Summary
The hedgehog (Hh) family of morphogens plays important instructional roles in the development of numerous metazoan structures [1]. Consistent with the role Hh homologs play in cell fate determination, aberrant Hh signaling results in numerous human pathologies. Hh signal transduction is initiated when Hh binds to its receptor Patched (Ptc) [2, 3], activating the transmembrane protein Smoothened (Smo)[4, 5]. Smo transmits its activation signal to a microtubule-associated Hedgehog signaling complex (HSC). At a minimum, the HSC consists of the Kinesin-related protein Costal2 (Cos2), the protein kinase Fused (Fu), and the transcription factor Cubitus interruptus (Ci) [6–11]. In response to HSC activation, the ratio between repressor and activator forms of Ci is altered, determining the expression levels of various Hh target genes [11–13]. The steps between Smo activation and signaling to the HSC have not been described. Here, we describe a functional interaction between Smo and Cos2, which is necessary for Hh signaling. We propose that this interaction is direct and allows for activation of Ci in response to Hh. This work fills in the last major gap in our understanding of the Hh signal transduction pathway by suggesting that no intermediate signal is required to connect Smo to the HSC.
Results and Discussion
Smoothened Associates Directly with the HSC through Cos2
In response to Hh, Smo is phosphorylated, stabilized, and relocalized to the plasma membrane [14–17]. The cause and effect of these processes are unknown, as are the mechanistic events by which Smo communicates with the intracellular Hh signaling components. Smo is a member of the heterotrimeric G protein-coupled receptor (GPCR) superfamily and, as such, was originally thought to signal through an associated G protein [4, 18]. However, to date there has been little evidence supporting a role for G proteins immediately downstream of Smo, suggesting that Smo may signal to the HSC through some novel mechanism. A chimeric GPCR containing the carboxyl-terminal cytoplasmic domain of Smo fused to the Frizzled1 receptor is capable of activating Hh signaling, albeit in a Wg-dependent manner [5]. The carboxyl-terminal domain of GPCRs is not normally coupled to G proteins, further supporting the idea that the Smo carboxyl-terminal domain signals to downstream components via a novel mechanism. Our recent genetic analysis of various Smo mutants indicated that stoichiometric interactions between Smo and Cos2 may regulate HSC activity [5]. To test this hypothesis, we immunoprecipitated Smo from wild-type Drosophila embryo lysate using antisera specific to either the amino terminus of Smo, the carboxy terminus of Smo, or a species-matched control IgG (Figure 1A). Both Cos2 and Fu coimmunoprecipitated specifically with both Smo antisera but were not detected in the control immunoprecipitation. These results indicate that a physical interaction may exist between Smo and members of the HSC.
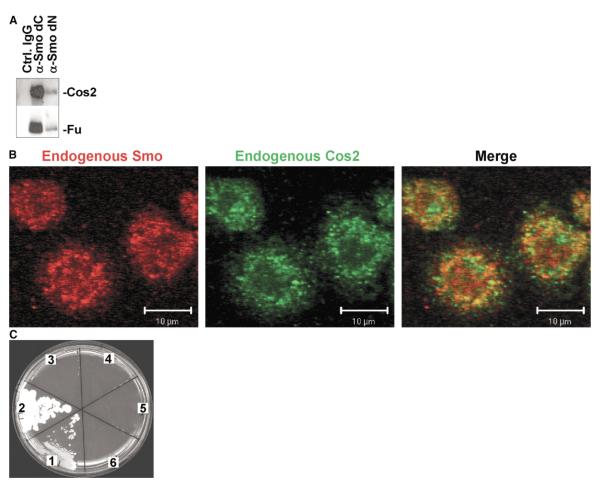
Figure 1.
Smo Associates with the HSC via Direct Interaction with Cos2 (A) The HSC associates with endogenous Smo. Wild-type Drosophila embryos were lysed and immunoprecipitated with antibodies generated to either amino- (dN), carboxyl-terminal (dC) regions of Smo, or control antibody. Cos2 and Fu immunoprecipitating with Smo are indicated. (B) Smo and Cos2 colocalize in Drosophila S2 cells. Endogenous Smo and Cos2 in S2 cells were stained using dC anti-Smo (Santa Cruz) and 5D6 anti-Cos2 mAbs, respectively. (C) Smo and Cos2 associate directly. Matings were performed between indicated yeast strains, then streaked on selective media in numbered sectors as follows: (1) pGAD-SmoC and pGBDU-Cos2, (2) pGAD-Fu-tail and pGBDU-Cos2, (3) pGAD-Fu-tail and pGBDU-antiCos2, (4) pGAD-SmoC and pGBDU-antiCos2, (5) pGAD-antiCos2 and pGBDU-SmoC, and (6) pGAD-Cos2 and pGBDU-SmoC. “Anti” indicates antisense orientation of the indicated cDNA.
To examine whether Smo and Cos2 localize similarly in cells, we visualized the two proteins in Drosophila S2 cells and wing imaginal discs using indirect immunofluorescence microscopy (Figures 1B and 2C). S2 cells were fixed, permeabilized, and stained using appropriate antibodies (Experimental Procedures). A significant amount of colocalization (66% of Cos2 colocalized with Smo; 77% of Smo colocalized with Cos2, see Experimental Procedures) between Smo and Cos2 is evident (merge, seen as yellow). As a control, we also quantitated the colocalization between Fu and Cos2 in S2 cells (M.A., Jr. and D.J.R, unpublished data) and found that ~90% of Cos2 colocalized with Fu, whereas ~50% of Fu colocalized with Cos2. Previously, we have provided significant biochemical evidence that Fu and Cos2 directly associate [24]. Therefore, the colocalization values for Smo and Cos2 are similar to the colocalization values of two proteins known to directly associate. Thus, our results are consistent with the hypothesis that a significant proportion of Smo and Cos2 colocalize.
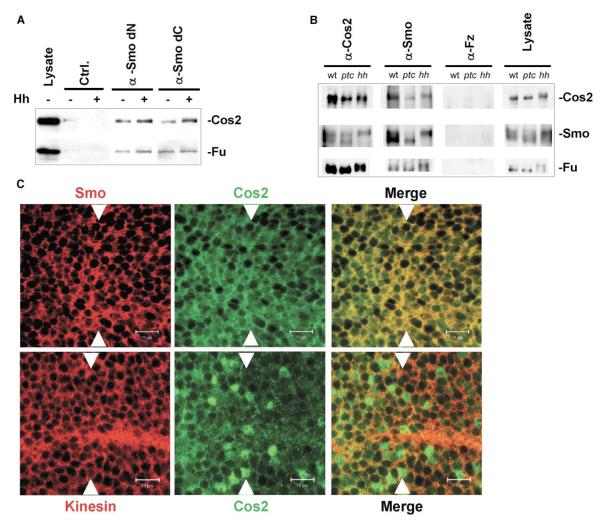
Figure 2.
Smo-Cos2 Association Is Not Modified in Response to Hh (A) Smo and Cos2 association in S2 cells is Hh insensitive. Postnuclear lysates were prepared from S2 cells transfected with Hh expression (pDA-Flag-HhN) or control vector. Smo was immunoprecipitated from these lysates using Smo dC or dN antibodies, as indicated. Immunoprecipitates were probed for the presence of Fu and Cos2. (B) Smo-Cos2 association is Hh insensitive in vivo. Drosophila embryos representing normal (wt), repressed (ptc), or activated (hh) Hh signaling were generated by expressing Ptc or Hh. Lysates from 25 mg of embryos were immunoprecipitated with either α-Cos2 5D6 mAb (Cos2 IP), Smo antibody (Smo IP), or Fz antibody (Fz IP). Western blots were probed for Smo (N-terminal antibody), Fu, and Cos2. Each protein has multiple isoforms, with the slower-migrating forms due to phosphorylation [6, 25]. The unmodified form of each protein is favored in the absence of Hh signaling (and in ptc-overexpressing embryos), while the phosphorylated forms appear with Hh exposure (hh lanes). (C) Smo-Cos2 colocalization is similar across the wing imaginal disc. Wild-type wing imaginal discs were immunostained with antibodies directed against Smo (red) and Cos2 (green) (top), or Kinesin (red) and Cos2 (green) (bottom). For each panel, anterior is to the left, and dorsal is toward the top. The anterior/posterior boundary is marked by arrowheads. The colocalization of Kinesin or Fz1 (data not shown) was minimal, emphasizing the specificity of the Smo-Cos2 interaction. Low-magnification images are also provided for comparison; see Figure S1.
To determine whether Cos2 and Smo could interact directly, we utilized a directed yeast two-hybrid assay (Figure 1C). The cytoplasmic carboxyl-terminal domain of Smo was used in the two-hybrid assay, as the signaling capabilities of Smo appear to reside within this domain [5]. We find that the carboxyl-terminal domain of Smo interacts with Cos2, though this interaction appears less efficient than that of Cos2 with Fu (compare sector 1 to sector 2). This interaction is specific and reproducible, as there is no growth when the open reading frame of Cos2 is inserted in the reverse orientation (sectors 4 and 5). These results demonstrate that the carboxyl-terminal domain of Smo is sufficient to associate with Cos2 and that this association appears to be direct. Combined with our immunoprecipitation and immunofluorescence data, our yeast two-hybrid results provide strong evidence that Smo and Cos2 directly associate and that the association occurs within the intracellular signaling portion of Smo.
The Smo Association with Cos2 Is Hh Independent
To determine whether Hh signaling would affect the Cos2-Smo interaction, we immunoprecipitated Smo from S2 cell lysates prepared from cells transfected with Hh expression [19] or control vectors (Figure 2A). Cos2 and Fu coimmunoprecipitate with Smo at similar levels regardless of Hh activation status. Phosphorylation-induced mobility shifts of Cos2 occurred in Hh-transfected cells, verifying that Hh signaling is intact (Figure 2A). The modest increase observed in Cos2 immunoprecipitating with Smo in response to Hh stimulation may be accounted for by Smo protein stabilization in response to Hh [15, 16]. Our results suggest that interactions between Smo, Cos2, and Fu are relatively stable and independent of Hh activation status.
To verify that Hh activation does not modify Smo-Cos2 association in vivo, we performed Smo immunoprecipitations from embryos engineered to overexpress Ptc, Hh, or neither (Figure 2B). Embryos overexpressing Ptc serve as a source of cells in which Hh signaling is inactive [20, 21] due to repression of Smo by Ptc, while embryos overexpressing Hh serve as a source of Hh-activated cells. Mobility shifts of Cos2, Fu, and Smo, which have previously been attributed to Hh-induced phosphorylation [9, 15, 25], confirm that Hh or Ptc have turned Hh signaling on or off in these embryos. We observe an equal amount of Cos2 coimmunoprecipitating with Smo from wild-type, Ptc, and Hh embryo lysates (Figure 2B). In two separate experiments, we estimated that 3% of Cos2, 4% of Fu, and 3%–8% of Smo were recovered in coimmunoprecipitates. By contrast, ~50% of Fu was recovered by Cos2 immunoprecipitation, while negligible amounts of Fu, Cos2, or Smo was recovered in Fz immunoprecipitates. These results are similar to Figure 2A and demonstrate that a small percentage of total Cos2 and Smo are associated in a high-affinity association, and the percentage associated does not change due to Hh signaling.
As a further test of whether Smo-Cos2 association could be regulated by Hh, we examined their degree of colocalization in Drosophila wing imaginal discs. Hh is expressed throughout the posterior compartment of the wing imaginal disc but diffuses into the anterior compartment to trigger a graded series of Hh activation states. We find that approximately 71% of total Cos2 colocalizes with Smo, while approximately 53% of total Smo colocalizes with Cos2. These percentages were similar regardless of whether we measured their colocalization in anterior (Hh responsive) or posterior (Hh producing but nonresponsive) compartments (Figure 2C, top). We observe approximately 20% colocalization between Cos2 and either Kinesin (Figure 2C, bottom) or Frizzled1 (data not shown). We interpret these latter results to indicate the percent localization one would observe between Cos2 and a protein that would localize to similar regions within a cell, but would not necessarily associate. These results support our proposal that the amount of Cos2 associating with Smo is not dramatically altered in response to activated Hh signaling. The apparent difference in the amount of Smo and Cos2 association detected using coimmunoprecipitation and coimmunofluorescence analysis may be explained by the fact that coimmunoprecipitation gives a lower limit for efficiency of associations, as only stable associations are detected. Colocalization by immunofluorescence gives an upper limit to associations by highlighting populations physically close to each other. Therefore, it is likely that the true extent of Cos2 and Smo association lies between the levels quantified by these two assays.
A Functional Interaction between Smo and Cos2 Is Required for Proper Hh Signaling
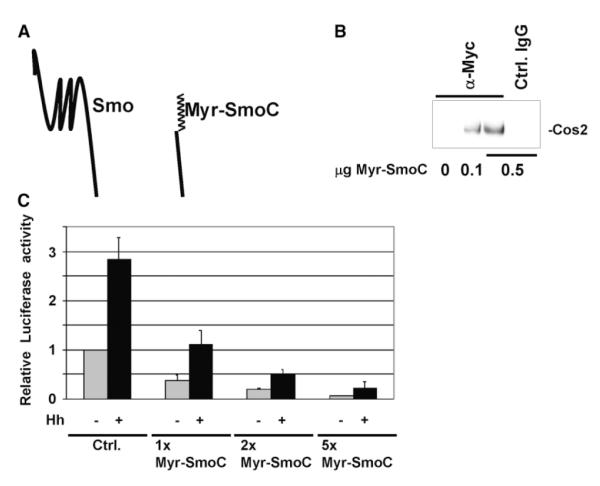
Expression of a chimera of SmoC fused to a myristate membrane-targeting sequence (Myr-SmoC) induces phenotypes in Drosophila similar to cos2 loss-of-function mutations; weak Hh responses are activated, while strong Hh responses are inhibited [5]. Myr-SmoC drives all Hh responses to a weak activation state in Drosophila and requires endogenous Smo to do so [5]. Although the mechanism by which Myr-SmoC acts is unknown, dosage dependence of the effect suggests that it interferes with signaling by competing with endogenous Smo for Cos2 [5]. We expressed a similar epitope-tagged construct (Figure 3A) in Clone 8 (Cl8) cells to test the hypothesis that Myr-SmoC interferes with signaling by binding to Cos2. Using a Myc epitope tag to specifically immunoprecipitate Myr-SmoC, we find that both Cos2 and Fu (not shown) associate with Myr-SmoC (Figure 3B). These data support our directed two-hybrid experiment, showing that the carboxyl-terminal domain of Smo is sufficient to interact with Cos2. Further, we find that Myr-SmoC functions as a potent inhibitor of Hh signaling, able to inhibit Hh-dependent transcription in a dose-dependent fashion (Figure 3C). Our results indicate that even in the absence of Hh, Ci activity is effectively reduced by Myr-SmoC. Thus, Myr-SmoC does not constitutively activate Ci in this reporter assay. We propose that Myr-SmoC can act as a dominant negative by binding endogenous Cos2. This argument is bolstered by genetic evidence showing that increasing Cos2 levels in vivo can suppress the overgrowth phenotype associated with expressing Myr-SmoC in flies [5]. Our results are consistent with the hypothesis that association between Smo and Cos2 is necessary for Hh signaling to be propagated to its ultimate effector, the transcription factor Ci.
Figure 3.
Cos2 Association with Smo Is Necessary for Hh Signal Transduction (A) Myr-SmoC diagram. Illustration of wild-type Smo and Myr-SmoC. Myr-SmoC contains an amino-terminal myristoylation signal, as well as a Myc epitope tag. (B) Myr-SmoC associates with Cos2. Myr-SmoC was immunoprecipitated, using anti-Myc beads (Sigma), from postnuclear lysates of Cl8 cells transfected with increasing amounts of pUAS-Myr-SmoC, as indicated. An IgG control immunoprecipitation was performed from the lysate of cells transfected with 0.5 μg of Myr-SmoC. Immunoprecipitates were separated by SDS-PAGE and immunoblotted for Cos2 and Fu (data not shown). (C) Myr-SmoC inhibits Hh activated transcription. 5 × 106 Cl8 cells were transfected with 0.1 μg ptcΔ136-Luc reporter, 0.02 μg pRL-TK transfection control, increasing amounts of pUAS-Myr-SmoC (0, 0.1, 0.2, and 0.5 μg) with pActGal4, and either 0.2 μg pDA-HhN-Flag (black columns) or a control vector (gray columns). Luciferase activity was normalized to a Renilla transfection control and is expressed relative to baseline ptcΔ136-Luc activity.
We propose two scenarios that may account for the observation that Smo and Cos2 association is not altered in response to Hh. The first possibility is that Smo and Cos2 may be held in an associated but inactive state in the absence of Hh stimulation, presumably through the function of Ptc. Hh stimulation would relieve Ptc-mediated repression of the Smo-Cos2 complex to allow Smo relocalization to the plasma membrane, as has previously been reported [17]. The Kinesin-like properties of Cos2 and its direct interaction with Smo may facilitate this relocalization. A second possibility is that the dynamics of association are changed in response to Hh, such that Smo and Cos2 association turns over more rapidly in the process of creating the active form of Ci.
Experimental Procedures
Immunoprecipitation from Embryo Lysates and Cells
Cell and embryo lysates were precleared for 30 min at 4°C with protein G beads (Sigma). 10 μg of α-Smo antibody (dC-20 or dN-17, Santa Cruz) or an isotype-matched control antibody was added for 1 hr at 4°C. Immune complexes were collected with protein G beads during a final 30 min incubation. Beads were collected by pulse centrifugation and then washed three times in a Triton X-100 buffer (1% Triton X-100, 150 mM NaCl, 25 mM Hepes, 0.5 mM DTT [pH 7.5]). Samples were resuspended in 2× Laemmli buffer, boiled for 10 min, and then analyzed by gel electrophoresis and immunoblot.
For Myr-SmoC immunoprecipitations, Clone 8 (Cl8) imaginal disc cells were transfected with increasing amounts of pUAS-myc-myr-SmoC [5] plus pAct-Gal4, with DNA content per transfection normalized with empty vector. Cells were lysed in NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris, 5 mM NaF [pH 8.0]). Postnuclear lysates were incubated with 20 μl of a 50% anti-myc agarose slurry (Sigma) or control rabbit sera plus protein A agarose (Sigma). Following incubation, beads were washed three times in 1% NP-40 lysis buffer and prepared for immunoblot analysis.
Immunoprecipitation from Transgenic Embryo Lysates
Transgenic embryos were collected from matings of hsp70::Gal4 females with UAS::Ptc males, y w males, or UAS::Hh males. Following 1 hr heatshock at 37°C to induce Gal4 expression, embryos were recovered for 8 hr at 25°C and then lysed by homogenization in Brij buffer (1% Brij-97, 50 mM EDTA, 25 mM NaF, 0.4 mM NaVO3, and PIC in PBS [pH 7.4]). Postnuclear lysates were precleared for 60 min with protein A beads (Pharmacia) and then incubated overnight at 4°C with Cos2 monoclonal, Smo polyclonal [14], or Fz1 1C11 monoclonal antibodies [22] as control. Immune complexes were collected on protein A (for Smo) or G (for Cos2) beads for 60 min at 4°C, then washed four times with Brij buffer and once with PBS. Complexes were resuspended in 2× Laemmli buffer plus 8M Urea, heated to 65°C for 5 min, and then analyzed by gel electrophoresis and immunoblot. Three different aliquots of lysate (0.2%, 1%, and 5%) and two dilutions of immunoprecipitations (100% and 10%) were immunobloted to estimate efficiencies of immunoprecipitation reactions (data not shown).
Immunofluorescence Microscopy
S2 cells transfected with pAct-3XHA-Cos2 and pUAS-Myr-Smo plus pAct-Gal4 and untransfected control cells were plated on ConA-treated slides [23] 2 hr prior to fixing with 4% formaldehyde. Cells were permeabilized and blocked with 5% normal goat sera or 5% BSA and 0.1% Triton X-100 in PBS for 1 hr prior to incubation with appropriate antibodies (1/500 mouse anti-myc [Covance], 2 μg/ml mouse monoclonal anti-Cos2 5D6 [24], 2 μg/ml goat anti-Smo dN17 [Santa Cruz], 2 μg/ml goat anti-Smo dC20 [Santa Cruz], diluted in PBGT [5% normal goat serum or BSA and 0.1% Tween-20 in PBS]). Cells were incubated with primary antibodies for 1 hr and then washed three times in PBGT. The appropriate fluorescent secondary antibodies were then applied to each sample for 1 hr (goat anti-mouse Alexa-546 or Alexa-488, donkey anti-goat Alexa-546 [all at 1:500 dilution in PBGT; Molecular Probes]). Experimental samples were blanked against control chambers. Confocal images were collected using the LSM-510 Confocal Laser Scanning Microscope (Zeiss) and processed using LSM Image Browser software (Zeiss) and Adobe Photoshop 6.0. The LSM-510 pinhole diameters for each fluorescence channel were set for 1 μm (S2 cells) or 1.6 μm (wing discs) thick image sections. All images were taken using a 63× oil-immersion objective at 1024 × 1024 pixel resolution.
Preparation and immunofluorescence microscopy of wing discs were performed as described [24] using mouse anti-Cos2 5D6 (4 μg/ml), rabbit anti-Smo (kindly provided by the Cohen laboratory) (1:100), and rabbit anti-kin01 (Cytoskeleton, Inc.) (2.5 μg/ml). Secondary antibodies included goat anti-mouse Alexa-488, goat anti-rabbit Alexa-546, and goat anti-rabbit Alexa-647 (pseudocolored red), all diluted at 1:250 in PBGT.
Colocalization analysis was performed using Metamorph 5.05 software. Percent colocalization values are defined as the percent of protein A pixels that colocalized with protein B pixels. Pixels were considered as a true signal by zeroing the signal threshold settings against appropriate control images. The control images were taken with the exact acquisition setting as the experimental images on the day each experiment was performed.
Supplementary Material
Acknowledgments
National Institutes of Health grants CA82628 (to D.J.R.) and GM4539 (to J.E.H) and NCI National Institutes of Health Training Grant 5-T32 ES07250 to S.K.O, L.M.S., and M.A., Jr. supported this work. M.A., Jr. is an Albert J. Ryan Fellow. We thank Dr. R. Fukunaga (Osaka University, Japan) for the HhN expression vector, Dr. P. Adler (University of Virginia) for the Frizzled1 antibody, Dr. P. Beachy (Johns Hopkins) for the Ptc reporter construct, Dr. M. Scott (Stanford University, School of Medicine) for UAS-Ptc flies, and Dr. P. Ingham (University of Sheffield) for UAS-Hh flies. We thank the University of Cincinnati, Department of Cell Biology Microscopy Core for their expert assistance. We also thank C. Nasrallah and J. Goetz for expert technical assistance.
Footnotes
Supplemental Data Supplemental Data including experimental procedures and a figure showing low-magnification images of Figure 2C are available at http://www.current-biology.com/cgi/content/full/13/22/1998/DC1/.
References
- 1.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 2.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 3.Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- 4.van den Heuvel M, Ingham PW. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature. 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 5.Hooper JE. Smoothened translates Hedgehog levels into distinct responses. Development. 2003;130:3951–3963. doi: 10.1242/dev.00594. [DOI] [PubMed] [Google Scholar]
- 6.Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- 7.Stegman MA, Vallance JE, Elangovan G, Sosinski J, Cheng Y, Robbins DJ. Identification of a tetrameric hedgehog signaling complex. J. Biol. Chem. 2000;275:21809–21812. doi: 10.1074/jbc.C000043200. [DOI] [PubMed] [Google Scholar]
- 8.Motzny CK, Holmgren R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 1995;52:137–150. doi: 10.1016/0925-4773(95)00397-j. [DOI] [PubMed] [Google Scholar]
- 9.Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- 10.Monnier V, Dussillol F, Alves G, Lamour-Isnard C, Plessis A. Suppressor of fused links fused and Cubitus interruptus on the hedgehog signalling pathway. Curr. Biol. 1998;8:583–586. doi: 10.1016/s0960-9822(98)70227-1. [DOI] [PubMed] [Google Scholar]
- 11.Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, Kornberg TB. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. [DOI] [PubMed] [Google Scholar]
- 12.Methot N, Basler K. An absolute requirement for Cubitus interruptus in Hedgehog signaling. Development. 2001;128:733–742. doi: 10.1242/dev.128.5.733. [DOI] [PubMed] [Google Scholar]
- 13.Kalderon D. Hedgehog signalling: Ci complex cuts and clasps. Curr. Biol. 1997;7:R759–R762. doi: 10.1016/s0960-9822(06)00398-8. [DOI] [PubMed] [Google Scholar]
- 14.Alcedo J, Zou Y, Noll M. Posttranscriptional regulation of smoothened is part of a self-correcting mechanism in the Hedgehog signaling system. Mol. Cell. 2000;6:457–465. doi: 10.1016/s1097-2765(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 15.Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 16.Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr. Biol. 2000;10:1315–1318. doi: 10.1016/s0960-9822(00)00755-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhu AJ, Zheng L, Suyama K, Scott MP. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 2003 doi: 10.1101/gad.1080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–232. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 19.Fukumoto T, Watanabe-Fukunaga R, Fujisawa K, Nagata S, Fukunaga R. The fused protein kinase regulates Hedgehog-stimulated transcriptional activation in Drosophila Schneider 2 cells. J. Biol. Chem. 2001;276:38441–38448. doi: 10.1074/jbc.M105871200. [DOI] [PubMed] [Google Scholar]
- 20.Schuske K, Hooper JE, Scott MP. patched over-expression causes loss of wingless expression in Drosophila embryos. Dev. Biol. 1994;164:300–311. doi: 10.1006/dbio.1994.1200. [DOI] [PubMed] [Google Scholar]
- 21.Ingham PW, Hidalgo A. Regulation of wingless transcription in the Drosophila embryo. Development. 1993;117:283–291. doi: 10.1242/dev.117.1.283. [DOI] [PubMed] [Google Scholar]
- 22.Krasnow RE, Adler PN. A single frizzled protein has a dual function in tissue polarity. Development. 1994;120:1883–1893. doi: 10.1242/dev.120.7.1883. [DOI] [PubMed] [Google Scholar]
- 23.Rogers SL, Rogers GC, Sharp DJ, Vale RD. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 2002;158:873–884. doi: 10.1083/jcb.200202032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ascano M, Jr., Nybakken KE, Sosinski J, Stegman MA, Robbins DJ. The carboxyl-terminal domain of the protein kinase fused can function as a dominant inhibitor of hedgehog signaling. Mol. Cell. Biol. 2002;22:1555–1566. doi: 10.1128/mcb.22.5.1555-1566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therond PP, Knight JD, Kornberg TB, Bishop JM. Phosphorylation of the fused protein kinase in response to signaling from hedgehog. Proc. Natl. Acad. Sci. USA. 1996;93:4224–4228. doi: 10.1073/pnas.93.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.