Abstract
We demonstrate a two-color, fiber-delivered picosecond source for coherent Raman scattering (CRS) imaging. The wavelength-tunable picosecond pump is generated by nonlinear spectral compression of a prechirped femtosecond pulse from a mode-locked titanium:sapphire (Ti:S) laser. The 1064 nm picosecond Stokes pulse is generated by an all-fiber time-lens source that is synchronized to the Ti:S laser. The pump and Stokes beams are combined in an optical fiber coupler, which serves not only as the delivery fiber but also as the nonlinear medium for spectral compression of the femtosecond pulse. CRS imaging of mouse skin is performed to demonstrate the practicality of this source.
Coherent Raman scattering (CRS) microscopy, with contrast from coherent anti-Stokes Raman scattering (CARS) [1] or stimulated Raman scattering (SRS) [2], allows label-free imaging of biological samples with endogenous image contrast based on vibrational spectroscopy. An important technical challenge in CRS microscopy is the requirement of two synchronized picosecond excitation sources. Current excitation sources for CRS include two mode-locked lasers with a phase-locked loop (PLL) and fine cavity adjustments to synchronize the pulses [3,4], optical parametric oscillators (OPOs) synchronously pumped by mode-locked lasers [2,5,6], and two-color sources based on fiber lasers and continuum generation [7], subsequent soliton self-frequency shift [8–10], or active modulation [11]. Synchronized mode-locked lasers and OPOs are bulky, costly, and environmentally sensitive, while previous fiber-based sources either had limited spectral resolution due to the broad spectral bandwidth of the femtosecond pulse [7,8] or suffer from low average power [9,10] or peak power due to the long pulse width [11].
Recently, we demonstrated a new (to our best knowledge) scheme for two-color, synchronized picosecond light sources for CRS imaging, based on the time-lens concept and its superb capability of being synchronized to any mode-locked lasers [12]. This technique also benefits from a robust all-fiber configuration and radiofrequency (RF) electronic tuning of the pulse delay for the temporal overlap between the two pulse trains. However, a picosecond wavelength-tunable source, e.g., a picosecond titanium:sapphire (Ti:S) laser, is needed for the pump due to the bandwidth limitation of CRS imaging, making the technique not applicable to a large number of labs with only femtosecond Ti:S lasers. Besides, free-space beam combining is needed for the two-color source, adding experimental complexity and significantly limiting its potential application to fiber endoscopy [13].
In this paper, we demonstrate a fiber-delivered two-color picosecond source for CARS imaging. The 817 nm pump pulse is generated by nonlinear spectral compression of a negatively prechirped pulse from a femtosecond Ti:S laser in a 2 × 2 fiber coupler. In the presence of self-phase modulation and negative prechirping, the spectrum of the pulse can be compressed by nonlinear propagation in an optical fiber [14,15]. Such a nonlinear spectral compression process retains the optical power in the original pulse, in contrast to spectral filtering. In our experiment, the 7.6 nm spectral bandwidth of the femtosecond Ti:S output is compressed to 0.68 nm, which is well suited for CRS imaging. Prechirping of the pulse is accomplished through a rotating cylindrical lens system, which offers tunable dispersion with spatial beam stability, a desirable feature for coupling light into fibers [16]. The 1064 nm Stokes pulse is generated by a synchronized, all-fiber time-lens source, and it also propagates through the 2 × 2 fiber coupler. We perform CARS imaging of mouse skin at the CH2 stretching frequency (2845 cm−1) to demonstrate the practicality of the source.
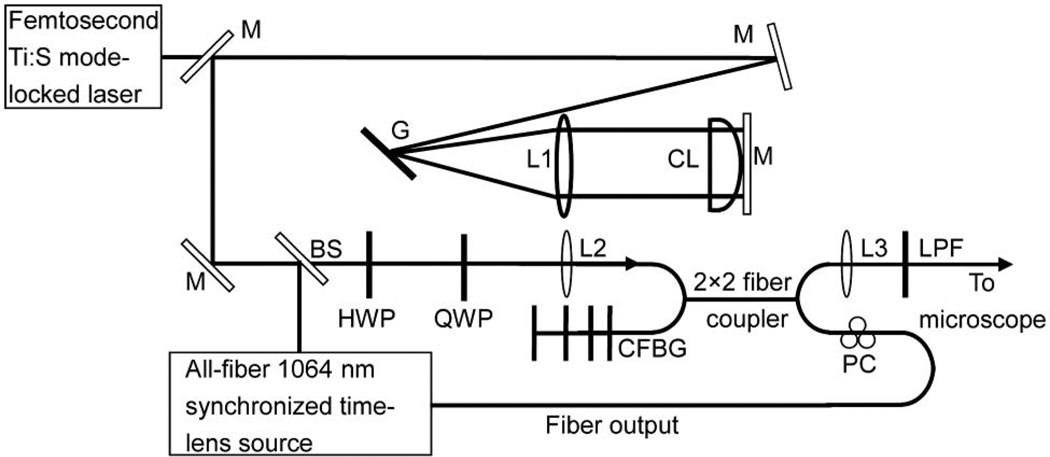
In our experiment (Fig. 1), the 817 nm femtosecond pulses are generated from a mode-locked Ti:S laser (Tsunami, Spectra-Physics). The prechirp of the femtosecond pulse is provided by a rotating cylindrical lens system in a 4f configuration, which consists of a 1800 lines/mm silver-coated grating, a f = 30 cm spherical lens, a f = 10 m concave cylindrical lens (SCX-50.8–5000.0-C, CVI Melles Griot) and a mirror. The cylindrical lens is mounted on a rotation stage for rotation in the plane perpendicular to the direction of light propagation. In our setup, for an incident angle of 44° onto the grating, the calculated maximum anomalous dispersion is −1.4 × 105 fs2 [16]. The prechirped 817 nm pulse is coupled into a 2 × 2 fiber coupler (FC1064-50B-FC, Thorlabs) for nonlinear spectral compression and fiber-optic beam combining. The total propagation length of the 817 nm pump pulse in the fiber is 107 cm. A half-wave plate and a quarter-wave plate are inserted before the coupling lens, to adjust the polarization of the output pump pulse after the fiber. The 1064 nm Stokes pulse is generated by an all-fiber time-lens source synchronized to the Ti:S laser, which has been described in detail in [12]. The RF signal for synchronization is provided by a high-speed photodiode that samples the 80 MHz Ti:S optical pulse train. A chirped fiber Bragg grating (CFBG) spliced to the fiber coupler (instead of a circulator in [12]) was used as an all-fiber dispersion compensator for the time-lens source. A polarization controller (PC) is used to adjust the polarization of the 1064 nm output from the fiber coupler, ensuring that the Stokes pulse is linearly polarized and parallel to that of the pump to maximize the CARS signal. A discrete RF delay (PDL-10A, Colby Instruments) and a continuously tunable RF delay provide a large tuning range (0.795 ns) to adjust the relative time delay between the pump and the Stokes pulses without any mechanical optical delay line. The 2 × 2 fiber coupler acts as a spatial beam combiner and delivery fiber for both the pump and the Stokes pulses, ensuring perfect spatial overlap of the two beams for CRS imaging. After collimation and an 800 nm long-pass filter (LPF), which removes the four-wave mixing background generated in the fiber coupler [17], the spatially and temporally overlapping pump and Stokes pulses are sent into a microscope (described in detail in [18]) for CARS imaging. The CARS signal is excited and collected in the epi-direction with a water immersion objective lens (XLUMPlanFl, Olympus, NA = 0.95).
Fig. 1.
Experimental setup of the fiber-delivered two-color picosecond source for CARS imaging. The 1064 nm synchronized time-lens source is described in detail in [12]: M, mirror; G, 1800 line/mm grating; L1, f = 30 cm concave lens; CL, f = 10 m concave cylindrical lens; BS, beam splitter; HWP, half-wave plate; QWP, quarter-wave plate; L2, aspheric lens; CFBG, chirped fiber Bragg grating; L3, collimating lens; LPF, 800 nm long-pass filter; PC, polarization controller.
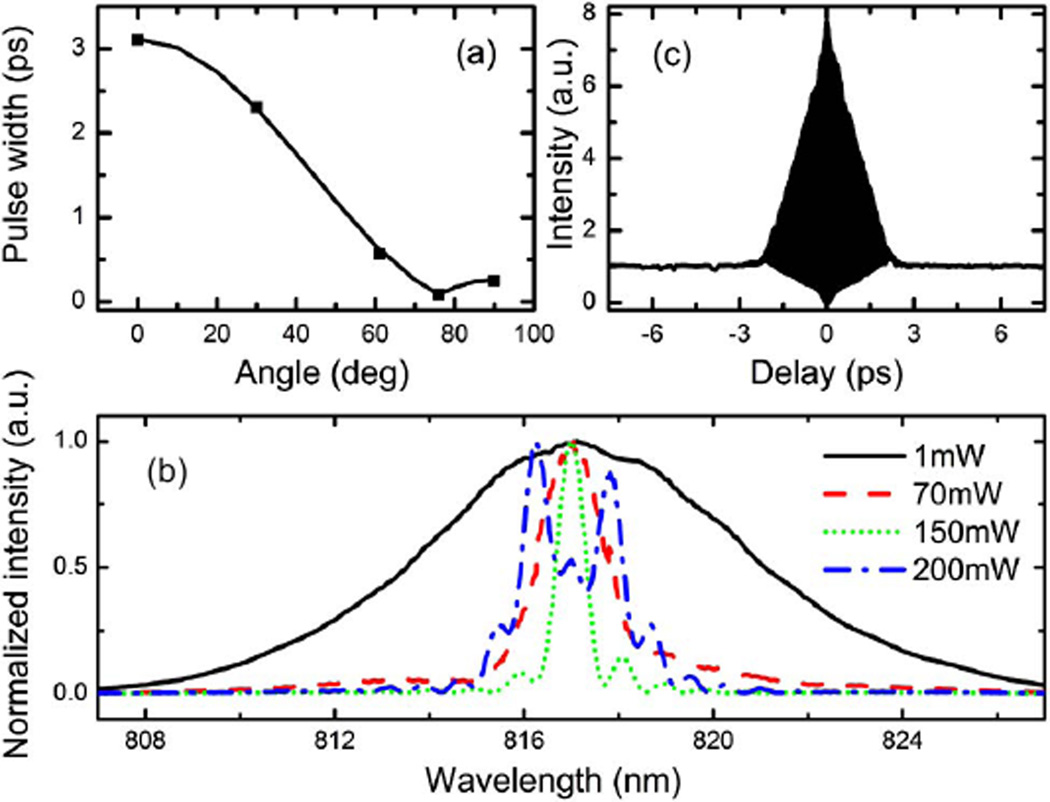
We first characterize the linear dispersion of the rotating cylindrical lens system before the fiber coupler. The pulse width as a function of the rotation angle of the cylindrical lens is measured [dots in Fig. 2(a)], and compared with theoretical calculation [solid curve in Fig. 2(a)] by assuming the maximum dispersion of −1.4 × 105 fs2 at 0°. The experimental and theoretical results agree well. Note that as the angle decreases (dispersion increases), the pulse width is compressed to its minimum at 76° due to other dispersive elements in the beam path (e.g., an optical isolator) before the cylindrical lens system. To maximize the power that can be spectrally compressed, the cylindrical lens is fixed at maximum dispersion (0°). The measured spectra of the 817 nm pulse as a function of the output power from the fiber coupler are shown in Fig. 2(b). As the power increases, the spectrum is progressively compressed and reaches its minimum of 0.68 nm at 150 mW output power [dotted curve in Fig. 2(b)]. This corresponds to 11.2 times spectral compression from that of linear propagation at low power [solid curve in Fig. 2(b)]. As the output power further increases, the spectrum broadens again [dashed–dotted curve in Fig. 2(b)] due to the maximum prechirp provided by the current system, which may be further increased by using a shorter focal length cylindrical lens. The maximum center wavelength fluctuation is below 0.1 nm. The pulse width at maximum spectral compression (i.e., at 150 mW output), deconvolved from the measured second-order interferometric autocorrelation trace [Fig. 2(c)], is 1.8 ps.
Fig. 2.
(Color online) (a) Measured (dots) and calculated (curve) pulse width as a function of the rotation angle of the cylindrical lens. (b) Measured 817 nm pump pulse spectra at various output power from the fiber coupler. (c) Measured second-order interferometric autocorrelation trace at 150 mW output power.
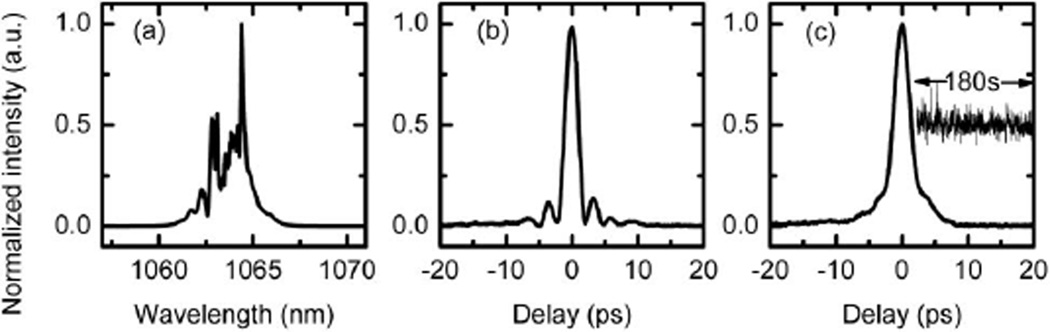
We then characterize the time-lens output and the synchronization performance. The measured spectral bandwidth (FWHM) is 1.7 nm [Fig. 3(a)]. For temporal profile characterization, we measured the cross correlation between the time-lens output and the 87 fs pulse directly from the Ti:S laser, using sum-frequency (SF) generation in a BBO crystal. The relative delay between the pulses is readily scanned by tuning the electronic RF delay line. Figure 3(b) shows that the pulse width of the time-lens output is 2.2 ps. The cross correlation between the time-lens output and the 1.8 ps spectrally compressed 817 nm pulse is shown in Fig. 3(c). The FWHM is 2.9 ps. To measure the relative timing jitter between the pump and Stokes pulses, we record the SF intensity fluctuation at the half-maximum of the cross-correlation trace [inset in Fig. 3(c)] [3,12]. The RMS timing jitter over a measurement time of 180s is 110 fs, only a small fraction of the pulse widths. The measured output power of the 1064 nm Stokes pulse is 110 mW.
Fig. 3.
(a) Measured spectrum of the time-lens output. Cross-correlation traces of the time-lens output with an 87 fs pulse (b) and the 150 mW, 1.8 ps pulse from the fiber coupler (c). (c) Inset, measured SF signal at the half-maximum of the cross-correlation trace over 180 s.
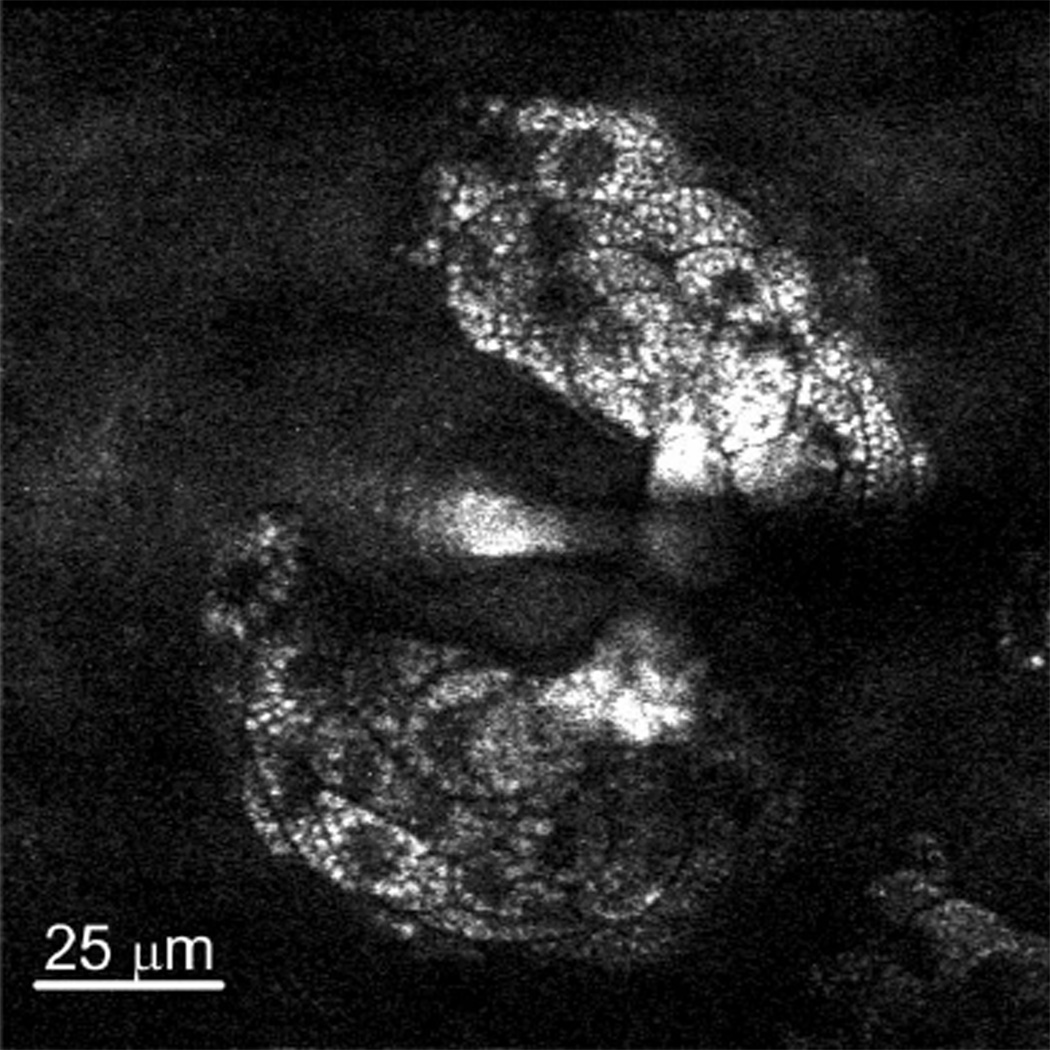
We perform CARS imaging of sebaceous glands at the CH2 stretching frequency in ex vivo mouse ear. Figure 4 highlights the practicality of the two-color, fiber-delivered picosecond source for CARS imaging. While CRS imaging of the CH2 bond is demonstrated, the wavelength tunability of the femtosecond Ti:S laser ensures that a wide range of stretching bands can be covered by the two-color source. Wavelength tuning can be easily accomplished through rotating the grating, while no other realignment is needed. For example, to excite the polyene stretching frequency at 1600 cm−1 by tuning the Ti:S laser to 909 nm [12], the grating is rotated by 7.6° and the corresponding incident angle is 51.6°.
Fig. 4.
CARS image of sebaceous glands at the CH2 stretching frequency in mouse ear: 512 × 512 pixels, 4 s/frame, no average.
In conclusion, we have demonstrated a fiber-delivered two-color, picosecond source for CARS imaging. A 2 × 2 fiber coupler spectrally compresses the pump pulse from a mode-locked femtosecond laser and spatially combines the pump and the Stokes pulses, the latter of which is generated by a synchronized, all-fiber time-lens source. The temporal overlap of the two pulses is electronically adjusted without any mechanical optical delay line, greatly facilitating the temporal alignment of the excitation beams for CRS imaging. Mouse skin imaging at the CH2 stretching frequency (2845 cm−1) is performed to demonstrate the practicality of this source. The combination of the all-fiber time-lens source and the nonlinear spectral compression of a femtosecond source in an optical fiber has the potential to make CRS imaging easily accessible to any researcher with a wavelength-tunable femtosecond source.
Acknowledgments
This research project is supported by the National Institutes of Health (NIH) under contract R21RR032392. We acknowledge X. S. Xie and C. W. Freudiger from Harvard University for valuable discussion of CARS imaging.
References
- 1.Zumbusch A, Holtom GR, Xie XS. Phys. Rev. Lett. 1999;82:4142. [Google Scholar]
- 2.Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, Tsai JC, Kang JX, Xie XS. Science. 2008;322:1857. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potma EO, Jones DJ, Cheng JX, Xie XS, Ye J. Opt. Lett. 2002;27:1168. doi: 10.1364/ol.27.001168. [DOI] [PubMed] [Google Scholar]
- 4.Ozeki Y, Kitagawa Y, Sumimura K, Nishizawa N, Umemura W, Kajiyama S, Fukui K, Itoh K. Opt. Express. 2010;18:13708. doi: 10.1364/OE.18.013708. [DOI] [PubMed] [Google Scholar]
- 5.Kieu K, Saar BG, Holtom GR, Xie XS, Wise FW. Opt. Lett. 2009;34:2051. doi: 10.1364/ol.34.002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Wang H, Slipchenko MN, Jung Y, Shi Y, Zhu J, Buhman KK, Cheng JX. Opt. Express. 2009;17:1282. doi: 10.1364/oe.17.001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svedberg F, Brackmann C, Hellerer T, Enejder AJ, Biomed J. Opt. 2010;15:026026. doi: 10.1117/1.3374039. [DOI] [PubMed] [Google Scholar]
- 8.Pegoraro AF, Ridsdale A, Moffatt DJ, Pezacki JP, Thomas BK, Fu L, Dong L, Fermann ME, Stolow A. Opt. Express. 2009;17:20700. doi: 10.1364/OE.17.020700. [DOI] [PubMed] [Google Scholar]
- 9.Krauss G, Hanke T, Sell A, Träutlein D, Leitenstorfer A, Selm R, Winterhalder M, Zumbusch A. Opt. Lett. 2009;34:2847. doi: 10.1364/OL.34.002847. [DOI] [PubMed] [Google Scholar]
- 10.Andresen ER, Berto P, Rigneault H. Opt. Lett. 2011;36:2387. doi: 10.1364/OL.36.002387. [DOI] [PubMed] [Google Scholar]
- 11.Bégin S, Burgoyne B, Mercier V, Villeneuve A, Vallée R, Côté D. Biomed. Opt. Express. 2011;2:1296. doi: 10.1364/BOE.2.001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Freudiger CW, Lee JH, Saar BG, Xie XS, Xu C. Opt. Express. 2010;18:24019. doi: 10.1364/OE.18.024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saar BG, Johnston RS, Freudiger CW, Xie XS, Seibel EJ. Opt. Lett. 2011;36:2396. doi: 10.1364/OL.36.002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberthaler M, Hopfel RA. Appl. Phys. Lett. 1993;63:1017. [Google Scholar]
- 15.Andresen ER, Dudley JM, Oron D, Finot C, Rigneault H. Opt. Lett. 2011;36:707. doi: 10.1364/OL.36.000707. [DOI] [PubMed] [Google Scholar]
- 16.Durst ME, Kobat D, Xu C. Opt. Lett. 2009;34:1195. doi: 10.1364/ol.34.001195. [DOI] [PubMed] [Google Scholar]
- 17.Balu M, Liu G, Chen Z, Tromberg BJ, Potma EO. Opt. Express. 2010;18:2380. doi: 10.1364/OE.18.002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobat D, Durst ME, Nishimura N, Wong AW, Schaffer CB, Xu C. Opt. Express. 2009;17:13354. doi: 10.1364/oe.17.013354. [DOI] [PubMed] [Google Scholar]