Abstract
PROBLEM
Our knowledge of the innate host-defenses in the vagina, a site where these defenses are essential to protecting the host upper reproductive tract from invasion by pathogens, is as yet rudimentary. Specifically, little is known about the pattern-recognition component of vaginal innate immunity, the relationship of pattern-recognition molecules to known cytokine levels, and the role of gonadal hormones in their regulation.
METHOD OF STUDY
We measured levels of Surfactant Protein-A (SP-A), a prototypic innate pattern-recognition protein, in vaginal fluid (VF) and correlated them with levels of IL-1β and IL-8, two cytokines known to be present in VF. Assays were carried out on VF collected over three consecutive cycles from ten healthy naturally cycling women who were sampled at three specific time points in the menstrual cycle. The three time points were chosen to enable correlation with distinct hormonal states.
RESULTS
Both SP-A and cytokines levels were highest 5–6 days after menses (p < 0.05) and were significantly lower at ovulation and mid-luteal phase.
CONCLUSION
SP-A, like other host-defense molecules in the reproductive tract, appears to be regulated by gonadal hormones.
Keywords: Female hormonal cycle, mucosal innate immunity, pattern recognition, vaginal host-defense, vaginal mucosa
Introduction
Mucosal surfaces of the body constitute an important site of interaction between the host and potential pathogens in the external environment. In this context, vaginal mucosa serves as a ‘gatekeeper’ to the upper genital tract, where a range of commensal microbes, semen, and other foreign substances are tolerated and where sexually transmitted pathogens can be eliminated1,2. The immune mechanisms that contribute to this process are just becoming identified and characterized2,3.
Innate immunity has an essential role in mucosal host defenses. Innate immune processes have broad-specificity and precede acquired immune responses both evolutionarily and functionally4. Recognition of molecular patterns on microbial surfaces by pattern recognition molecules is an important component of the innate immune response. Pattern recognition proteins can both promote elimination of pathogens directly and mobilize acquired immune responses. The collagenous lectins (collectins) are a class of pattern recognition molecules that utilize a C-type lectin carbohydrate recognition domain (CRD)5,6. Surfactant Protein-A (SP-A), a prototypic collectin was first discovered as a component of pulmonary surfactant7,8. Since then, SP-A has been found to have an essential host defense function, as illustrated in studies of SP-A null mice. In a sterile environment, homozygous mice null for SP-A reproduce normally and appear to have normal respiratory function, but succumb to pulmonary infection in a non-sterile environment9. SP-A’s role in host-defense extends to a number of other sites of interaction between the internal and external environment7–10.
SP-A has been shown to influence numerous innate immune functions: It facilitates phagocytosis by opsonizing bacteria, fungi and viruses and can provide a link between innate and adaptive immunity by modulating differentiation and chemotaxis of cells of monocytic origin, including dendritic cells11–15. Importantly, SP-A can participate in both pro-inflammatory and anti-inflammatory responses. In vitro studies demonstrate that SP-A causes an increase in IL-1 and IL-8 production in THP-1 cells that have not been pathogen-stimulated13. Contribution of SP-A to these responses is context-dependent and differs depending on whether it is tested in a basal or pathogen-stimulated state.
There is good evidence that in both humans and laboratory animals ovarian hormones influence host-defenses in the upper reproductive tract16,17. There are, however, only a few studies of the role of hormones in the regulation of immune functions in the lower genital tract of humans3. An influence of gonadal hormones on cytokines in VF is suggested by reports where IL-1β and IL-8 levels are down-regulated at mid-cycle during the estrogen-dominant stage of the cycle3. Changes in pattern recognition proteins in relation to cyclic hormonal changes in the lower genital tract have not been examined.
We first identified SP-A in vaginal mucosa of pre-menopausal and post-menopausal women, demonstrated its presence in VF and provided immunocytochemical evidence that it is produced in the parabasal layer. The goal of the present study was to test the hypothesis that SP-A levels in VF are influenced by gonadal hormones and to examine whether cytokine levels correlate with SP-A levels. The two cytokines we chose to examine are IL-1β and IL-8, both of which have been consistently included in female reproductive tract studies and which have been shown to be responsive to SP-A in vitro13. Further, IL-1β has a role in the generation of rapid and potent immune responses, and IL-8 is a secondary pro-inflammatory mediator that can demonstrate a long-lasting increase in production after initial stimulus3,18. We present herein the first description of in vivo SP-A and cytokine concentration in healthy women over the monthly hormonal cycle.
Methods
Study design
VF was prospectively collected by saline lavage from ten healthy reproductive age women without infection or symptoms of inflammation and reporting normal menstrual cycles of 25–32 days.
Inclusion and exclusion criteria
In order to study normal vaginal host defenses we excluded women with genital irritation, discharge, odor, or a history suggestive of genital tract inflammatory conditions, such as, spontaneous preterm birth, pelvic inflammatory disease, recurrent episodes of vaginosis/vaginitis and superficial or deep dyspareunia. In order to insure normal monthly gonadal hormonal levels women were excluded who were found not to have undergone a luteinizing hormone (LH) surge as defined below. Histories of lower genital tract health were supported by physical examination and vaginal gram smear at each visit. Study subjects were required to refrain from sexual intercourse for 24 hours before sample collection.
Sampling
Women with normal menstrual cycles of 25 to 32 days were sampled at three time points characteristic of distinct hormonal states (diagramed in Figure 1)19: 1) day 5–6 after the first day of the last menstrual period for an early follicular phase sample; 2) within 12–24 hours of LH surge, determined by a qualitative urine test (OvuQuick One-Step Ovulation Predictor by Conception Technologies, Inc.) for an ovulatory phase sample; and 3) day 7–9 following LH surge for a luteal phase sample. Each time point was sampled three times over three cycles. Ovulation predictor tests were confirmed to be positive by the study coordinator.
Figure 1. Timing of vaginal fluid sample collection in relation to ovarian cycle in women with natural cycles.
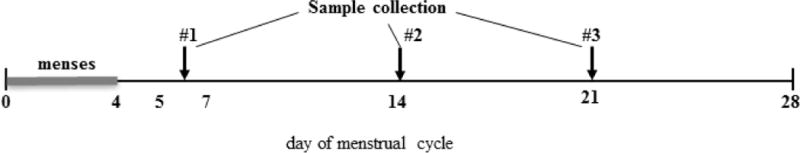
The day-5/6 sample (#1) was collected immediately after the end of menses; day-14 sample (#2) was collected within 12–24 hours after urine luteinizing hormone (LH) test positivity; and day-21 sample (#3) was collected 7–9 days following LH surge.
Sampling times were selected to reflect distinct hormonal states. Sampling at day 5–6 of the cycle was conducted at a time when average serum estradiol is 30–60 pg/ml and progesterone is minimal (< 1 ng/ml). Sampling at the ovulatory phase was conducted just after LH surge, at a time when serum estradiol levels reach a peak and serum progesterone is low (2.5 to 3 ng/ml). Mid-luteal phase samples were obtained at a time when serum progesterone is at its peak and serum estradiol levels are relatively high20. Serum estrogen and progesterone levels were not measured but inferred by confirmation of LH surge.
Vaginal fluid was obtained by placing a #2 Pederson speculum in the vagina and instilling 2 ml PBS into the speculum. A 3 ml pipette was used to rinse vaginal walls 2 times, and the lavage fluid was then withdrawn from the speculum. VF was placed in a −80° C freezer within 3 minutes of collection. A cotton swab was used to collect a Gram smear and fungal smear prior to every VF sampling. Gram and fungal smears were analyzed by the institution’s clinical microbiology laboratory, certified by the College of American Pathology and by internal semi-annual certification. White blood cell counts (WBC) and polymorphonuclear (PMN) leukocyte counts were taken from Gram smear reports, as were Nugent scores. Nugent scores of 7 or greater (on a scale of 0 to 10) indicate increasing severity of bacterial vaginosis. The Nugent method is a standardized method of scoring that provides improved inter-center reliability over Spiegel criteria and also provides information on gradations of disturbance of vaginal flora21.
SP-A and cytokine enzyme-linked immunosorbent assay (ELISA)
SP-A in VF was assayed by indirect ELISA using a rabbit polyclonal IgG directed against human SP-A. The method for purifying human SP-A for use as a protein standard has been described previously15. The antibody recognizes SP-A with high affinity and does not cross-react significantly with SP-D or mannose binding lectin (MBL), another human collectin. For SP-A ELISA, flat-bottom Immulon 2 ELISA plates (Dynex; Chantilly, VA) were used. Standard wells were coated overnight at 4°C with serial dilutions of SP-A ranging from 50 ng to 0.05 ng of SP-A in 100 μL of carbonate-bicarbonate buffer (pH 9.6). VF samples were diluted by adding 5 μL of VF sample to 995 μL of coating buffer and applying 100 μL of the diluted VF to duplicate wells on the plate. Plates were washed and proteins were blocked with 0.5% Tween-20 in PBS. One hundred μL of PBS, 0.05% Tween-20 containing 0.2 ng of anti-SP-A IgG was added to each well. After 1 hr at 37°C, the plate was washed and 100 μL of goat anti-rabbit IgG conjugated with horseradish peroxidase (Bio-Rad; Hercules, CA; diluted 1:2500) was added to each well. After incubating for 1 hr at 37°C, color was developed with an O-phenylene diamine dihydrochloride substrate, and the reaction terminated by the addition of sulfuric acid. Optical density was measured immediately at 490 nm, and SP-A concentrations were calculated using Sigma Plot (SPSS; Chicago, IL) by comparison of the samples with the SP-A standard curve. ELISA assays were repeated in 18 samples; the average normalization value was 1.066.
IL-1β and IL-8 concentrations were measured as previously reported with a double-sandwich ELISA following the manufacturers instruction (R&D Systems, McKinley Place, NE)22. VF samples were diluted and optical density measured as described above.
Statistical analysis
SP-A concentration was analyzed in relation to cycle phase and cytokine levels in two ways. First, all subjects at a specific phase of the cycle were analyzed as a group in order to determine if distinct hormonal states were associated with SP-A and cytokine levels. Differences in grouped SP-A and cytokines levels at one phase of the natural menstrual cycle compared to another phase were examined with a repeated measures analysis of variance using a Bonferroni correction. Second, individual SP-A and cytokine levels within a single individual’s samples were analyzed at each phase of the cycle in order to determine whether increases in SP-A correlated with increases or decreases in cytokines. Correlation between individual SP-A and cytokine levels, and the significance of the difference between 2 correlation curves, e.g., the correlation between an individual’s SP-A level and their IL-8 level determined in the ovulatory phase, compared with the correlation determined in the luteal phase, was tested using the Mixed Procedure23,24. This test employs a general linear mixed models technique to estimate correlations in a longitudinal data wherein there is a need to assess correlation in settings where multiple measurements are made on each of the variables of interest. Significance was accepted for p values of < 0.05.
Results
Subject characteristic
Characteristics of study subjects are described in Table I. Mean cycle length was 27 days. We did not assess the number of sexual partners, in part, because of the difficulty of verifying responses. All subjects, however, reported that they were sexually active.
Table.
Study subject characteristics
| Demographic Information | Natural cycles (n = 10) |
|---|---|
| Mean Age (range) | 39.1 (30–47) |
| Mean Cycle length (range) | 27.2 (25–32) |
| Marital Status | 80% Married |
| Mean Pregnancies (range) | 2 (1–3) |
| Mean VF WBCs (range) | 0.26 (0–4) |
| Mean VF PMNs (range) | 0.244 (0–4) |
| Mean Nugent score (range) | 2.425 (0–10) |
White blood cell (WBC) and polymorphonuclear cell (PMN) counts were taken from Gram smears performed by the institution’s clinical microbiology laboratory.
Vaginal fluid SP-A is highest in the early follicular phase
We sampled VF by saline lavage in 10 healthy asymptomatic women having natural cycles at three points in the monthly cycle depicted in Figure 1, over the course of 3 months (9 samples per subject; total of 90 samples). The concentration of SP-A was significantly higher in VF obtained at the early follicular phase of the cycle than at the ovulatory and luteal phases (p = 0.017, follicular vs. ovulatory; p = 0.0093, follicular vs. luteal) (Figure 2). The SP-A levels during the ovulatory and luteal phases were not significantly different from each other.
Figure 2. Vaginal fluid SP-A is highest in the early follicular phase of the cycle.
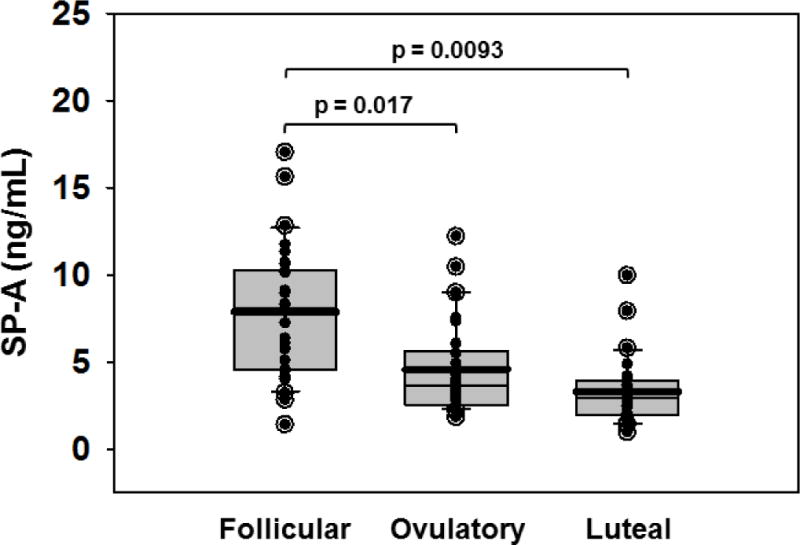
Vaginal fluid collected from 10 healthy women over the course of 3 months by lavage with 2ml normal saline was assayed by indirect ELISA. The open circles with solid centers represent outliers; solid circles are all other data points. The upper whisker in each cycle phase indicates the 90th percentile; the lower whisker indicates the 10th percentile. The upper edge of each box indicates the 75th percentile; the lower edge of each box indicates the 25th percentile. The thin line in each box indicates the median; the thick line in each box indicates the mean.
Vaginal fluid IL-1β and IL-8 are highest in women in the follicular phase of natural cycles
To determine whether increased SP-A in the follicular phase was associated with an increase or decrease in inflammatory cytokines in the follicular-phase, we measured IL-1β and IL-8 levels in the same samples of VF in which SP-A was measured. We found a significantly higher average IL-1β (p =< 0.018) and IL-8 (p =< 0.011) concentration in the early follicular phase when compared with levels in the ovulatory phase (Figure 3). There were no significant differences between the other phases of the cycle for either IL-1β or IL-8 (IL-1β: p =< 0.48, follicular vs. luteal; p =< 0.94, luteal vs. ovulatory; IL-8: p =< 0.73, follicular vs. luteal; p =< 0.49, luteal vs. ovulatory) (Figure 3).
Figure 3. Vaginal fluid cytokines are increased in the early follicular phase of the cycle.
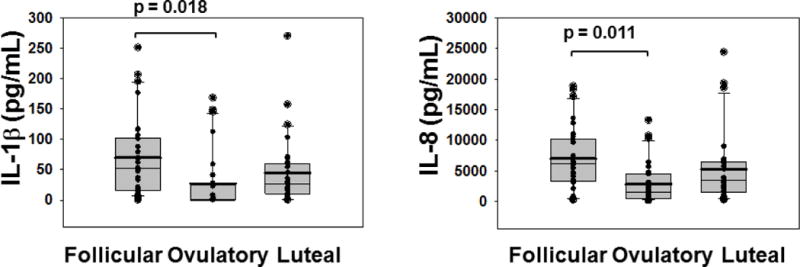
Vaginal fluid samples, identical to the samples assayed for SP-A in Figure 2, were collected from 10 healthy women over the course of 3 months by lavage with 2 ml normal saline and assayed for cytokines by double sandwich ELISA. The open circles with solid centers represent outliers; solid circles are all other data points. The upper whisker in each cycle phase indicates the 90th percentile; the lower whisker indicates the 10th percentile. The upper edge of each box indicates the 75th percentile; the lower edge of each box indicates the 25th percentile. The thin line in each box indicates the median; the thick line in each box indicates the mean.
The relationship between individual SP-A levels and cytokine levels in VF of women with natural cycles
We considered whether an effect of SP-A on cytokine levels could be suggested by examining the level of SP-A and cytokine within an individual sample. To look for a correlation between SP-A and cytokines the individual follicular, ovulatory or luteal SP-A level was plotted as an independent variable and cytokine concentration as a dependent variable. Within a specific phase of the ovarian cycle this assessment generates a curve, the slope of which correlates the level of a cytokine with a specific level of SP-A for each subject at each time of sampling. In the case of IL-8 (Figure 4) the slope of the curve depicting the SP-A associated increase in IL-8 in the luteal phase is significantly greater than that of the ovulatory phase (p =< 0.0028), indicating that there may be a stronger association between SP-A and IL-8 in the luteal phase. Note the progesterone is approximately four fold higher in the mid-luteal phase than in the ovulatory phase when progesterone levels are minimal, and estradiol levels are approximately 30% lower in the mid-luteal phase compared to the estradiol peak in the ovulatory phase20. Either, or both, of these hormonal changes could be causally associated with the stronger association of SP-A and IL-8 in the mid-luteal phase. The SP-A associated increase in IL-8 in the follicular phase of the cycle was identical to that of the ovulatory phase (data not shown). In the case of IL-1β (data not shown) the shallow slopes of all three phases of the natural cycle suggest little association between IL-1β and SP-A levels in any of the three phases of the natural cycle.
Figure 4. Correlation between individual sample SP-A concentration and IL-8 concentration in vaginal fluid at 3 phases (follicular (●), ovulatory (◆), and luteal (○)) of the natural hormonal cycle.
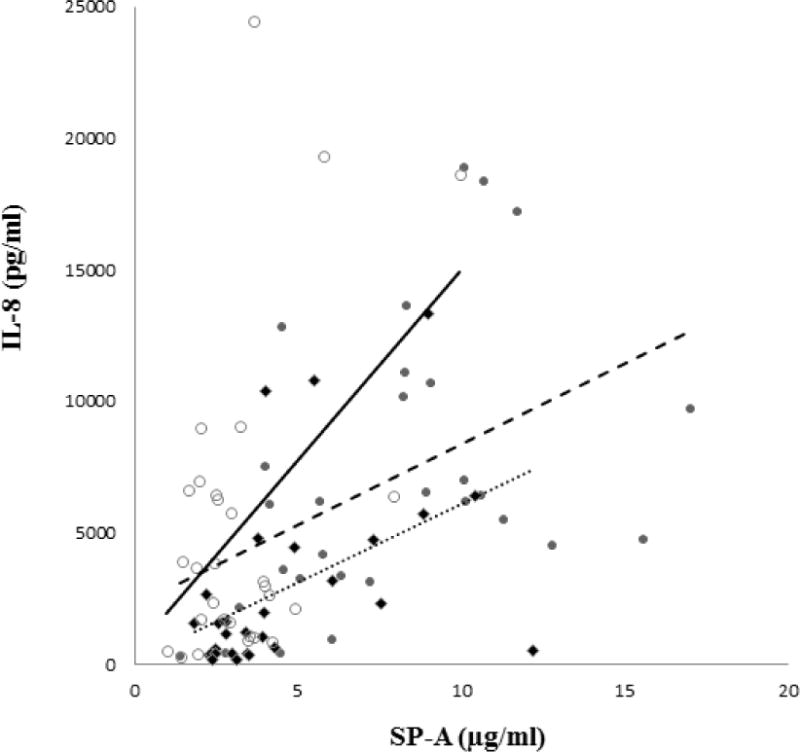
Ten subjects were sampled at specific time points during three cycles, three times each cycle, as described in Figure 1. Each marker represents SP-A and IL-8 concentration at a single sampling for each subject. Note that the natural cycle luteal phase slope (solid line) is steep, while the natural cycle follicular phase (dashed line) and ovulatory phase (dotted line) slopes are shallow.
Discussion
Previously we reported that SP-A is produced in the parabasal layer of the vaginal mucosa, is sequestered in the superficial layer and is present in the VF10. Here we document cyclic changes in the concentration of SP-A in VF as measured in a lavage that samples the layer of fluid covering the vagina. Physiologically, successful reproduction requires a balance between the immune system’s protective function against pathogens with a need for immune tolerance, in order to accommodate commensals, sperm and the fetal allograft. In this context, the finding of higher levels of SP-A after menstruation is consistent with the known protective function of this host-defense molecule: post-menses is when vaginal flora is unstable, vaginal mucosa is thinned and the endometrial epithelium is being re-established, and hence, it is a time of increased susceptibility to infection. Our finding that highest levels of IL-1β and IL-8 coincide with highest levels of SP-A suggests a similar protective role for these cytokines at a time of increased vulnerability to infection. In contrast, significantly lower levels of VF SP-A in the luteal phase coincide with the timing of implantation when immune tolerance is required.
It is interesting to note that in a parallel study of SP-A and cytokines in users of oral contraceptives, SP-A and IL-1 β and IL-8 levels were highest 2 to 3 days into the pill-free period when estrogen and progestin levels are low (data not shown). All participants took combined oral contraceptives containing ethinyl estradiol and various progestins in a cyclic manner. Together this information supports the notion that gonadal hormones suppress SP-A production.
It is reasonable to propose that coordination of host defense with the requirements of reproduction occurs through the actions of gonadal hormones. Our findings support this notion. Specifically, they indicate that SP-A production may be suppressed by estrogen and/or progesterone. This interpretation is supported by the finding that SP-A levels: a) are highest when both estrogen and progesterone levels are lowest; b) are significantly lower at the time of the estrogen surge; and c) are maintained at a low level during the luteal phase when both estrogen and progesterone levels are high19,25. A suppressive role for estrogens on SP-A production is supported by a recent study in which treatment of pregnant baboons with the aromatase inhibitor CGS20267 (that resulted in a 95% reduction in umbilical vein estrogen level) was shown to be associated with a 40% increase in SP-A levels in fetal lung25. The mechanism by which female steroid hormones might regulate human SP-A production remains to be determined.
Collagenous lectins, of which SP-A is a prototype, play a protective role on at least two levels, through pattern recognition and through their ability to mobilize cells that participate in the adaptive immune response. The first of these actions have to take place at the surface of the vagina, the most superficial layer, the layer that was sampled in this study. The second action has to take place where antigen-presenting cells reside. In the vagina, dendritic cells and macrophages that fulfill this function are present in the parabasal layer and lamina propria1,2. These two functionally important layers, the superficial epithelia and the parabasal layer, are connected by series of micro-channels permeating the squamous mucosa26. As we documented previously, it is in this deeper parabasal layer that SP-A is produced10. Hence, sampling VF provides a non-invasive approach to study processes deep in the mucosa.
Innate immune molecules such as SP-A are recognized to provide the first line of defense against invasion of the host by pathogens5–7. Their functions include an ability to recruit adaptive components of host defenses12,27. An example of this is evidence obtained in laboratory studies of SP-A’s actions on cytokine production.
Specifically, addition of SP-A to macrophage-like cells in vitro has been shown to result in a dose-dependent increase in IL-1β, IL-8 and other cytokines13,14,27. Our finding that both SP-A and IL-1β and IL-8 levels are highest in the early follicular phase when there is maximum need for protection suggest a similar role for SP-A also in vivo. Further examination of SP-A’s effects in the complex milieu of the vaginal mucosa will likely require studies of a multi-component organotypic in vitro test system as well as in vivo studies of innate and acquired immune components of host-defenses.
To the best of our knowledge, this is the first study in which SP-A and cytokine levels were measured during distinct hormonal phases of the ovarian cycle. This information both enhances our knowledge of hormonal influences on innate immunity in the healthy female reproductive tract and provides a baseline for study of inflammatory and infectious processes and mucosal immunity by non-invasive methods in vivo.
Acknowledgments
This work was supported by a grant to J.F. from the NIH (HL-34788) and to C.M. from the National Vulvodynia Association.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- ECL
enhanced chemiluminescence
- IgG
immunoglobulin G
- IL-1β
Interleukin-1 beta
- IL-8
Interleukin-8
- LH
luteinizing hormone
- MBL
mannose binding lectin
- SP-A
Surfactant Protein A
- SP-D
Surfactant Protein-D
- VF
vaginal fluid
References
- 1.Witkin SS. Immunology of the vagina. Clin Obstet Gynecol. 1993;36:122–128. doi: 10.1097/00003081-199303000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutinho A, Poltorack A. Innate immunity: from lymphocyte mitogens to Toll-like receptors and back. Current opinion in immunology. 2003;15:599–602. doi: 10.1016/j.coi.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochimica et biophysica acta. 2002;1572:387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 6.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annual review of immunology. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 7.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4:252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright JR. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 9.George CL, Goss KL, Meyerholz DK, Lamb FS, Snyder JM. Surfactant-associated protein A provides critical immunoprotection in neonatal mice. Infection and immunity. 2008;76:380–390. doi: 10.1128/IAI.01043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, Weisz J. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology. 2004;111:91–99. doi: 10.1111/j.1365-2567.2003.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koptides M, Umstead TM, Floros J, Phelps DS. Surfactant protein A activates NF-kappa B in the THP-1 monocytic cell line. The American journal of physiology. 1997;273:L382–388. doi: 10.1152/ajplung.1997.273.2.L382. [DOI] [PubMed] [Google Scholar]
- 12.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. American journal of physiology. 2003;284:L232–241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 13.Kremlev SG, Umstead TM, Phelps DS. Surfactant protein A regulates cytokine production in the monocytic cell line THP-1. The American journal of physiology. 1997;272:L996–1004. doi: 10.1152/ajplung.1997.272.5.L996. [DOI] [PubMed] [Google Scholar]
- 14.Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. The American journal of physiology. 1994;267:L712–719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- 15.Kremlev SG, Umstead TM, Phelps DS. Effects of surfactant protein A and surfactant lipids on lymphocyte proliferation in vitro. The American journal of physiology. 1994;267:L357–364. doi: 10.1152/ajplung.1994.267.4.L357. [DOI] [PubMed] [Google Scholar]
- 16.Wira CR, Rossoll RM, Young RC. Polarized uterine epithelial cells preferentially present antigen at the basolateral surface: role of stromal cells in regulating class II-mediated epithelial cell antigen presentation. J Immunol. 2005;175:1795–1804. doi: 10.4049/jimmunol.175.3.1795. [DOI] [PubMed] [Google Scholar]
- 17.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 18.Fichorova RN. Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr. 2004;37(Suppl 3):S184–S193. [PMC free article] [PubMed] [Google Scholar]
- 19.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 20.Speroff LGR, Kase NG. Clinical gynecologic endocrinology and infertility. Baltimore: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 21.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Combined SP-A-bleomycin effect on cytokines by THP-1 cells: impact of surfactant lipids on this effect. American journal of physiology. 2002;283:L94–L102. doi: 10.1152/ajplung.00434.2001. [DOI] [PubMed] [Google Scholar]
- 23.Hamlett A, Ryan L, Serrano-Trespalacios P, Wolfinger R. Mixed models for assessing correlation in the presence of replication. J Air Waste Manag Assoc. 2003;53:442–450. doi: 10.1080/10473289.2003.10466174. [DOI] [PubMed] [Google Scholar]
- 24.Chakraborty H, Helms RW, Sen PK, Cohen MS. Estimating correlation by using a general linear mixed model: evaluation of the relationship between the concentration of HIV-1 RNA in blood and semen. Stat Med. 2003;22:1457–1464. doi: 10.1002/sim.1505. [DOI] [PubMed] [Google Scholar]
- 25.Pepe GJ, Ballard PL, Albrecht ED. Fetal lung maturation in estrogen-deprived baboons. The Journal of clinical endocrinology and metabolism. 2003;88:471–477. doi: 10.1210/jc.2001-010228. [DOI] [PubMed] [Google Scholar]
- 26.Roig de V-L, Burgos MH. Migration of lymphocytes in the normal human vagina. Am J Obstet Gynecol. 1968;102:1094–1101. doi: 10.1016/0002-9378(68)90398-0. [DOI] [PubMed] [Google Scholar]
- 27.Meloni F, Alberti A, Bulgheroni A, Lupi A, Paschetto E, Marone Bianco A, Rodi G, Fietta A, Luisetti M, Baritussio A. Surfactant apoprotein A modulates interleukin-8 and monocyte chemotactic peptide-1 production. Eur Respir J. 2002;19:1128–1135. doi: 10.1183/09031936.02.00211102. [DOI] [PubMed] [Google Scholar]


