Abstract
Chemical examination of the methanolic extract of the leaflets of Cycas circinalis L. led to the isolation of one new biflavonoid, (2S, 2″S)-2,3,2″,3″-tetrahydro-4′,4‴-di-O-methylamentoflavone (tetrahydroisoginkgetin; 2), and 15 known compounds, 11 of which are reported for the first time from C. circinalis. Chromatographic separation of the chloroform extract of C. revoluta Thunb. leaflets afforded 12 compounds, seven of which are reported for the first time from this species. The isolated compounds from both species include 14 biflavonoids, three lignans, three flavan-3-ols, two flavone-C-glucosides, two nor-isoprenoids, and one flavanone. This is the first report of NMR and CD data of 2,3,2″,3″-tetrahydro-4′-O-methyl- and 2,3-dihydro-4′-O-methyl-amentoflavone (6) and (7). The effect of O-methylation on the chemical shifts of the neighboring carbons in the 13C NMR spectra of the dihydro- and tetrahydro-amentoflavone skeletons provides a tool to identify the location of the methoxy groups. Compounds 2, 6, and 18 displayed moderate antibacterial activity against Staphylococcus aureus (IC50 values of 3.8, 9.6, and 8.2 μM, respectively) and methicillin-resistant S. aureus (MRSA; IC50 values of 5.9, 12.5, and 11.5 μM, respectively).
Keywords: Cycadaceae, Cycas circinalis, Cycas revolute, biflavonoid, lignin, flavan-3-ol, nor-isoprenoid, flavone-C-glucoside, antibacterial activity
Introduction
Cycas is the only currently known genus of the family Cycadaceae, order Cycadales. Cycas revoluta Thunb is the most widespread species of the genus Cycas and is known as sago Cycas or king sago palm [1], while C. circinalis L is known as queen sago palm. This genus is native to eastern and southeastern Asia and is cultivated in many tropical and subtropical areas for ornamental purposes [2]. The Chinese utilize the seeds of C. revoluta as an antirheumatic, expectorant, and tonic. The terminal shoots are utilized as an astringent diuretic [3]. The very young leaves are edible and the juice of tender leaves is useful for the treatment of flatulence and vomiting [4]. It was also reported that a tincture of C. revoluta leaves contains inhibitors of cytochrome P-450 aromatase and thus may be efficacious in treating estrogen-dependent carcinoma [5]. Most of the research on the title plants focused on the seeds which produce neurotoxic metabolites [6–8]. Little information could be traced regarding the isolation of secondary metabolites from the leaves. In a previous chemical investigation of the leaves, a series of biflavonoids including amentoflavone (11), hinokiflavone (25), their dihydro derivatives (18, 8), podocarpusflavone A (19), isoginkgetin (9), and bilobetin (10) were identified [9]. Some phenolic acids were detected by TLC [10]. In this paper, we report the isolation of a new biflavonoid, (2S,2″S)-2,3,2″,3″-tetrahydro-4′,4‴-di-O-methylamentoflavone (2,3,2″,3″-tetrahydroisoginkgetin) (2), together with 24 known compounds belonging to the biflavonoid (4, 6 – 11, 17 – 19, 21, 22, 25), lignan (5, 20, 23), flavan-3-ol (12 – 14), flavone-C-glucoside (15, 16), nor-isoprenoid (1, 24), and flavanone (3) classes of plant secondary metabolites. We also report the antimicrobial activity of the three biflavonoids 2, 6, and 18. Compounds 6 and 7 were reported as new compounds from Selaginella uncinata [11], but ours is the first report of their NMR, CD, and antimicrobial data.
Materials and Methods
General experimental procedures
NMR spectra were recorded on a Bruker DRX NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C with a 3 mm direct carbon probe. NMR samples were dissolved in acetone-d6 (biflavonoids), DMSO-d6 (flavone glycosides), methanol-d4 (flavan-3-ols), and CDCl3 (lignans and nor-isoprenoids). Chemical shifts were standardized to the solvent resonances (CDCl3 7.24 ppm, CD3OD 4.78, 3.31ppm, acetone-d6 2.05 ppm, and DMSO-d6 2.5ppm). UV spectra were recorded on a Varian Cary 50 Bio UV-Visible spectrometer and CD on Olis DSM 20 instrument. IR spectra were measured in CHCl3 on an ATI Mattson Genesis series FT-IR spectrophotometer, whereas optical rotations were acquired with a 589–546 Rudolph Research Analytical Autopol IV automatic polarimeter. Accurate mass measurements were carried out on an Agilent HPLC 1100 series instrument equipped with a diode array detector and a mass detector in series (Agilent Technologies). The time-of-flight mass detector (model G1969A) was equipped with an electrospray ionization interface and controlled by Aligent software (Aligent Mass Hunter Work Station, A.02.01). HPLC was done using a Delta Prep 4000 (Waters Corporation) equipped with a dual wavelength detector Model 2487 adjusted at 210 and 330 nm.
Materials for CC were silica gel (32–63 μm; Dynamic Adsorbents Inc.), Sephadex LH-20 (40 – 70 μm; GE Healthcare Bio-Science AB), polyamide resin, and C-18 silica gel (40 – 63 μm; Sorbent Technology Co.). The preparative HPLC column was from Phenomenex Luna C18 (2) (100 A 250 × 15.00 mm, 5 μ).
Plant material
C. circinalis and C. revoluta leaflets were collected in May–August 2007 in the National Research Center in Giza, Egypt and identified by Mrs T. Labib, head specialist for plant identification, El-Orman Public Garden, Cairo, Egypt. They were also identified by the herbarium of the Faculty of Sciences, Cairo University.
Extraction and isolation
The shade-dried and powdered leaflets of C. circinalis (700 g) and C. revoluta (1500 g) were separately extracted with 80% MeOH by percolation. The solvent was evaporated under reduced pressure at 40°C to yield 100 and 170 g of crude extracts, respectively. The aqueous MeOH extracts were suspended in H2O and partitioned with petroleum ether, CHCl3, EtOAc, and n-BuOH saturated with H2O. The C. circinalis CHCl3 extract (A, 3 g) was subjected to CC on silica gel (90 g, 30 × 3.8 cm) and eluted successively with a gradient of n-hexane - CHCl3 then CHCl3 - MeOH mixtures of increasing polarities. The n-hexane - CHCl3 (20:80) eluate (A1, 900 mg) was rechromatographed on silica gel (30g, 15 × 3.5 cm), eluted with CHCl3 – MeOH, then purified on RP-HPLC using H2O + 0.05% formic acid (A) and MeOH + 0.05% formic acid (B) in a gradient mode: A/B 60/40 - 50/50; 10 min, 50/50 - 25/75; 5 min, 25/75 - 0/100; 10 min, 0:100; 10 min with a flow rate of 10 mL/min to give 1 (tR = 10.43 min, 6.5 mg), 2 (tR = 23.35 min, 15 mg), 3 (tR = 20.01 min, 14 mg), and 4 (tR = 23.52 min, 12 mg). The CHCl3- MeOH (90:10) eluate (A2, 0.8 g) was purified on RP-HPLC using the same method to give 5 (tR = 12.99 min, 5mg), 6 (tR = 20.83 min, 35 mg), 7 (tR = 21.90 min, 18 mg), 8 (tR = 22.80 min, 60 mg), and 9 (tR = 24.50 min, 28 mg). The C. circinalis EtOAc extract (B, 2 g) was subjected to CC on silica gel (60g, 30 × 3cm) eluted successively with gradient CHCl3 - EtOAc mixtures and rechromatographed over silica gel with n-hexane - EtOAc and Sephadex LH-20 with MeOH to afford 10 (4 mg), 11(30 mg), 12 (15 mg), 13 (6 mg), and 14 (40 mg). The C. circinalis n-BuOH extract (C, 8 g) was fractionated on polyamide with H2O - MeOH mixtures and then on a C-18 silica gel column and RP-HPLC to obtain 15 (6.5 mg) and 16 (20 mg). The C. revoluta CHCl3 extract (D, 8 g) was subjected to CC on silica gel (150 g, 60 × 3cm) and eluted successively with gradient n-hexane - EtOAc in 5% increments to afford four subfractions. The n-hexane-EtOAc (50:50, D1, 200 mg) eluate was rechromatographed on silica gel with gradient CHCl3 - MeOH and then purified on Sephadex LH-20 with MeOH to give 3 (45 mg). The n-hexane - EtOAc (40:60 - 30:70, D2, 630 mg) eluate was rechromatographed on silica gel with gradient CHCl3 – MeOH, then purified on Sephadex LH-20 with MeOH to afford 17 (16 mg) and 18 (30 mg). The n-hexane - EtOAc (25:75, D3, 500 mg) eluate was rechromatographed on silica gel with gradient CHCl3 - MeOH, then RP-HPLC using H2O + 0.05% formic acid (A) and MeOH + 0.05% formic acid (B) in a gradient mode: A/B 80/20 - 60/40; 15 min, 60/40 - 20/80; 25 min with a flow rate of 10 mL/min to afford 18 (tR = 23.52 min, 8 mg), 19 (tR = 27.28 min, 6 mg), and 8 (tR = 28.49 min, 24 mg). The n-hexane - EtOAc (20:80 - 0:100, D4, 800 mg) eluate was rechromatographed on silica gel with gradient CH2Cl2 - Me2CO in 5% increments to afford three subfractions. The CH2Cl2 - Me2CO (65:35, D4.1, 150 mg) eluate was repurified on silica gel with CHCl3 - MeOH, then RP-HPLC to give 20 (tR = 12.71 min, 13 mg), 21(tR = 32.09 min, 3 mg), and 22 (tR = 30.22 min, 2 mg). The CH2Cl2 - Me2CO (55:45, D4.2, 350 mg) eluate was repurified on silica gel with CHCl3 - MeOH, Sephadex LH-20 with MeOH, and RP-HPLC to afford 23 (tR = 13.52 min, 6 mg) and 24 (tR = 7.34 min, 12 mg). The CH2Cl2 - Me2CO (35:65, D4.3, 100 mg) eluate was filtered through Sephadex LH-20 with MeOH, then purified with RP-HPLC to give 11(tR = 22.07 min, 60 mg) and 25 (tR = 27.96 min, 5 mg).
Antimicrobial assay
All organisms were obtained from the American Type Culture Collection (ATCC) and included the fungi Candida albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, Cryptococcus neoformans ATCC 90113, and Aspergillus fumigatus ATCC 204305, as well as the bacteria Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 33591 (MRSA), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853, and Mycobacterium intracellulare ATCC 23068. Susceptibility testing was performed using a modified version of the Clinical and Laboratory Standards Institute (CLSI; formerly National Committee for Clinical Laboratory Standards - NCCLS) methods [12–15]. M. intracellulare was tested using a modified method of Franzblau et al. [16]. Samples were serially-diluted in 20% DMSO/saline and transferred in duplicate to 96 well flat bottom microplates. Microbial inocula were prepared by correcting the OD630 of microbe suspensions in incubation broth to afford final target inocula. Drug controls [ciprofloxacin (ICN Biomedicals) for bacteria and amphotericin B (ICN Biomedicals) for fungi] were included in each assay. All organisms were read at either 530 nm using the Biotek Powerwave XS plate reader (Bio-Tek Instruments) or 544ex/590em (M. intracellulare, A. fumigatus) using the Polarstar Galaxy Plate Reader (BMG LabTechnologies) prior to and after incubation. Minimum fungicidal or bactericidal concentrations were determined by removing 5 μL from each clear well, transferring to agar, and incubating. The Minimum Fungicidal Concentration/Minimum Bactericidal Concentration (MFC/MBC) is defined as the lowest test concentration that kills the organism and allows no growth on agar.
Characterization
(2S,2″S)-2,3,2″,3″-tetrahydro-4′,4‴-di-O-methylamentoflavone [(2S,2″S)-2,3,2″,3″-tetrahydroisoginkgetin] (2): yellowish white powder; [α]25D −28.0 (MeOH, c 0.20), UV (MeOH) λmax = 290 and 330 nm; CD (MeOH) [θ]293.2 −6.79 × 10, [θ]330 +1.49 × 10; IR (KBr) νmax 3326, 2926, 2853, 1713,1638, 1515, 1462, 1341, 1307, 1253, 1181, 1159, 1087, 1028 cm−1; HR-MSD-TOF (ES negative-ion mode): m/z 569.1590 [M-H]− and 1139.3056 [2M-H]− (calculated for m/z 569.1447 [M(C32H26O10)-H]− and 1139.2973 [2M(C64H52O20)-H]−, respectively; 1H and 13C NMR data: see Tables 1 and 2.
Table 1.
The 13C NMR (100 MHz) data of compounds 2, 6, and 7 (Me2CO-d6)
| No. | 2 δ*C |
6 δC |
7 δC |
|---|---|---|---|
| 2 | 78.57, 78.44a | 78.79, 78.53 | 79.08, 79.00 |
| 3 | 42.46, 41.87 | 42.53, 41.79 a | 43.06, 42.70 |
| 4 | 196.2 | 196.2 | 196.23, 196.20 |
| 5 | 164.44, 164.42 | 164.4 | 164.4 |
| 6 | 95.9 | 95.79, 95.96 | 96.1 |
| 7 | 166.65, 166.67 | 166.8 | 166.5 |
| 8 | 95.07 | 95.04, 95.07 | 95.1 |
| 9 | 163.5 | 163.5 b | 163.5a |
| 10 | 102.25, 102.29 | 102.22,102.25 c | 102.3 |
| 1′ | 130.33, 130,44 | 130.45, 130.33 | 130.91, 130.76 |
| 2′ | 131.28, 131.32 | 131.26, 131.35 | 131.50, 131.31 |
| 3′ | 121.8 | 121.8 | 121.02, 120.93 |
| 4′ | 158.36, 157.98 | 158.0, 158.3 | 158.30, 158.26 |
| 5′ | 110.86, 110.94 | 110.90, 110.90 | 111.23, 111.15 |
| 6′ | 127.09, 127,50 | 127.50, 127.09 | 127.99, 127.95 |
| 2″ | 79.1a | 79.1 | 164.01, 163.98 |
| 3″ | 42.78, 43.01 | 43.03, 42.72 a | 102.68, 102.63 |
| 4″ | 196.7 | 196.9 | 182.6 |
| 5″ | 163.7 | 163.4 b | 161.5 |
| 6″ | 96.1 | 96.02,96.06 | 98.8 |
| 7″ | 164.09, 164.17 | 164.01, 164.11 | 161.4a |
| 8″ | 105.82, 105.95 | 105.82, 105.95 | 104.7 |
| 9″ | 160.3 | 160.4 | 154.8 |
| 10″ | 102.51, 102.54 | 102.52, 102.57 c | 104.5 |
| 1‴ | 130.83, 131.19 | 130.03, 129.67 | 122.3 |
| 2‴ | 127.92, 127.60 | 127.70, 128.04 | 128.24, 128.19 |
| 3‴ | 113.78, 113.75 | 115.20, 115.15 | 115.9 |
| 4‴ | 159.76, 159.77 | 157.53, 157.64 | 161.0 |
| 5‴ | 113.78, 113.75 | 115.20, 115.15 | 115.9 |
| 6‴ | 127.92, 127.60 | 127.70, 128.04 | 128.24, 128.19 |
| 4′-O-CH3 | 54.65, 54.63 | 55.11, 55.03 | 55.2 |
| 4‴-OCH3 | 55.12, 55.05 | ----------------- | -------------- |
Chemical shift values in ppm.
Values having similar superscripts in the same column may be interchanged.
Table 2.
| No. | 2* δH |
6* δH |
7 δH |
|---|---|---|---|
| 2 | 5.58 – 5.41 m | 5.57 – 5.41 m | 5.60 dt (13.0, 2.8) |
| 3 | 3.23 – 3.07 m, H-3ax 2.91 – 2.60 m, H-3eq |
3.24 – 3.06 m, H-3ax 2.89 – 2.62 m, H-3eq |
3.38 – 3.21 m, H-3ax 2.90 – 2.73 m, H-3eq |
| 6 | 5.96–6.01 m | 5.99 – 5.92 m | 5.96 s |
| 8 | 5.96–6.01 m | 6.01 d (2.1) | 5.99 s |
| 2′ | 7.47 – 7.40 m | 7.49 – 7.40 m | 7.71 – 7.62 m |
| 5′ | 7.04 dd (8.7, 4.9) | 7.04 dd (8.8, 4.2) | 7.24 d (9.1) |
| 6′ | 7.47 – 7.40 m | 7.49 – 7.40 m | 7.71 – 7.62 m |
| 2″ | 5.58 – 5.41 m | 5.57 – 5.41 m | ------------------------ |
| 3″ | 3.23 – 3.07 m, H-3ax 2.91 – 2.60 m, H-3eq |
3.24 – 3.06 m, H-3ax 2.89 – 2.62 m, H-3eq |
6.65 s |
| 6″ | 6.12 d (4.6) | 6.12 d (4.9) | 6.43 s |
| 2‴ | 7.37 d (8.7) | 7.28 dd (8.5, 3.2) | 7.60 dd (8.0, 5.2) |
| 3‴ | 6.90 t (8.7) | 6.87 – 6.75 m | 6.97 – 6.85 m |
| 5‴ | 6.90 t (8.7) | 6.87 – 6.75 m | 6.97 – 6.85 m |
| 6‴ | 7.37 d (8.7) | 7.28 dd (8.5, 3.2) | 7.60 dd (8.0, 5.2) |
| 5-OH | 12.21 s | 12.21 s | 12.18 s |
| 5″-OH | 12.30 s | 12.31 s | 13.12 s |
| 4′-OCH3 | 3.82 s, 3.77 s | 3.79 d (13.2) | 3.79 s |
| 4‴–OCH3 | 3.79 s, 3.77 s | ------------------------ | ------------------------ |
Chemical shift values in ppm and J values (in Hz) are presented in parentheses.
The assignments are based on DEPT, HMQC, HMBC and NOE experiments.
Multiplicity is complicated because of rotational isomerism.
(2S,2″S)-2,3,2″,3″-tetrahydro-4′-O-methylamentoflavone [(2S,2″S)-2,3,2″,3″-tetrahydrobilobetin] (6): yellowish white powder; [α]25D −2.0 (MeOH, c 0.15); UV (MeOH) λmax = 291 and 332 nm; CD (MeOH) [θ]291.5 - 5.46 × 10, [θ]330 + 1.1 × 10; HR-MSD-TOF (ES negative-ion mode): m/z 555.1467 [M-H]− and 1111.2777 [2M-H]− (calculated for m/z 555.1291 [M(C31H24O10)-H]− and 1111.2660 [2M(C62H48O20)-H]−, respectively). 1H and 13C NMR data: see Tables 1 and 2.
(2S)-2,3-dihydro-4′-O-methylamentoflavone [(2S)-2,3-dihydrobilobetin] (7): yellow powder; [α]25D +11.2 (MeOH, c 0.29); UV (MeOH) λmax = 289 and 330 nm; CD (MeOH) [θ]293.2 - 3.17 × 10, [θ]331 + 8.07; HR-MSD-TOF (ES negative-ion mode): m/z 553.1273 [M-H]− and m/z 1107.2427 [2M-H]− (calculated for m/z 553.1134 [M(C31H22O10)-H]− and 1107.2347 [2M(C62H44O20)-H]−, respectively). 1H and 13C NMR data: see Tables 1 and 2.
Supporting Information
1H NMR, 13C NMR, and HR-MS spectra of compounds 2, 6, and 7 are available as Supporting Information.
Results and Discussion
The CHCl3 soluble fraction of the MeOH extract of the leaflets of C. circinalis was subjected to various chromatography steps to afford (−)-loliolide (1) [17, 18], the new (2S,2″S)-2,3,2″,3″-tetrahydroisoginkgetin) (2), (2S)-naringenin (3) [19], (2S,2″S)-2,3-dihydro-4′,4‴-di-O-methylamentoflavone (4) [20], (+)-(7S,8R)-dihydrodehydrodiconiferyl alcohol (3′-methylcedrusin) (5) [21], (2S,2″S)-2,3,2″,3″-tetrahydro-4′-O-methylamentoflavone (6), (2S)-2,3-dihydro-4′-O-methylamentoflavone (7), (2S)-2,3-dihydrohinokiflavone (8) [22], and 4′,4‴-di-O-methylamentoflavone (isoginkgetin) (9) [22]. The EtOAc soluble fraction was subjected to column chromatography sequentially over silica gel and Sephadex LH-20 to obtain 4′-O-methylamentoflavone (bilobetin; 10) [22, 23], amentoflavone (11) [22], epicatechin (12) [24], epigallocatechin (13) [25, 26], and gallocatechin (14) [26]. Fractionation of the n-BuOH soluble fraction on polyamide-6, RP-18, and RP-HPLC afforded vicenin-2 (violanthin) (15) [27], and 2″-glucosylvitexin (16) [28, 29]. The CHCl3 soluble fraction of the methanolic extract of C. revoluta leaflets was subjected to column chromatography over silica gel followed by Sephadex LH-20 and RP-HPLC to afford (2S)-naringenin (3), (2S)-2,3-dihydrohinokiflavone (8), amentoflavone (11), (2S,2″S)-2,3,2″,3″-tetrahydroamentoflavone (17) [30], (2S)-2,3-dihydroamentoflavone (18) [22], podocarpusflavone A (19) [22, 31], (+)-lariciresinol (20) [32], (2S)-2,3-dihydroisocryptomerin (21) [33], (2S,2″S)-2,3,2″,3″-tetrahydrohinokiflavone (22) [34], (+)-isolaricerisinol (23) [35], (6S,7E,9S)-6,9-dihydroxy-4,7-megastigmadien-3-one (vomifoliol) (24) [36], and hinokiflavone (25) [22]. The structures of these compounds were established by analysis of their physical and spectroscopic data in comparison with reported data.
Compound 2
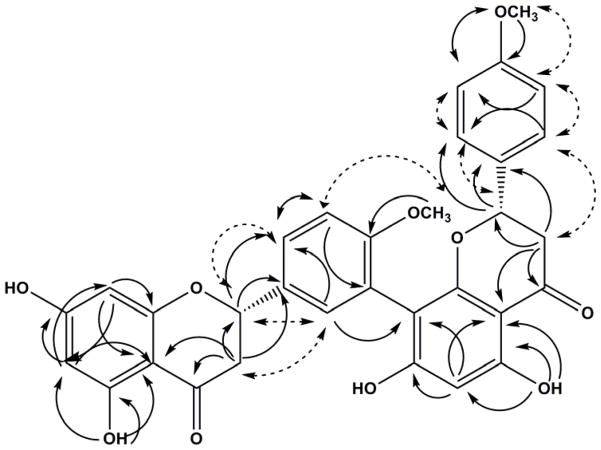
The MSD-TOF spectrum of compound 2 displayed ions at m/z 569.1590 [M-H]− and m/z 1139.3056 [2M-H]− which were consistent with a molecular formula C32H26O10. The IR spectrum of 2 indicated a broad OH absorption band at 3326 cm−1 and C=O at 1638 cm−1, while the UV spectrum showed absorption maxima at 290 and 330 nm, characteristic of a flavanone derivative [37]. The 1H and 13C NMR spectra of compound 2 at 294° K showed a pair of rotamers (1:1) due to the restricted rotation about the interflavanyl bond. At this temperature the signals were duplicated hence rendering the assignment of resonances to the individual rotamers not feasible. The biflavanone structure of 2 was evident from the presence of two duplicated benzylic oxymethine carbon signals in the 13C NMR spectrum at δC 78.58, 78.45 (C-2), and 79.07 (C-2″), two duplicated methylene carbon signals at δC 42.45, 41.87 (C-3) and 43.01, 42.79 (C-3″), as well as two carbonyl carbon signals at δC 196.28 (C-4) and 196.71, 196.73 (C-4″). The 1H NMR spectrum further supported the biflavanone structure of 2 via two sets of ABX spin patterns of rings C and F at δH 2.60–2.91 (m, H-3eq, H-3″eq), 3.07–3.23 (m, H-3ax, H-3″ax) and 5.41–5.58 (m, H-2, H-2″), and two D2O-exchangeable hydrogen-bonded hydroxy groups at δH 12.21(1H, s, OH-5) and 12.30 (1H, s, OH-5″). The 1H NMR spectrum also showed three signals corresponding to two methoxy groups at δH 3.77, 3.79, and 3.82, signals corresponding to H-6 and H-8 of ring A at δH 5.96, 5.98, 6.01, and H-6″ of ring D at δH 6.13 and 6.12. The AA′ BB′ spin pattern of ring E resonated at δH 6.90 (t, J = 8.7 Hz, H-3‴, H-5‴) and 7.37 (d, J = 8.7 Hz, H-2‴, H-6‴). The ABM spin pattern of ring B resonated at δH 7.04 (dd, J = 8.7, 4.9 Hz, H-5′) and 7.47 – 7.40 (m, H-2′, H-6′). The assignment of these protons was confirmed by Heteronuclear Multiple Quantum Correlation (HMQC) and Heteronuclear Multiple Bond Correlation (HMBC) experiments. The HMBC spectrum of 2 confirmed the involvement of C-3′ (B-ring) and C-8″ (D-ring) in the interflavanyl linkage via the 3JCH correlations of H-5′ (δH 7.04) with C-3′ (δC 121.75) and the 3JCH correlations of H-6″ (δH 6.13 and 6.12) and H-2′ (δH 7.40–7.47) with C-8″ (δC 105.83 and 105.95) thus indicating compound 2 as a member of the amentoflavone class of biflavonoids. The location of the methoxy groups was confirmed by the 3JCH correlations between 4′-OCH3 (δH 3.82) and 4‴-OCH3 (δH 3.79) with C-4′ (δC 157.98, 158.36) and C-4‴ (δC 159.76, 159.87), respectively. It was also confirmed by the Nuclear Overhauser Effect (NOE) between the 4‴-OCH3 (δH 3.79) and H-3‴ and H-5‴ (δH 6.90), as well as between 4′-OCH3 (δH 3.82) and H-5′ (δH 7.04). The CD spectrum of 2 showed high amplitude negative and low amplitude positive Cotton effects for the π → π* and n → π* transitions at 293 and 330 nm, respectively, which permitted the assignment of (2S,2″S) absolute configuration [38, 39]. Based on this data, compound 2 was unambiguously assigned as (2S,2″S)-2,3,2″,3″-tetrahydro-4′,4‴-di-O-methylamentoflavone [(2S,2″S)-2,3,2″,3″-tetrahydroisoginkgetin].
Compound 6
The MSD-TOF spectrum of compound 6 displayed ions at m/z 555.1467 [M-H]− and m/z 1111.2777 [2M-H]− which were consistent with a molecular formula C31H24O10. The compound showed similar structural features to compound 2 except for possessing only one methoxy group. The location of the methoxy group was defined at C-4′ since the methoxy protons at δH 3.97 showed NOE correlations to H-5′ (δH 7.04). The presence of rotational isomers was also evident in both the 1H and 13C NMR spectra. The CD spectrum showed high amplitude negative and low amplitude positive Cotton effects for the π → π* and n → π* transitions at 291 and 330 nm, respectively, which permitted the assignment of (2S,2″S) absolute configuration. Compound 6 was thus identified as (2S,2″S)-2,3,2″,3″-tetrahydro-4′-O-methylamentoflavone [(2S,2″S)-2,3,2″,3″-tetrahydrobilobetin].
Compound 7
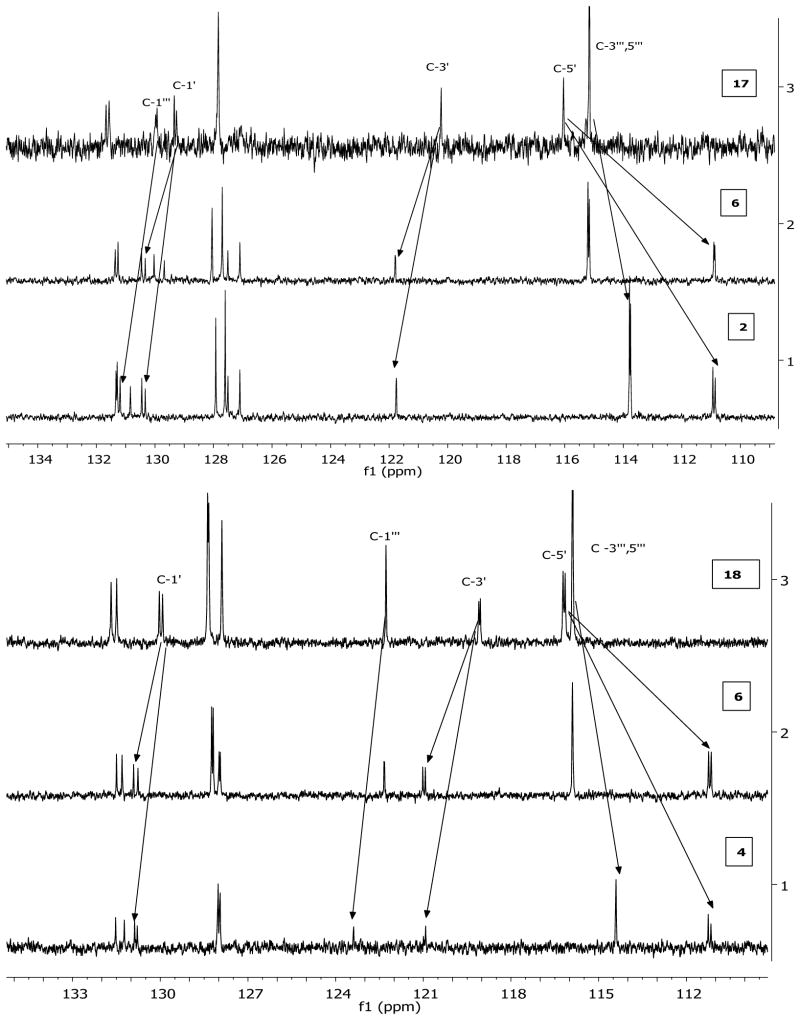
The molecular formula C31H22O10 was established via MSD-TOF as it showed ions at m/z 553.1273 [M-H]− and m/z 1107.2427 [2M-H]−. The compound showed similar structural features to compound 18 except for possessing an additional methoxy group which was located at C-4′ from HMBC and NOE data. The CD spectrum showed high amplitude negative and low amplitude positive Cotton effects for the π → π* and n → π* transitions at 293 and 331 nm, respectively, which permitted the assignment of an (2S) absolute configuration. Compound 7 was thus identified as (2S)-2,3-dihydro-4′-O-methylamentoflavone ((2S)-2,3-dihydrobilobetin). The O-methylation induced shifts in the carbon resonances in the 13C NMR spectra of the dihydro- and tetrahydro-amentoflavone skeletons (Fig. 3, Table 3) are similar to those previously reported for amentoflavone [22]. In addition, 4′-O –methylation induced a shift of + (1.5 – 2.0) ppm for C-3′. The absolute configurations reported for compounds 1 and 24 [40, 41] were confirmed by X-ray crystallography.
Fig. 3.
Comparison between the 13C NMR spectra (110–135 ppm) of some of the isolated biflavonoids.
Table 3.
Shifts in the 13C NMR data observed in compounds 2, 4, 6, and 7 compared to the phenolic derivatives 17 and 18
| Compound | 13C NMR Shifts Observed (ppm) | Indication | ||||
|---|---|---|---|---|---|---|
| C-5′ | C-1′ | C-3‴ | C-3‴, 5‴ | C-1‴ | ||
| 2 | −5 | +1 | +1.6 | −1.4 | +1.2 | 4′- and 4‴-O-methylation |
| 4 | −5 | +0.8 | +1.9 | −1.5 | +1 | 4′- and 4‴-O-methylation |
| 6 | −4.8 | +1 | +1.6 | 4′-O-methylation | ||
| 7 | −5 | +0.8 | +1.9 | 4′-O-methylation | ||
The isolated biflavonoids were tested for antimicrobial activity. None showed antifungal, antimalarial, or antileishmanial activity. Compounds 2, 6, and 18 displayed moderate antibacterial activity against Staphylococcus aureus (IC50 values of 3.8, 9.6, and 8.2 μM, respectively) and methicillin-resistant S. aureus (MRSA; IC50 values of 5.9, 12.5, and 11.5 μM, respectively). All three compounds possess the amentoflavone-type skeleton. Compound 2 is approximately 11 times less potent than ciprofloxacin against S. aureus and 21 times less potent than ciprofloxacin against MRSA.
Supplementary Material
Fig. 1.
Chemical structures of compounds from C. circinalis and C. revoluta leaflets.
Fig. 2.
Important HMBC (single-head arrow) and NOESY (double-head dashed arrow) correlations of compound 2.
Table 4.
Antibacterial activity displayed by isolated biflavonoids
| Compound | Staphylococcus aureus | MRSA | ||||
|---|---|---|---|---|---|---|
| IC50 | MIC | MBC | IC50 | MIC | MBC | |
| 2 | 3.9 | 17.5 | NA | 5.9 | NA | NA |
| 6 | 9.7 | 35.9 | NA | 12.5 | 35.9 | NA |
| 18 | 8.2 | 37.0 | NA | 11.5 | 37.0 | NA |
| Ciprofloxacin | 0.3 | 0.8 | 3.0 | 0.3 | 0.8 | 3.0 |
IC50 = the test concentration (μM) that afford 50% inhibition relative to controls.
MIC (minimum inhibitory concentration) is the lowest test concentration (μM) that allows no detectable growth.
MBC (minimum bactericidal concentration) is the lowest test concentration (μM) that kills the organism.
NA = not active at the highest test concentration of 20μg/mL (35.0, 35.9, and 37.0 μM, respectively).
Acknowledgments
A. Moawad would like to thank the Egyptian government for a fellowship through the Ministry of Higher Education and Scientific Research, J. P. J. Marais for recording CD spectra, B. Avula for recording the MS spectra, and P. Carvalho and F.R. Fronczek for resolving the crystal structures. We also thank Ms. Marsha Wright for biological testing. This work was supported by the NIH, NIAID, Division of AIDS, Grant No. AI 27094 and the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
Footnotes
Supporting information available online at http://www.thieme-connect.de/ejournals/toc/plantamedica
References
- 1.Duke JA. CRC handbook of medicinal herbs. Boca Raton, Florida: CRC Press; 1985. [Google Scholar]
- 2.Jones D. Cycads of the World. Washington D. C: Simthsonian Institution Press; 1993. [Google Scholar]
- 3.Duke JA, Ayensu ES. Medicinal plants of China. 1. Algonac, Michigan: Reference Publications; 1985. [Google Scholar]
- 4.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants (including the supplement) New Delhi: Council of Scientific and Industrial Research; 1986. [Google Scholar]
- 5.Kowalska MT, Itzhak Y, Puett D. Presence of aromatase inhibitors in Cycads. J Ethnopharmacol. 1995;47:113–116. doi: 10.1016/0378-8741(95)01259-g. [DOI] [PubMed] [Google Scholar]
- 6.Chang HO, Brownson DM, Mabry TJ. Screening for non-protein amino acids in seeds of the Guam cycad, Cycas circinalis, by an improved GC-MS method. Planta Med. 1995;61:66–70. doi: 10.1055/s-2006-958002. [DOI] [PubMed] [Google Scholar]
- 7.Whiting MG. Neurotoxicity of cycads, an annotated bibliography for the years 1929–1989. Lyonia. 1989;2:201–270. [Google Scholar]
- 8.Li CJ, Brownson MD, Mabry T, Perera C, Bell EA. Nonprotein amino acids from seeds of Cycas circinalis and Phaseolus vulgaris. Phytochemistry. 1996;42:443–445. doi: 10.1016/0031-9422(95)00851-9. [DOI] [PubMed] [Google Scholar]
- 9.Varshney AK, Mah T, Khan NU, Rahman W, Hwa CW, Okigawa M, Kawano N. Biflavones from Cycas revoluta, C. circinalis, and C. rumphii. Indian J Chem. 1973;11:1209–1214. [Google Scholar]
- 10.Wallace JW. A survey for benzoic and cinnamic acid of the Cycadaceae. Am J Bot. 1972;59:1–4. [Google Scholar]
- 11.Yao X, Wang N, Fan M, Zheng J, Wu L, Liu H, Ding A, Gao H, Dai Y. Application of flavone derivatives as antioxidation and anti-hypoxia drug or food and their preparation. CN 101361733 A 20090211 Faming Zhuanli Shenqing Gongkai Shuomingshu. 2009
- 12.NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A2. 15 Vol. 22. Wayne: National Committee on Clinical Laboratory Standards; 2002. [Google Scholar]
- 13.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, seventh edition M7-A7. 2 Vol. 26. Wayne: National Committee on Clinical Laboratory Standards; 2006. [Google Scholar]
- 14.NCCLS. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic Actinomycetes; tentative standard—approved standard, M24-A. 18. Vol. 23. Wayne: National Committee on Clinical Laboratory Standards; 2003. [PubMed] [Google Scholar]
- 15.NCCLS. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, M38-A. 16 Vol. 22. Wayne: National Committee on Clinical Laboratory Standards; 2002. [Google Scholar]
- 16.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park KE, Kim YA, Jung HA, Lee HJ, Ahn J-W, Lee B-J, Soe Y. Three norisoprenoids from the brown alga Sargassum thunbergii. J Korean Chem Soc. 2004;48:394–398. [Google Scholar]
- 18.Kimura J, Maki N. New loliolide derivatives from the brown alga Undaria pinnatifida. J Nat Prod. 2002;65:57–58. doi: 10.1021/np0103057. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim ARS. Sulfation of naringenin by Cunninghamella elegans. Phytochemistry. 2000;53:209–212. doi: 10.1016/s0031-9422(99)00487-2. [DOI] [PubMed] [Google Scholar]
- 20.Cheng KT, Hsu FL, Chen SH, Hsieh PK, Huang HS, Lee CK, Lee MH. New constituent from Podocarpus macrophyllus var. macrophyllus shows anti-tyrosinase effect and regulates tyrosinase-related proteins and mRNA in human epidermal melanocytes. Chem Pharm Bull. 2007;55:757–761. doi: 10.1248/cpb.55.757. [DOI] [PubMed] [Google Scholar]
- 21.Seidel V, Bailleul F, Waterman PG. Novel oligorhamnosides from the stem bark of Cleistopholis glauca. J Nat Prod. 2000;63:6–11. doi: 10.1021/np9901478. [DOI] [PubMed] [Google Scholar]
- 22.Markham KR, Shepard C, Geiger H. 13C NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry. 1987;26:3335–3337. [Google Scholar]
- 23.Silva GL, Chai H, Gupta MP, Farnsworth NR, Cordell GA, Pezzuto JM, Beecher CWW, Kinghorn AD. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry. 1995;40:129–134. doi: 10.1016/0031-9422(95)00212-p. [DOI] [PubMed] [Google Scholar]
- 24.Cren-Olivé C, Wieruszeski JM, Maes E, Rolando C. Catechin and epicatechin deprotonation followed by 13C NMR. Tetrahedron Lett. 2002;43:4545–4549. [Google Scholar]
- 25.Foo LY, Lu Y, Molan AL, Woodfield DR, McNabb WC. The phenols and prodelphinidins of white clover flowers. Phytochemistry. 2000;54:539–548. doi: 10.1016/s0031-9422(00)00124-2. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Kondou K, Kouno I. Oxidation and epimerization of epigallocatechin in banana fruits. Phytochemistry. 2000;53:311–316. doi: 10.1016/s0031-9422(99)00533-6. [DOI] [PubMed] [Google Scholar]
- 27.Sato S, Akiya T, Nishizawa H, Suzuki T. Total synthesis of three naturally occurring 6,8-di-C-glycosylflavonoids: phloretin, naringenin, and apigenin bis-C-β-D-glucosides. Carbohydr Res. 2006;341:964–970. doi: 10.1016/j.carres.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Markham KR, Webby RF, Vilain C. 7-O-methyl-(2R:3R)-dihydroquercetin 5-O-β-D-glucoside and other flavonoids from Podocarpus nivalis. Phytochemistry. 1984;23:2049–2052. [Google Scholar]
- 29.Pauli GF, Junior P. Phenolic glycosides from Adonis aleppica. Phytochemistry. 1995;38:1245–1250. [Google Scholar]
- 30.Ahmed I, Ishratullah K, Ilyas M, Rahman W, Seligmann O, Wagner H. Tetrahydroamentoflvone from nuts of Semecarpus prainii. Phytochemistry. 1981;20:1169–1170. [Google Scholar]
- 31.Suarez AI, Beth DM, Monache FD, Compagnone RS. Biflavonoids from Podocalyx loranthoides. Fitoterapia. 2003;74:473–475. doi: 10.1016/s0367-326x(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 32.Xie LH, Akao T, Hamasaki K, Deyama T, Hattori M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem Pharm Bull. 2003;5:508–515. doi: 10.1248/cpb.51.508. [DOI] [PubMed] [Google Scholar]
- 33.Lin LC, Chou CJ. Three new biflavonoids from Selaginella delicatula. Chin Pharm J (Taipei) 2000;52:211–218. [Google Scholar]
- 34.Rani MS, Rao CV, Gunasekar D, Blond A, Bodo B. A biflavonoid from Cycas beddomei. Phytochemistry. 1998;47:319–321. [Google Scholar]
- 35.Jutiviboonsuk A, Zhang H, Tan GT, Ma C, Hung NV, Cuong NM, Bunyapraphatsara N, Soejarto DD, Fong HHS. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry. 2005;66:2745–2751. doi: 10.1016/j.phytochem.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Calis I, Kuruüzüm-Uz A, Lorenzetto PA, Ruedi P. (6S)-hydroxy-3-oxo-α-ionol glucosides from Capparis spinosa fruits. Phytochemistry. 2002;59:451–457. doi: 10.1016/s0031-9422(01)00399-5. [DOI] [PubMed] [Google Scholar]
- 37.Mabry TJ, Markham KR, Thomas MB. The systematic identification of flavonoids. Heidelberg-New York: Springer-Verlag; 1970. p. 354. [Google Scholar]
- 38.Gaffield W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: Determination of aglycone chirality in flavanone glycosides. Tetrahedron. 1970;26:4093–4108. [Google Scholar]
- 39.Ding Y, Li XC, Ferreira D. Theoretical calculation of electronic circular dichroism of the rotationally restricted 3,8″-biflavonoid morelloflavone. J Org Chem. 2007;72:9010–9017. doi: 10.1021/jo071134z. [DOI] [PubMed] [Google Scholar]
- 40.Chen JY, Chen JM, Xiao PG, Wu N, Lu Y. Crystal structure of loliolide [5,6,7α-tetrahydro-6-hydroxy-4,4,7α-trimethyl-2(4H)-benzofuranone] Jiegou Huaxue. 1997;16:335–337. [Google Scholar]
- 41.Jong TT, Jean MY. Constituents of Houttuyniae cordata and the crystal structure of vomifoliol. J Chin Chem Soc (Taipei, Taiwan) 1993;40:399–402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.