Abstract
Rebound insomnia, worsened sleep when discontinuing use of a hypnotic, is reported in some short-term studies. No study has prospectively assessed, using patient reports or nocturnal polysomnography (NPSG), the likelihood of rebound insomnia with chronic hypnotic use. The objectives of this study was to assess in primary insomniacs the likelihood of experiencing rebound insomnia and a withdrawal syndrome on repeated placebo substitutions over 12 months of nightly zolpidem use. A group of 33 primary insomniacs, without psychiatric disorders or drug and alcohol abuse, 32–65 years old, 15 men and 18 women, were randomized to take zolpidem 10 mg (n = 17) or placebo (n = 16) nightly for 12 months. In probes during months 1, 4, and 12, placebo was substituted for 7 consecutive nights in both the zolpidem and placebo groups. NPSGs were collected and Tyrer Bezodiazepine Withdrawal Symptom Questionnaires were completed on the first two discontinuation nights. Rebound insomnia was not observed on the first two and the seventh discontinuation nights and its likelihood did not increase over the 12 months of nightly zolpidem use. Some individuals did show rebound insomnia, approximately 30–40% of participants, but the percentage of ‘rebounders’ did not differ between the placebo and zolpidem groups and did not increase across 12 months. No clinically significant withdrawal symptoms on the Tyrer were observed on the discontinuation nights over the 12 months of nightly use. Chronic nightly hypnotic use at therapeutic doses by primary insomniacs does not lead to rebound insomnia or withdrawal symptoms.
Keywords: Chronic nightly use, rebound insomnia, withdrawal syndrome, zolpidem
Introduction
Rebound insomnia is defined as worsened sleep relative to baseline when discontinuing a hypnotic (Kales and Scharf, 1978). It was first reported in discontinuing the benzodiazepine hypnotic triazolam and was found to last for one or two nights after abrupt discontinuation (Kales and Scharf, 1978). Rebound insomnia is more likely at higher doses; it was seen with a 0.50 mg dose of triazolam, but not a 0.25 mg dose (Roehrs et al., 1986, 1990). The 0.50 mg dose (e.g. a high dose above the therapeutic dose) provided no greater hypnotic efficacy than the 0.25 mg dose, but did show rebound insomnia. It has been reported with most short- and intermediate-acting hypnotic benzodiazepines using nocturnal polysomnographic (NPSG) or self-report measures of sleep, but not with long-acting drugs due to the gradual decline in plasma concentration inherent in their pharmacokinetics (Kales et al., 1986; Roehrs et al., 1990).
The literature on rebound insomnia after discontinuation of clinical doses of non-benzodiazepine (i.e. zolpidem, zopiclone, zaleplon, eszopiclone), benzodiazepine receptor agonist hypnotics (BzRAs) is mixed. Rebound in NPSG-defined sleep was found on the first night of discontinuation following one month of nightly 0.5 mg triazolam, but not 10 mg zolpidem (Ware et al., 1997). However, it should be noted that these are not equipotent doses and rebound insomnia, as noted above is dose dependent. The 10 mg zolpidem dose is equipotent to 0.25 mg triazolam and 20 mg zolpidem to 0.50 mg triazolam (Roehrs et al., 1994). A three-week NPSG study found a one-night worsening of latency to persistent sleep (LPS), wake after sleep onset (WASO) and total sleep time (TST) after discontinuing a 12.5 mg modified release formulation of zolpidem versus placebo (Roth et al., 2006). In another study TST and sleep efficiency (SE) were worsened on the first night after discontinuing one month of triazolam 0.25 mg, but not 7.5 mg zopiclone or 10 mg zolpidem (Voderholzer et al., 2002).
Studies have reported that rebound insomnia, when shown, is not present in all insomniacs. One night of worsened, self-reported sleep was found in 20% of patients after two weeks of zolpidem 10 mg, but not gaboxadol 5, 10, or 15 mg (Hajak et al., 2009). Self-ratings on visual analogue scales of sleep latency, nocturnal awakenings and total sleep time were done during a no-pill discontinuation after a 28-night treatment of 0.25 mg triazolam, 7.5 mg zopiclone, 1.0 mg flunitrazepam or placebo (Hajak et al., 1998). Rebound insomnia, defined as worsened ratings relative to pre-treatment in at least one of the three parameters, appeared in 22% of patients overall. The overall rate was higher in the placebo group than any of the active treatment groups and those patients who did not respond to treatment had higher rebound rates than responders. The observation of rebound insomnia following a no-pill discontinuation in placebo-treated patients replicates an earlier one-week NPSG study that found rebound after a no-pill discontinuation in the placebo group (Roehrs et al., 1992).
The relation of rebound insomnia to a withdrawal syndrome is unclear. Rebound insomnia is the exacerbation of a patient’s original symptom, while a withdrawal syndrome is the expression of a new symptom complex. In short-term studies in which rebound was induced, no new symptoms, beyond sleep disturbance, were observed (Roehrs et al., 1990). However, animal studies have demonstrated physical dependence with benzodiazepines and in patients chronic use of therapeutic doses of anxiolytics is associated with reports of withdrawal signs and symptoms when discontinued (Griffiths and Johnson, 2005). All of the previous rebound insomnia studies of hypnotics have been short-term studies. Whether rebound insomnia will develop with chronic use of clinical doses of a hypnotic and whether it will develop into a complete withdrawal syndrome after discontinuation of the chronic use is unknown. The present study was designed to address these issues.
This prospective, double-blind, study of primary insomniacs assessed the likelihood of rebound insomnia during 12 months of nightly use of zolpidem versus placebo. The presence of rebound insomnia was assessed in months 1, 4, and 12 of this double-blind, placebo-controlled trial by substituting placebo in the zolpidem group for seven nights and collecting NPSGs on the first two and seventh nights. The presence of withdrawal symptoms on the first two placebo substitution nights was assessed with the Tyrer Benzodiazepine Withdrawal Symptom Questionnaire (Tyrer et al., 1990).
Method
Participants
People between 21 and 70 years old with difficulty falling asleep and/or staying asleep were recruited through newspaper advertisements. The study was completed by 33 individuals, 15 men and 18 women, aged 32–64 years, meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM IV-TR) criteria for primary insomnia (see Table 1 for participant demographics). After signing the consent and receiving at least one night of medication 25 discontinued the study, 15 from the placebo group and 10 from the zolpidem group (see Table 2). The majority of participant loss occurred in months 1–4. The major reasons for discontinuation were non-compliance and scheduling conflicts. All were in good physical and psychiatric health based on medical, psychiatric, drug use history and physical examination, as well as, screening blood and urine laboratory analyses (see below). The Institutional Review Board of the Henry Ford Health System approved the study protocol. All provided informed consent and were paid for participation.
Table 1.
Demographics and drug use history of study groups.
| Placebo | Zolpidem | ||
|---|---|---|---|
| N | 17 | 16 | |
| Average age (range) | 52 (34–70) | 51.5 (23–68) | |
| Gender | Males: 8 | Males: 7 | |
| Females: 9 | Females: 9 | ||
| Insomnia measures | |||
| Reported sleep time (hrs) | 5.76±0.26 | 5.56±0.34 | |
| ±SD | 6.0 | 5.75 | |
| NPSG sleep time (hrs) mean ±SD; | 5.82±0.19 | 6.03±0.17 | |
| median | 5.90 | 6.14 | |
| LPS NPSG (min)±SD | 45.25±10.45 | 38.44±7.92 | |
| (median; range) | (32.5; 6–169) | (27; 6.5–114.5) | |
| WASO NPSG (min) ±SD | 106.66±10.80 | 98.18±8.37 | |
| (range) | (111; 38.5–185.5) | (96.5; 37.5–171) | |
| Age of insomnia onset | 40.67 | 35.77 | |
| (median; range) | (41; 20–60) | (35; 12–55) | |
| Duration of insomnia (yrs) | 11.47 | 18.66 | |
| (median; range) | (8; 1–31) | (11.5; 3–43) | |
| Self-reported alcohol and drug consumption | |||
| Alcohol beverages (No. drinks/week: No. self-reporting) | 0–1: 11 | 0–1: 9 | |
| 2–6: 3 | 2–6: 5 | ||
| 7–14: 2 | 7–14: 1 | ||
| 16: 1 | 16: 1 | ||
| Caffeinated products (No. drinks/week: No. self-reporting) |
0–1: 2 | 0–1: 5 | |
| 2–6: 4 | 2–6: 5 | ||
| 7–14: 6 | 7–14: 1 | ||
| 15 or more: 5 | 15 or more: 5 | ||
| Previous illicit drug history | No drug history: 12 | No drug history: 13 | |
| Marijuana use | Marijuana use | ||
| ≥2 yrs ago: 2 | ≥2yrs ago: 0 | ||
| Marijuana use | Marijuana use | ||
| ≥20 yrs ago: 3 | ≥20 yrs ago: 3 | ||
| Current nicotine usage | Nonsmokers: 15 | Nonsmokers: 15 | |
| ≤15 cigarettes/day: 2 | ≤10 cigarettes/day: 1 | ||
Table 2.
Participants discontinuing after consent and one night of medication.
| Placebo |
Zolpidem |
||||
|---|---|---|---|---|---|
| Number | Month of withdrawal | Reason | Number | Month of withdrawal | Reason |
| 3 | Month 1 (Night 2) | Dislike electrodes, skin irritation | 2 | Month 1 (Night 2) | Caffeine withdrawal symptoms |
| Life events | Month 1 | Anxiety symptoms/sense of dread |
|||
| Noncompliant | |||||
| 5 | Month 1 (Nights 8–9) | Noncompliant (3) | |||
| Scheduling conflicts | 2 | Month 2 | Scheduling conflicts (2) | ||
| Life events | |||||
| 4 | Month 2 | Heart attack | 2 | Month 3 | Scheduling conflicts |
| No longer interested | 1 | Month 4 | Scheduling conflicts | ||
| Noncompliant (2) | |||||
| 1 | Month 3 | Noncompliant | 1 | Month 5 | Scheduling conflicts |
| 2 | Month 4 | Scheduling conflicts (2) | 2 | Month 6 | Scheduling conflicts |
| Job moved out of state | |||||
Life events: personal and/or family problems; Scheduling conflicts: inability to coordinate research study into work, family, school, and/or social life.
Experimental design
This was a mixed design, double-blind, placebo-controlled, study with a between group comparison of primary insomniacs randomly assigned to use zolpidem 10 mg (5 mg for those >60 years) or placebo nightly for 12 months. During the study, participants returned to the laboratory for a one week placebo discontinuation in months 1, 4, and 12, which constituted the within subjects variable. NPSGs were collected and the Tyrer Bezodiazepine Withdrawal Symptom Questionnaire completed on the first two nights.
Procedures
General health and psychiatric screening
Respondents to advertisements for persons ‘with difficulty falling asleep and/or staying asleep’ were interviewed by telephone regarding their insomnia, general health, past and current psychiatric, alcohol, and drug abuse histories. Those passing the telephone screen were scheduled for a clinic visit during which they gave informed consent, provided a medical and drug-taking history, including prescribed medications, legal and/or illegal recreational drugs, underwent a physical examination, and provided blood and urine samples. Those with any acute or unstable illness including conditions making it unsafe for the person to participate, conditions with a potential to impact sleep (i.e. acute pain, respiratory disorders), and conditions which could interact with the pharmacokinetics or pharmacodynamics (e.g. moderate to severe liver disease, heavy smoking) of zolpidem were excluded, as were those with chronic illnesses including renal failure, liver disease, seizures, and dementing illnesses. Participants with current psychiatric disorders, anxiety, depression, and schizophrenia, as identified by the Structured Clinical Interview for DSM-IV-TR (SCID) were also excluded.
The blood and urine panel also included testing for drugs including opiates, benzodiazepines, and stimulants. Participants with a history of substance (drug or alcohol) use disorders or current use of central nervous system acting medications at screening were excluded. Participants reporting any use of illegal drugs within the past 2 years also were excluded. Participants who reported consuming >14 standard (1oz) alcoholic drinks per week, caffeine consumption >300 mg/day, and smoking during the night (11pm–7am) were excluded (see Table 1 for drug use histories).
Sleep disorders screen
Each participant underwent a sleep-wake history, sleep disorders evaluation, and NPSG. Each qualified for a DSM-IVR diagnosis of primary insomnia. As part of the sleep-wake history, participants completed a two-week sleep diary, which was used to determine their habitual bedtime and the screening NPSG bedtime. For the screening NPSG bedtime, the midpoint of their bedtime reported on the two-week sleep diary was determined and 4 hours were added to each side of the diary-reported bedtime midpoint to create an 8 hour bedtime, which would not disrupt circadian rhythms.
After the screening physical and laboratory tests, the participants underwent the screening 8-hour NPSG. In addition to the clinical DSM-IVR primary insomnia diagnosis, all participants were also required to show sleep efficiencies of ≤85% (total sleep time/time in bed) and no other primary sleep disorders on a single night of NPSG. The additional NPSG sleep efficiency criterion was used because in our previous short-term study insomniacs with objective sleep disturbance were those at greatest risk for hypnotic self-administration (Roehrs et al., 1992). Participants with respiratory disturbances (apnea hypopnea index [AHI] >10) or leg movements (periodic limb movement arousal index [PLMAI] >5) were excluded from the study.
The standard Rechtschaffen and Kales (1968) methods for recording of sleep were used. The NPSGs obtained included standard central (C3–A2) and occipital (Oz–A2) electroencephalograms (EEGs), bilateral horizontal electrooculograms (EOG), submental electromyogram (EMG), and electrocardiogram (ECG) recorded with a V5 lead. In addition, on the screening night airflow was monitored with oral and nasal thermistors and leg movements were monitored with electrodes placed over the left tibialis muscles; respiration and tibialis EMG recordings were scored for apnea and leg movement events and event frequencies were tabulated (Coleman 1982; Bornstein 1982). Those with ≥10 sleep disordered breathing events or ≥10 periodic leg movements with EEG arousals per hour of sleep were excluded. After screening, subsequent NPSGs excluded airflow and leg monitoring. All NPSGs were scored in 30-sec epochs according to the standards of Rechtschaffen and Kales (1968). Scorers maintained 90% inter-rater reliability.
Medication preparation
Zolpidem 10 mg, or 5 mg for participants >60 years old, and placebo was prepared by the HFHS Research Pharmacy in size No. 1 capsules with lactose added. Participants were instructed to take their designated medication nightly 30 min before bedtime. Capsules were dispensed at monthly clinic visits.
Weekly telephone monitoring
Using an automated voice interactive telephone system participants were instructed to call an 800 telephone number each week on a set day. Failure to call on the set day was followed with a staff-initiated call. These weekly calls were a 10 item questionnaire regarding the participant’s sleep the past week, which included a question about the number of capsules taken the past week. These reports were confirmed with capsule counts taken at the monthly clinic visits.
Rebound insomnia assessments
For the rebound insomnia assessments of months 1, 4, and 12 each participant spent 2 consecutive nights in the sleep laboratory, the next 4 nights at home and a final night in the laboratory (a total of 7 nights). Participants in the active drug group received placebo and those in the placebo group remained on placebo. Eight-hour NPSGs were collected on the three laboratory nights. The primary dependent measure for assessment of rebound insomnia was sleep efficiency (SE: sleep time/time in bed) and the secondary measures were latency to persistent sleep (LPS: 10 min of continuous sleep), and wake after sleep onset (WASO: min of wake after LPS).
Withdrawal syndrome assessments
On the placebo substitution nights of months 1, 4, and 12 the Tyrer Bezodiazepine Withdrawal Symptom Questionnaire, a 20 symptom rating scale was administered each morning after arising (Tyrer et al., 1990). Each symptom was rated on a scale of: 0, no; 1, yes moderate; or 2, yes severe. A total score ≥20 is considered clinically significant (Tyrer et al., 1990).
Statistical analyses
The self-reports of the number of capsules taken while at home (only one per night) were compiled for each month and expressed as a percentage of the number of nights per month. These compliance data were compared between groups with a chi square. For each of the month 1, 4, and 12 rebound assessments the three primary and secondary sleep outcome variables were used. We powered this study based on our earlier studies assessing rebound as a function of dose (Roehrs et al., 1986) and of the duration of administration (Merlotti et al., 1991) in which sample sizes were n = 12 on active drug. Those studies showed rebound with powers of .93 and greater. The present study was powered to detect an increase in wake time of 10 min and greater with a power of .80 in sample sizes of n = 15 per group. Mixed design multivariate analysis of variance (MANOVAs) were used to compare groups with nights 1, 2, and 7 and months 1, 4, and 12 as two repeated within group variables. The total Tyrer scores were analyzed with similar mixed design MANOVAs.
Results
The demographics, insomnia history, and drug use history of the study groups are presented in Table 1. The groups did not differ in age and sex and in self-reported or NPSG-defined sleep times. Their self-reported sleep times were consistent with their laboratory screening NPSG-defined sleep times. They reported, and showed on NPSG, both sleep onset and maintenance problems. While the zolpidem group reported an earlier age of onset and longer duration of insomnia symptoms, because of the variability in each group, the groups did not differ statistically. The groups had similar alcohol and drug use histories.
Table 3 presents the monthly medication compliance data for the groups. On weekly telephone interviews, done during the non-laboratory weeks, participants reported taking between 73% and 89% of the single nightly capsules each month while at home. The percentages of capsules taken did not vary systematically over the 12 study months and the groups did not differ in the average percentage of capsules used over the 12 months (placebo: 81 ± 0.04% vs zolpidem: 84 ± 0.03%).
Table 3.
Treatment compliance of study groups by months.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 76 | 85 | 82 | 78 | 85 | 73 | 79 | 81 | 82 | 82 | 87 | 85 |
| Zolpidem | 81 | 83 | 88 | 85 | 82 | 77 | 87 | 80 | 84 | 89 | 82 | 83 |
| Total | 79 | 84 | 85 | 82 | 83 | 75 | 84 | 80 | 84 | 86 | 84 | 84 |
Percentage of nights the study medication was taken while sleeping at home as compiled by month from the weekly phone interviews
The three outcome variables for assessment of rebound insomnia on the three discontinuation nights in months 1, 4, and 12 for the placebo and zolpidem groups are presented in Table 4. As seen in Table 4, mean total sleep time on all discontinuation nights in both groups was never less than that of the screening total sleep time. Similarly, latency to persistent sleep did not increase above that of the screen night and wake after sleep onset did not increase above that of the screening night. As is also seen, these three measures did not worsen from month 1 to month 12.
Table 4.
Sleep measures on screening and the three discontinuation nights ofmonths 1, 4, and 12.
| Placebo |
Zolpidem |
|||||||
|---|---|---|---|---|---|---|---|---|
| Scrn | Discon1 | Discon2 | Discon3 | Scrn | Discon1 | Discon2 | Discon3 | |
| Month 1 | ||||||||
| TST | 350.80 (44.87) |
385.1 (47.17) |
361.3 (83.48) |
383.5 (66.20) |
358.2 (50.47) |
380.0 (66.29) |
408.0 (38.75) |
396.0 (53.81) |
| LPS | 42.65 (38.85) |
19.03 (19.37) |
26.80 (30.63) |
30.38 (31.22) |
45.18 (37.95) |
25.53 (24.58) |
21.94 (12.89) |
15.25 (15.18) |
| WASO | 104.9 (45.77) |
81.65 (44.27) |
82.60 (42.34) |
80.41 (51.70) |
100.9 (40.88) |
80.68 (55.56) |
58.00 (35.57) |
74.47 (55.17) |
| Month 4 | ||||||||
| TST | 350.8 (44.87) |
359.37 (75.98) |
355.6 (80.45) |
382.03 (47.18) |
358.2 (50.47) |
384.6 (58.55) |
407.9 (439.79) |
395.91 (76.05) |
| LPS | 42.65 (38.85) |
31.53 (29.97) |
34.60 (43.07) |
47.81 (104.89) |
45.18 (37.95) |
29.00 (22.73) |
28.22 (30.23) |
39.81 (61.15) |
| WASO | 104.9 (45.77) |
97.53 (56.17) |
106.9 (79.73) |
84.44 (49.49) |
100.9 (40.88) |
82.22 (50.90) |
49.78 (24.12) |
56.66 (47.58) |
| Month 12 | ||||||||
| TST | 350.8 (44.87) |
376.1 (71.16) |
374.8 (54.24) |
355 (90.20) |
358.2 (50.47) |
373.03 (67.27) |
377.5 (66.45) |
414.56 (42.53) |
| LPS | 42.65 (38.85) |
36.85 (65.79) |
25.59 (26.80) |
37.03 (45.92) |
45.18 (37.95) |
29.59 (39.02) |
26.53 (31.01) |
17.34 (13.18) |
| WASO | 104.9 (45.77) |
81.09 (51.76) |
88.68 (40.30) |
100.49 (73.33) |
100.9 (40.88) |
94.16 (59.92) |
80.18 (56.74) |
54.66 (36.80) |
Data means (SD) in minutes. For comparison purposes the screening data shown for month 1 are repeated at month 4 and 12. Comparing across columns within a row, total sleep times less than screening and latency to persistent sleep and wake during sleep times greater than screening would reflect rebound insomnia. Discon, discontinuation night; LPS, latency to persistent sleep; Scrn, screen night; TST, total sleep time; WASO, wake after sleep onset.
Interestingly, there was a significant improvement in total sleep time, latency to persistent sleep, and wake after sleep onset on the seventh discontinuation night in the zolpidem group compared with the placebo group (see Table 4). The improvement on each measure appeared as a trend (a triple interaction: p <0.07) on the omnibus three factor MANOVAs (group, months, and nights). Separate two factor MANOVAs (groups and nights) for the data of months 1, 4, and 12 revealed a significant improvement on night 7 for each measure in month 12 only (nights by group interaction: p <0.01). Sleep time was increased, and latency to persistent sleep and wake after sleep onset were both reduced on month 12, night 7 in the zolpidem group relative to the placebo group.
Table 5 presents the three outcome variables on the screen and discontinuation nights in months 1 and 4 for the participants that withdrew from the study. Nineteen participants remained in the study through the month 1 assessment and of these, 5 continued through the month 4 assessment. There was no differential participant withdrawal from the placebo versus zolpidem groups. As for the study completers, there was no worsening of any of the outcome variables relative to the screening night in the withdrawn participants.
Table 5.
Discontinuation nights of withdrawn participants at months 1 and 4.
| Placebo (N=10) |
Zolpidem (N=9) |
|||||
|---|---|---|---|---|---|---|
| Month 1 | Scrn | Discon1* | Discon2 (N=8) | Scrn | Discon1 | Discon2 |
| TST | 380.05 (36.04) |
384.50 (84.47) |
404.38 (22.64) |
404.56 (60.29) |
378.56 (45.62) |
407.00 (35.98) |
| LPS | 26.90 (28.27) |
50.44 (51.37) |
51.50 (77.51) |
33.28 (17.89) |
27.44 (31.85) |
23.056 (15.94) |
| WASO | 79.95 (31.22) |
63.438 (63.09) |
31.50 (10.55) |
88.89 (51.08) |
78.67 (50.65 |
59.72 (33.79) |
| Placebo (N=3) |
Placebo (N=2) |
|||||
|---|---|---|---|---|---|---|
| Month 4 | Scrn | Discon1 | Discon2 | Scrn | Discon1 | Discon2 |
| TST | 380.05 (36.04) |
341.33 (95.53) |
367.00 (116.32) |
404.56 (60.29) |
397.50 (2.12) |
421.50 (3.54) |
| LPS | 26.90 (28.27) |
17.67 (14.29) |
26.17 (13.38) |
33.28 (17.89) |
11.25 (12.37) |
4.75 (3.89) |
| WASO | 79.95 (31.22) |
125.00 (102.98) |
97.67 (111.90) |
88.89 (51.08) |
77.00 (10.61) |
56.00 (4.95) |
Data means (SD) in minutes. *indicates the withdrawal of 2 participants prior to discon2, both due to noncompliance. Discon, discontinuation night; LPS, latency to persistent sleep; Scrn, screen night; TST, total sleep time; WASO, wake after sleep onset.
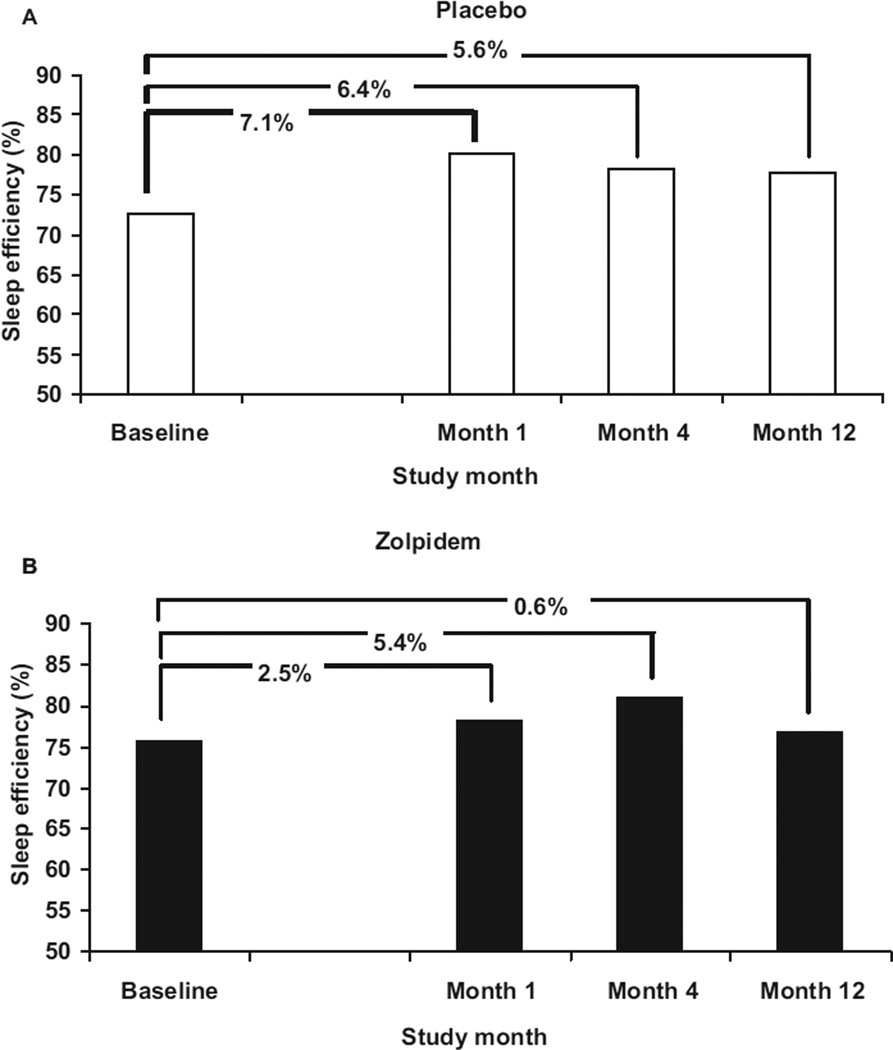
Each subject’s change in sleep efficiency (total sleep time/time in bed) relative to their screening sleep efficiency was calculated for the first discontinuation night of months 1, 4, and 12 (month ‘x’ minus screening). In Figure 1, the mean sleep efficiency and change in sleep efficiency is presented for the placebo (Panel A) and the zolpidem (Panel B) groups. A negative change score would reflect rebound insomnia. Change scores in month 1 did not differ between placebo and zolpidem groups (+7.1% vs +2.5%), month 4 (+6.4% vs +5.4%), and month 12 (+5.6% vs +0.6%) (overall p = 0.492).
Figure 1.
Sleep efficiency (total sleep time/time in bed) at baseline and on the first discontinuation night in months 1, 4, and 12 for the placebo and zolpidem groups. Values are the difference scores versus screening (month ′x′ minus screening). A negative score would reflect rebound insomnia.
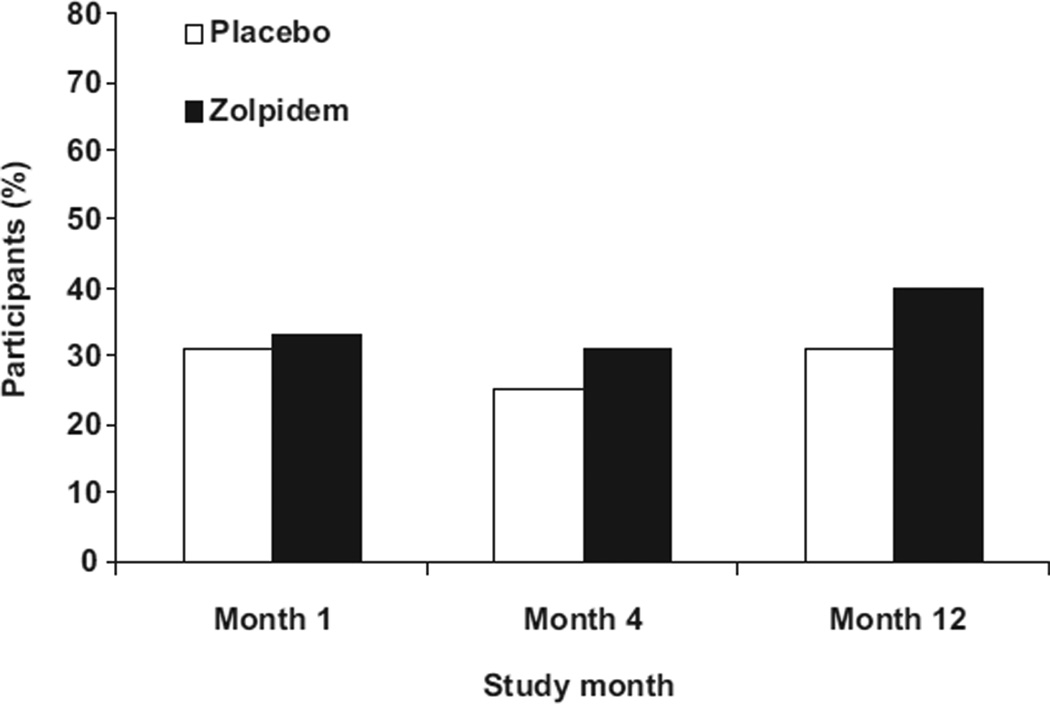
Some subjects in each group did show rebound insomnia and Figure 2 presents the percent of subjects who slept more poorly (night ‘x’ month ‘x’ total sleep time > screening total sleep time) on the discontinuation night than the screening night. The groups did not differ in the percent of subjects showing rebound insomnia at any time point (month 1: 31% vs 33%; month 4: 25% vs 31%; month 12: 31% vs 40%) (overall p = 0.85). The percentages did not vary significantly from month 1 to 12.
Figure 2.
Percentage of participants whose sleep efficiency (total sleep time/time in bed) was worse than screening on the first discontinuation night of month 1, 4, and 12.
The total Tyrer scores on nights 1 and 2 of month 1, 4, and 12 for the placebo and zolpidem groups are presented in Table 6. At none of the time points did the mean score approach the clinically significant score of ≥20, no single subject had a total score >10 on any one of the nights, and 96% of the scores for a given participant on a given night were <5. Overall the scores did not differ between the zolpidem and placebo groups or between night 1 and night 2 (NS: main effects of groups and nights). There was a main effect of months (F = 9.73, p <0.001) with the month 12 score across both treatment groups being greater than month 4 and month 4 greater than month 1. There also was a significant month by group interaction (F = 5.45, p <0.005) with the zolpidem group score being greater than that of the placebo group in month 4, but not month 1 or month 12. Separate analyses comparing groups on each of the 20 symptoms during month 4 showed significantly higher ratings of ‘sore eyes’ on night 1 and ‘sensitivity to light’ on night 2 in the zolpidem group compared with the placebo group. There were no other group differences on any of the remaining 19 symptoms on night 1 or 2.
Table 6.
Total Tyrer Withdrawal Scores on the two discontinuation nights of months 1, 4, and 12 for placebo and zolpidem groups.
| Discontinuation night | Placebo |
Zolpidem |
||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| Month 1 | 1.15 (1.13) | 1.10 (1.17) | 1.75 (2.29) | 1.40 (1.23) |
| Month 4 | 1.15 (1.42) | 0.90 (1.07) | 2.65 (2.94) | 1.95 (2.26) |
| Month 12 | 2.55 (1.85) | 1.25 (1.52) | 2.30 (2.68) | 2.10 (2.25) |
Data are means (SD).
Discussion
To our knowledge this is the first NPSG study to have assessed rebound insomnia repeatedly in the same participants and over 12 months of nightly use of a BzRA hypnotic. No rebound insomnia was observed on either of the discontinuation nights and the likelihood of rebound insomnia did not increase relative to placebo over the 12 months of nightly zolpidem use. Some individual insomniacs did show rebound insomnia on the discontinuation nights, approximately 30–40% of participants. But the percentage of ‘rebounders’ did not differ between the placebo and zolpidem groups and did not increase systematically across the 12 months. The results of this study also showed that no clinically significant withdrawal symptoms were observed on the discontinuation nights over the 12 months of nightly use.
The results of this study confirm the short-term studies that have failed to find rebound insomnia with a 10 mg dose of zolpidem (Ware et al., 1997; Voderholzer et al., 2002). The one study that reported rebound insomnia with zolpidem utilized an extended release preparation which is a total 12.5 mg dose (Roth et al., 2006). This again supports the finding that dose is critical to the risk of rebound insomnia. Our earlier studies with triazolam found rebound at a 0.50 mg dose, but not the 0.25 mg dose (Roehrs et al., 1992). It may be that a 10 mg dose of zolpidem is the threshold dose beyond which rebound insomnia is more likely to occur.
These rebound insomnia results extend those of a previous short-term study regarding the impact of duration of use (Merlotti et al., 1991). Rebound insomnia severity as function of duration of administration was assessed by administering the 0.50 mg dose of triazolam for 1, 6, or 12 consecutive nights. Sleep efficiency was reduced on the first discontinuation night after triazolam compared with placebo, but the severity of the rebound did not differ after 1, 6 or 12 consecutive nights of administration. Here we report that the likelihood of rebound with a clinical dose of zolpidem (10 mg) does not increase relative to placebo across 1, 4, and 12 months of nightly hypnotic use.
Some participants did show poorer sleep than baseline during the two discontinuation nights of months 1, 4, and 12. This suggests that there may be some individual differences in the expression of rebound insomnia. The present study with its three separate discontinuations for each participant (i.e. month 1, 4, and 12) will allow us to assess the repeatability of rebound within an individual and to look for possible predictors of the likelihood of experiencing rebound insomnia. Such analyses are being conducted currently for a future report.
One limitation of the present study is that it did not include a positive control which many of the short-term studies included, typically triazolam 0.50 mg. Given the chronic nature of this study we considered it unethical to administer, for 12 months, a treatment known in short-term studies to produce adverse effects. The sensitivity of this experiment to detect changes in NPSG sleep, and potentially worsened sleep, can be evaluated through the efficacy assessments done in this study during month 1 and month 8. We reported that zolpidem relative to placebo significantly increased total sleep time by 48 min in month 1 and 42 min in month 8 (Randall et al., 2010). Moreover, this study showed significantly improved sleep on discontinuation night 7 in month 12 in the zolpidem group relative to the placebo group.
The Tyrer Withdrawal Symptom ratings clearly indicate that a clinically significant withdrawal is not associated with 12 months of nightly use of zolpidem. The mean total Tyrer scores never exceeded a mean of 3 on any of the 6 discontinuation nights (i.e. 2 each in month 1, 4, and 12) and scores of greater than 20 are considered to be clinically significant. Importantly, there was no zolpidem group specific systematic increase in scores across the 12 months. Interestingly, there was a main effect of months indicating that there was an overall increase in Tyrer ratings over the 12 months. The scores averaged across groups were: month 1: 1.4, month 4: 1.7, and month 12: 2.1, increases of 0.3 and 0.4 from months 1 to 4 and 4 to 12. The reason(s) for this non-specific increase across months is not clear. This is especially perplexing as the placebo subjects simply kept taking placebo during the ‘discontinuation’ week.
While overall there were no drug group differences in the expression of withdrawal symptoms, a drug group difference was seen on the two discontinuation nights of month 4. The higher Tyrer scores of the zolpidem group in month 4 can be attributed to higher ratings of symptoms reflecting increased light sensitivity. Spontaneous written participant comments suggest this may have been an artifact of laboratory awakening procedures with the abrupt illumination of bright overhead room lights at the time of arising. Month 4 nights 1 and 2 were the first return to the laboratory for participants after 3 months of sleeping at home and for the zolpidem group, as opposed to the placebo group, continuous sleep throughout their 8-hour bedtimes at home. This may have made them more sensitive to an abrupt bright light awakening on the first nights back in the laboratory. Clearly, this finding needs replication and further clarification.
These findings of an absence of clinically significant withdrawal suggest that 12 months of BzRA hypnotic use by primary insomniacs at a clinical dose is not sufficient for development of dependence and withdrawal. In an analysis of patients from general practice and outpatient psychiatric clinics the duration of BzRA use in patients showing dependence ranged from 18 to 90 months with the mean duration 30 months (Kan et al., 2004). Thus, the 12 months of this study may not have approached the threshold for dependence development. Another important variable for dependence liability, dose, was carefully monitored in this study with weekly telephone reporting of nightly use and also monthly dispensing of medication. In an analysis of case reports of zolpidem dependence, dose was an important factor with the daily dose range being 40 mg to 1120 mg (i.e. four times and greater than the clinical dose) (Victorri-Vigneau et al., 2007).
Another important factor in evaluating physical dependence liability of BzRAs is likely to be duration of receptor occupancy. The reports of withdrawal associated with BzRA use typically are for anxiolytics, which are long-acting and thereby BzRA receptors are occupied for the full 24 hours. Most hypnotics, and specifically zolpidem, are short-acting and thus receptors are occupied for approximately a third of the 24 hour day. Thus, longer durations of nightly hypnotic use may be required to produce physical dependence on a hypnotic as compared with an anxiolytic.
It also has been suggested that the repeated daily receptor occupation followed by non-occupation (i.e. 8 hours on, 16 hours off) may operate as a kindling process to sensitize receptors and thus induce a withdrawal (Allison and Pratt, 2004). However, if kindling is the critical process, the present data would suggest one year of nightly kindling is not sufficient to induce rebound insomnia and withdrawal symptoms. This explanation seems less feasible, given the short kindling exposure typically used in animal studies.
It must be recognized that the participants of this study were carefully screened.
Any history of drug or alcohol abuse was an exclusion criterion in this study. Other medical, psychiatric, and primary sleep disorders were ruled out as well. The probable difference between the results of this study and reports of BzRA dependence in the general population is the patient population being treated and not the medication. The clinician may consider the patients of this study to be highly non-representative of their insomnia patients. However, these data do set the best case limits and inform the clinician of the conditions under which chronic hypnotic use is relatively safe.
Acknowledgments
Funding
NIDA grant number R01DA17355 awarded to TAR.
Footnotes
Conflict of interest
T Roehrs: consultant – Sanofi, Evotec; grantee – Takeda. S Randall, E Harris, and R Maan: none. T Roth: consultant – Actelion, Addrenex, Cephalon, Eisai, Intec, Merck, Pfizer, Sanofi, Sepracor, Shire, Somaxon, TransOral; speaker – Cephalon, Sanofi; grantee – Merck. This was not an industry supported study.
References
- Allison C, Pratt JA. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol Ther. 2004;98:171–195. doi: 10.1016/s0163-7258(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Bornstein SK. Respiratory monitoring during sleep: polysomnography. In: Guilleminault C, editor. Sleeping and Waking Disorders: Indications and Techniques. Menlo Park, CA: Addison-Wesley Publishing Co; 1982. pp. 183–212. [Google Scholar]
- Coleman RM. Periodic movements I sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, editor. Sleeping and Waking Disorders: Indications and Techniques. Menlo Park, CA: Addison-Wesley Publishing Co; 1982. pp. 265–296. [Google Scholar]
- Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry . 2005;9(66 Suppl):31–41. [PubMed] [Google Scholar]
- Hajak G, Clarenback P, Fischer W, Rodenbeck A, Bandelow B, Broocks A, et al. Rebound insomnia after hypnotic withdrawal in insomniac outpatients. Eur Arch Psychiatry Clin Neurosci. 1998;248:148–156. doi: 10.1007/s004060050032. [DOI] [PubMed] [Google Scholar]
- Hajak G, Hedner J, Eglin M, Loft H, Storustovu S, Lutofl S, et al. A 2-week efficacy and safety study of gaboxadol and zolpidem using electronic diaries in primary insomnia outpatients. Sleep Med. 2009;10:705–712. doi: 10.1016/j.sleep.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Kales A, Scharf MMB. Rebound insomnia: a new clinical syndrome. Science. 1978;201:1039–1041. doi: 10.1126/science.684426. [DOI] [PubMed] [Google Scholar]
- Kales A, Bixler EO, Vela-Bueno A, Soldatos C, Niklaus DE, Manfredi RL. Comparison of short and long half-life b3enzodiazepine hypnbotics: triazolam and quazepam. Clin Pharmacol Ther. 1986;40:378–386. doi: 10.1038/clpt.1986.194. [DOI] [PubMed] [Google Scholar]
- Kan CC, Hilbedrink SR, Breteier MHM. Determination of the main risk factor for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiatry. 2004;45:88–94. doi: 10.1016/j.comppsych.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Merlotti L, Roehrs T, Zorick F, Roth T. Rebound insomnia: Duration of use and individual differences. J Clin Psychopharm. 1991;11:368–373. [PubMed] [Google Scholar]
- Randall S, Roehrs T, Harris E, Maan R, Roth T. Chronic use of zolpidem is not associated with loss of efficacy. Sleep. 2010;33:A221. [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- Roehrs T, Merlotti L, Zorick F, Roth T. Rebound insomnia in normals and patients with insomnia after abrupt and tapered discontinuation. Psychopharmacology. 1992;108:67–71. doi: 10.1007/BF02245287. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Merlotti L, Zorick F, Roth T. Sedative, memory, and performance effects of hypnotics. Psychopharmacology. 1994;116:130–134. doi: 10.1007/BF02245054. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Zorick F, Wittig R, Roth T. Dose determinants of rebound insomnia. Br J Clin Pharmacol. 1986;22:143–147. doi: 10.1111/j.1365-2125.1986.tb05241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs TA, Vogel G, Roth T. Rebound insomnia: Its determinants and significance. Am J Med. 1990;88:43S–60S. doi: 10.1016/s0002-9343(13)80001-0. [DOI] [PubMed] [Google Scholar]
- Roth T, Soubrane C, Titeux L, Walsh JK. Efficacy and safety of zolpidem-MR: A double-blind, placebo-controlled study in adults with primary insomnia. Sleep Med. 2006;7:397–406. doi: 10.1016/j.sleep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Murphy S, Riley P. The benzodiazepine withdrawal symptom questionnaire. J Affect Dis. 1990;19:5361. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- Ware JC, Walsh JK, Scharf MB, Roehrs T, Roth T, Vogel GW. Minimal rebound insomnia after treatment with 10-mg zolpidem. Clin Neuropharm. 1997;20:116–125. doi: 10.1097/00002826-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Victorri-Vigneau C, Dailly E, Veyrac G, Joillet P. Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol. 2007;64:198–209. doi: 10.1111/j.1365-2125.2007.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voderholzer U, Riemann D, Hornyak M, Backhaus J, Feige B, Berger M, et al. A double-blind, randomized and placebo-controlled study on the polysomnographic withdrawal effects of zopiclone, zolpidem and triazolam in healthy subjects. Eur Arch Psychiatry Clin Neurosci. 2002;251:117–123. doi: 10.1007/s004060170045. [DOI] [PubMed] [Google Scholar]