Abstract
Background
Although recombinant adeno-associated virus serotype 2 (AAV2) vectors have gained attention owing to their safety and efficacy in number of Phase I/II clinical trials, their transduction efficiency in hematopoietic stem cells (HSCs) has been reported to be low. Only a handful of additional AAV serotype vectors have been evaluated, and comparative analyses of their transduction efficiency in HSCs from different species have not been performed.
Methods
Here, we evaluated the transduction efficiency of all available AAV serotype vectors (AAV1 through AAV10) in primary mouse, cynomolgus monkey, and human HSCs, respectively. The transduction efficiency of the optimized AAV vectors was also evaluated in human HSCs in a murine xenograft model in vivo.
Results
We observed that although there are only six amino acid differences between AAV1 and AAV6, AAV1, but not AAV6, transduce mouse HSCs cells well, whereas AAV6, but not AAV1, transduce human HSCs well. None of the 10 serotypes transduce cynomolgus monkey HSCs in vitro. We also evaluated the transduction efficiency of AAV6 vectors containing mutations in surface-exposed tyrosine residues, and observed that tyrosine (Y) to phenylalanine (F) point mutations in residues 445, 705, and 731, led to a significant increase in transgene expression in human HSCs in vitro and in a mouse xenograft model in vivo.
Discussion
These studies suggest that the tyrosine-mutant AAV6 serotype vectors are the most promising vectors for transducing human HSCs, and that it is possible to further increase the transduction efficiency of these vectors for their potential use in HSC-based gene therapy in humans.
Keywords: AAV vectors, hematopoietic stem cells, gene transfer, gene expression
Introduction
Transplantation of patient-derived, genetically-modified autologous hematopoietic stem cells (HSCs) is, to date, the most promising alternative to allogeneic transplantation to potentially cure certain genetic diseases such as β -thalassemia and sickle cell disease. Indeed, in a recent clinical trial, a recombinant lentiviral vector-mediated β -globin gene transfer in an adult patient with severe β-thalassaemia led to transfusion-independence [1]. However, the observed therapeutic benefit was also accompanied by transcriptional activation of a cellular proto-oncogene, HMGA2, following lentiviral vector integration, leading to clonal expansion of myeloid cells. Thus, it is clear that alternatives to lentiviral vectors are needed. The safety as well as clinical efficacy of recombinant vectors based on a non-pathogenic human virus, the adeno-associated virus 2 (AAV2) has attracted attention [2, 3]. However, controversies abound with reference to the transduction efficiency of AAV2 vectors in human HSCs. For example, we and others have reported that AAV2 vectors efficiently transduce human CD34+ cells at relatively low multiplicities of infection (MOIs) [4-9], whereas some have reported that successful transduction of human CD34+ cells by AAV2 vectors requires exceedingly high (>106) MOIs [10, 11]. One group claimed that the alleged transduction of human CD34+ cells by AAV2 vectors is due to contaminants [12]. The underlying basis of these discrepancies has been reviewed previously [13, 14].
In recent years, a number of additional AAV serotype vectors have become available, but only a handful of these vectors have been evaluated for their ability to transduce human HSCs. For example, Handa et al. [15] reported that AAV3 vectors did not show any advantage over AAV2 vectors; Schuhmann et al. [16] documented that AAV2 vectors transduce human cord blood CD34+ cells more efficiently than AAV3 and AAV5 vectors; and Veldwijk et al. [17] documented that AAV6 was the most efficient serotype among AAV1 through AAV6 for human CD34+ cells. However, all previous studies have been limited to in vitro conditions only. We have previously reported that AAV1 vectors mediate the highest levels of transgene expression among AAV1, AAV2, AAV4, AAV5, AAV7, AAV8, and AAV10 serotypes in murine HSCs in vivo [18, 19]. We and others have also documented that site-directed mutagenesis of surface-exposed tyrosine residues on AAV serotype capsids leads to higher transduction efficiency both in vitro and in vivo in various cell types [20-25].
In our present studies, we systematically evaluated the transduction efficiency of the 10 available AAV serotype vectors in primary HSCs from mice, cynomolgus monkeys, and humans, respectively. We report here that: (i) AAV1 vectors transduce primary murine HSCs most efficiently; (ii) None of the 10 AAV serotype vectors transduce cynomolgus monkey HSCs well in vitro; (iii) AAV6 vectors are the most efficient in transducing primary human HSCs, both in vitro and in a mouse xenograft model in vivo; and (iv) The transduction efficiency of AAV6 vectors is further augmented following site-directed mutagenesis of specific surface-exposed tyrosine residues, both in vitro and in vivo. These studies suggest that the optimized AAV6 vectors may prove to be a safe and effective alternative to lentiviral vectors for their potential use in HSC-based gene therapy in humans.
Methods
Plasmids
All AAV serotype vectors are encapsidated using the AAV2 inverted terminal repeats (ITRs) and rep sequences, and these plasmids are designated as pATGrep/cap or pACGrep/cap, in which ATG and ACG denote the start codon for Rep78/68 proteins. Xiao and Samulski reported that mutation of the start codon of rep78/68 from ATG to ACG could up regulate AAV packaging efficiency [26]. pACG2/6 was constructed by replacing the fragment between Xba I and Nco I on pATG2/6 by the fragment between Xba I and Nco I on pACG2/2. pACG2/1 - pACG2/6 were kind gifts from Dr. R. Jude Samulski, University of North Carolina at Chapel Hill, NC, and pACG2/7 - pACG2/10 were generously provided by Dr. James M. Wilson, University of Pennsylvania, Philadelphia, PA. Y to F capsid mutants were generated with pACG2/6 using QuikChange® II Site-Directed Mutagenesis Kit (Stratagene) as described previously [20]. Surface-exposed tyrosine residues are described in Supplementary Table 4, and primers containing sequence changes for introducing point mutations and amino acid changes are detailed in Supplementary Table 5. PCR was performed according to the manufacturer’s instructions. All mutants were sequence-screened before use.
AAV vector production
Viral vectors were packaged using a protocol described previously [18]. Briefly, HEK 293 cells were co-transfected by three plasmids in the presence of Polyethyleneimine (PEI, linear, MW 25,000, Polyscinces, Inc.), and medium was replaced 4 hrs post-transfection [20]. Cells were harvested at 72 hrs post-transfection, subjected to 3 rounds of freeze-thaw, digested with Benzonase (Invitrogen) and purified by iodixanol (Sigma) gradient ultracentrifugation followed by ion exchange chromatography using HiTrap SP HP for AAV2 and HiTrap Q HP for all other serotypes (GE Healthcare) or purified through two rounds of cesium chloride gradient centrifugation. Titers were determined by quantitative DNA slot blot using 32P-labeled specific DNA probes as previously described [20] or titered using a Taqman qPCR assay (21).
Mice
Four month-old male C57BL/6 mice were purchased from the Jackson Laboratory and maintained in the University of Florida Animal Care Facility. Six- to 8 week-old male NOD.CB17-Prkdcscid/NcrCrl (NOD/SCID) mice for xeno-transplantation were maintained in a specific pathogen-free (SPF) facility at the Animal Resources Center (City of Hope). All experimental protocols involving animals were approved by each of the local Institutional Animal Care and Use Committee guidelines.
Cell isolation and culture
Human embryonic kidney 293 (HEK293) and human erythroleukemia K562 cells were obtained from American Type Culture Collections (Manassas, VA) and maintained in Dulbecco’s-modified Eagle’s medium (DMEM; Lonza, Walkersville, MD), or Iscove’s-modified Dulbecco’s medium (IMDM; Irvine Scientific, Santa Ana, CA) supplemented with 10% fetal bovine serum (FBS; Sigma, St. Louis, MO), 100 μg/ml of penicillin and 100 U/ml of streptomycin (Invitrogen, Grand Island, NY) at 37°C. Human bone marrow CD34+ and umbilical cord blood CD34+ cells were either purchased from AllCells or obtained under a City of Hope Institutional Review Board approved protocol isolated as described by Chatterjee et al. [7], and maintained in either StemSpan SFEM medium (Stem Cell Technologies, Inc) or Iscove’s modified Dulbecco’s medium (IMDM; Irvine Scientific, Santa Ana, CA) with 10 ng/ml of rhIL6 (Peprotech), 10 ng/ml of rhIL3 (Peprotech), and 1 ng/ml of rhSCF (R&D Systems). CD34+ cell purity was determined by flow cytometry and was ≥ 90%. Mouse HSCs, which were positive for Sca-1, c-kit and negative for lineage markers (c-kit+, Sca-1+, lin-) were isolated from 4-month-old male C57BL/6 mice as previously reported (20-22) with slight modification. Briefly, femurs and tibias were obtained from C57BL/6 mice, and bone marrow cells were flushed from the bones with phosphate-buffered saline (PBS). The lineage-negative cells were isolated by immunomagnetic separation using Lineage Cell Detection Cocktail (MACS). APC-conjugated CD117 (c-kit) antibody and Alexa fluor 700 conjugated Sca-1 antibody (MACS) were used during fluorescence-activated cell sorting (FACS). Mouse c-kit+, Sca-1+, lin- cells were maintained and infected in IMDM in the presence of 10 ng/ml of mIL6, 10 ng/ml of mIL3, and 1 ng/ml of mSCF. Cynomolgus monkey (Macaca fascicularis) CD34+ cells were isolated from bone marrow from 2 monkeys according to established immunoselection protocols and cryopreserved using a controlled rate cryopreservation protocol in aliquots prior to use [27]. Cells were recovered and maintained in IMDM in the presence of 10% FBS, 10 ng/ml of rhIL6, rhIL3 and 1 ng/ml of rhSCF for 4 hrs before infection.
Visualization of AAV vectors localization using confocal microscopy
The method used to label AAV vectors with cyanine 3 (Cy3) (Amersham Life Sciences, Pittsburgh, Pa.) was modified from a previously published protocol [28]. Briefly, purified vectors were concentrated with Microcon YM-100 Centrifugal Filter unit (Millipore). Then the dye in sodium carbonate solution (pH 9.3, final concentration 0.1 M) was added. After incubating for 1.5 hrs at room temperature (RT) with gentle shaking, the vector-dye mixture was dialyzed with 20K MWCO Slide-A-Lyzer dialysis® Cassettes (PIERCE) in PBS overnight to remove the unconjugated dye. Vectors were then collected and titered before use. Since cord blood CD34+ cells are suspension cells, to minimize the loss of cells during the procedure, we used poly L-lysine (Sigma-Aldrich) to coat the acid washed cover slips and slides to provide higher adhesion. Cells were seeded in poly-L-lysine treated cover slips in 24- well plates the day before infection, and then infected at 2×104 vector genomes (vgs)/cell for the indicated time length. The medium was removed and cells were washed with PBS and fixed by 4% paraformaldehyde (PFA) (USB Corp.) for 8 minutes at RT. Cover slips with cells attached were then transferred to slides and mounted with ProLong® Gold antifade reagent with DAPI (Invitrogen). Images were acquired on Leica TCS SP5 confocal microscopy using oil immersed 63 × objective lens.
Ex vivo transduction of HSCs
Frozen human cord blood CD34+ cells were recovered and seeded in 96-well or 12- well plates (BD Falcon™) with either StemSpan SFEM or complete IMDM containing 10 ng/ml of rhIL6, 10 ng/ml of rhIL3 and 1 ng/ml of rhSCF for 2 hrs before infection to allow the cells to recover. Infection of human CD34+ cells was performed in the same medium unless otherwise specified. Different transduction conditions were compared in some experiments. To evaluate the effect of FBS, equivalent numbers of CD34+ cells from same lots were transduced either in the absence or the presence of 10% FBS in the above medium. To determine the influence of the duration time of infection, cells were infected either for 2 hrs or 16 hrs, and to examine the impact of different combinations of cytokines, equivalent numbers of CD34+ cells were transduced either under Condition 1: IMDM containing 10 ng/ml of rhFlt3, 10 ng/ml of rhTPO and 1 ng/ml of rhSCF, or Condition 2: IMDM containing 10 ng/ml of rhIL6, 10 ng/ml of rhIL3 and 1 ng/ml of rhSCF. Cynomolgus monkey CD34+ cells were infected in the presence of 10 ng/ml of rhIL6, 10 ng/ml of rhIL3 and 1 ng/ml of rhSCF. Mouse stem cells were infected in the presence of 10 ng/ml of mIL6, 10 ng/ml of mIL3 and 1 ng/ml of mSCF. Cells were infected at various viral particles/cell ratios at 37°C for 2 hrs or 16 hrs. Mock-infected or infected cells were infused into recipient mice or examined for transgene expression by fluorescence microscopy or by flow cytometry (Acurri C6) 48-72 hrs post-infection.
Xeno-transplantations
All experiments were performed under protocols approved by the City of Hope Institutional Animal Care and Use Committee. Transplantations were performed as described [29]. Briefly, 6-8 week old male NOD.CB17-Prkdcscid/NCrCrl (NOD/SCID) mice (Charles River, Wilmington, MA) were maintained in a specific pathogen free facility at the Animal Resources Center, City of Hope Medical Center and placed on sulfamethoxazole & trimethoprim oral pediatric antibiotic (Hi-Tech Pharmacal Co., Inc., Amityville, NY) (10 ml/500 ml H2O) for at least 48 hrs before transplant. Mice were sub-lethally irradiated with 350cGy from a 137Cs source and allowed to recover for a minimum of 4 hrs prior to transplantation. Approximately 106 CD34+ cells were infused via the tail vein in a volume of 200 μl. 3-5 mice were transplanted per vector group. Femoral marrow and the spleen were harvested for analysis from each mouse at 16 to 22 weeks post-transplantation. Each group consisted of 3 to 10 mice.
In vivo imaging
Luciferase expression in xeno-transplanted mice was measured by serial biweekly bioluminescent imaging using a Xenogen In Vivo Imaging System (Caliper Life Sciences, Hopkinton, MA) starting 4 weeks post-transplantation as described previously [21]. Briefly, mice were anesthetized with oxygen containing 4% isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO) for induction, and 2.5% for maintenance. Luciferin (Caliper Life Sciences, Hopkinton, MA) was injected intraperitoneally at a dose of 0.15 mg/gram of mouse weight. Photons were accumulated over a five-minute exposure from the ventral aspect, ten minutes post-injection. Living Image 3.0 software (Caliper Life Sciences, Hopkinton, MA) was used to calculate light emission.
Flow cytometric analysis
In vitro expression was analyzed 22 hrs after rAAV transduction in cells were washed with PBS containing 5% fetal calf serum (FCS), 0.1% sodium azide PBS (Mediatech, Manassas, VA) solution before analysis on a Cyan ADP Flow Cytometer (Dako, Denmark). Engraftment of human cells in bone marrow and spleen of xenografted mice was analyzed as described previously [29]. Lineage distribution was assessed in bone marrow and spleen cell suspensions following staining with human specific FITC-conjugated anti-CD45 (Becton Dickinson, Mountain View, CA).
rAAV frequency detection
The frequency of rAAV genomes in frequencies were detected in marrow cells of transplant recipients by quantitative real-time PCR with vector-specific primers and probe on a 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) as previously described [21]. The single-copy human gene ApoB, served to quantitate human cell equivalents and as template integrity controls [29].
Results and Discussion
Transduction efficiency of different AAV serotype vectors in murine, monkey, and human HSCs in vitro
We and others have previously reported that the transduction efficiency of conventional single-stranded AAV vectors in murine and human HSCs is negatively affected due to the rate-limiting step of viral second-strand DNA synthesis [16-19]. Thus, in the present study, we evaluated the transduction efficiency of the 10 serotypes using self-complementary (sc) AAV vectors containing the enhanced green fluorescent protein (EGFP) reporter gene driven by the chicken β-actin promoter (CBAp). Primary murine Sca-1+, c-kit+, lin- cells (>80% purity; >95% viability) from C57BL/6 mice, CD34+ cells from cynomolgus monkeys blood (~90% purity, ~90% viability), and CD34+ cells from human umbilical cord blood or bone marrow (~94% purity, ~98% viability) were either mock-transduced, or transduced with scAAV-CBAp-EGFP serotype vectors as described above. Transgene expression was analyzed 48-72 hrs post-infection by fluorescence microscopy and FACScan analyses. These data are summarized in Table 1, and the results from a representative experiment with human CD34+ cells are shown in Figure 1. Although the transduction efficiency was low, AAV1 serotype vectors were the most efficient in mediating transgene expression in murine HSCs, consistent with previously published studies [18, 19]. It is intriguing that AAV6 differs from AAV1 in only 6 amino acids [23, 24], but AAV6 vectors failed to transduce murine HSCs in vitro.
Table 1.
Transduction efficiency of scAAV serotype vectors in mouse, monkey, and human HSCs in vitro.
| %EGFP-positive cells | |||
|---|---|---|---|
| Vector | Murinea | Monkeyb | Humanc |
| Mock | 1.4±0.3 | 1.48 | 2.9±1.0 |
| scAAV1 | 6.1±2.1 | 1.88 | 4.5±3.4 |
| scAAV2 | 0.7±0.1 | 3.10 | 10.8±9.7 |
| scAAV3 | 0.8±0.3 | 2.56 | 4.2±2.7 |
| scAAV4 | 1.3±0.1 | 1.62 | 3.6±1.6 |
| scAAV5 | 1.0±0.9 | 1.46 | 3.1±1.7 |
| scAAV6 | 1.4±1.0 | 2.50 | 29.0±26.4 |
| scAAV7 | 1.2±0.2 | 2.42 | 3.5±2.6 |
| scAAV8 | 1.7±1.1 | 1.70 | 4.1±3.3 |
| scAAV9 | 1.0±0.7 | 1.96 | 3.3±2.2 |
| scAAV10 | 0.7±0.1 | 2.50 | 2.5±1.2 |
Cells were isolated from C57B/L mice as described in Methods. The cell purity was examined by FACScan using Alexa Fluor 700 conjugated anti-Sca-1 and APC-conjugated anti c-kit antibodies before infection, and was ~80%. The cell livability was examined by trypan blue-staining, and was ~95%. Cells were transduced in serum-free IMDM containing 1 ng/ml of mSCF, 10 ng/ml of mIL6 and 10 ng/ml of mIL3. n=3. Data are shown as average ± SD.
Primary cynomolgus monkey CD34+ cells were isolated as described previously [27]. Data from pooled cells from 4 monkeys from one representative experiment are shown. In a second experiment, the transduction efficiencies of the wild-type and tyrosine-mutant AAV6 vectors [40] were also evaluated (see Supplementary Table 1).
Primary human bone marrow- or umbilical cord blood-derived CD34+ cells were either obtained commercially (AllCells, Inc.), or isolated as described by Chatterjee et al. [7], and maintained in either StemSpan SFEM medium (Stem Cell Technologies, Inc) or Iscove’s modified Dulbecco’s medium (IMDM; Irvine Scientific, Santa Ana, CA) with 10 ng/ml of rhIL6 (Peprotech), 10 ng/ml of rhIL3 (Peprotech), and 1 ng/ml of rhSCF (R&D Systems, Minneapolis, MN). CD34+ cell purity was determined by flow cytometry and was ≥ 90%. Vectors were incubated with cells overnight in the presence of 10 ng/ml of each hIL6 and hIL3 and 1 ng/ml of rhSCF. Purity and viability of cord blood CD34+ cells were analyzed by PI staining and PE-conjugated anti CD34+ antibody following FACScan prior to transduction. n=11, Data are shown as average ± SD. Representative data from several experiments (see Fig. 2 and Supplementary Table 2).
Figure 1.
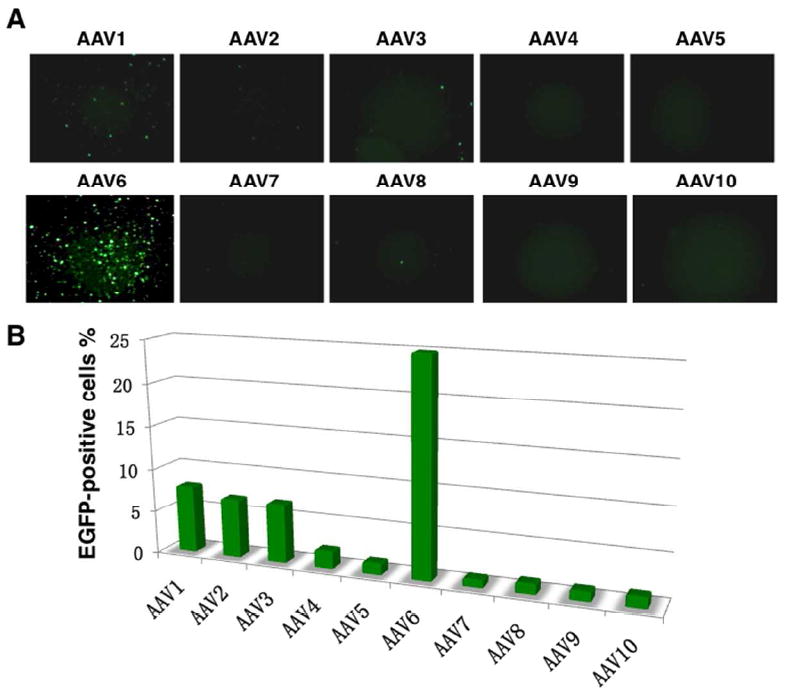
Comparative analyses of the transduction efficiencies of scAAV1 through AAV10 serotype vectors in primary human CD34+ cells. (A) Equivalent numbers of cells from a single donor were infected with of scAAV serotype vectors at 2×104 vgs/cell under identical culture conditions in IMDM containing 10% FBS, 10 ng/ml of hIL6, 10 ng/ml of hIL3 and 1 ng/ml of rhSCF for 16 hrs. Transgene expression was evaluated 48 hrs post-transduction by fluorescence microscopy and the data were quantitated by flow cytometry (B).
Similarly, AAV2 vectors transduced CD34+ cells from cynomolgus monkeys at low levels comparable to those reported for rhesus monkeys [30], and tyrosine-mutations did not significantly enhance the transduction efficiency of scAAV6 vectors in these cells (Supplementary Table 1). Interestingly, of the 10 serotypes evaluated, AAV6 vectors transduced human CD34+ cells most efficiently. The lack of enhanced functional transgene expression in mouse or cynomolgus monkey HSCs, compared with human CD34+ cells, suggests species-specific differences. Since differences in the transduction efficiency of scAAV6 vectors with different donor cells were clearly evident, all subsequent studies were carried out with scAAV6 serotype vectors and human CD34+ cells as follows.
Transduction efficiency of scAAV6 vectors in human CD34+ cells: Effects of donor variation and culture conditions in vitro
We have previously reported a significant donor variation in the transduction efficiency of ssAAV2 vectors in primary human bone marrow-derived CD34+ cells [31]. During the course of these studies, we observed a similar donor variation in the transduction efficiency of scAAV6 in human cord blood-derived CD34+ cells. These results are shown in Figure 2A. Whereas the transgene expression mediated by scAAV2 vectors ranged between 2-30%, the percentage of EGFP-positive cells transduced by scAAV6 vectors ranged between 6-87% of cells from several different donors (n=11). No significant variation in the transduction efficiency was observed in K562 cells using the same viral vector stocks, and when CD34+ cells from pooled cord blood from 10 to 15 donors were used, there was less variation than that observed in CD34+ from a single donor (data not shown). The results of transduction experiments performed under a number of conditions are shown in Figure 2B-C and Figure 3A-C, and summarized in Supplementary Table 2. Although it is possible the observed variation is due to factors such as the use of cryopreserved versus freshly isolated cells, and the purity of the different CD34+ cell populations, these data suggest that similar to AAV2 vectors, the wide range of transduction efficiencies of AAV6 vectors is due to differential levels of expression of the putative receptor/co-receptor on these cells.
Figure 2.
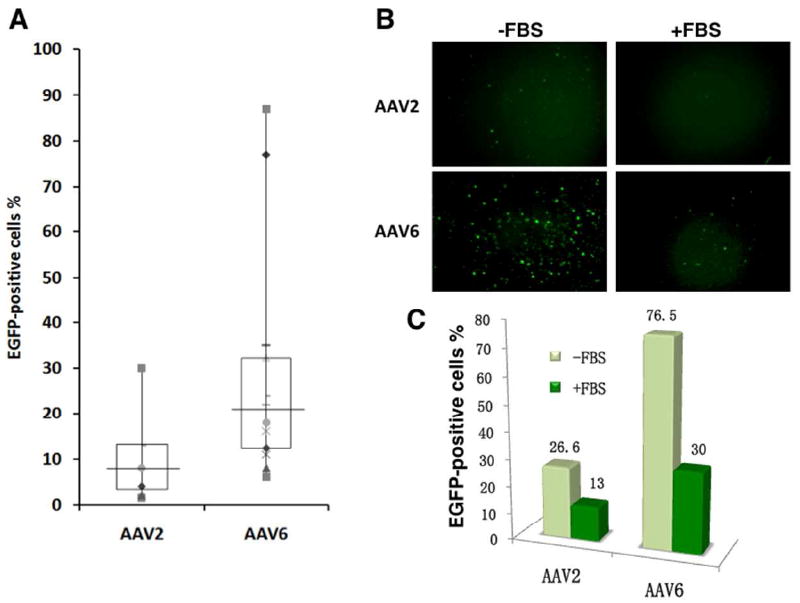
Comparative analyses of the transduction efficiencies of scAAV2 and AAV6 serotype vectors in primary human CD34+ cells from multiple donors. (A) Equivalent numbers of cells from various donors were infected with 2×104 vgs/cell of scAAV2 or AAV6 vectors and transgene expression was evaluated as described above. The boxes represent the upper quartile and lower quartile of the values; the horizontal lines indicate the median values; and different markers represent individual experiments. (B, C) Equivalent numbers of CD34+ cells from the same lots were transduced with scAAV2 or scAAV6 vectors as described above, either in the absence or the presence of 10% FBS. Transgene expression was evaluated 72 hrs post-transduction by fluorescence microscopy, and quantitated by flow cytometry.
Figure 3.
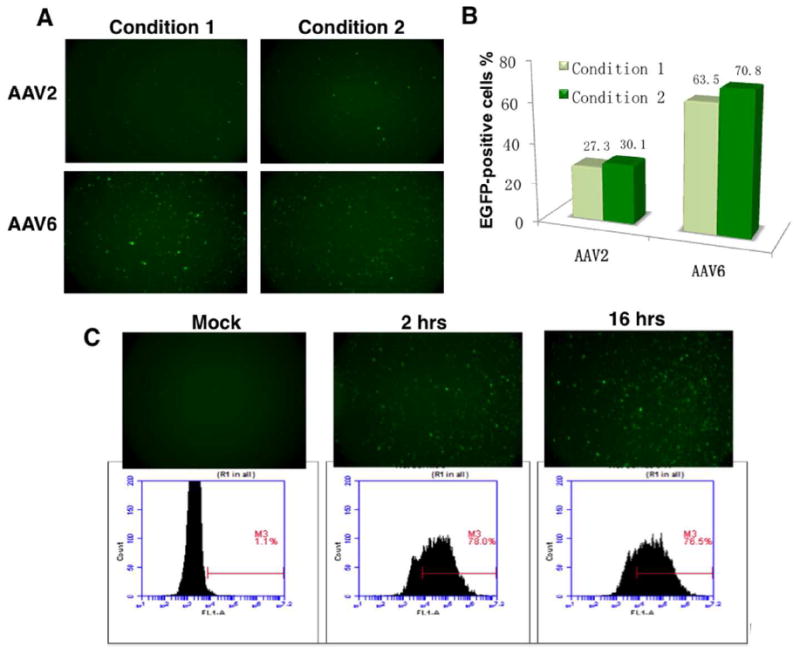
Transduction efficiencies of scAAV2 and AAV6 serotype vectors in primary human CD34+ cells under different culture conditions. (A, B) Equivalent numbers of CD34+ cells were transduced with scAAV2 or scAAV6 vectors in the presence of 10 ng/ml of rhFlt3, 10 ng/ml of rhTPO and 1 ng/ml of rhSCF for 2 hrs (Condition 1), or in the presence of 10 ng/ml of rhIL6, 10 ng/ml of rhIL3 and 1 ng/ml for 2 hrs (Condition 2). Transgene expression was evaluated 72 hrs post-transduction by fluorescence microscopy, and quantitated as described above. (C) Equivalent numbers of CD34+ cells were either mock-infected, or infected with scAAV6 vectors for either 2 or 16 hrs. Transgene expression was evaluated 72 hrs post-infection by flow cytometry as described above.
Although EGFR was recently identified to be the cellular receptor for AAV6 [32], in our studies, pre-treatment of CD34+ cells with EGF had no effect on the transduction efficiency of AAV6 vectors, and K562 cells, which are known to lack expression of EGFR [33], could be efficiently transduced by AAV6 vectors (data not shown). More recently, Denard et al. [34], reported that galectin 3-binding protein in human sera agglutinates AAV6 vectors, which results in decreased transduction efficiency of these vectors. We also observed significant inhibition in AAV6 vector-mediated transgene expression in the presence of serum (Figure 2B-C). The identity of the putative receptor for AAV6 vectors in human CD34+ cells, remains elusive, and additional studies are clearly warranted. In our preliminary studies, we have observed that treating K562 cells with Proteinase K dramatically decreased AAV6 vector-mediated transduction, but no significant change was observed following treatment with Phospholipase A2 (unpublished results). This implies that AAV6 uses a protein rather than a lipid on K562 cells for binding and entry into these cells. In addition, since AAV6 is known to have evolved from recombination between AAV1 and AAV2, and since AAV2 uses heparan sulfate proteoglycan (HSPG) as the primary cellular receptor (32), we have examined the level of HSPG expression on K562 surface by FACS. Although we observed abundant expression of HSPG on K562 cells, AAV6 infection could not be inhibited by heparin, suggesting AAV6 either does not use HSPG as a receptor, or HSPG is not the only receptor for AAV6. Furthermore, we have investigated the effect of inhibitors of O-linked and N-linked glycosylation on the transduction efficiency of AAV6 vectors in K562 cells. Although the O-glycan inhibitors do not significantly reduce the transduction efficiency, the treatment of cells with Tunicamycin for 24 hrs, which blocks the synthesis of all N-linked glycoproteins on the cell surface, markedly reduces transduction efficiency. Similar studies with primary human HSCs are currently underway to confirm whether AAV6 requires N-linked glycans for efficient transduction of these cells as well, which may eventually lead to the identification of the true receptor for AAV6 in human HSCs.
Efficient entry of scAAV6 vectors in human CD34+ cells
To examine whether the ability to enter human CD34+ cells was the primary reason for the observed efficient transduction of these cells by AAV6 serotype vectors, scAAV1, scAAV3, scAAV6, and scAAV9 serotype vectors were labeled with the fluorescent dye, Cy3, and incubated with CD34+ cells as described above. Typical images at 0.5 μm were captured, and Cy3-positive cells were enumerated under a confocal microscope from a total of 100 cells. Representative images are presented in Figure 4. Less than 10% of Cy3-positive cells were observed in AAV1 and AAV9 vector groups, and the percentages of Cy3-positive cells ranged between 8-12 in AAV2 and AAV3 vector groups. Interestingly, ~60% of CD34+ cells transduced with AAV6 vectors were Cy3-positive (summarized in Supplementary Table 3). These data suggest that AAV6 vectors enter human CD34+ cells significantly more efficiently than do other AAV serotypes. However, we also observed that most of the Cy3-labeled AAV6 vectors accumulated in the perinuclear region 2 hrs post infection (Figure 4B), consistent with findings with AAV2 serotype vectors [35, 36]. Thus, inefficient translocation of AAV6 vectors into nuclei might be an additional rate-limiting step for optimal transduction of HSCs by these vectors. We also observed that in some cells, the signal from Cy3 was observed in the nuclei. Since Cy3 was conjugated with the capsid, the appearance of Cy3 in the nuclei implies that the failure of a fraction of the vectors to undergo uncoating might be an additional rate-limiting step for transgene expression in HSCs mediated by AAV6 vectors.
Figure 4.
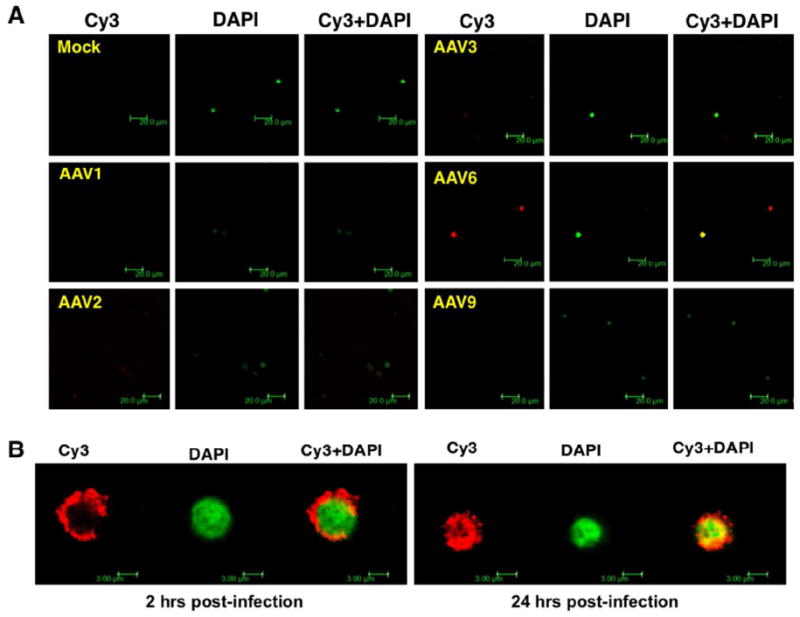
Confocal microscopy for intracellular trafficking of scAAV serotype vectors in human CD34+ cells. Cells were seeded in 24 well plates on poly-L-lysine-coated coverslips, and were either mock-infected, or infected with Cy3-labeled scAAV1, scAAV2, scAAV3, scAAV6, and scAAV9 vectors for 2 hrs (A). Cells were fixed with 4% paraformaldehyde for 8 min at room temperature and nuclei were stained with DAPI for 10 min at room temperature. Images were acquired with a Leica TCS-SP5 laser scanning confocal microscope under an oil-immersed ×63 objective lens. A z-stack was collected with step size of 0.5 μm until Cy3-signal intensity became faint under the laser. The co-localization analyses were performed by velocity software. To facilitate comparisons, the color from DAPI counterstaining was converted into green. The scale bar was calibrated to show the size of the cells (Magnification, × 107.1) (B). Representative confocal images for intracellular trafficking of scAAV6 vectors in human CD34+ cells at 2 and 24 hrs post-transduction. Images were acquired as described above (Magnification, ×806.4).
Enhanced transduction by tyrosine-mutant AAV6 vectors in human CD34+ cells in vitro
We have recently shown that mutations of the seven surface-exposed tyrosine residues in AAV2 capsids facilitate viral nuclear transport by limiting proteasome-mediated degradation, leading to high-efficiency transduction [20]. Since six of seven of these tyrosine residues are highly conserved in AAV6 (Supplementary Table 4), we generated point-mutations in each of the six tyrosine residues (Y252F, Y273F, Y445F, Y701F, Y705F, Y731F) using specific primer-pairs (Supplementary Table 5). scAAV6-CBAp-EGFP vectors containing the wild-type (WT) and each one of the six tyrosine-mutant vectors were evaluated for their transduction potential in primary human CD34+ cells at 5×103 vgs/cell (Figure 5A). The transduction efficiency of two of the tyrosine-mutant vectors [705F>Y731F] was significantly higher compared with the WT scAAV6 vectors.
Figure 5.
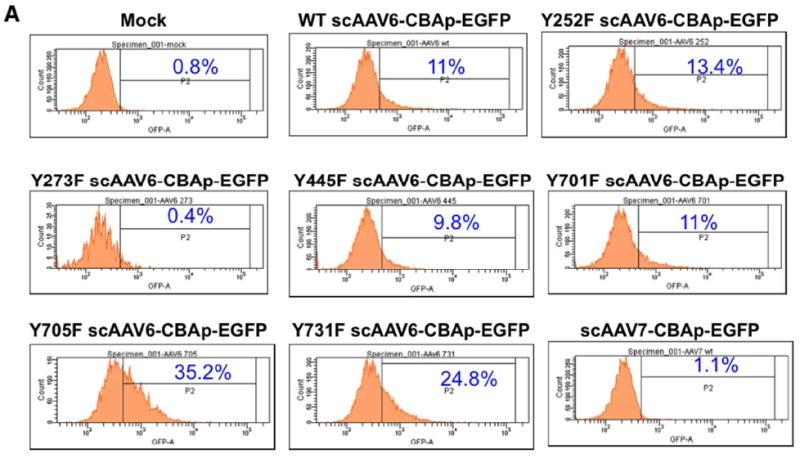
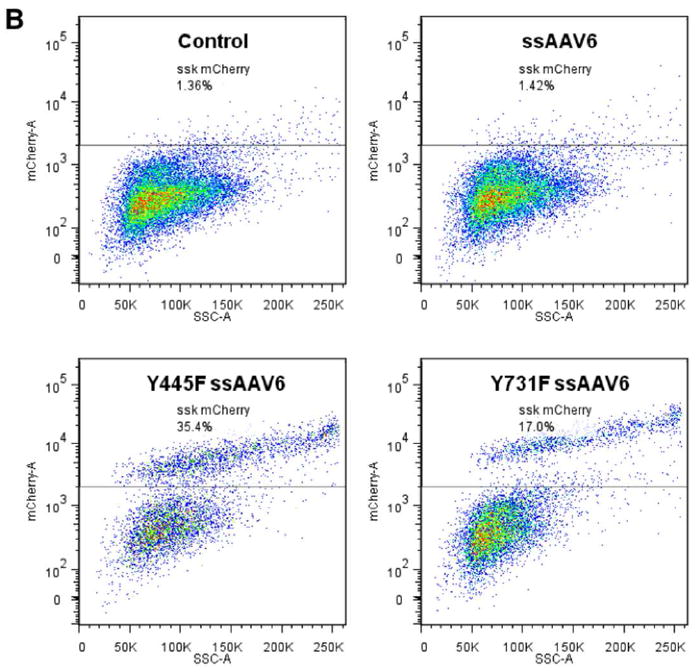
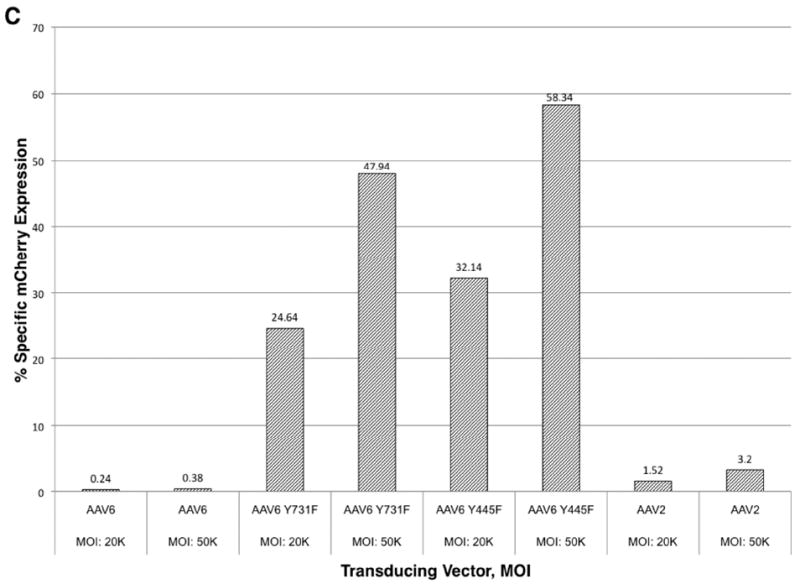
Transduction efficiencies of WT and tyrosine-mutant AAV6 serotype vectors in primary human CD34+ cells. (A) Equivalent numbers of CD34+ cells were either mock-transduced, or transduced with WT and tyrosine-mutant scAAV6-CBAp- EGFP vectors at 5×103 vgs/cells, and transgene expression was evaluated 72 hrs post-infection by flow cytometry as described above. scAAV7-CBAp-EGFP vectors were used as a negative control. (B, C) Equivalent numbers of CD34+ cells were either mock-transduced, or transduced with WT and tyrosine-mutant ssAAV6-CBAp-mCherry vectors at 2×104 or 5×104 vgs/cells. WT ssAAV2 vectors were used as appropriate controls. Transgene expression was evaluated at 46 hrs postinfection by flow cytometry, and quantitated as described above.
We also evaluated the transduction efficiency of WT and tyrosine-mutant ssAAV6 vectors using a different reporter gene, mCherry. WT and two tyrosine-mutant ssAAV6-CBAp-mCherry vectors (Y445F and Y731F) were used to transduce human CD34+ cells either at 2×104 or 5×104 vgs/cell, and transgene expression was evaluated at 46 hrs post-transduction. Whereas the WT ssAAV6 and AAV2 vectors were inefficient in mediating transgene expression, the transduction efficiency of two of the tyrosine-mutant vectors was shown to range between 24-58% (Figure 5B, C).
Enhanced in vivo transduction mediated by tyrosine-mutant ssAAV6 vectors in immune-deficient mice xeno-transplanted with human CD34+ cells
We evaluated the ability of WT and two tyrosine-mutant ssAAV6 vectors to transduce long-term in vivo engrafting human cord blood stem cells in a humanized NOD-SCID xenograft mouse model (Figure 6A). The vectors encoded the firefly luciferase (Luc) gene under the control of the CBA promoter in a single stranded AAV2 genome. Cord blood CD34+ cells transduced with WT and mutant AAV6 vectors all supported long-term engraftment and hematopoiesis. In vivo transgene expression measured by real time bioluminescent imaging revealed that CD34+ cells transduced with Y731F-ssAAV6 vectors supported the highest level of long term in vivo transduction, up to 22 weeks, the end of the experiment (Figure 6B). Y445F-ssAAV6 vectors appeared to be less efficient in supporting in vivo transduction than the WT ssAAV6 vectors. Comparison with the standard ssAAV2 vectors revealed that both WT and mutant ssAAV6 vectors were more efficient in supporting long-term in vivo transduction.
Figure 6.
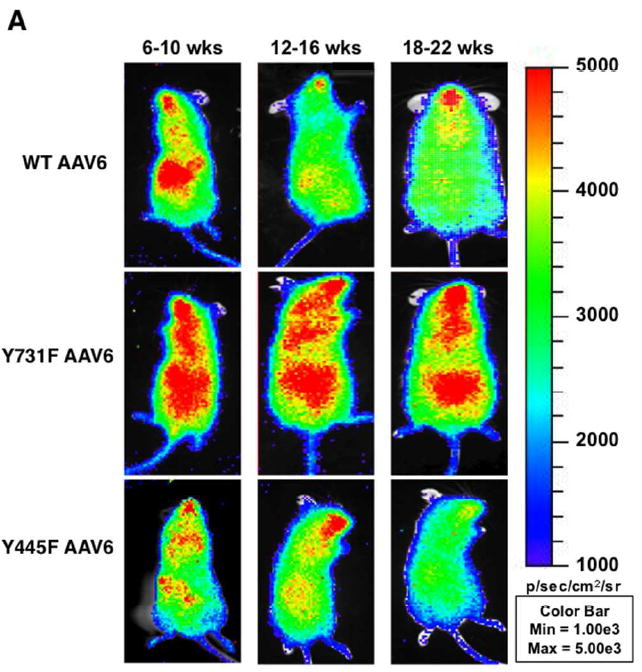
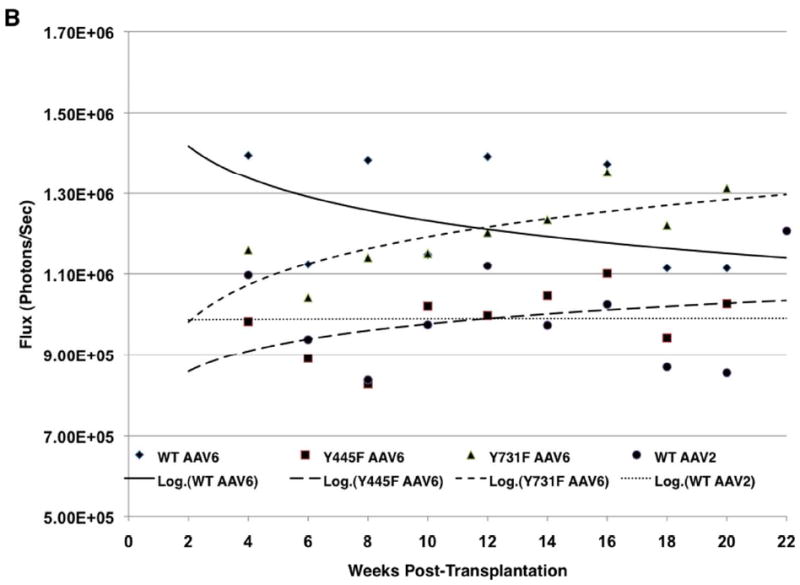
Transduction efficiencies of WT and tyrosine-mutant ssAAV6 serotype vectors in primary human CD34+ cells in NOD/SCID xenograft mice in vivo. (A) Whole-body bioluminescent imaging was performed as described previously [21]. Representative mice from different time intervals post-transplantation are shown for each capsid type. Serial in vivo bioluminescent imaging showing luciferase expression in NOD/SCID mice transplanted with human CD34+ cells transduced with rAAV Human cord blood CD34+ cells were transduced with the indicated vectors (WT AAV2: n=3; WT AAV6: n=3; Y445F AAV6: n=8; Y731F: n=10). (B). Xeno-transplanted mice were imaged biweekly following luciferin administration. Results are shown for 3-10 mice per group. Each mouse was transplanted with cells pooled from 1-5 CB samples. Luciferase expression is depicted as flux (photons/second) over time.
Analysis of the CD45+ human cell populations in the marrow of xeno-transplanted mice at the time of harvest at 18-22 weeks post-transplantation revealed that 45-63% of cells were of human origin indicating successful long-term engraftment and a lack of toxicity of AAV transduction (Figure 7A). Sixteen to 28% of spleen cells were also found to be of human origin (Figure 7B), consistent with trafficking and/or seeding of the input human CD34+ cells and their progeny.
Figure 7.
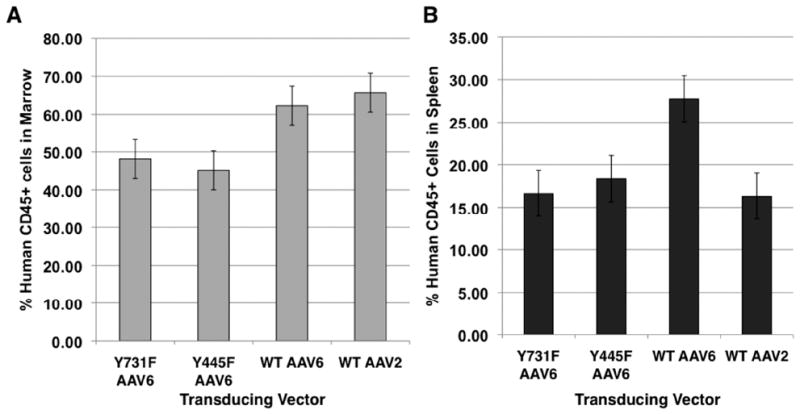
Engraftment of human cells in the marrow (A) and spleen (B) of NOD/SCID mice. Mononuclear cells from the marrow and spleen of transplant recipients were analyzed for human CD45 expression at the time of harvest 16-22 weeks post-transplantation. The presence of human CD45+ cells indicated human cell engraftment.
To estimate the long-term persistence of vector genomes in the marrow, we quantitated the frequency of the ssAAV-Luc genome relative to a single copy cellular gene ApoB in high molecular weight marrow DNA using a quantitative Taqman real time PCR assay at the time of harvest of xeno-transplant recipients. The average frequency of Y731F-AAV6 and Y445F-AAV6 vectors were found to be somewhat higher than the WT AAV6 vectors (Table 2). These results collectively indicate that human CD34+ cells transduced with WT AAV6 and Y-F mutant AAV6 vectors are capable of supporting long-term engraftment and hematopoiesis in vivo with no associated toxicity. The relative persistence of rAAV genomes in the marrow of transplant recipients at 16-22 weeks post-transplantation indicated that these vectors are capable of mediating stable transduction of long-term in vivo engrafting stem cells.
Table 2.
Frequency of rAAV genomes in the marrow of long-term xeno-tranplanted recipient mice in vivo
| Transducing vector | Genome copies/cell |
|---|---|
| WT AAV2 | 0.003 |
| WT AAV6 | 0.065 |
| Y445F AAV6 | 0.112 |
| Y731F AAV6 | 0.127 |
rAAV genome copies were quantitated in the femoral marrow of xeno-transplanted mice at the time of harvest, the end of the experiment at 16-22 weeks post-transplantation. High molecular weight DNA was analyzed by qPCR using luciferase-specific primers and probes. Human cell equivalents in the samples were quantitated using a qPCR assay using humanspecific Apo B primers and probes (29). The values shown represent the average of each vector group.
Further studies are warranted to evaluate whether double-, triple, or multiple tyrosine-mutant AAV6 vectors would mediate further augmented in vivo transgene expression in human CD34+ cells, as has been observed with multiple tyrosine-mutant AAV2 vectors [37]. We have also reported recently that site-directed mutagenesis of specific surface-exposed serine and threonine residues on AAV2 capsid increases the transduction efficiency of these vectors in [38, 39]. Since these serine residues are conserved in AAV6 as well, it remains possible that by combining both tyrosine- and serine-mutations in AAV6 capsid, the transduction efficiency of AAV6 serotype vectors in human CD34+ cells can be further improved [40]. Overall, these studies demonstrate the feasibility of the use of optimized scAAV6 vectors for achieving high-efficiency transduction of HSCs as well as their potential use in gene therapy applications for hematological disorders.
Supplementary Material
Acknowledgments
We thank Drs. R. Jude Samulski, James M. Wilson, and Xiao Xiao for their kind gifts of recombinant AAV plasmids. This research was supported in part by a research grant from the Fanconi Anemia Research Fund, Inc., (to LZ); a Natural Science Foundation of China (NSFC) grant 30971299 (to MT), and Public Health Service grants R01 HL-065770, HL-076901, P01 DK-058327 (Project 1), and R01 HL-09870 from the National Institutes of Health, and an Institutional grant from the Children’s Miracle Network (to AS). GRJ was supported in part by an ‘Overseas Associate Fellowship-2006’ from the Department of Biotechnology, Government of India. The following City of Hope Cancer Center cores were utilized in this study: Animal Resources Center, Flow Cytometry Core, In Vivo Imaging Core, and DNA Sequencing Core.
References
- 1.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature. 2010;467(7313):318–22. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Therapy. 2008;15(11):858–63. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 3.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nature Reviews Genetics. 2011;12(5):341–55. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 4.Zhou SZ, Cooper S, Kang LY, et al. Adeno-associated virus 2-mediated high efficiency gene transfer into immature and mature subsets of hematopoietic progenitor cells in human umbilical cord blood. The Journal of Experimental Medicine. 1994;179(6):1867–75. doi: 10.1084/jem.179.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman S, Xiao X, Donahue RE, et al. Recombinant adeno-associated virus-mediated gene transfer into hematopoietic progenitor cells. Blood. 1994;84(5):1492–500. [PubMed] [Google Scholar]
- 6.Fisher-Adams G, Wong KK, Jr, Podsakoff G, et al. Integration of adeno-associated virus vectors in CD34+ human hematopoietic progenitor cells after transduction. Blood. 1996;88(2):492–504. [PubMed] [Google Scholar]
- 7.Chatterjee S, Li W, Wong CA, et al. Transduction of primitive human marrow and cord blood-derived hematopoietic progenitor cells with adeno-associated virus vectors. Blood. 1999;93(6):1882–94. [PubMed] [Google Scholar]
- 8.Tian J, Wang F, Xue JF, et al. Recombinant AAV2-mediated beta-globin expression in human fetal hematopoietic cells from the aborted fetuses with beta-thalassemia major. International Journal of Hematology. 2011;93(6):691–9. doi: 10.1007/s12185-011-0823-x. [DOI] [PubMed] [Google Scholar]
- 9.Paz H, Wong CA, Li W, et al. Quiescent subpopulations of human CD34-positive hematopoietic stem cells are preferred targets for stable recombinant adenoassociated virus type 2 transduction. Human Gene Therapy. 2007;18(7):614–26. doi: 10.1089/hum.2006.188. [DOI] [PubMed] [Google Scholar]
- 10.Nathwani AC, Hanawa H, Vandergriff J, et al. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38- subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Therapy. 2000;7(3):183–95. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- 11.Malik P, McQuiston SA, Yu XJ, et al. Recombinant adeno-associated virus mediates a high level of gene transfer but less efficient integration in the K562 human hematopoietic cell line. J Virol. 1997;71(3):1776–83. doi: 10.1128/jvi.71.3.1776-1783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander IE, Russell DW, Miller AD. Transfer of contaminants in adeno-associated virus vector stocks can mimic transduction and lead to artifactual results. Human Gene Therapy. 1997;8(16):1911–20. doi: 10.1089/hum.1997.8.16-1911. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A. Obstacles to human hematopoietic stem cell transduction by recombinant adeno-associated virus 2 vectors. Journal of Cellular Biochemistry. 2002;38:39–45. doi: 10.1002/jcb.10053. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava A. Hematopoietic stem cell transduction by recombinant adeno-associated virus vectors: problems and solutions. Human Gene Therapy. 2005;16(7):792–8. doi: 10.1089/hum.2005.16.792. [DOI] [PubMed] [Google Scholar]
- 15.Handa A, Muramatsu S, Qiu J, et al. Adeno-associated virus (AAV)-3-based vectors transduce haematopoietic cells not susceptible to transduction with AAV-2-based vectors. The Journal of General Virology. 2000;81(Pt 8):2077–84. doi: 10.1099/0022-1317-81-8-2077. [DOI] [PubMed] [Google Scholar]
- 16.Schuhmann NK, Pozzoli O, Sallach J, et al. Gene transfer into human cord blood-derived CD34(+) cells by adeno-associated viral vectors. Experimental Hematology. 2010;38(9):707–17. doi: 10.1016/j.exphem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Veldwijk MR, Sellner L, Stiefelhagen M, et al. Pseudotyped recombinant adenoassociated viral vectors mediate efficient gene transfer into primary human CD34(+) peripheral blood progenitor cells. Cytotherapy. 2010;12(1):107–12. doi: 10.3109/14653240903348293. [DOI] [PubMed] [Google Scholar]
- 18.Zhong L, Li W, Li Y, et al. Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Human Gene Therapy. 2006;17(3):321–33. doi: 10.1089/hum.2006.17.321. [DOI] [PubMed] [Google Scholar]
- 19.Maina N, Han Z, Li X, et al. Recombinant self-complementary adeno-associated virus serotype vector-mediated hematopoietic stem cell transduction and lineage-restricted, long-term transgene expression in a murine serial bone marrow transplantation model. Human Gene Therapy. 2008;19(4):376–83. doi: 10.1089/hum.2007.143. [DOI] [PubMed] [Google Scholar]
- 20.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105(22):7827–32. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauss MA, Smith LJ, Zhong L, et al. Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2. Human Gene Therapy. 2010;21(9):1129–36. doi: 10.1089/hum.2010.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng B, Ling C, Dai Y, et al. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene Therapy. 2012;19:375–84. doi: 10.1038/gt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao C, Zhang W, Yuan Z, et al. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Human Gene Therapy. 2010;21(10):1343–8. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ussher JE, Taylor JA. Optimized transduction of human monocyte-derived dendritic cells by recombinant adeno-associated virus serotype 6. Human Gene Therapy. 2010;21(12):1675–86. doi: 10.1089/hum.2010.087. [DOI] [PubMed] [Google Scholar]
- 25.Dalkara D, Byrne LC, Lee T, et al. Enhanced gene delivery to the neonatal retina through systemic administration of tyrosine-mutated AAV9. Gene Therapy. 2012;19:176–81. doi: 10.1038/gt.2011.163. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. Journal of Virology. 1998;72(3):2224–32. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CC, Fletcher MD, Tarantal AF. Effect of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+ and hematopoietic progenitor cells. Pediatric Research. 2005;58(2):315–22. doi: 10.1203/01.PDR.0000169975.30339.32. [DOI] [PubMed] [Google Scholar]
- 28.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. The Journal of Neuroscience. 2007;27(37):9928–40. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santat L, Paz H, Wong C, et al. Recombinant AAV2 transduction of primitive human hematopoietic stem cells capable of serial engraftment in immune-deficient mice. Proc Natl Acad Sci USA. 2005;102(31):11053–8. doi: 10.1073/pnas.0502902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schimmenti S, Boesen J, Claassen EA, et al. Long-term genetic modification of rhesus monkey hematopoietic cells following transplantation of adenoassociated virus vector-transduced CD34+ cells. Human Gene Therapy. 1998;9(18):2727–34. doi: 10.1089/hum.1998.9.18-2727. [DOI] [PubMed] [Google Scholar]
- 31.Ponnazhagan S, Mukherjee P, Wang XS, et al. Adeno-associated virus type 2-mediated transduction in primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. Journal of Virology. 1997;71(11):8262–7. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weller ML, Amornphimoltham P, Schmidt M, et al. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nature Medicine. 2010;16(6):662–4. doi: 10.1038/nm.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen H, Hsuan J, Clark S, et al. Expression of epidermal-growth-factor receptor in the K562 cell line by transfection. Altered receptor biochemistry The Biochemical Journal. 1990;271(3):785–90. doi: 10.1042/bj2710785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denard J, Beley C, Kotin R, et al. Human galectin 3 binding protein interacts with recombinant adeno-associated virus type 6. Journal of virology. 2012;86(12):6620–31. doi: 10.1128/JVI.00297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao W, Warrington KH, Jr, Hearing P, et al. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. Journal of Virology. 2002;76(22):11505–17. doi: 10.1128/JVI.76.22.11505-11517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao PJ, Samulski RJ. Cytoplasmic trafficking, endosomal escape, and peri-nuclear accumulation of AAV2 particles are facilitated by microtubule network. Journal of Virology. 2012 doi: 10.1128/JVI.00935-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markusic DM, Herzog RW, Aslanidi GV, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Molecular Therapy. 2010;18(12):2048–56. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aslanidi GV, Rivers AE, Ortiz L, et al. High-efficiency transduction of human monocyte-derived dendritic cells by capsid-modified recombinant AAV2 vectors. Vaccine. 2012;30(26):3908–17. doi: 10.1016/j.vaccine.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aslanidi GV, Rivers AE, Ortiz L, et al. Optimization of recombinant adeno-associated virus 2 (AAV2) vectors for gene therapy: The final threshold? PloS One. 2013;8(3):e59142. doi: 10.1371/journal.pone.0059142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song L, Li X, Jayandharan GR, et al. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PloS One. 2013;8(3):e58757. doi: 10.1371/journal.pone.0058757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


