDAH 2774 cells to low concentrations of CDDO-Me after knocking down AKT. As shown in Figure 6A, there was some increase in the sensitivity of OVCAR-5 control cells to CDDO-Me at 0.625 and 1.25 μM following the transfection protocol which included culturing cells in serum-free medium for 48 h, as opposed to the lack of sensitivity at these concentrations as seen in Figure 1A. Transfection with siRNA-AKT increased the sensitivity of both OVCAR-5 and MDAH 2774 cells to CDDO-Me at 0.156 μM to 1.25 μM compared to non-transfected control cells (p<0.05). Some increase in the sensitivity of cells transfected with non-targeting siRNA was also seen at these low concentrations of CDDO-Me, which correlated with non-specific reduction in AKT protein following transfection with non-targeting siRNA.
Figure 6.
Knocking down AKT sensitizes ovarian cancer cells to CDDO-Me. A: OVCAR-5 and MDAH 2774 cells were transfected with siRNA-AKT or scrambled siRNA sequence for 48 h and levels of AKT were measured by Western blotting. The sensitivity of control and transfected cells to low concentrations of CDDO-Me was determined at 72 h by MTS assay. Similar results were obtained in two independent experiments. B: Cellular lysates of OVCAR-5 and MDAH 2774 cells treated with CDDO-Me as described were analyzed by Western blotting for BCL-2, BCL-xL, cIAP, survivin and β-actin (loading control). Bar graphs reflect change in signal strength compared to the control. Similar results were obtained in two independent experiments. *p<0.05 compared to control and non-targeting siRNA transfected cells.
Next we examined whether inhibition of the AKT/NF-κB axis impacts the expression of NF-κB-regulated antiapoptotic BCL-2, BCL-xL, cIAP1 and survivin. As shown in Figure 6B, the level of BCL-2, BCL-xL, cIAP1 and survivin was reduced in OVCAR-5 and MDAH 2774 cells treated with CDDO-Me in a concentration-related manner. These data indicated that CDDO-Me kills ovarian cancer cells by inhibiting the expression of AKT/NF-κB-regulated antiapoptotic proteins.
Discussion
Aberration of apoptosis has been implicated in tumor development and resistance to anticancer therapies including chemotherapy and ionizing radiation (20). Thus, promotion of apoptosis could potentially lead to the regression and improved prognosis of chemorefractory disease. Oleanolic acid is a naturally occurring triterpenoid with antitumorigenic activity (5, 6). Synthetic analogs of oleanolic acid including CDDO-Me exhibit stronger anti-inflammatory and antiproliferative activity than oleanolic acid (7, 8) CDDOs have shown strong antitumorigenic activity against a wide range of cancer cells both in vitro (9–12) and in vivo (16–18). Since the response of ovarian cancer cells to CDDO-Me has not been reported before, we tested the antitumor activity of CDDO-Me against four human ovarian cancer cell lines. We provide evidence that CDDO-Me has potent growth inhibitory and apoptosis-inducing effects on human ovarian cancer cells. CDDO-Me inhibited the growth of each of the four ovarian cancer lines tested at concentrations ranging from 2.5 to 10 μM. As a matter of fact, in three cell lines (e.g. OVCAR-5, MDAH 2774 and OVCAR-3) the growth-inhibitory effect reached a plateau at 5 to 10 μM CDDO-Me. Although the mechanisms of the anticancer effects of CDDOs are not fully understood, cancer cell differentiation and activation of caspase-dependent and -independent apoptosis contribute to the antitumor activity of CDDOs. Indeed, increased binding of annexin V due to the externalization of phosphatidylserine and cleavage of PARP-1 indicate that induction of apoptosis is part of the mechanism by which CDDO-Me inhibits the growth of ovarian cancer cells.
Two major pathways of apoptotic cell death program have been identified, namely receptor-mediated (extrinsic) and chemical-induced mitochondrial (intrinsic) pathway of apoptosis (21). In both cases, caspases, a family of cysteine proteases, play an important role in the apoptotic cell death. In the extrinsic pathway, binding of the death ligands (e.g. TNF-α, FASL, TRAIL) with their cognate receptors activates initiator caspase-8 which then cleaves and activates effector caspases-3, -6 and -7 leading to apoptosis (22). In chemical-induced (e.g. chemotherapeutic agents) apoptosis (intrinsic pathway), undefined signals induce the release of cytochrome c from mitochondria, which in conjunction with APAF-1 causes activation of initiator caspase-9. Activated caspase-9, in turn, activates effector caspases-3, -6 and -7 (21). CDDO-Me caused the cleavage of the most apical initiator procaspase-8 and the effector procaspase-3 in ovarian cancer cells. The cleavage of these procaspases suggested that death receptor-signaling pathway (extrinsic) of apoptosis plays a role in the apoptotic death of ovarian cancer cells by CDDO-Me. Whether CDDO-Me increases the expression of death receptors DR4 and DR5 remains to be determined. CDDO-Me also induce the cleavage of procaspase-9 and mitochondrial depolarization, indicating that the intrinsic pathway of apoptosis is also involved in CDDO-Me-induced apoptosis. The induction of both pathways of apoptosis by CDDO-Me in ovarian cancer cells is consistent with previous reports showing similar effects of CDDOs on other tumor types (23).
Phosphatidylinositol-3 kinase/AKT (PI3K/AKT) is a major signal transduction pathway that controls cell proliferation, survival, apoptosis, and malignant transformation and it is frequently hyperactivated in most type of cancer (24). Activated AKT promotes cell growth and survival by inactivating downstream substrates such as BAD, procaspase-9, and Forkhead transcription factors. Antiapoptotic NF-κB and progrowth mTOR signaling pathways are downstream targets of activated PI3K/AKT. The NF-κB family of transcription factors controls the expression of genes involved in immune, inflammatory and oncogenic responses. It also plays a critical role in the resistance of cancer cells to anticancer therapies by protecting them from apoptosis (25). mTOR is a serine-threonine kinase, which controls cell growth, survival, ribogenesis and is a target for novel anticancer agents (26). CDDO-Me inhibited the expression of p-AKT, NF-κB and p-mTOR in OVCAR-5 and MDAH-2774 cells, showing that expression of each of the three major growth and survival-promoting signaling proteins are inhibited by CDDO-Me in ovarian cancer cells. NF-κB-regulated BCL-2, BCL-xL, cIAP1 and survivin are major antiapoptotic proteins. We conclude from these findings that prosurvival signaling proteins and antiapoptotic cellular proteins regulated by them are potential targets of CDDO-Me. This conclusion is supported by the finding that inhibition of AKT, which controls the activation of both mTOR and NF-κB, increases the sensitivity of ovarian cancer cells to CDDO-Me. Thus, CDDO-Me is a promising agent worthy of development for the treatment/prevention of ovarian cancer.
Acknowledgments
This work was supported by NIH grant R01CA130948.
References
- 1.American Cancer Society. Cancer Facts and Figures 2010. Atlanta, GA, USA: [Accessed April 14, 2011.]. [Google Scholar]
- 2.Berek JS. Ovarian Cancer. In: Berek JS, Hacker NF, editors. Practical Gynecologic Oncology. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 443–451. [Google Scholar]
- 3.Du Bois A, Neijt JP, Thigpen JT. First-line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer A new standard of care? Ann Oncol. 1999;10:35–41. doi: 10.1023/a:1008355317514. [DOI] [PubMed] [Google Scholar]
- 4.Neijt JP, Engelholm SA, Tuxen MK, Sorensen PG, Hansen M, Sessa C, de Swart CA, Hirsch FR, Lund B, van Houwelingen HC. Exploratory Phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- 5.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 6.Yu LJ, Ma RD, Wang Yq, Nishino H, Takayasu J, He WZ, Chang M, Zhen J, Liu WS, Fan SX. Potent anti-tumorigenic effect of tubeimoside 1 isolated from the bulb of Bolbostemma paniculatum (Maxim) Franquet. Int J Cancer. 1992;50:635–638. doi: 10.1002/ijc.2910500425. [DOI] [PubMed] [Google Scholar]
- 7.Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Biorg Med Chem Lett. 1998;8:2711–2714. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 8.Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Suh N, Wang Y, Sporn MB, Gribble GW. Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000;43:4233–4246. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Deeb D, Jiang H, Liu Y, Dulchavsky SA, Gautam SC. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-κB and Notch1 signaling. J Neuro Oncol. 2007;84:147–157. doi: 10.1007/s11060-007-9364-9. [DOI] [PubMed] [Google Scholar]
- 10.Deeb D, Gao X, Jiang H, Janic B, Arbab AS, Rojanasakul Y, Dulchavsky SA, Gautam SC. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem Pharmacol. 2010;79:350–360. doi: 10.1016/j.bcp.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao X, Deeb D, Arbab AS, Dulchavsky SA, Gautam SC. Synthetic triterpenoids inhibit growth, induce apoptosis and suppress Akt, mTOR and NF-κB signaling proteins in colorectal cancer cells. Anticancer Res. 2010;30:785–792. [PMC free article] [PubMed] [Google Scholar]
- 12.Konopleva M, Tsao T, Estrov Z, Lee RM, Wang RY, Jackson CE, McQueen T, Monaco G, Munsell M, Belmont J, Kantarjian H, Sporn MB, Andreeff M. The synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1,9-dien-28-oic acid induces caspase-dependent and -independent apoptosis in acute myelogenous leukemia. Cancer Res. 2004;64:7927–7935. doi: 10.1158/0008-5472.CAN-03-2402. [DOI] [PubMed] [Google Scholar]
- 13.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-κB activation through direct inhibition of IkB kinase β. Mol Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]
- 14.Konopleva M, Contractor R, Kurinna SM, Chen W, Andreeff M, Ruvolo PP. The novel triterpenoid CDDO-Me suppresses MAPK pathways and promotes p38 activation in acute myeloid leukemia cells. Leukemia. 2005;19:1350–1354. doi: 10.1038/sj.leu.2403828. [DOI] [PubMed] [Google Scholar]
- 15.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and NRF2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 16.Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, Honda T, Gribble GW, Sporn MB, Kensler TW. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 17.Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporan MB. The synthetic triterpenoid CDDO-methyl ester and CDDO-amide prevent lung cancer induced by vinyl carbomate in A/J mice. Cancer Res. 2007;67:2414–2419. doi: 10.1158/0008-5472.CAN-06-4534. [DOI] [PubMed] [Google Scholar]
- 18.Deeb D, Gao X, Liu Y, Jiang D, Divine G, Arbab SA, Dulchavsky SA, Gautam SC. Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival-signaling. Carcinogenesis. 2011;32(5):756–764. doi: 10.1093/carcin/bgr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan Z, Ames R, Ryan M, Hornicek FJ, Mankin H, Seiden MV. CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and STAT3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother Pharrmacol. 2009;63:681–689. doi: 10.1007/s00280-008-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- 22.Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science. 1998;281:305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda T, Sporn M, Honda T, Gribble GW, Kufe D. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003;63:5551–5558. [PubMed] [Google Scholar]
- 24.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 25.Mayo MW, Baldwin AS. The transcription factor NF-κB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta. 2000;1470:M55–M62. doi: 10.1016/s0304-419x(00)00002-0. [DOI] [PubMed] [Google Scholar]
- 26.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncology. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]