Abstract
Protein kinase D (PKD) participates in activation of the transcription factor NF-κB (nuclear factor κB) in cells exposed to oxidative stress, leading to increased cellular survival. We previously demonstrated that phosphorylation of PKD at Tyr463 in the PH (pleckstrin homology) domain is mediated by the Src-Abl pathway and that it is necessary for PKD activation and subsequent NF-κB induction. Here we show that activation of PKD in response to oxidative stress requires two sequential signaling events, i.e., phosphorylation of Tyr463 by Abl, which in turn promotes a second step, phosphorylation of the PKD activation loop (Ser738/Ser742). We show that this is mediated by PKCδ (protein kinase Cδ), a kinase that is activated by Src in response to oxidative stress. We also show that other PKCs, including PKCɛ and PKCζ, do not participate in PKD activation or NF-κB induction. We propose a model in which two coordinated signaling events are required for PKD activation. Tyrosine phosphorylation in the PH domain at Tyr463, mediated by the Src-Abl pathway, which in turn facilitates the phosphorylation of Ser738/Ser742 in the activation loop, mediated by the Src-PKCδ pathway. Once active, the signal is relayed to the activation of NF-κB in oxidative stress responses.
The activation of the inducible transcription factor NF-κB (nuclear factor κB) is important for many cellular responses because it participates in the activation of genes such as interleukin-1 and TNF-α (tumor necrosis factor alpha), which contribute to efficient immune and inflammatory responses. NF-κB activation has also been shown to control both pro- and antiapoptotic signaling (20, 36, 49). Moreover, numerous studies have shown that in a variety of cell types, NF-κB acts as a sensor for oxidative stress (21, 29, 34). For example, cells exposed to H2O2, or induced to produce intracellular reactive oxygen species (ROS), show potent NF-κB activation (12, 42).
Activation of NF-κB is regulated by multiple distinct upstream signaling pathways, and the contribution of each pathway to NF-κB induction is dependent on the nature of the cellular stimulus (20). However, one feature common to many NF-κB-activating pathways is that they converge at the level of the IκB kinase (IKK) signalosome, a multiprotein complex necessary for phosphorylation and down-regulation of the NF-κB inhibitory protein IκB. Although the signaling pathways acting upstream of the IKK complex used by proinflammatory cytokines such as TNF are well characterized, the signaling cascades that mediate oxidative stress-induced NF-κB activation have remained elusive (20, 29, 41). Recent studies have shown that activation of NF-κB in response to oxidative stress is concomitant with tyrosine phosphorylation of a number of signaling intermediates in the canonical IKK/NF-κB pathway (12, 42). As an example, we recently showed that in response to oxidative stress, activation of the Src-Abl signaling module converges on the serine/threonine kinase PKD (protein kinase D), which in turn stimulates activation of NF-κB (42). Moreover, tyrosine phosphorylation of PKD at Tyr463 in the pleckstrin homology (PH) domain is the key event that controls NF-κB induction in response to ROS. Once active, PKD participates in signal relay to NF-κB via activation of the canonical IKK complex and IκB degradation.
The serine/threonine kinase PKD, originally described as a novel PKC (nPKC) and named PKCμ, is now known to comprise a distinct family of kinases that includes two other members, PKD2 and PKCν/PKD3 (51). In the kinome, the PKD family is more closely related to the calcium-calmodulin kinase superfamily than it is to the PKC family (32, 37, 51). Activation of PKD by extracellular stimuli has been shown to be mediated by a PKC-dependent pathway (58). For example, nPKC isoforms such as PKCθ and PKCη (3, 55) have been shown to bind the PKD PH domain and promote the phosphorylation of PKD at its activation loop serine residues (Ser738 and Ser742 in human PKD, Ser744 and Ser748 in murine PKD). PKD does not autophosphorylate its own activation loop, but phosphorylation of these residues is a prerequisite for kinase activity (53). However, thus far the mechanism by which PKCs control PKD activation, as well as the specific PKC isoforms used by specific stimuli in the PKD activation pathway, is not well known. Recently, Waldron et al. described an attractive model in which direct phosphorylation of the activation loop by PKCɛ facilitates release of the autoinhibitory PH domain, leading to increased PKD activity (54). Our own studies have revealed that phosphorylation of Tyr463 in the PH domain promotes PKD activation, specifically in response to oxidative stress, but not in response to other PKD-activating stimuli such as PDGF (platelet-derived growth factor) or bradykinin (40). Thus, the PKD PH domain appears to play a critical role in the activation of the kinase in response to multiple stimuli.
Here we show that PKD Tyr463 and Ser738/Ser742 phosphorylation is essential for kinase activity and subsequent NF-κB activation in response to oxidative stress. Activation loop phosphorylation of PKD is mediated by the PKC pathway, specifically, the nPKCδ isoform and not other PKCs. We propose a model in which two distinct signaling pathways converge at the level of PKD; the Src-Abl pathway, which controls Tyr463 phosphorylation, and PKCδ, which controls activation loop phosphorylation. Synergistic activation of both pathways is required for efficient signal relay to NF-κB in cells exposed to ROS.
MATERIALS AND METHODS
Cell culture, antibodies, and reagents.
HeLa cells were purchased from the American Type Culture Collection (Manassas, Va.) and maintained in high-glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. HeLa cells stably expressing the NF-κB and β-galactosidase (β-gal) reporter genes have been described previously (42). Anti-PKCδ, anti-PKCɛ, anti-PKCζ, anti-PKD, anti-Abl, and anti-phospho-PKCδ (pThr507) antibodies were from Santa Cruz (Santa Cruz, Calif.); anti-β-actin and anti-FLAG antibodies were from Sigma (St. Louis, Mo.); anti-Src and anti-phosphotyrosine (4G10) antibodies were from Upstate Biotechnology (Lake Placid, N.Y.); and anti-phospho-PKD (pSer744/748 in murine PKD, pS738/S742 in human PKD) antibodies were from Cell Signaling Technologies (Beverly, Mass.). Anti-hemagglutinin (anti-HA) antibody was purified from the 12CA5 hybridoma. H2O2 (30%) was from Fisher Scientific (Pittsburgh, Pa.). BSO (dl-buthionine-[S,R]-sulfoximine) was from Sigma. Rottlerin and PP2 were from Biomol (Plymouth Meeting, Pa.), myristoylated PKC inhibitor 19-27 was from Calbiochem (La Jolla, Calif.), and STI-571 was from Novartis (Basel, Switzerland). The PKD-specific substrate peptide used was AALVRQMSVAFFFK. Recombinant PKD was expressed in insect cells after infection with baculovirus harboring HA-tagged PKD in pFAST-Bac (Invitrogen Life Technologies, Carlsbad, Calif.) and purified on an Ni-nitrilotriacetic acid affinity column. Purified active Abl and PKCδ were from Calbiochem. Superfect (Qiagen, Valencia, Calif.) or TransIT HeLa Monster reagent (Mirus, Madison, Wis.) was used for transient transfections of HeLa cells, and TransIT 293 transfection reagent was used for transient transfections of human embryonic kidney (HEK) 293E cells in accordance with the manufacturer's instructions.
Expression plasmids and RNA interference (RNAi) constructs.
The cloning of an expression vector for N-terminal HA-tagged human PKD has been described previously (42). All expression plasmids for mutant PKDs are based on this construct. The PKD.K612W, PKD.Y463F, PKD.Y463E, and PKD.ΔPH mutant proteins have already been described (40, 42). Mutagenesis was carried out by PCR with QuikChange (Stratagene, La Jolla, Calif.) for the following mutant forms: PKD.SS744/748EE, 5′-ATTGGAGAGAAGGAGTTCCGGAGGGAGGTGGTGGGTACC-3′ and 5′-GGTACCCACCACCTCCCTCCGGAACTCCTTCTCTCCAAT-3′; PKD.SS744/748AA, 5′-ATTGGAGAGAAGGCTTTCCGGAGGGCAGTGGTGGGTACC-3′ and 5′-GGTACCCACCACTGCCCTCCGGAAAGCCTTCTCTCCAAT-3′. Constructs were verified by DNA sequencing. Expression plasmids for PKCɛ or PKCζ have been described previously (6, 7). Expression plasmids for PKCδ (wild type, kinase dead [K376A], or constitutively active [R144/145A]) were obtained from Shigeo Ohno, (Yokohama City University, Yokohama, Japan) or Mary Reyland (University of Colorado, Denver). Wild-type and kinase-dead Abl expression constructs were from Naomi Rosenberg (Tufts University, Boston, Mass.). All of the other expression plasmids used have already been described (40, 42).
To silence the expression of PKCδ, PKCζ, and PKCɛ, pSUPER.PKC RNAi vectors were constructed by ligation of the following oligonucleotide pairs to pSUPER (5): 5′-GATCCCCGACAAGATCATCGGCAGATTTCAAGAGAATCTGCCGATGATCTTGTCTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAGACAAGATCATCGGCAGATTCTCTTGAAATCTGCCGATGATCTTGTCGGG-3′ for PKCδ, 5′-GATCCCCGATGGAGGAAGCTGTACCGTTCAAGAGACGGTACAGCTTCCTCCATCTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAGATGGAGGAAGCTGTACCGTCTCTTGAACGGTACAGCTTCCTCCATCGGG-3′ for PKCζ, and 5′-GATCCCCGATCGAGCTGGCTGTCTTTTTCAAGAGAAAAGACAGCCAGCTCGATCTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAA GATCGAGCTGGCTGTCTTTTCTCTTGAAAAAGACAGCCAGCTCGATCGGG-3′ for PKCɛ. To silence the expression of PKD2, a pSUPER.PKD2 RNAi vector was constructed by ligation of the following oligonucleotide pair to pSUPER (5): 5′-GATCCCCACATGACCCCACGTCGGCCTTCAAGAGAGGCCGACGTGGGGTCATGTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAACATGACCCCACGTCGGCCTCTCTTGAAGGCCGACGTGGGGTCATGTGGG-3′. The vector used to silence the expression of PKD1 (pSuper.PKD RNAi construct) has been described previously (42).
Immunoblotting and immunoprecipitation.
Cells were lysed in lysis buffer (50 mM Tris/HCl [pH 7.4], 1% Triton X-100, 150 mM NaCl, 5 mM EDTA [pH 7.4]) plus protease inhibitor cocktail (Sigma-Aldrich, St. Louis, Mo.), and lysates were used for immunoblot analysis or proteins of interest were immunoprecipitated by a 1-h incubation with the respective antibody (2 μg), followed by a 30-min incubation with protein A/G-agarose (Santa Cruz). Immune complexes were washed five times with TBS (50 mM Tris/HCl [pH 7.4], 150 mM NaCl) and resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) or subjected to in vitro kinase assays.
PKC kinase and PKD protein kinase assays. (i) PKC kinase assays.
After immunoprecipitation, 20 μl of kinase buffer (50 mM Tris/HCl [pH 7.4], 10 mM MgCl2, 2 mM dithiothreitol) was added to the precipitates and the kinase reaction was carried out for 30 min at room temperature after adding 10 μl of a kinase substrate mixture (1 μg of myelin basic protein [MBP], 50 μM ATP, 10 μCi of [γ-32P]ATP in kinase buffer or without MBP for autophosphorylation assays). To terminate the reaction, 30 μl of 2× SDS sample buffer was added and the samples were resolved by SDS-PAGE. Gels were Coomassie stained and then dried and analyzed on a Molecular Imager (Bio-Rad, Hercules, Calif.).
(ii) PKD protein kinase assays.
After immunoprecipitation (with anti-PKD antibody for endogenous PKD and anti-HA antibody for transfected PKD) and washing, 20 μl of kinase buffer (50 mM Tris/HCl [pH 7.4], 10 mM MgCl2, 2 mM dithiothreitol) was added to the precipitates and the kinase reaction was carried out for 20 min after adding 10 μl of a kinase substrate mixture (150 μM PKD-specific substrate peptide, 50 μM ATP, 10 μCi of [γ-32P]ATP in kinase buffer). To terminate the reaction, the samples were centrifuged and the supernatants were spotted onto P81 phosphocellulose paper (Whatman, Clifton, N.J.). The papers were washed three times with 0.75% phosphoric acid and once with acetone and dried, and activity was determined by liquid scintillation counting.
RNAi.
HeLa cells were transfected with pSUPER or pSUPER-RNAi with the TransIT HeLa Monster reagent (Mirus). HEK 293E cells were transfected with the TransIT 293 transfection reagent (Mirus). In all experiments, the cells were transfected at 30% confluency. Transfection efficiencies (95 to 100%) were controlled with a green fluorescent protein expression vector. Experiments were performed 48 h after initial transfection. Reduced expression of target proteins was evaluated by immunoblotting.
Reporter gene assays.
Cells were transiently cotransfected with an NF-κB-reporter construct (NF-κB-luc [luciferase], 5 μg), 1 μg of pCS2-(n) β-gal, and the cDNA of interest (1 μg) with Superfect (Qiagen). Assays for luc and β-gal activities were performed on total cell lysates with standard assays, and measurements were made with a luminometer. luc activity was normalized to β-gal activity. Protein expression was controlled by immunoblot analysis. For reporter gene assays, in which RNAi was used, HeLa cells stably transfected with the NF-κB-luc reporter and the β-gal reporter were used as previously described (42).
RESULTS
Oxidative stress-mediated activation of the Src/Abl pathway leads to PKD activation loop phosphorylation.
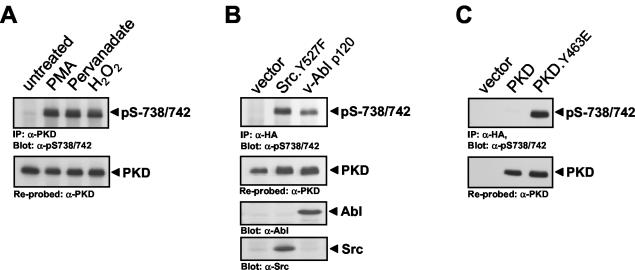
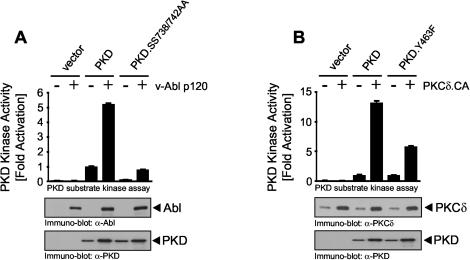
Since stimulation of cells with growth factors such as PDGF stimulates the phosphorylation of PKD at activation loop residues Ser738 and Ser742, leading to increased kinase activity (18), we investigated whether activation loop phosphorylation is also required for oxidative stress-dependent PKD activation. Exposure of HeLa cells to oxidative stress (H2O2) or pervanadate significantly increased PKD Ser738/Ser742 phosphorylation compared to control PMA (phorbol 12-myristate 13-acetate) stimulation (Fig. 1A). Ser738/Ser742 phosphorylation was also induced in cells transiently transfected with active alleles of Src (Src.Y527F) and Abl (v-Abl p120), which act upstream of PKD by inducing Tyr463 phosphorylation (Fig. 1B). Interestingly, a mutant PKD that is predicted to mimic the net effect of phosphorylation at Tyr463 (PKD.Y463E) and is constitutively active in the absence of extracellular stimuli (42) is also constitutively phosphorylated at Ser738/Ser742 (Fig. 1C). This suggests that a negative charge at Tyr463 in the PH domain induces a conformational change that releases autoinhibition and permits activation loop phosphorylation by upstream kinases.
FIG. 1.
PKD activation loop phosphorylation is promoted by Tyr463 phosphorylation. (A) HeLa cells were treated for 10 min with 100 nM PMA, 75 μM pervanadate, or 10 μM H2O2. (B) HeLa cells were cotransfected with HA-tagged PKD and either the vector alone, active Src, or active Abl. (C) PKD or mutant protein PKD.Y463E was overexpressed, and PKD was immunoprecipitated (IP; anti-PKD antibody in panel A, anti-HA antibody in panels B and C) and immunoblotted with an antibody that recognizes the phosphorylated PKD activation loop (anti-pS738/742 antibody). All results are typical of three independent experiments.
Ser738/Ser742 and Tyr463 phosphorylation contributes to PKD activity.
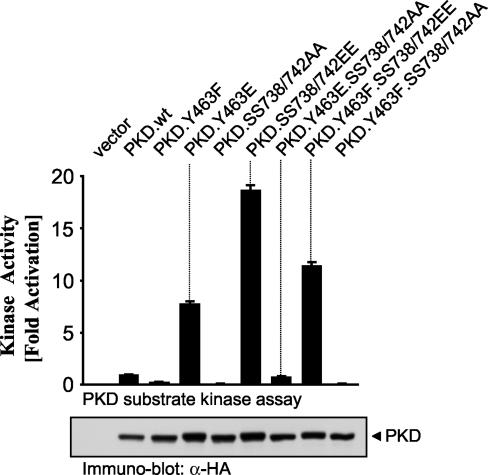
To examine the relationship between PH domain phosphorylation and activation loop phosphorylation in controlling PKD activity, we generated a series of mutant PKDs with Ala or Glu mutations at Tyr463 and Ser738/Ser742 (Fig. 2). The mutant proteins were transiently expressed in HeLa cells, and their activities toward a specific PKD substrate peptide were measured in immune complex kinase assays. As previously shown (42), the PKD.Y463E mutant enzyme has elevated activity compared with that of wild-type PKD. However, Ala substitution of Ser738/Ser742 in the context of Y463E results in complete loss of PKD kinase activity (Fig. 2, PKD.Y463E.SS738/742AA). This shows that PKD activity stimulated by Tyr463 phosphorylation also requires activation loop phosphorylation. Secondly, a mutant protein in which the activation loop residues are replaced with negatively charged Glu is also constitutively active, as originally shown by Iglesias et al. (19) (Fig. 2, PKD.SS738/742EE). However, mutation of Tyr463 to nonphosphorylatable Ala in the context of SS738/742EE retains considerable kinase activity (Fig. 2, Y463F.SS738/742EE).
FIG. 2.
Phosphorylation of Tyr463 and Ser738/Ser742 controls PKD activity. The indicated mutant PKDs were transiently expressed in HeLa cells and immunoprecipitated (anti-HA antibody), and a PKD substrate peptide kinase assay was performed. Immunoblot assays of immunoprecipitated PKD were used to monitor the expression of all mutant PKDs. The results are typical of three independent experiments.
These data are indicative of two sequential activation steps, whereby Tyr463 phosphorylation is permissive for the subsequent activation loop phosphorylation, an event absolutely required for PKD activity (18). The fact that the Y463E mutant protein is not as active as the SS738/742EE mutant protein may be explained by the fact that, under these conditions, the PKD activation loop upstream kinase is likely not fully active. Moreover, we have detected a small but reproducible increase in Tyr463 phosphorylation when the PKD.SS738/742EE mutant protein is expressed in cells, and this argues for a basal activity of Abl, the Tyr463 kinase, in untreated cells (data not shown). This may explain why the PKD.Y463F.SS738/742EE mutant protein is less active than the PKD.SS738/742EE mutant protein. Taken together, these observations point to a critical role for both PH domain phosphorylation (Tyr463) and activation loop phosphorylation (Ser738 and Ser742) in controlling PKD activity.
Ser738/Ser742 phosphorylation and Tyr463 phosphorylation contribute to NF-κB activation.
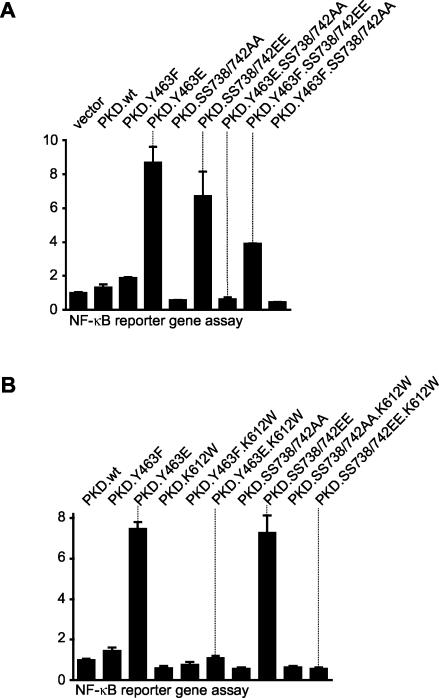
We next evaluated the contributions of Ser738/Ser742 phosphorylation and Tyr463 phosphorylation to the activation of NF-κB. Since the constitutively active PKD.Y463E mutant protein stimulates NF-κB in the absence of extracellular stimuli (42), we evaluated the contribution of PKD activation loop phosphorylation in this event. With the mutant proteins described above, we found that PKD.Y463E potently induced maximal NF-κB activity compared to control wild-type PKD (Fig. 3A). Moreover, the Y463E mutant protein was as potent as the activation loop SS738/742EE mutant protein in activating NF-κB. Mutation of the activation loop serines to nonphosphorylatable Ala in the context of the Y463E mutant protein completely attenuated NF-κB activation (PKD.Y463E.SS738/742AA, Fig. 3A), consistent with the complete loss of PKD activity observed with this mutant protein (Fig. 2). Interestingly, mutation of Tyr463 to a nonphosphorylatable Phe in the context of a negative charge at the activation loop also attenuated NF-κB activity, although this was not complete (PKD.Y463F.SS738/742EE, Fig. 3A). This points to an important but not exclusive role for PKD activation loop phosphorylation in stimulating NF-κB activity.
FIG. 3.
Contributions of the phosphorylation of Tyr463 and Ser738/Ser742 and kinase activity to NF-κB activation. HeLa cells were transfected with NF-κB luc and β-gal reporter genes and either the control vector alone or the indicated mutant PKDs. luc and β-gal reporter gene assays were then performed. Protein expression was controlled in immunoblot assays against PKD (anti-HA antibody) (data not shown). All results are typical of three independent experiments.
Finally, all of the mutant proteins used in Fig. 3A were further mutated to kinase-inactive alleles by introducing a Trp into the critical PKD ATP-binding site at Lys612. Activation of NF-κB in cells expressing either PKD.Y463E or PKD.SS738/742EE was completely eliminated in the context of a K612W mutation (Fig. 3B). Therefore, PKD kinase activity is a prerequisite for NF-κB activation.
PKCδ is activated in response to oxidative stress and interacts with PKD.
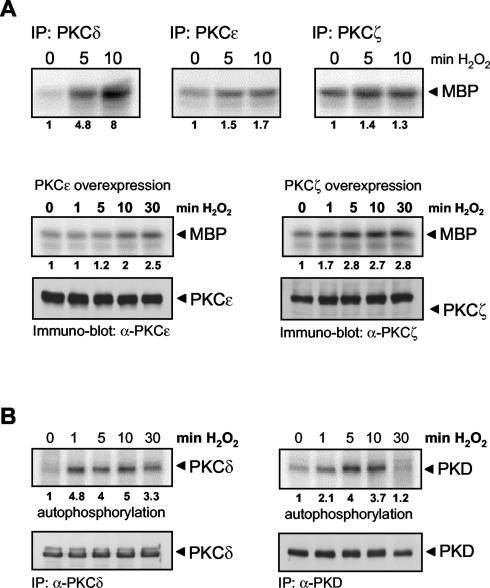
Previous studies have shown that certain nPKC isoforms, particularly PKCɛ and PKCη, act upstream of PKD by phosphorylating the PKD activation loop at Ser378/Ser742 (3, 50, 55). In order to determine whether PKCs also regulate PKD phosphorylation and activation in response to oxidative stress, we first evaluated which novel and atypical PKCs are expressed in HeLa cells. By reverse transcriptase PCR and immunoblotting with specific antibodies, we found that HeLa cells express PKCδ, PKCɛ, and PKCζ (data not shown). We therefore evaluated which of these PKCs are activated in response to oxidative stress. Interestingly, only PKCδ was significantly (five- to eightfold) activated by oxidative stress, as judged by immune complex kinase assays (Fig. 4A) or autophosphorylation assays (Fig. 4B, left side). In contrast, PKCζ or PKCɛ revealed little or no increased kinase activity in response to stimulation of HeLa cells by oxidative stress, even when overexpressed (Fig. 4A). Moreover, the kinetics of PKCδ activation induced by oxidative stress closely correlated with those of PKD, which was evident after 1 min of stimulation, with a peak of activity at 5 to 10 min (Fig. 4B).
FIG. 4.
PKCδ activation by oxidative stress. (A) HeLa cells were treated with H2O2 (10 μM) in a time-dependent manner as indicated, and PKCs were immunoprecipitated (IP) with specific antibodies, after which substrate phosphorylation kinase assays were performed. In overexpression experiments (bottom), protein expression was controlled by immunoblotting against PKCɛ and PKCζ. (B) HeLa cells were treated with H2O2 (10 μM) in a time-dependent manner as indicated, PKCδ or PKD were immunoprecipitated, and autophosphorylation kinase assays were performed. Immunoblot assays of immunoprecipitated PKCδ or PKD were used to monitor equal amounts of endogenous protein. All results are typical of three independent experiments.
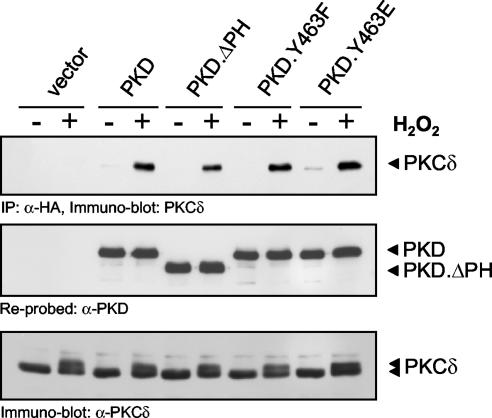
These data suggest that PKCδ is an upstream kinase for PKD. We further investigated this and determined whether a PKCδ-PKD complex exists in cells. Coimmunoprecipitation experiments revealed that following stimulation of HeLa cells with H2O2, endogenous PKCδ and PKD form a complex (data not shown). This was also observed upon expression of an HA-tagged PKD allele, which coimmunoprecipitated with endogenous PKCδ only after H2O2 stimulation (Fig. 5). Interestingly, deletion of the PKD PH domain, as well as mutation of Tyr463, did not significantly abolish this interaction, suggesting that the interaction between PKCδ and PKD is independent of a functional PH domain.
FIG. 5.
Oxidative stress-induced association between PKCδ and PKD. HeLa cells were transfected with the indicated mutant PKDs, and PKD was immunoprecipitated (IP; anti-HA antibody). The immunoprecipitates were resolved by SDS-PAGE and immunoblotted for coimmunoprecipitated endogenous PKCδ. Immunoblots were reprobed to evaluate equivalent PKD immunoprecipitation. Immunoblot assays of cell lysates were also used to monitor endogenous expression of PKCδ. All results are typical of three independent experiments.
PKCδ is the upstream kinase for PKD in oxidative stress signaling.
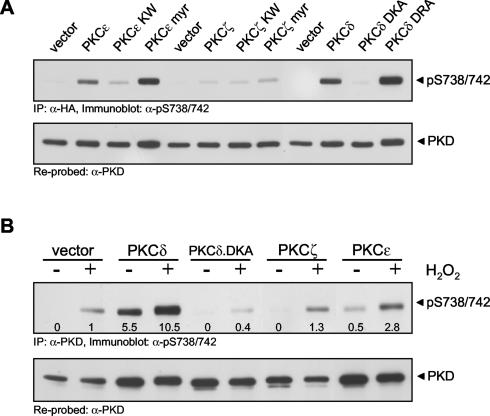
To determine which PKC isoform(s) is responsible for PKD activation, we first transiently expressed wild-type, constitutively active, and kinase-inactive alleles of PKCδ, PKCɛ, and PKCζ and evaluated activation loop phosphorylation of cotransfected PKD. Both wild-type and constitutively active PKCδ and PKCɛ induced Ser738/Ser742 phosphorylation in cells, whereas PKCζ did not (Fig. 6A). Interestingly, in cells exposed to oxidative stress, expression of the kinase-inactive PKCδ allele significantly blocked PKD Ser738/Ser742 phosphorylation compared to that in cells transfected with the control vector alone (Fig. 6B). This suggested that the PKCδ.DKA mutant protein acts in a dominant-negative manner. Conversely, expression of wild-type PKCδ significantly potentiated oxidative stress-stimulated PKD S738/S742 phosphorylation by 10-fold compared to that in control cells, whereas the wild-type PKCζ allele had no effect and wild-type PKCɛ had a modest effect (2.8-fold, Fig. 6B).
FIG. 6.
PKCδ is a PKD upstream kinase in oxidative stress signaling. (A) PKD was expressed together with the indicated PKCɛ, PKCδ, or PKCζ mutant protein (myr, myristoylated; KW, kinase dead; DKA, kinase dead; DRA, constitutively active) in HeLa cells. PKD was immunoprecipitated (IP; anti-HA antibody) and immunoblotted with the phosphospecific PKD activation loop anti-pS738/742 antibody. (B) HeLa cells were transfected with the control vector, wild-type or kinase-inactive PKCδ (DKA), wild-type PKCɛ, or PKCζ along with PKD, and cells were treated with H2O2 (10 μM, 10 min). PKD was immunoprecipitated (anti-PKD antibody) and immunoblotted with anti-pS738/742 antibody. Equal expression of the proteins was monitored by immunoblot analysis (data not shown). All results are typical of three independent experiments.
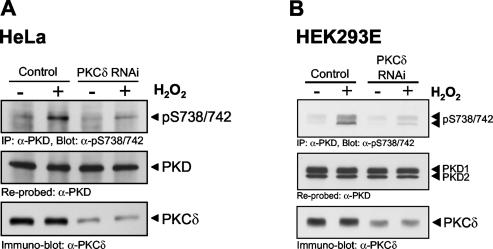
Because of the potential nonspecific effects observed with dominant-negative alleles of kinases such as PKCδ, we used RNAi to specifically silence PKCδ expression. A PKCδ-specific RNAi sequence, cloned in the pSUPER RNAi expression vector, significantly silenced endogenous PKCδ protein by approximately 80% in transiently transfected HeLa cells (Fig. 7A). In the same experiment, phosphorylation of PKD at Ser738/Ser742 was significantly reduced in PKCδ RNAi construct-transfected cells, compared to that in control cells transfected with pSUPER alone. To address the generality of oxidative stress-induced PKD activation loop phosphorylation by PKCδ, we conducted a similar experiment with HEK 293E cells, in which we had previously shown tyrosine phosphorylation and activation of PKD by H2O2 (42). Transient silencing of PKCδ by RNAi reduced PKCδ protein levels, with a concomitant decrease in the H2O2-stimulated phosphorylation of PKD at S738/S742 (Fig. 7B). Note that the PKCδ RNAi construct was specific to this PKC isoform and did not cause silencing of either PKCɛ or PKCζ (data not shown). These data point to an exclusive role for PKCδ in the oxidative stress signaling pathway which leads to PKD phosphorylation at Ser738/Ser742.
FIG. 7.
Oxidative stress-mediated PKD phosphorylation is blocked by PKCδ RNAi. HeLa or HEK 293E cells were transfected with a pSUPER-PKCδ RNAi construct or a control vector. After 48 h, cells were stimulated with H2O2 (10 μM, 10 min). Endogenous PKD was immunoprecipitated (IP; anti-PKD antibody) and analyzed for activation loop phosphorylation (anti-pS738/742 antibody). The blot was reprobed against PKD. Silencing of endogenous PKCδ was monitored by immunoblot analysis. All results are typical of three independent experiments.
PKCδ directly phosphorylates and activates PKD.
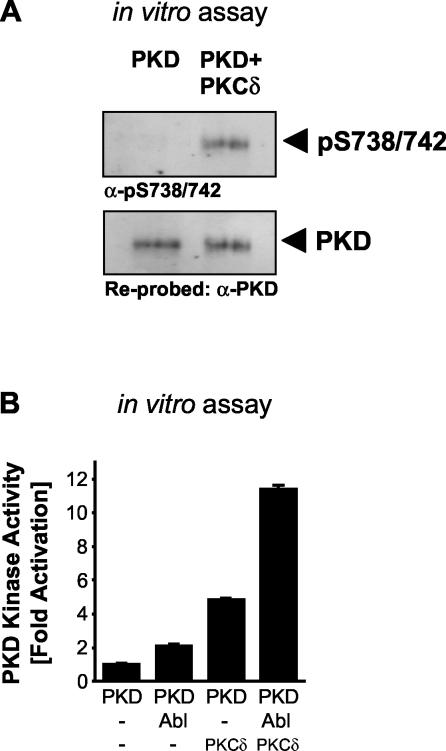
We next determined whether PKCδ is a direct upstream kinase for the PKD activation loop. Purified, recombinant PKCδ directly phosphorylated PKD at Ser738/Ser742 in vitro (Fig. 8A). We then investigated the consequence of this phosphorylation on PKD activity. We previously showed that phosphorylation of PKD at Tyr463 by Abl in vitro results in increased PKD activity (40). With purified, recombinant PKCδ, PKD, and Abl, we found that incubation of PKD with either Abl or PKCδ alone resulted in a two- to fourfold increase in PKD activity, as judged by the phosphorylation of the PKD-specific substrate peptide. However, incubation of both Abl and PKCδ together with PKD resulted in a synergistic, 11-fold increase in PKD activity compared to that of the control (Fig. 8B). This provides further evidence that phosphorylation of both Tyr463, mediated by Abl, and Ser738/Ser742, mediated by PKCδ, is required to achieve maximal PKD activity.
FIG. 8.
Direct phosphorylation of PKD by PKCδ stimulates PKD. (A) An in vitro kinase reaction was performed with purified recombinant PKD and PKCδ (250 ng of PKD, 100 ng of PKCδ, 100 μM unlabeled ATP). PKD then was analyzed for activation loop phosphorylation (anti-pS738/742 antibody). The blot was stripped and reprobed against PKD. (B) PKD, PKCδ, or Abl was added alone or in combination, as indicated, to an unlabeled in vitro kinase assay mixture (200 ng of PKD, 100 ng of PKCδ and/or 20 ng of Abl, 150 μM unlabeled ATP). PKD was immunoprecipitated, and after extensive washing in kinase buffer, a PKD substrate peptide assay was performed as described in Materials and Methods. All results are typical of two independent experiments.
To further investigate the contribution of Y463 phosphorylation in PKD activation loop phosphorylation downstream of Abl (the Y463 kinase) and PKCδ (the S738/S742 kinase), we transiently expressed HeLa cells with either wild-type PKD or the activation loop mutant protein PKD.SS738/742AA, either alone or cotransfected with activated Abl, followed by immune complex PKD kinase assays. As predicted, activated Abl potently induced PKD activity, but this was completely blocked in the activation loop mutant protein (Fig. 9A). This again shows that Y463 phosphorylation alone cannot support PKD activity, which requires activation loop phosphorylation even in the presence of pY463. In a parallel experiment, we probed the requirement for Y463 phosphorylation in the presence of functional S738/S742 phosphorylation. Again, either wild-type PKD or the nonphosphorylatable PKD.Y463F mutant protein was transfected alone or with activated PKCδ (the S738/S742 kinase). Whereas wild-type PKD was potently activated by PKCδ.CA, the Y463F mutant protein was significantly impaired (approximately 50% inhibition, Fig. 9B). Therefore, Y463 phosphorylation is still required to achieve maximal PKD activity even when S738/S742 is phosphorylated in cells. These data further corroborate the model in which phosphorylation of both Y463 and S738/S742 is required to achieve maximal PKD activity in the oxidative stress, Abl, and PKCδ pathway.
FIG. 9.
Y463 phosphorylation and activation loop phosphorylation contribute to PKD activation. (A) HeLa cells were cotransfected with active Abl (v-Abl p120) and with a control vector, wild-type PKD, or mutant protein PKD.SS738/742AA. PKD was immunoprecipitated (anti-HA antibody), and a substrate kinase assay was performed. Expression of proteins was monitored by immunoblot analysis. (B) HeLa cells were cotransfected with active PKCδ (PKCδ.CA, PKCδ.R144/145A), a control vector, wild-type PKD, or mutant protein PKD.Y463F. PKD was immunoprecipitated (anti-HA antibody), and a substrate kinase assay was performed. Expression of proteins was monitored by immunoblot analysis. All results are typical of three independent experiments.
Src-dependent activation of PKCδ in oxidative stress signaling.
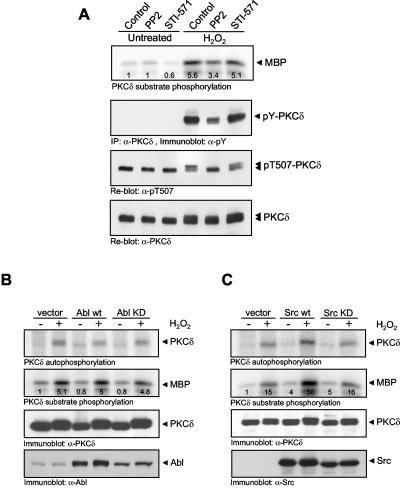
The data thus far demonstrate that PKCδ plays an exclusive role in PKD activation loop phosphorylation, leading to its activation in cells exposed to oxidative stress. We therefore next asked the following question: how is PKCδ activated in response to oxidative stress? Previous studies have shown that in response to oxidative stress, PKCδ associates with Abl (44) and that tyrosine phosphorylation of PKCδ increases its activity (24, 26, 30). However, it is not entirely clear if Abl is a direct upstream kinase for PKCδ. To investigate this further, we used the Src kinase inhibitor PP2 and the Abl inhibitor STI-571. Interestingly, PP2 reduced PKCδ tyrosine phosphorylation, as well as kinase activity (toward MBP) in response to oxidative stress (Fig. 10A). Conversely, inhibition of Abl by STI-571 had no effect on PKCδ activity or tyrosine phosphorylation (Fig. 10A). In addition, neither inhibitor had any effect on the phosphorylation of the PKCδ activation loop residue Thr507, which is phosphorylated by PDK-1 (phosphoinositide-dependent kinase 1). Note that the appearance of a more slowly migrating PKCδ species in response to H2O2 in the anti-pT507 immunoblot is likely due to tyrosine phosphorylation by Src, because this mobility shift is also blocked in cells treated with PP2. Moreover, we did not detect any measurable changes in the phosphorylation of PKCδ at its activation loop (Thr507).
FIG. 10.
Src is upstream of PKCδ in oxidative stress signaling. (A) HeLa cells were treated with PP2 (40 μM) or STI-571 (5 μM) or left untreated for 1 h. Cells were then stimulated with H2O2 (10 μM, 10 min) as indicated. PKCδ was immunoprecipitated, and a substrate kinase assay was performed or PKCδ was immunoblotted with anti-pTyr (4G10), anti-pThr507, or anti-PKCδ antibody. (B, C) PKCδ and the vector alone, wild-type (wt) or kinase-inactive Abl (B), or wild-type or kinase-inactive Src (C) were coexpressed. Cells were stimulated with H2O2 (10 μM, 10 min), PKCδ was immunoprecipitated, and a substrate phosphorylation kinase assay was performed. Protein expression was verified by immunoblotting with anti-Abl, anti-Src, and anti-PKCδ antibodies. All results are typical of two independent experiments.
To reinforce the results obtained with PP2 and STI-571, we transiently transfected HeLa cells with wild-type Src and Abl, as well as kinase-inactive alleles of both Src and Abl, and evaluated PKCδ activity. Wild-type Abl was not able to potentiate PKCδ MBP kinase activity stimulated by H2O2, and the kinase-inactive allele also did not block this response (Fig. 10B). Conversely, a wild-type Src allele potentiated H2O2-stimulated PKCδ activity, whereas the kinase-inactive allele did not (Fig. 10C). These data show that in response to oxidative stress, PKCδ is activated primarily downstream of Src, whereas Abl does not appear to play a major role in PKCδ activation.
PKCδ mediates PKD-dependent NF-κB activation in oxidative stress signaling.
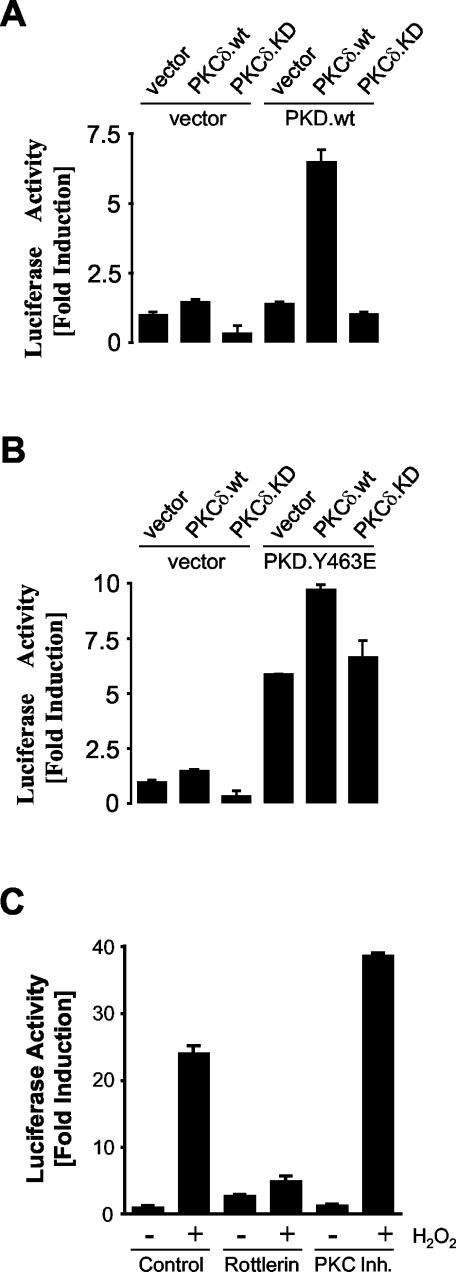
Since phosphorylation of the PKD activation loop is required for NF-κB activation (Fig. 3), we next investigated the role of PKCδ in this pathway. Firstly, coexpression of wild-type PKCδ with wild-type PKD potently induced NF-κB activity, as measured with the NF-κB-luc reporter (Fig. 11A). This effect was not observed when the kinase-inactive PKCδ allele was used. Secondly, NF-κB activity induced by expression of the constitutively active PKD.Y463E mutant protein was further stimulated by coexpression of wild-type PKCδ, but again not kinase-inactive PKCδ (Fig. 11B). This lends additional support to the notion that although the PKD.Y463E mutant protein shows constitutive kinase activity and constitutive Ser738/Ser742 phosphorylation, the endogenous basal PKCδ activity is not capable of stimulating maximal phosphorylation of the PKD activation loop in the absence of cell stimulation. Thus, coexpression of exogenous PKCδ further stimulates PKD.Y463E-dependent NF-κB activity.
FIG. 11.
Role of PKCδ in oxidative stress-induced NF-κB activation. (A) HeLa cells were cotransfected with NF-κB luc and β-gal reporters and with the control vector, PKD, PKCδ, or kinase-inactive PKCδ as indicated. (B) HeLa cells were cotransfected with NF-κB luc and β-gal reporter genes and the control vector, PKD.Y463E, PKCδ, or kinase-inactive PKCδ. (C) HeLa cells were transfected with NF-κB luc and β-gal reporter genes, treated with inhibitors (rottlerin at 10 μM or myristoylated PKC inhibitor [Inh.] 19-27 at 10 μM) for 6 h, and then stimulated with 500 nM H2O2 for 16 h. luc and β-gal reporter gene assays were performed. Protein expression was controlled by immunoblotting with anti-PKCδ and anti-PKD antibodies (data not shown). All results are typical of three independent experiments.
We also used two chemical inhibitors to further define the role of PKCs in NF-κB activation. Rottlerin has been described and used as a PKCδ-selective inhibitor (14). However, studies have shown that rottlerin does not directly inhibit PKCδ (9) and most likely acts to block its activity because it is a potent uncoupler of the mitochondrial ATP electron transport chain (39). Therefore, in rottlerin-treated cells, PKCδ activity is indirectly inhibited, and this correlates with inhibition of NF-κB activation in response to oxidative stress (Fig. 11C). More compelling was the fact that the myristoylated PKC inhibitor 19-27, which blocks the activity of conventional PKCα and PKCβ, had no effect on H2O2-stimulated NF-κB activation.
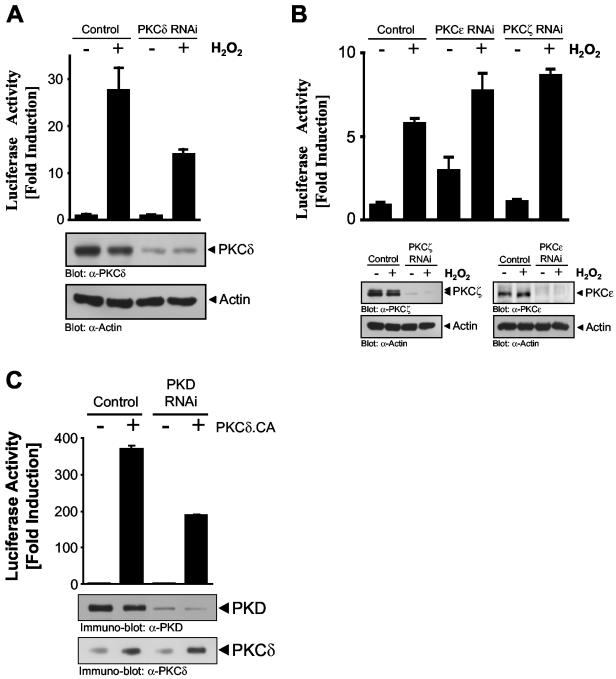
Finally, we used PKCδ RNAi to silence PKCδ and demonstrate a specific role for this nPKC isoform in oxidative stress-dependent NF-κB activation. HeLa cells transiently transfected with a PKCδ RNAi construct and stimulated with H2O2 showed a significant reduction in NF-κB activation, concomitant with loss of PKCδ protein (Fig. 12A). Conversely, cells transfected with a PKCɛ or PKCζ RNAi construct showed no reduction in NF-κB luc activity, despite a nearly complete silencing of PKCɛ and PKCζ protein (Fig. 12B). Finally, to probe a potential role of PKCδ in NF-κB activation which may be independent of PKD, we transiently expressed the activated PKCδ.CA allele, either alone or in combination with a PKD RNAi construct, and measured NF-κB luc activity. The PKD RNAi construct blocked NF-κB induction by activated PKCδ by approximately 50%, suggesting that the remaining NF-κB activity could be due to PKD-independent pathways activated downstream of PKCδ. A cautionary note must be made, however, because in this and other experiments we have not been able to completely and quantitatively silence PKD expression to undetectable levels, and thus, the remaining NF-κB activity seen in this experiment may indeed be due to the residual PKD. This issue can only be addressed by the use of cells with the gene for PKD deleted by homologous recombination, which are not yet available. Nonetheless, the data in Fig. 12 strongly argue that PKCɛ, PKCζ, and conventional PKCs do not participate in the NF-κB activation pathway in oxidative stress signaling. Rather, PKCδ, acting upstream of PKD, is responsible for signal relay to the NF-κB module.
FIG. 12.
PKCδ RNAi blocks oxidative stress-induced NF-κB activation. (A, B) HeLa cells stably expressing the NF-κB luc and β-gal reporters (42) were transfected for 48 h with pSUPER (control), a pSUPER.PKCδ RNAi construct (PKCδ RNAi), a pSUPER.PKCɛ RNAi construct (PKCε RNAi), or a pSUPER.PKCζ RNAi construct (PKCζ RNAi) and treated with H2O2 (500 nM, 16 h), and luc reporter gene assays were performed. Protein expression was controlled by immunoblotting with anti-PKCδ, anti-PKCɛ, anti-PKCζ, or anti-actin (loading control) antibody. (C) HeLa cells stably expressing the NF-κB luc and β-gal reporters were transfected for 48 h with pSUPER (control) or pSUPER PKD1/2 and either the vector alone or the constitutively active PKCδ allele (PKCδ.CA), and luc and β-gal reporter gene assays were performed. Protein expression was monitored by immunoblotting with anti-PKD and anti-PKCδ antibodies. All results are typical of three independent experiments.
Intracellular ROS generation promotes PKD and NF-κB activation through PKCδ.
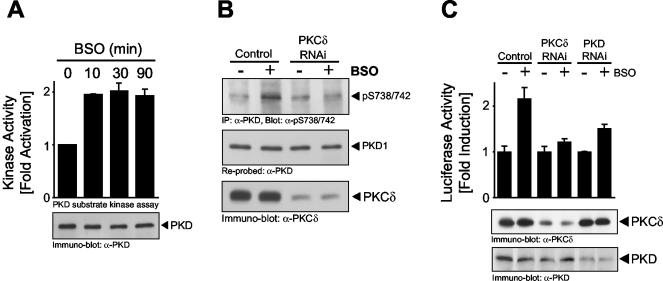
Although numerous studies have shown that exposure of cells to H2O2 induces intracellular ROS generation (13), and also that exposure of cells to extracellular ROS occurs in human pathologies such as cancer and inflammation (11, 46), we wanted to more directly implicate intracellular ROS release in the activation of the PKCδ/PKD/NF-κB pathway. Glutathione (GSH) is the major intracellular thiol and ROS scavenger (33), and its synthesis is controlled by glutamylcysteine synthetase. BSO is a compound that causes GSH depletion by selectivity inhibiting glutamylcysteine synthetase, such that the net effect is an increase in intracellular ROS (1). HeLa cells exposed to BSO revealed an increase in PKD activity as measured in immune complex kinase assays (Fig. 13A). As expected, BSO also promoted increased PKD activation loop phosphorylation at S738/S742, and this was blocked by specific silencing with the PKCδ RNAi construct (Fig. 13B). This is consistent with a role for PKCδ in oxidative stress-mediated PKD regulation at the activation loop. Finally, BSO also promoted NF-κB activation and, as predicted, this activation was blocked by both PKD and PKCδ RNAi constructs, with a concomitant reduction in the expression of both proteins (Fig. 13C). Thus, release of intracellular ROS leads to PKD activation, which requires PKCδ, and the signal is then relayed to NF-κB.
FIG. 13.
Intracellular oxidative stress activates the PKCδ/PKD/NF-κB pathway. (A) HeLa cells were stimulated with BSO (500 μM) for the indicated times. PKD was immunoprecipitated, and a substrate kinase assay was performed. PKD expression was revealed by immunoblotting. (B) HeLa cells were transfected with a pSUPER.PKCδ RNAi construct or the control vector. After 48 h, cells were stimulated with BSO (500 μM, 15 min). Endogenous PKD was immunoprecipitated (anti-PKD antibody) and analyzed for activation loop phosphorylation (anti-pS738/742 antibody). The blot was reprobed against PKD. Silencing of endogenous PKCδ was monitored by immunoblot analysis. (C) HeLa cells were transfected for 48 h with pSUPER (control), a pSUPER.PKCδ RNAi construct (PKCδ RNAi), or a pSUPER.PKD RNAi construct-pSuper.PKD2 RNAi construct combination (PKD RNAi). After 24 h, a second transfection with the NF-κB luc and β-gal reporters was performed. PKD and PKCδ expression was controlled by immunoblotting. All results are typical of three independent experiments.
DISCUSSION
Activation of the serine/threonine kinase PKD controls a number of cellular responses, including cell growth and DNA synthesis, Golgi organization, B- and T-cell function, and cellular survival (15, 51). In addition to activation by polypeptide growth factors such as PDGF, PKD is also potently activated by oxidative stress and ROS (42, 52). Activation of PKD by oxidative stress in turn activates NF-κB through the canonical IKK/IκB pathway (42). Despite numerous reports demonstrating activation of NF-κB in response to extracellular and intracellular ROS generation, the notion that NF-κB is a sensor for oxidative stress was recently challenged by a report which suggested that ROS do not mediate NF-κB activation (16). However, this is potentially misleading because the report actually provided evidence that the antioxidants N-acetyl-l-cysteine and pyrrolidine dithiocarbamate, which have previously been used to block TNF-mediated ROS production and consequently NF-κB activation, actually block NF-κB by lowering the affinity of TNF receptors for ligand (in the case of N-acetyl-l-cysteine) or blocking IκB-ubiquitin ligase activity in a cell-free system (in the case of pyrrolidine dithiocarbamate). In fact, numerous studies have shown that exposure of cells to oxidative stress, such as H2O2, or intracellular ROS generation leads to a potent NF-κB response, regardless of antioxidant activity. As an example, BSO, which depletes the ROS scavenger GSH, elevates intracellular ROS with a concomitant activation of PKD and NF-κB (Fig. 13). Our own studies have also shown that in response to oxidative stress (H2O2), a hierarchical Src-Abl signaling pathway is activated. Abl then directly phosphorylates PKD at Tyr463 in the PH domain. We also showed that Tyr463 phosphorylation is restricted to oxidative stress signaling and that upon Tyr463 activation, PKD is activated and relays the signal to NF-κB. The net consequence is increased survival of cells exposed to oxidative stress (42). However, because it is well known that PKD activity is also controlled by activation loop phosphorylation, we turned our attention to the mechanisms by which PKD activation loop phosphorylation controls NF-κB activity and the role of Tyr463 phosphorylation in this event. The data presented here point to a central role played by phosphorylation of both Tyr463 and Ser738/Ser742 for efficient activation of NF-κB in response to oxidative stress.
Synergistic action of PKD Tyr463 and Ser738/Ser742 phosphorylation.
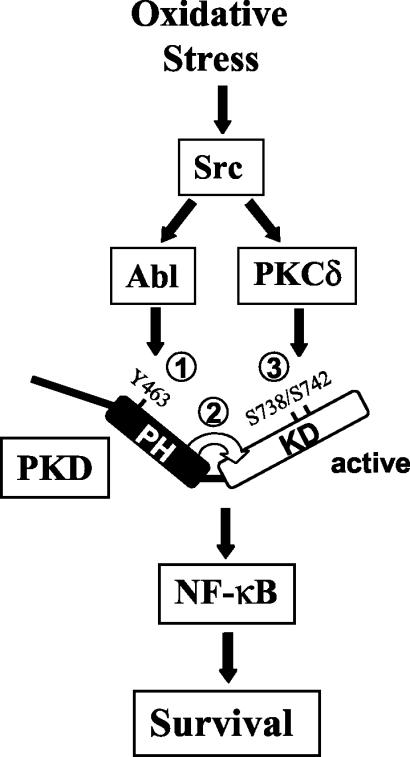
It is well known that the PKD PH domain acts in an autoinhibitory manner toward the catalytic kinase domain, because a mutant protein with the PKD PH domain deleted is constitutively active (18). A recent study by the Rozengurt laboratory demonstrated that following growth factor stimulation, phosphorylation of PKD activation loop residues Ser738 and Ser742 promotes release of the PH domain (54). They also showed that PKCɛ was responsible for PKC activation loop phosphorylation. Our present results also point to an important role for the PKD PH domain in controlling kinase activity but suggest an alternative model for the activation of PKD, specifically in response to oxidative stress signaling. We propose that in cells exposed to ROS, Abl-mediated phosphorylation of PKD at Tyr463 facilitates a conformational change in the PH domain that results in exposure of the activation loop residues, now accessible to the upstream kinase, in this case, PKCδ (Fig. 14). The key findings that lend support to this model are as follows: firstly, a Y463E mutant protein is constitutively phosphorylated at the activation loop (Fig. 1C); secondly, a PKD.Y463E.SS738/744AA mutant protein is impaired in kinase activity (Fig. 2); thirdly, Abl and PKCδ synergistically activate PKD in vitro (Fig. 8C); finally, a PKD Y463F mutant protein shows impaired kinase activity even when activation loop phosphorylation is induced by expression of activated PKCδ (Fig. 9B). Thus, phosphorylation of both Tyr463 and Ser738/Ser742 is required to achieve maximal PKD activity, both in vitro and in cells. Importantly, we also propose that the mechanism by which PH domain phosphorylation controls accessibility to the activation loop is restricted to oxidative stress signaling. This is because in response to PDGF or bradykinin, for example, Tyr463 phosphorylation does not occur, and there is also no induction of NF-κB (42). Therefore, in response to mitogens, activation of PKD may be initiated by activation loop phosphorylation, as proposed by Waldron and colleagues (54). At any rate, one feature common to both mechanisms is the crucial role played by the PH domain. Ultimately, validation of these models currently suggested by biochemical analyses will have to come from a structural analysis of PKD by crystallographic studies.
FIG. 14.
Proposed model of PKD activation in oxidative stress signaling. Oxidative stress activates PKD by two Src-mediated signaling events. Src lies upstream of both Abl and PKCδ and induces Abl-mediated phosphorylation of PKD at Tyr463 in the PH domain (step 1). This facilitates release of the PH domain, which exposes the catalytic kinase domain and activation loop residues (step 2), which are in turn directly phosphorylated by PKCδ (step 3). This results in fully active PKD, which relays the signal for the activation of NF-κB in cells exposed to oxidative stress.
PKCδ is the upstream kinase for PKD in oxidative stress signaling.
Our studies point to an important and exclusive role for PKCδ in the activation of PKD, which is then relayed to NF-κB in cells exposed to H2O2. Moreover, we show that other PKCs, most notably, PKCɛ and PKCζ, do not participate in PKD activation loop phosphorylation and activation (Fig. 6), nor do they contribute to oxidative stress-mediated NF-κB induction (Fig. 12). This is of particular interest because previous reports have shown that, at least in response to mitogens, PKCɛ acts upstream of PKD and that in vitro, PKCɛ can efficiently phosphorylate PKD at Ser738/Ser742 (54). Thus, unlike PKCδ, PKCɛ is not a relevant upstream kinase for the PKD activation loop in oxidative stress signaling. This is despite the fact that PKCɛ is expressed in HeLa cells and also shows a modest increase in activity following exposure of cells to H2O2 (Fig. 4). Moreover, although PKCη and PKCɛ have been shown to associate with PKD through its PH domain (52), we find that the association between PKCδ and PKD, which occurs in response to H2O2 stimulation, does not require a functional PH domain (Fig. 5). One possible explanation for the inability of PKCɛ to regulate PKD is that the distinct cellular location of PKCδ and PKCɛ determines their accessibility to PKD. This is currently under investigation.
With a combination of active and inactive PKCδ, PKCɛ, and PKCζ alleles, as well as chemical inhibitors and isoform-specific RNAi constructs, we have shown that PKCδ plays a selective role in PKD activation leading to NF-κB induction. Previous studies have addressed the role of various PKC isoforms in regulating the activation of NF-κB. For example, nPKCθ is crucial for the activation of NF-κB in T and B cells (2, 8, 25, 31, 45). Conventional PKCβ has been shown to control IKK activation, IκB degradation, and NF-κB induction in B cells stimulated through the B-cell receptor (38, 43). Similarly, PMA-stimulated NF-κB activation has been shown to be mediated by PKCɛ (17), whereas PKCδ appears to participate in TNF-induced NF-κB induction (22, 48). However, in the latter case, it appears that cleavage of PKCδ by caspase 3 is required and subsequently has a proapoptotic function (4). In contrast, in oxidative stress signaling, PKCδ acts upstream of NF-κB by participating in the activation of PKD, which leads to increased cell survival in cells exposed to oxidative stress (42). Finally, studies by the Moscat laboratory have also indicated that atypical PKCζ participates in the activation of NF-κB, such that PKCζ-deficient fibroblasts show impaired TNF-dependent NF-κB activation (28). Moreover, PKCζ was recently shown to directly phosphorylate Ser311 in the RelA subunit of NF-κB, and this was required for NF-κB antiapoptotic signaling (10). However, with PKCζ-specific RNAi, we have shown that, at least in the oxidative stress pathway, PKCζ is dispensable for NF-κB activation (Fig. 12).
Src is upstream of Abl and PKCδ in oxidative stress signaling.
Several PKC isoforms are activated by oxidative stress (23). In the case of PKCδ, studies have shown that in cells stimulated with H2O2, PKCδ is activated by tyrosine phosphorylation (24, 26, 30). It has also been shown that oxidative stress promotes the association between PKCδ and Abl (44) and also that activation of PKCδ in response to genotoxic agents is mediated by Abl (56). However, activation of PKCδ by Abl in oxidative stress signaling has not been reported. This has been further complicated by the fact that Src has also been shown to interact with PKCδ and thus to have the potential to regulate PKCδ tyrosine phosphorylation and activity (47, 57). We therefore systematically analyzed the contributions of both Src and Abl to the activation of PKCδ in oxidative stress signaling (Fig. 10). Our results demonstrate that PKCδ tyrosine phosphorylation and activation in response to oxidative stress require Src, but not Abl, activity (Fig. 10). Therefore, Src appears to play a key role in the regulation of PKD because it is required for activation of Abl and also participates in the activation of PKCδ in response to H2O2. These two signals are integrated at the level of PKD, whereby Abl directly phosphorylates Tyr463 in the PH domain, and PKCδ directly phosphorylates the activation loop Ser738/Ser742. Finally, it is also interesting that increased PKCδ activity was not due to increased PKCδ activation loop phosphorylation, because we could not detect measurable increases in Thr507 phosphorylation. Thr507 is phosphorylated by PDK-1 in the phosphoinositide 3-kinase pathway (27), and although activation of PDK-1 has been reported to occur in response to oxidative stress (35), this does not appear to be sufficient to mediate increased Thr507 phosphorylation. This is also consistent with the fact that phosphoinositide 3-kinase does not participate in the activation of PKD. Rather, the key event that controls PKCδ tyrosine phosphorylation appears to be Src activation.
In summary, we have shown that activation of PKD by oxidative stress requires two coordinated signaling events, the first of which is Tyr463 phosphorylation, which is mediated by the Src-Abl signaling pathway; this facilitates the second step, phosphorylation of the activation loop Ser738/Ser742, which is mediated by the Src-PKCδ pathway. Both events are required for full PKD activity, which in turn stimulates the activation of NF-κB in oxidative stress responses. The remaining challenge is to ascribe specific substrates of PKD that are responsible for signal relay to the IKK-NF-κB module. This is currently under investigation in our laboratory.
Acknowledgments
We thank R. Agami, J. Brugge, S. Ohno, M. Reyland, N. Rosenberg, and B. Schaffhausen for generously providing expression plasmids. We also thank E. Buchdunger (Novartis Inc.) for providing STI-571 and members of the Toker laboratory for insightful discussions.
This work was supported by grants from the National Institutes of Health (CA 75134, A.T.) and the Deutsche Forschungsgemeinschaft (STO 439/1-1, P.S.).
REFERENCES
- 1.Aruoma, O. I., B. Halliwell, B. M. Hoey, and J. Butler. 1989. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 6:593-597. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, B., N. Krumbock, F. Fresser, F. Hochholdinger, M. Spitaler, A. Simm, F. Uberall, B. Schraven, and G. Baier. 2001. Complex formation and cooperation of protein kinase Cθ and Akt1/protein kinase Bα in the NF-κB transactivation cascade in Jurkat T cells. J. Biol. Chem. 276:31627-31634. [DOI] [PubMed] [Google Scholar]
- 3.Brandlin, I., S. Hubner, T. Eiseler, M. Martinez-Moya, A. Horschinek, A. Hausser, G. Link, S. Rupp, P. Storz, K. Pfizenmaier, and F. J. Johannes. 2002. Protein kinase C (PKC)η-mediated PKCμ activation modulates ERK and JNK signal pathways. J. Biol. Chem. 277:6490-6496. [DOI] [PubMed] [Google Scholar]
- 4.Brodie, C., and P. M. Blumberg. 2003. Regulation of cell apoptosis by protein kinase Cδ. Apoptosis 8:19-27. [DOI] [PubMed] [Google Scholar]
- 5.Brummelkamp, T. R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550-553. [DOI] [PubMed] [Google Scholar]
- 6.Cenni, V., H. Doppler, E. D. Sonnenburg, N. Maraldi, A. C. Newton, and A. Toker. 2002. Regulation of novel protein kinase Cɛ by phosphorylation. Biochem. J. 363:537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, M. M., W. Hou, J. Johnson, L. K. Graham, M. H. Lee, C. S. Chen, A. C. Newton, B. S. Schaffhausen, and A. Toker. 1998. Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr. Biol. 8:1069-1077. [DOI] [PubMed] [Google Scholar]
- 8.Coudronniere, N., M. Villalba, N. Englund, and A. Altman. 2000. NF-κB activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-θ. Proc. Natl. Acad. Sci. USA 97:3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duran, A., M. T. Diaz-Meco, and J. Moscat. 2003. Essential role of RelA Ser311 phosphorylation by ζPKC in NF-κB transcriptional activation. EMBO J. 22:3910-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englert, R. P., and E. Shacter. 2002. Distinct modes of cell death induced by different reactive oxygen species: amino acyl chloramines mediate hypochlorous acid-induced apoptosis. J. Biol. Chem. 277:20518-20526. [DOI] [PubMed] [Google Scholar]
- 12.Fan, C., Q. Li, D. Ross, and J. F. Engelhardt. 2003. Tyrosine phosphorylation of IκBα activates NFκB through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J. Biol. Chem. 278:2072-2080. [DOI] [PubMed] [Google Scholar]
- 13.Finkel, T. 2003. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15:247-254. [DOI] [PubMed] [Google Scholar]
- 14.Gschwendt, M., H. J. Muller, K. Kielbassa, R. Zang, W. Kittstein, G. Rincke, and F. Marks. 1994. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199:93-98. [DOI] [PubMed] [Google Scholar]
- 15.Hausser, A., G. Link, L. Bamberg, A. Burzlaff, S. Lutz, K. Pfizenmaier, and F. J. Johannes. 2002. Structural requirements for localization and activation of protein kinase Cμ (PKCμ) at the Golgi compartment. J. Cell Biol. 156:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa, M., H. Miyashita, I. Sakamoto, M. Kitagawa, H. Tanaka, H. Yasuda, M. Karin, and K. Kikugawa. 2003. Evidence that reactive oxygen species do not mediate NF-κB activation. EMBO J. 22:3356-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano, M., S. Hirai, K. Mizuno, S. Osada, M. Hosaka, and S. Ohno. 1995. A protein kinase C isozyme, nPKCɛ, is involved in the activation of NF-κB by 12-O-tetradecanoylphorbol-13-acetate (TPA) in rat 3Y1 fibroblasts. Biochem. Biophys. Res. Commun. 206:429-436. [DOI] [PubMed] [Google Scholar]
- 18.Iglesias, T., and E. Rozengurt. 1998. Protein kinase D activation by mutations within its pleckstrin homology domain. J. Biol. Chem. 273:410-416. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias, T., R. T. Waldron, and E. Rozengurt. 1998. Identification of in vivo phosphorylation sites required for protein kinase D activation. J. Biol. Chem. 273:27662-27667. [DOI] [PubMed] [Google Scholar]
- 20.Karin, M. 1999. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18:6867-6874. [DOI] [PubMed] [Google Scholar]
- 21.Karin, M., T. Takahashi, P. Kapahi, M. Delhase, Y. Chen, C. Makris, D. Rothwarf, V. Baud, G. Natoli, F. Guido, and N. Li. 2001. Oxidative stress and gene expression: the AP-1 and NF-κB connections. Biofactors 15:87-89. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick, L. E., J. Y. Lee, K. M. Haines, D. E. Campbell, K. E. Sullivan, and H. M. Korchak. 2002. A role for PKC-δ and PI 3-kinase in TNF-α-mediated antiapoptotic signaling in the human neutrophil. Am. J. Physiol. Cell Physiol. 283:C48-C57. [DOI] [PubMed] [Google Scholar]
- 23.Konishi, H., M. Tanaka, Y. Takemura, H. Matsuzaki, Y. Ono, U. Kikkawa, and Y. Nishizuka. 1997. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 94:11233-11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konishi, H., E. Yamauchi, H. Taniguchi, T. Yamamoto, H. Matsuzaki, Y. Takemura, K. Ohmae, U. Kikkawa, and Y. Nishizuka. 2001. Phosphorylation sites of protein kinase Cδ in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci. USA 98:6587-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krappmann, D., A. Patke, V. Heissmeyer, and C. Scheidereit. 2001. B-cell receptor- and phorbol ester-induced NF-κB and c-Jun N-terminal kinase activation in B cells requires novel protein kinase C's. Mol. Cell. Biol. 21:6640-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronfeld, I., G. Kazimirsky, P. S. Lorenzo, S. H. Garfield, P. M. Blumberg, and C. Brodie. 2000. Phosphorylation of protein kinase Cδ on distinct tyrosine residues regulates specific cellular functions. J. Biol. Chem. 275:35491-35498. [DOI] [PubMed] [Google Scholar]
- 27.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 28.Leitges, M., L. Sanz, P. Martin, A. Duran, U. Braun, J. F. Garcia, F. Camacho, M. T. Diaz-Meco, P. D. Rennert, and J. Moscat. 2001. Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol. Cell 8:771-780. [DOI] [PubMed] [Google Scholar]
- 29.Li, N., and M. Karin. 1999. Is NF-κB the sensor of oxidative stress? FASEB J. 13:1137-1143. [PubMed] [Google Scholar]
- 30.Li, W., H. Mischak, J. C. Yu, L. M. Wang, J. F. Mushinski, M. A. Heidaran, and J. H. Pierce. 1994. Tyrosine phosphorylation of protein kinase C-δ in response to its activation. J. Biol. Chem. 269:2349-2352. [PubMed] [Google Scholar]
- 31.Lin, X., A. O'Mahony, Y. Mu, R. Geleziunas, and W. C. Greene. 2000. Protein kinase C-θ participates in NF-κB activation induced by CD3-CD28 costimulation through selective activation of IκB kinase β. Mol. Cell. Biol. 20:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 298:1912-1934. [DOI] [PubMed] [Google Scholar]
- 33.Meister, A., and M. E. Anderson. 1983. Glutathione. Annu. Rev. Biochem. 52:711-760. [DOI] [PubMed] [Google Scholar]
- 34.Mercurio, F., and A. M. Manning. 1999. NF-κB as a primary regulator of the stress response. Oncogene 18:6163-6171. [DOI] [PubMed] [Google Scholar]
- 35.Prasad, N., R. S. Topping, D. Zhou, and S. J. Decker. 2000. Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1). Biochemistry 39:6929-6935. [DOI] [PubMed] [Google Scholar]
- 36.Roebuck, K. A. 1999. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-κB (review). Int. J. Mol. Med. 4:223-230. [DOI] [PubMed] [Google Scholar]
- 37.Rykx, A., L. De Kimpe, S. Mikhalap, T. Vantus, T. Seufferlein, J. R. Vandenheede, and J. Van Lint. 2003. Protein kinase D: a family affair. FEBS Lett. 546:81-86. [DOI] [PubMed] [Google Scholar]
- 38.Saijo, K., I. Mecklenbrauker, A. Santana, M. Leitger, C. Schmedt, and A. Tarakhovsky. 2002. Protein kinase Cβ controls nuclear factor κB activation in B cells through selective regulation of the IκB kinase α. J. Exp. Med. 195:1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soltoff, S. P. 2001. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cδ tyrosine phosphorylation. J. Biol. Chem. 276:37986-37992. [DOI] [PubMed] [Google Scholar]
- 40.Storz, P., H. Doppler, F. J. Johannes, and A. Toker. 2003. Tyrosine phosphorylation of protein kinase D in the pleckstrin homology domain leads to activation. J. Biol. Chem. 278:17969-17976. [DOI] [PubMed] [Google Scholar]
- 41.Storz, P., and A. Toker. 2003. NF-κB signaling—an alternate pathway for oxidative stress responses. Cell Cycle 2:9-10. [DOI] [PubMed] [Google Scholar]
- 42.Storz, P., and A. Toker. 2003. Protein kinase D mediates a stress-induced NF-κB activation and survival pathway. EMBO J. 22:109-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su, T. T., B. Guo, Y. Kawakami, K. Sommer, K. Chae, L. A. Humphries, R. M. Kato, S. Kang, L. Patrone, R. Wall, M. Teitell, M. Leitges, T. Kawakami, and D. J. Rawlings. 2002. PKC-β controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3:780-786. [DOI] [PubMed] [Google Scholar]
- 44.Sun, X., F. Wu, R. Datta, S. Kharbanda, and D. Kufe. 2000. Interaction between protein kinase Cδ and the c-Abl tyrosine kinase in the cellular response to oxidative stress. J. Biol. Chem. 275:7470-7473. [DOI] [PubMed] [Google Scholar]
- 45.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 46.Tan, S., Y. Sagara, Y. Liu, P. Maher, and D. Schubert. 1998. The regulation of reactive oxygen species production during programmed cell death. J. Cell Biol. 141:1423-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapia, J. A., L. J. Garcia-Marin, and R. T. Jensen. 2003. Cholecystokinin-stimulated protein kinase C-δ kinase activation, tyrosine phosphorylation and translocation is mediated by Src tyrosine kinases in pancreatic acinar cells. J. Biol. Chem. 278:35220-35230. [DOI] [PubMed]
- 48.Vancurova, I., V. Miskolci, and D. Davidson. 2001. NF-κB activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cδ. Correlation to nuclear IκBα. J. Biol. Chem. 276:19746-19752. [DOI] [PubMed] [Google Scholar]
- 49.van den Berg, R., G. R. Haenen, H. van den Berg, and A. Bast. 2001. Transcription factor NF-κB as a potential biomarker for oxidative stress. Br. J. Nutr. 86: (Suppl. 1):S121-S127. [DOI] [PubMed] [Google Scholar]
- 50.Van Lint, J., Y. Ni, M. Valius, W. Merlevede, and J. R. Vandenheede. 1998. Platelet-derived growth factor stimulates protein kinase D through the activation of phospholipase Cγ and protein kinase C. J. Biol. Chem. 273:7038-7043. [DOI] [PubMed] [Google Scholar]
- 51.Van Lint, J., A. Rykx, Y. Maeda, T. Vantus, S. Sturany, V. Malhotra, J. R. Vandenheede, and T. Seufferlein. 2002. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 12:193-200. [DOI] [PubMed] [Google Scholar]
- 52.Waldron, R. T., T. Iglesias, and E. Rozengurt. 1999. The pleckstrin homology domain of protein kinase D interacts preferentially with the η isoform of protein kinase C. J. Biol. Chem. 274:9224-9230. [DOI] [PubMed] [Google Scholar]
- 53.Waldron, R. T., O. Rey, T. Iglesias, T. Tugal, D. Cantrell, and E. Rozengurt. 2001. Activation loop Ser744 and Ser748 in protein kinase D are transphosphorylated in vivo. J. Biol. Chem. 276:32606-32615. [DOI] [PubMed] [Google Scholar]
- 54.Waldron, R. T., and E. Rozengurt. 2003. Protein kinase C phosphorylates protein kinase D activation loop Ser744 and Ser748 and releases autoinhibition by the pleckstrin homology domain. J. Biol. Chem. 278:154-163. [DOI] [PubMed] [Google Scholar]
- 55.Yuan, J., D. Bae, D. Cantrell, A. E. Nel, and E. Rozengurt. 2002. Protein kinase D is a downstream target of protein kinase Cθ○. Biochem. Biophys. Res. Commun. 291:444-452. [DOI] [PubMed] [Google Scholar]
- 56.Yuan, Z. M., T. Utsugisawa, T. Ishiko, S. Nakada, Y. Huang, S. Kharbanda, R. Weichselbaum, and D. Kufe. 1998. Activation of protein kinase Cδ by the c-Abl tyrosine kinase in response to ionizing radiation. Oncogene 16:1643-1648. [DOI] [PubMed] [Google Scholar]
- 57.Zang, Q., P. Frankel, and D. A. Foster. 1995. Selective activation of protein kinase C isoforms by v-Src. Cell Growth Differ. 6:1367-1373. [PubMed] [Google Scholar]
- 58.Zugaza, J. L., J. Sinnett-Smith, J. Van Lint, and E. Rozengurt. 1996. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 15:6220-6230. [PMC free article] [PubMed] [Google Scholar]