Abstract
Transcriptional control mediated by the cyclic AMP-responsive element (CRE) represents an important mechanism of gene regulation. To test our hypothesis that increased inducible cyclic AMP early repressor (ICER) Iγ inhibits function of CRE-binding proteins and thus disrupts CRE-mediated transcription in pancreatic β cells, we generated transgenic mice with β-cell-directed expression of ICER Iγ, a powerful repressor that is greatly increased in diabetes. Three transgenic lines clearly show that increased ICER Iγ expression in β cells results in early severe diabetes. From birth islets were severely disorganized with a significantly increased proportion of α cells throughout the islet. Diabetes results from the combined effects of impaired insulin expression and a decreased number of β cells. The decrease in β cells appears to result from impaired proliferation rather than from increased apoptosis after birth. Cyclin A gene expression is impaired by the strong inhibition of ICER; the suppression of cyclin A results in a substantially decreased proliferation of β cells in the postnatal period. These results suggest that CRE and CRE-binding factors have an important role in pancreatic β-cell physiology not only directly by regulation of gene trans-activation but also indirectly by regulation of β-cell mass.
Transcriptional control of pancreatic hormone genes is mediated by specific combinations of positive- and negative-acting factors (4, 10-12, 16, 40, 51, 54) through multiple cis-acting elements that are influenced by changes in glucose or cyclic AMP levels (19, 42, 45, 46, 55). One of the elements, the cyclic AMP-responsive element (CRE), has been shown to be an important determinant of gene expression in pancreatic islet cells (6, 13, 18, 30, 34, 43, 44, 47). While the function of its transcriptional activators has been studied extensively, much less is known about the actions and the physiological importance of transcriptional repressors.
CRE modulator (CREM) is a unique gene that generates by alternative splicing both transcriptional activators and repressors (14). These products show cell-, tissue-, and development-specific patterns of expression and play key physiological and developmental roles in regulating gene transcription through CREs (7). One of the isoforms, inducible cyclic AMP early repressor (ICER), is transcribed from an intronic promoter of the CREM gene and consists of only a DNA-binding domain (DBD) (41). The activity of ICER is greatly influenced by its intracellular concentration, because once increased it downregulates its own expression, establishing an autoregulatory feedback loop (15). The induction of this powerful repressor is important for the transient nature of cyclic AMP-induced gene expression (8). ICER has been shown to be dramatically upregulated by treatment with forskolin (39) but not by Ca2+ (35). Recently, it was reported that ICER expression in pancreatic β cells was powerfully induced by glucagon (27) and increased in diabetes (28). The binding activity of ICER is more efficient than that of CREM activator, enabling it to competitively block the binding of the CREM activator or other members of the CREB/ATF family (29), such as CREB, ΔCREB, and CRE-BP1 (17, 21, 31, 38).
Here we further examined the roles of CRE and CRE-binding activator in the pancreatic β cell by cell-specific overexpression of repressor ICER Iγ in transgenic (Tg) mice. Overexpressed ICER Iγ can compete with endogenous CRE-binding activators to block CRE-mediated transcription and result in diabetes.
MATERIALS AND METHODS
Generation of ICER Iγ Tg mice.
The pCMV-ICER Iγ expression plasmid carrying the cDNA for rat ICER Iγ has been described previously (29). ICER Iγ cDNA (encoding amino acids 1 to 108 [TAA]) was excised from pCMV-ICER Iγ as an EcoRI/EcoRI fragment and was inserted into exon 3 of rabbit β-globin downstream of the human insulin promoter in the Tg plasmid pIns-1 (25). The transgene cassettes (Fig. 2A) were excised from the resulting pIns-ICER Iγ plasmid by restriction enzyme digestion to exclude plasmid-derived sequences, and linearized cassettes were microinjected into fertilized eggs of C57BL/6 × C57BL/6 mice (Japan SLC Inc., Nagoya, Japan). Tg mice were identified by PCR analysis of tail DNA. The transgene copy number in founder mice was estimated from Southern blot signal intensity compared to indicator bands of the endogenous gene by using NIH Image. Signal intensity was obtained as PICT files by a slide scanner (Polascan 35; Polaroid, Tokyo, Japan), and the PICT file was opened in gray-scale mode by NIH Image. In all experiments, non-Tg littermates (wild type [WT]) were used for controls. All mice were handled in accordance with the guidelines for animal experiments of Kyoto University. The data presented here are from males only (line Tg23 except where noted), but similar results were obtained in females.
FIG. 2.
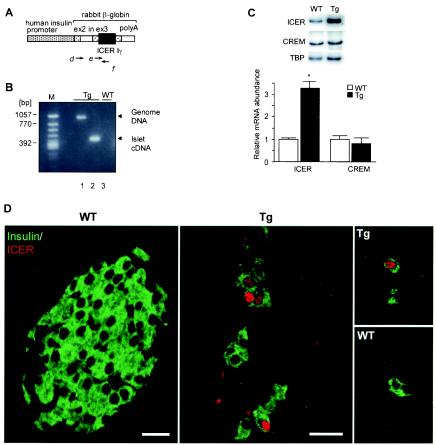
Generating ICER Iγ Tg mice. (A) The transgene contains the human insulin promoter, an intron (in), a part of exons 2 and 3 (ex2 and ex3), a poly(A) signal from the rabbit β-globin gene, and ICER Iγ cDNA. The transcribed mRNA contains ex2, ex3, and ICER Iγ but is translated into only the ICER Iγ protein. (B) Analysis of expression of the transgene in pancreatic islets was performed using primers d and f. Shorter PCR products show correct expression of the transgene in which the intron was spliced into Tg mouse islet cDNA (n = 10) (lane 2). Tg mouse genomic DNA with an intron and WT mouse islet cDNA without any transgene fragments were used as controls (lanes 1 and 3, respectively) (line Tg23). (C) The ICER Iγ and CREM activator mRNA levels in islet cells were determined by semiquantitative PCR (line Tg23). After normalization to the control gene, the mRNA levels are expressed relative to the level of WT mRNA. TBP, TATA-binding protein. *, P < 0.05. (D) For determination of ICER protein expression in β cells, dual staining of pancreatic sections at day 7 with anti-insulin and anti-ICER antibody was analyzed by confocal microscopy (line Tg23). ICER protein (red) was present in nuclei of insulin-positive β cells (green) in Tg but not WT mice. Bars, 10 μm.
DNA analysis.
Southern blot assays were performed with genomic DNA digested with EcoRI. A probe in exons γ, H, and Ia of the ICER gene was hybridized to a fragment specific for the transgene. Genotyping by PCR was performed with genomic DNA and oligonucleotide primers e and f (Fig. 2A), designed to hybridize to rabbit β-globin and exon H of the ICER gene. The transgene-specific products were amplified with 40 cycles of PCR and visualized with ethidium bromide in 1.5% agarose gels.
Measurements of blood glucose levels and serum parameters.
Blood glucose levels were determined by an enzyme-electrode method with Glutest (Sanwa Kagaku Kenkyusho, Nagoya, Japan) on whole blood taken from the tail vein. For other parameters, blood was withdrawn from the heart immediately before isolation of the pancreatic islets under pentobarbital anesthesia. Serum parameters were determined using the following enzyme-linked immunosorbent assay kits: insulin (Morinaga Institute of Biological Science, Yokohama, Japan), ketones (Sanwa Kagaku Kenkyusho), and glucagon (Yanaihara Institute Inc., Shizuoka, Japan).
Oral glucose tolerance test (OGTT) and insulin release test.
After a 16-h fast 10-week-old male mice (n = 10 in each group) were loaded with glucose (1 g kg of body weight−1) by gavage. Blood samples were collected from the tail vein at 0, 15, 30, 60, 90, and 120 min for glucose; serum insulin concentrations were determined for samples at 0, 15, and 30 min.
Isolation of pancreatic islets, insulin secretion, and content.
Pancreatic islets from Tg (n = 5) and WT (n = 5) mice at 12 weeks of age were isolated by collagenase digestion, followed by purification on Ficoll gradients. Insulin secretion, insulin content, and DNA content of freshly isolated islets matched for size were assessed using the batch incubation method, radioimmunoassay, and fluorometric assays, respectively, as described previously (23). Three independent isolations and/or experiments were performed.
RNA isolation, reverse transcription-PCR, and Northern blotting.
Total RNA was extracted from freshly isolated islets of Tg (n = 10) and WT (n = 10) mice at 10 weeks of age with Trizol reagent (GIBCO) according to the manufacturer's instructions and used as a template for cDNA synthesis (Superscript reverse transcriptase [Life Technologies]) and for Northern blotting. Mouse pancreatic islet cDNA was amplified by PCR with rabbit β-globin and ICER-specific oligonucleotides. To exclude any amplification product derived from genomic DNA that could contaminate the RNA preparation, total RNA without reverse transcription was amplified as a negative control. To determine the expression of the transgene, the primers d and f (Fig. 2A) were designed to span an intron of the rabbit β-globin gene to distinguish transgene mRNA and genomic DNA. Since the transgene construct carried a rabbit β-globin intron-splice signal, correct expression of the transgene could be identified by a smaller PCR product lacking the inserted β-globin intron sequence compared with the PCR from genomic DNA. Northern blotting was performed with a rat insulin cDNA probe with total islet RNA (20 μg). Probe labeling and detection were performed according to the protocol given in the Gene Images random prime labeling and detection system (Amersham Pharmacia Biotech).
Semiquantitative PCR.
The mRNA expression in islets of WT and Tg mice was quantified by semiquantitative PCR as described previously (28). Isoform-specific primers a to c were set to determine the expression of CREM and ICER Iγ (Fig. 1A). The number of amplification cycles was selected such that the amplification of each sequence was in the exponential phase of the amplification curve. For ICER Iγ, CREM activator, TATA-binding protein, and cyclin A mRNA, the exponential portion of the curve corresponds to 37 cycles. The amount of [α-32P]dCTP incorporated into each amplimer was measured on a PhosphorImager and quantified with ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.). TATA-binding protein was used to control for differences in overall cDNA concentration and experimental variations between samples. The amount of each specific product was expressed relative to this internal control, giving a specific product/control gene ratio for each sample.
FIG. 1.
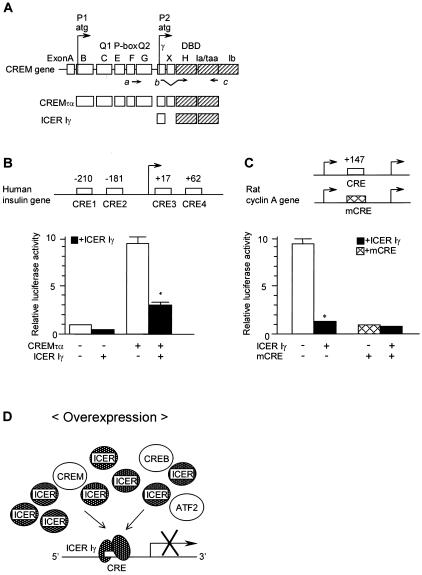
(A) Schematic presentation of the structure of the CREM gene and isoforms. CREMτα contains activation domains (Q1, Q2, P box, and the DBD) and functions as an activator. ICER Iγ is transcribed from an alternative intronic promoter (P2) and contains only the DBD. The arrows, a to c, represent the positions of synthetic oligonucleotide primers used to detect specific isoforms. (B and C) Transient-expression studies analyzing the effect of ICER Iγ in CRE-mediated transcription. ICER Iγ or CREMτα expression plasmids were transfected into HIT-T15 cells (pancreatic β-cell line) with the luciferase reporter plasmid containing the human insulin gene promoter (B) or the rat cyclin A promoter gene (C). To determine if ICER Iγ represses not only basal promoter activity but also CREMτα-induced promoter activity, the same amount of ICER Iγ expression plasmid was cotransfected with the expression plasmid of CREMτα. Solid bars represent cotransfection with ICER Iγ. These transfections were repeated more than three times. *, P < 0.05. (D) The hypothesis of this study is that increased ICER Iγ can compete with other members of the CREB/ATF family and disrupt CRE-mediated transcription.
Cell culture and transfection.
Cell culture and transient transfection in HIT-T15 cells (hamster pancreatic β-cell line) were performed as described previously (29). The cells were transfected using Lipofectamine (GIBCO) with a mixture of the luciferase reporter plasmid, containing the human insulin gene promoter or the rat cyclin A gene promoter (kindly provided by K. Oda and A. Takeuchi, Science University of Tokyo, Chiba, Japan [53]), CREMτα or ICER Iγ expression plasmid (29), and internal control p-act-β-gal. Forty-eight hours after transfection, the cells were harvested and cell extracts were prepared for luciferase assays and β-galactosidase assays. β-Galactosidase assays were performed for internal control. Luciferase activity was normalized to the background activity obtained from the transfection of the promoterless luciferase plasmid in the same experiments. Transfection experiments were repeated more than three times. The data presented here are from rat cyclin A only, but similar results were obtained using mouse cyclin A promoter (kindly provided by M. Schorpp-Kistner, Deutsches Krebsforschungszentrum Heidelberg, Heidelberg, Germany [2]).
Immunohistochemistry.
WT (n = 5 for each age except 7 days, for which n = 7) and Tg (n = 5 for each age except 7 days, for which n = 9) mouse pancreases were fixed in 10% buffered formalin, embedded in paraffin, and cut in serial (5-μm) sections. The following primary antibodies were used: anti-ICER (antiserum α-CREM S4; 1:500; kindly provided by J. F. Habener, Massachusetts General Hospital, Howard Hughes Medical Institute, Boston, Mass. [5, 27]), anti-insulin (1:500; DAKO); antiglucagon (1:350; OAL-123; Otsuka Assay Laboratory, Tokushima, Japan, or Linco); anti-islet amyloid polypeptide (IAPP; 1:50; gift of C. B. Verchere, VA Medical Center, Seattle, Wash.), anti-cyclin A (1:50; Santa Cruz Biotechnology), and anti-Ki67 (1:200; PharMingen). Primary antibody was detected by immunofluorescence labeling with fluorescein isothiocyanate-conjugated or Texas red-conjugated secondary antibodies or by immunoperoxidase with biotin-labeled secondary antibodies. Staining was visualized with alkaline phosphatase substrate (Vector Laboratories, Burlingame, Calif.) or diaminobenzidine. For proliferation studies, pancreatic sections of 7-day-old mice were double immunostained for insulin and Ki67; all (insulin-positive) islets per single section per animal were photographed using a Zeiss LSM 410 confocal microscope. Images of all insulin-positive cells were evaluated for expression of the cell cycle marker Ki67. For apoptosis studies, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed according to the manufacturer's instructions (in situ cell death detection POD kit; Roche) on pancreatic sections of 7-day-old mice (n = 4 for each group).
RESULTS
Blocking CRE-mediated transcription by ICER Iγ.
A schematic representation of the structure of the CREM isoforms is shown in Fig. 1A. The CREM gene consists of 10 exons, A to I, including functional domains, two glutamine-rich domains (Q1 and Q2), the phosphorylation domain (P box), and the DBD. CREMτα is a full-length isoform and contains activation domains, thus functioning as an activator (14), but ICER Iγ is transcribed from an alternative intronic promoter (P2) and consists only of DBD. A remarkable reduction in insulin and cyclin A gene transcription was observed when CREs were mutated in both humans (9, 30) and rodents (2, 44, 53), so transcription factors that bind to the CRE might play an important role in the regulation of these two genes. To test the effect of CREM and ICER Iγ on insulin and cyclin A gene transcription, HIT-T15 cells were transfected with a luciferase reporter plasmid containing the human insulin promoter or the rat cyclin A promoter (Fig. 1B and C). The insulin-luciferase reporter contains four functional CREs. CREMτα produced high levels of luciferase activity, while ICER Iγ repressed activity. When cotransfected, ICER Iγ substantially repressed CREMτα-induced activity (Fig. 1B). The rat cyclin A-luciferase reporter gene contained one CRE, and again, ICER Iγ repressed promoter activity (Fig. 1C). In the presence of mutated CRE, no effect of ICER Iγ was observed, suggesting that ICER Iγ competes and blocks the activities of transcriptional activators through CRE (Fig. 1D).
Production of ICER Iγ Tg mice.
To test our hypothesis that increased ICER Iγ blocks CRE-mediated transcription in vivo, we generated Tg mice expressing ICER Iγ in pancreatic β cells (Fig. 2A). Three mice carrying the transgene were identified among 62 potential founders. These mice were used to establish three ICER Iγ Tg lines, designated Tg7, Tg12, and Tg23 (Table 1). Reverse transcription-PCR with primers d and f to detect transgene expression showed a shorter PCR product (Fig. 2B, lane 2) for the transgene than for the genomic DNA template (lane 1) due to the excision of the rabbit β-globin intron sequence. To compare the mRNA levels of ICER Iγ and CREM activator in Tg and WT mouse islets, semiquantitative PCR was carried out using primers a to c (Fig. 1A). The expression level of ICER Iγ in islets of Tg mice was increased (Fig. 2C), but there was no significant difference in the expression of CREM activator between Tg and WT mice. To determine ICER protein expression, pancreatic sections of 7-day-old mice were double stained with anti-insulin and anti-ICER antibodies. ICER protein (red) was present only in nuclei of β cells (green) in islets and single insulin-positive cells in Tg mouse pancreas but was at an undetectable level in WT mouse pancreas (Fig. 2D).
TABLE 1.
Comparison of serum parameters and body weights at 12 weeks of age among ICER Iγ Tg mouse linesc
| Mouse line | Copy no. | Body wt (g)
|
Body glucose concn (mg/dl)
|
Plasma insulin concn (pg/ml)
|
|||
|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | ||
| Control | 0 | 33.2 ± 0.9 | 21.7 ± 0.7 | 153 ± 4.1 | 126 ± 9.9 | 1,350.3 ± 194.5 | 1,480.7 ± 19.4 |
| Tg7 | 4 | 25.8 ± 1.7a | 21.8 ± 0.6 | 527 ± 52.8a | 355 ± 61.7b | 350.3 ± 80.0a | 422.4 ± 130a |
| Tg12 | 4 | 22.7 ± 2.6a | 22.5 ± 0.9 | 528 ± 62.1a | 336 ± 93.2b | 545.6 ± 183.7b | 654.6 ± 84.2b |
| Tg23 | 6 | 21.7 ± 2.4a | 22.6 ± 1.0 | 551 ± 31.1a | 420 ± 59.5b | 278.1 ± 111.2a | 445.5 ± 39.7a |
P < 0.05 versus control.
P < 0.001 versus control.
Three mice positive for the transgene were founders of three ICER Iγ Tg lines, Tg7, Tg12, and Tg23. Results are means ± standard errors of at least 10 animals from each line. M, male; F, female.
ICER Iγ transgene expression results in severe diabetes.
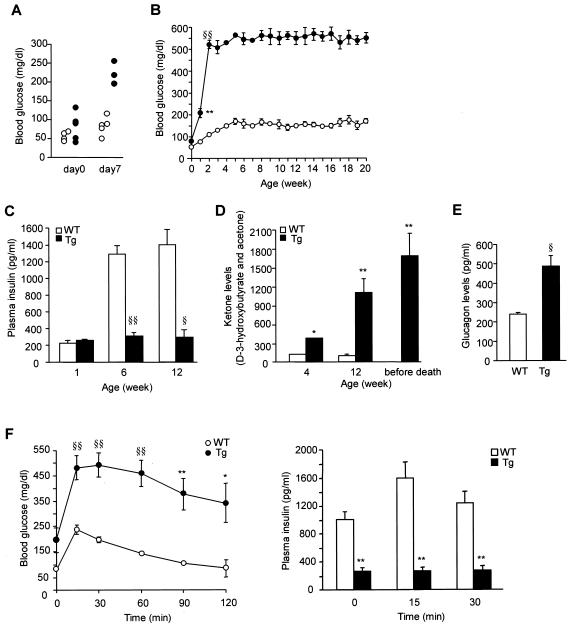
ICER Iγ Tg mice develop severe diabetes early in life. Blood glucose levels are normal at birth (day 0) (80 ± 21 mg/dl) but moderately elevated at day 7 (209 ± 21 mg/dl) (Fig. 3A) and further elevated by 2 weeks of age; blood glucose levels remained high until death at 20 to 36 weeks (Fig. 3B). Plasma insulin concentrations were extremely low, being only 20% of WT level at 6 weeks (WT versus Tg mice, 1,282 ± 87 versus 288 ± 45 pg/ml, respectively; P < 0.001) (Fig. 3C). Blood ketone levels were markedly elevated at 6 and 12 weeks of age and increased further before death (Fig. 3D). The plasma glucagon levels were also quite high (Fig. 3E). Upon oral glucose challenge (OGTT), blood glucose levels were substantially increased at 15 min and gradually declined to basal levels by 120 min in WT mice but remained abnormally high throughout the 120-min period in Tg mice (Fig. 3F, left). Glucose levels were about twice as high in Tg as in WT mice at all time points. The plasma insulin response following OGTT was also markedly impaired in Tg mice, being reduced by 70 to 80% at all time points (Fig. 3F, right).
FIG. 3.
Severe diabetes in ICER Iγ Tg mice. (A and B) Blood glucose levels; (C) fed plasma insulin levels; (D) blood ketone levels; (E) plasma glucagon levels (line Tg23); (F) OGTT and insulin release in ICER Iγ Tg mice at 10 weeks of age (line Tg12). Tg (n = 10) and WT (n = 10) mice were fasted for 16 h before the study and then were given glucose (1 g kg of body weight−1). Left, plasma blood glucose levels at indicated times; right, plasma insulin levels at 0, 15, and 30 min after oral glucose load. Solid and open bars or circles represent Tg and WT mice, respectively. *, P < 0.05; **, P < 0.01; §, P < 0.005; §§, P < 0.001.
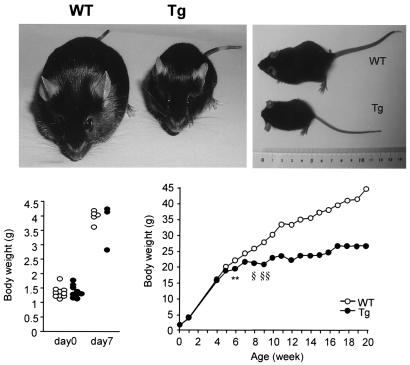
We compared serum parameters and body weights in males and females of the three Tg lines (Table 1) at 12 weeks of age. Although lines Tg7 and Tg12 had four copies of the transgene and line Tg23 had six copies, there were no significant differences in weight or blood glucose or plasma insulin levels among the three Tg lines. All were hyperglycemic with decreased plasma insulin levels, suggesting that the amount of ICER in each line was effective. This in vivo finding is consistent with the in vitro effect of ICER expression on insulin promoter activity in HIT-T15 cells (29). Body weight was markedly reduced in the male Tg mice at 12 weeks of age, but in the female mice only by 20 weeks of age. In a longitudinal study of Tg23 male mice, body weight was similar to that of controls until 4 weeks of age, at which point Tg mice failed to gain weight, with only slight growth after 8 weeks of age (Fig. 4).
FIG. 4.
Body weights of male mice (line Tg23). (Top) Photograph of male Tg mouse and control littermate (WT) at 12 weeks of age. (Bottom) Body weights of male WT (open circles) and Tg (solid circles) mice were similar to those of WT mice at days 0 and 7. The growth curve of male mice from day 0 to 20 weeks of age (n = 10) is shown. **, P < 0.01; at 6 weeks, §, P < 0.005; after 9 weeks, §§, P < 0.001.
Together, these results show that overexpression of ICER Iγ in pancreatic β cells is associated with early severe diabetes.
Isolated islets from ICER Iγ Tg mice.
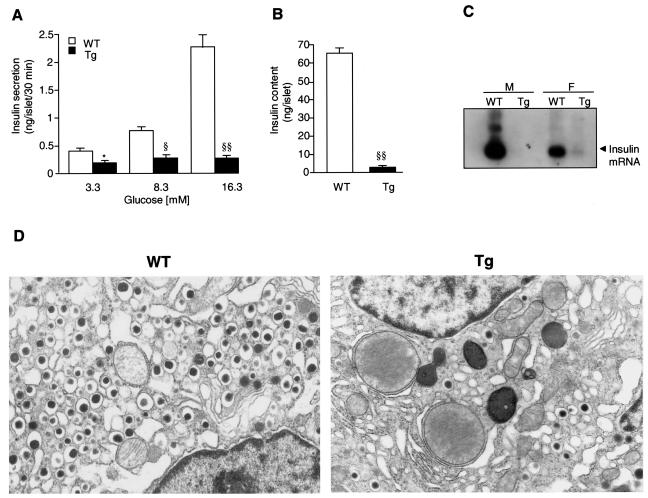
In static incubations isolated WT mouse islets had dose-dependent glucose-induced insulin secretion, but Tg mouse islets had greatly reduced insulin secretion (Fig. 5A). In comparison to isolated islets matched for size, Tg mouse islets were only 18% reduced in DNA level but 95% reduced in insulin content (WT versus Tg mice, 65.2 ± 3.7 versus 2.8 ± 0.55 ng of insulin/islet, respectively) (Fig. 5B). In islets isolated from 10-week-old mice, insulin mRNA expression was significantly reduced in Tg mice, with a weak band observed only in female Tg mice (Fig. 5C). Ultrastructural analysis showed a markedly reduced number of insulin secretory granules within individual β cells of Tg mice (Fig. 5D) compared to WT mice.
FIG. 5.
Insulin secretion and β-cell morphology of isolated islets (line Tg23). (A and B) Glucose-stimulated insulin secretion (A) and insulin content (B) were examined using islets freshly isolated from 12-week-old male Tg (n = 5) and WT (n = 5) mice. These islets were matched for size. Three independent isolations and/or experiments were performed. *, P < 0.05; §, P < 0.005; §§, P < 0.001. (C) Northern blot analysis of insulin mRNA levels in male (M) or female (F) mice. Total islet RNA (20 μg) freshly extracted from isolated islets of 10-week-old Tg (n = 10) and WT (n = 10) mice was used. (D) β-cell morphology as shown by electron microscopy at 36 weeks of age. Control β cells (left) contained numerous mature insulin secretory granules surrounded by clear wide halos. In contrast, there were markedly fewer insulin secretory granules within β cells in Tg mice (right).
ICER Iγ Tg mice exhibit abnormal islet morphology.
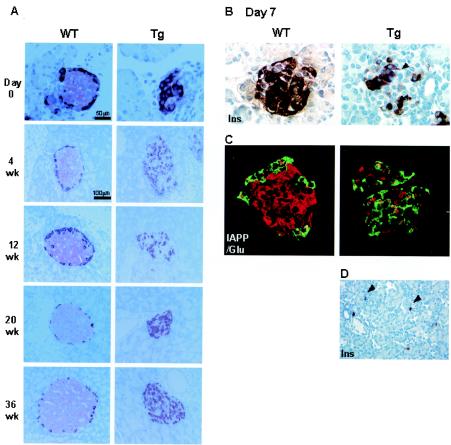
To assess islet morphology, pancreatic sections from 0-day-old (nondiabetic condition) to 36-week-old (severe diabetes) mice were immunostained for insulin and glucagon. In controls, the typical islet morphology of a core of insulin-positive cells with a mantle of glucagon-positive cells was seen (Fig. 6A). In Tg mice, however, even at day 0, there were far fewer insulin-positive cells, so the islets appeared severely disorganized with an increased proportion of glucagon-positive cells; this pattern did not differ with age. At 7 days of age, when animals were becoming hyperglycemic, differential expression of insulin by immunostaining was observed within the same islet (Fig. 6B), with some cells having clear staining and others having almost none. To demonstrate the presence of degranulated β cells, IAPP was used as a β-cell marker (Fig. 6C). Again, β cells were reduced in number and most of the islet was glucagon positive. A few cells coexpressing IAPP and glucagon were present in Tg mice. These morphological abnormalities were already apparent at day 0 and in the late fetal period (data not shown), indicating that these changes are a direct result of the increased ICER Iγ and not due to hyperglycemia.
FIG. 6.
Islet morphology (line Tg23). (A) Immunohistochemical staining of pancreatic sections from 0-day-old (nondiabetic condition) to 36-week-old (severe diabetes) mice with anti-insulin and antiglucagon antibodies. In Tg mice far fewer insulin-positive (pink) cells are present even at day 0, so the islets appear severely disorganized with a significantly increased proportion of glucagon-positive (red) cells, indicating that these changes result directly from the increased ICER Iγ and are not due secondarily to hyperglycemia. (B) Reduced insulin-positive cells in Tg mice (arrowhead) at 7 days. Additionally in the Tg mouse there is a marked variation in the amount of insulin per cell (degranulation) as shown by the insulin staining. (C) Dual staining of IAPP (red) as a β-cell marker and glucagon (green) was analyzed by confocal microscopy. (D) Scattered singlets-doublets of insulin-positive cells (arrowheads) in Tg mice. Magnification, ×1,000 (B and C) and ×400 (D).
ICER Iγ Tg mice exhibit reduced β-cell proliferation.
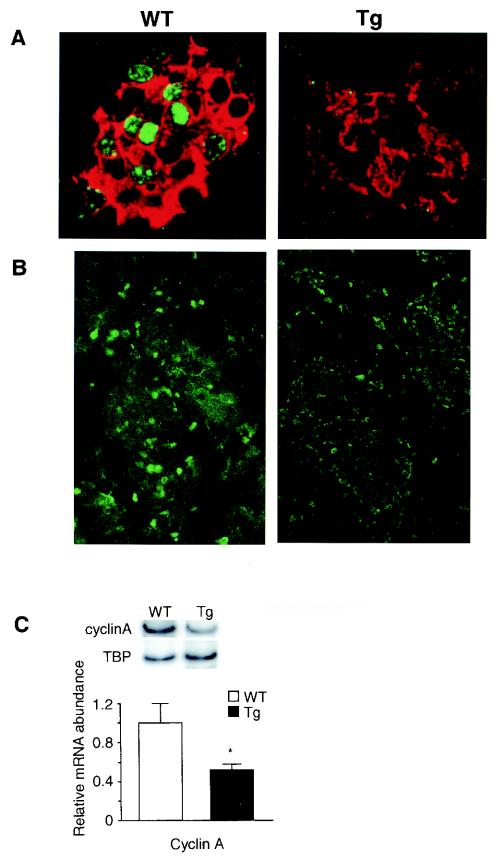
Since β-cell mass is regulated by a balance of cell renewal (neogenesis from precursors and proliferation of β cells) and cell loss through apoptosis, these determinants of β-cell mass were examined. While it is difficult to assess neogenesis, scattered singlets-doublets of insulin-positive cells were as frequent in Tg as WT mice at 7 days of age (Fig. 6D), suggesting that neogenesis of β cells was not impaired. Using TUNEL staining, no morphological evidence of increased apoptosis at 7 days of age was seen (data not shown). To evaluate β-cell proliferation, dual staining of Ki67 and insulin was performed on 7-day-old mouse pancreases. Ki67 protein was detected in many nuclei of insulin-positive cells in WT mice but in no insulin-positive cells in Tg mice (WT mice, 12 islets, 160 Ki67-positive, insulin-positive cells of 512 insulin-positive cells; Tg mice, 5 islets, 0 Ki67-positive, insulin-positive cells of 103 insulin-positive cells) (Fig. 7A). Since both CREM and ICER have been reported to regulate the cell cycle by modulating cyclin A gene expression (43) (Fig. 1C), cyclin A expression was measured. At day 7, normally a time of active islet proliferation, cyclin A protein, as detected by immunostaining, was seen in most nuclei in WT mouse islets but in only a few cells of Tg mouse islets (Fig. 7B) even though there was no difference in cyclin A immunostaining in acinar cells between Tg and WT mice. In agreement with this, cyclin A mRNA levels, determined by semiquantitative PCR, were decreased in Tg mouse islets (Fig. 7C). These data suggest that ICER Iγ represses cyclin A gene transcription in β cells, which in turn may limit β-cell proliferation. The neonatal period is one of enhanced replication and neogenesis and active apoptosis in normal rodent pancreas, but in these ICER Tg mice the decreased number of β cells is likely to be due to the decreased proliferation rather than increased apoptosis.
FIG. 7.
Cyclin A expression and proliferation in islets at day 7 of age (line Tg23). (A) Dual staining of Ki67 (green) as a marker of cell proliferation and insulin (red) was analyzed by confocal microscopy. Ki67 was detected in 30% of the nuclei of β cells in WT mice but in few nuclei, if any, in Tg mice. (B) At day 7, normally a time of active cell proliferation, cyclin A protein was detected in most of the nuclei in islets in WT mice but in only a few islets of Tg mice. Magnification, ×1,000 (A) and ×400 (B). (C) Cyclin A mRNA level determined by semiquantitative PCR with islet cDNA is greatly reduced in Tg mouse islets. TBP, TATA-binding protein. *, P < 0.05.
DISCUSSION
First, we demonstrated that CREMτα functions as an activator of insulin and cyclin A promoters, whereas ICER Iγ acts as a strong repressor. Previously, we have reported that the efficiency of binding of ICER Iγ to CRE was higher than that of CREMτα and so it competitively inhibited the binding of CREMτα (29). Those in vitro results suggested that increased ICER Iγ in pancreatic β cells could compete with endogenous members of the CREB/ATF family such as CREM (7), CREB (18), CRE-BP1 (31, 38), and ATF (21) and disrupt CRE-mediated insulin and cyclin A gene transcription in vivo. To test our hypothesis, we directly assessed in vivo the effects of increasing ICER Iγ in pancreatic β cells.
Since it is important to determine the specificity for the effect of ICER Iγ overexpression in pancreatic β cells, we established three Tg lines. Although the three lines contained different copy numbers of the transgene, all had normal blood glucose levels at birth, but within days their blood glucose levels increased dramatically and the mice developed severe diabetes characterized by polydipsia, polyuria, reduced serum insulin levels, and elevated ketone and glucagon levels. There was a profound depletion of insulin cells already during the late fetal period, preceding any hyperglycemia, which was evident only by 7 days of age. Insulin secretion from islets was markedly reduced, as were insulin content and mRNA levels. Since, as we previously have shown, the levels of β-cell-specific transcripts, such as GLUT2, glucokinase, PDX-1, and IAPP, are downregulated by hyperglycemia (33, 36, 37), normalization of insulin gene expression to another β-cell-specific gene would not be valid. The islet deficits are partly due to the depletion of β cells, but some of the reductions are direct effects of the overexpression of ICER. The insulin promoter luciferase assays showed that ICER directly repressed insulin gene transcription. In addition, as shown by electron microscopy insulin granules were remarkably decreased in individual β cells, thereby confirming the inhibitory effect of ICER on insulin expression. Thus, in this Tg model, diabetes resulted from a decreased number of β cells, further compounded by impaired insulin expression in individual β cells.
After birth, replication of preexisting β cells is the main means of expanding the β-cell population (24). Islets in late fetal life have an equal proportion of β and non-β cells, but after the perinatal burst of replication, the β cells comprise about 75% of the islet. This increase in proportion results from the discordance of proliferation between the β and non-β endocrine cells in the perinatal period. CREM and CREB have been reported to be involved in the regulation of cell proliferation and regeneration in the liver (8, 52). One effect may be through cyclin A, whose expression at S phase and association with cdc2 are required for cell proliferation (48, 56). The induction of the cyclin A gene is limited to a short period of the cell cycle during which the expression levels of phosphorylated CREM and CREB are high while that of ICER is low (9). Furthermore, a moderate amount of cyclin A is required for normal cell cycle progression, since the G1/S transition is accelerated by cyclin A overexpression (49). Therefore, it is possible that overexpressed ICER had inhibitory effects on the cell cycle and its progression. Consistent with the ability of ICER to suppress cyclin A promoter activity (Fig. 1C), cyclin A mRNA and protein levels were markedly reduced in islets of Tg mice compared to WT mice, but not in acinar cells. Furthermore, using double immunostaining for insulin and Ki67, a marker for β-cell proliferation, we found few, if any, β cells in the replicative cycle at 7 days of age even though about 30% of the β cells of the WT mice were in the cell cycle. These results suggest that reduction of cyclin A expression by ICER may limit β-cell proliferation. In addition, the presence of singlets-doublets of insulin-positive cells scattered throughout the pancreas suggested that neogenesis was still occurring. No evidence of abnormally increased apoptosis was seen at this time, although it cannot be ruled out that there was increased apoptosis earlier, possibly in the fetal period. Therefore, decreased replication is the likely basis of depletion of β cells within Tg mouse islets.
During the preparation of this paper, a report on dominant-negative A-CREB Tg mice showed an increased apoptosis via IRS2 reduction in β cells, leading to a late-onset, mild diabetes (32). A-CREB is a dominant-negative inhibitor of CREB, constructed by replacing the CREB basic region with an acidic amphipathic protein sequence; it is only 82 amino acids long whereas the full-length CREB is 342 amino acids (1). These mice have normal blood glucose levels and islet architecture until 8 weeks of age, after which apoptosis leads to a decreased β-cell number and mild diabetes by 12 weeks of age. In contrast, our ICER mice developed early severe diabetes with markedly reduced insulin secretion and islet insulin content and a reduced number of β cells. What is striking about our Tg mouse model was that the animals became hyperglycemic by 7 days of age, and islet morphology was stable with marked reduction of β cells from an early age. The stability of islet morphology is more consistent with an impaired proliferation than with increased apoptosis. With increased apoptosis and no compensatory increase in replication, there should be a steady decline in the proportion of β cells in the islets, yet this is not what we observed. In fact apoptosis, as shown by TUNEL staining, was not increased at this early age, and β-cell replication, which is normally active at this age, was severely decreased in our ICER Tg mice. Thus, the reduced number of β cells in our Tg mice is more consistent with a decreased proliferation than with increased apoptosis.
The discrepancy between our data and those for A-CREB Tg mice must be based on the different mechanisms of inhibition of ICER and A-CREB. ICER has a strong binding ability (29), and a small amount of ICER efficiently competes and prevents binding of activators. While ICER homodimer formation is more favored than that of the heterodimer ICER-CREM activator, ICER also forms heterodimers with all CREM proteins and CREB, generating nonactivating dimers (41). Thus, ICER occupies DNA-binding sites (CREs) as either a homodimer or an inactive heterodimer and blocks promoter activity by preventing the binding of other members of the basic leucine zipper (B-Zip) proteins, such as CREB, CREM, ATF, Jun, and Fos. In contrast, A-CREB as a homodimer is less stable than a CREB homodimer but selectively forms with CREB a very stable heterodimer that is 3,300-fold more stable than a CREB homodimer (1). However, A-CREB is unable to form heterodimers with other B-Zip proteins and so selectively prevents the binding of CREB to DNA (1). Considering that other B-Zip proteins, such as ATF-2, ATF-3, ATF-4, Jun, and Fos, can form heterodimers with each other and bind to CRE to activate gene transcription (20, 22, 50), it is possible that these B-Zip proteins could function in CREB-independent signaling pathways or compensate for CREB function in CREB-dependent signaling pathways in the A-CREB Tg mice, as was observed with CREB-knockout mice (3, 26). Taken together, these different mechanisms of repression may account for the very different, but complementary, findings from the work of Jhala et al. (32).
In conclusion, we found that inhibition of CRE-binding activator function in pancreatic β cells by the repressor ICER Iγ suppresses insulin and cyclin A expression. The early severe diabetes in this model probably results from both the depleted insulin expression in individual β cells and the decreased number of β cells resulting from their impaired proliferation. Dynamic changes in ICER Iγ, from transient induction to its own suppression, are likely to be important for pancreatic β-cell physiology both directly by regulation of gene expression and indirectly by regulation of β-cell mass.
Acknowledgments
We thank Kinichiro Oda (Science University of Tokyo, Chiba, Japan) and Arata Takeuchi (Chiba University, Chiba, Japan) for providing the rat cyclin A promoter plasmid, Marina Schorpp-Kistner (Deutsches Krebsforschungszentrum, Heidelberg, Germany) for providing the mouse cyclin A promoter plasmid, and Joel. F. Habener (Massachusetts General Hospital, Howard Hughes Medical Institute, Boston, Mass.) for providing S4-CREM antibody. We thank Gordon C. Weir (Joslin Diabetes Center) and Shunsuke Ishii (Riken Tsukuba Institute, Japan) for critical reading of the manuscript and Hirofumi Noguchi and Rafael Nesher (Joslin Diabetes Center; Hebrew University Hadassah Medical Center, Jerusalem, Israel) for helpful discussions. We also thank Sarah Yasui and Shun Nawata for technical assistance, Haruyasu Kohda and Makio Fujioka for electron microscopy, Oogi Inada for frequent help with mouse care, Hiroshi Kanamori and Hidenori Arai for providing 7-day-old mouse pancreases, Chris Cahill for confocal microscopy, and Sonya Yokoff for fluorescent dual-color immunostaining.
This study was supported by in part by Grants-in-Aid for Scientific Research and for Creative Scientific Research (NP10NPO201) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by grants from the Research for the Future Program from the Japan Society for the Promotion of Science (JSPS-RFTF97I00201), the Joslin NIH DERC Advanced Microscopy Core, and the Diabetes and Wellness Research Foundation. A.I. is the recipient of a fellowship and grant from the Yamanouchi Foundation 2002 and from the Manpei Suzuki Diabetes Foundation 2002.
REFERENCES
- 1.Ahn, S., M. Olive, S. Aggarwal, D. Krylov, D. D. Ginty, and C. Vinson. 1998. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol. Cell. Biol. 18:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrecht, S., A. Kolbus, B. Hartenstein, P. Angel, and M. Schorpp-Kistner. 2002. Cell cycle promoting activity of JunB through cyclin A activation. J. Biol. Chem. 277:35961-35968. [DOI] [PubMed] [Google Scholar]
- 3.Bleckmann, S. C., J. A. Blendy, D. Rudolph, A. P. Monaghan, W. Schmid, and G. Schutz. 2002. Activating transcription factor 1 and CREB are important for cell survival during early mouse development. Mol. Cell. Biol. 22:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boam, D. S., A. R. Clark, and K. Docherty. 1990. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J. Biol. Chem. 265:8285-8296. [PubMed] [Google Scholar]
- 5.Bodor, J., A. L. Spetz, J. L. Strominger, and J. F. Habener. 1996. cAMP inducibility of transcriptional repressor ICER in developing and mature human T lymphocytes. Proc. Natl. Acad. Sci. USA 93:3536-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel, P. B., W. H. Walker, and J. F. Habener. 1998. Cyclic AMP signaling and gene regulation. Annu. Rev. Nutr. 18:353-383. [DOI] [PubMed] [Google Scholar]
- 7.De Cesare, D., and P. Sassone-Corsi. 2000. Transcriptional regulation by cyclic AMP-responsive factors. Prog. Nucleic Acids Res. Mol. Biol. 64:343-369. [DOI] [PubMed] [Google Scholar]
- 8.Della Fazia, M. A., G. Servillo, and P. Sassone-Corsi. 1997. Cyclic AMP signaling and cellular proliferation: regulation of CREB and CREM. FEBS Lett. 410:22-24. [DOI] [PubMed] [Google Scholar]
- 9.Desdouets, C., G. Matestic, C. A. Molina, N. S. Foulkes, P. Sassone-Corsi, C. Brechot, and J. Sobczak-Thepot. 1995. Cell cycle regulation of cyclin A gene expression by the cyclic AMP-responsive transcription factors CREB and CREM. Mol. Cell. Biol. 15:3301-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docherty, K. 1992. R. D. Lawrence Lecture. The regulation of insulin gene expression. Diabet. Med. 9:792-798. [DOI] [PubMed] [Google Scholar]
- 11.Docherty, K., and A. R. Clark. 1994. Nutrient regulation of insulin gene expression. FASEB J. 8:20-27. [DOI] [PubMed] [Google Scholar]
- 12.Edlund, T., M. D. Walker, P. J. Barr, and W. J. Rutter. 1985. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5′ flanking elements. Science 230:912-916. [DOI] [PubMed] [Google Scholar]
- 13.Fimia, G. M., and P. Sassone-Corsi. 2001. Cyclic AMP signalling. Cell Sci. 114:1971-1972. [DOI] [PubMed] [Google Scholar]
- 14.Foulkes, N. S., E. Borrelli, and P. Sassone-Corsi. 1991. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell 64:739-749. [DOI] [PubMed] [Google Scholar]
- 15.Foulkes, N. S., J. Borjigin, S. H. Snyder, and P. Sassone-Corsi. 1996. Transcriptional control of circadian hormone synthesis via the CREM feedback loop. Proc. Natl. Acad. Sci. USA 93:14140-14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.German, M., S. Ashcroft, K. Docherty, H. Edlund, T. Edlund, S. Goodison, H. Imura, G. Kennedy, O. Madsen, and D. Melloul. 1995. The insulin gene promoter. A simplified nomenclature. Diabetes 44:1002-1004. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez, G. A., and M. R. Montminy. 1989. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59:675-680. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, G. A., K. Yamamoto, W. H. Fischer, K. Karr, P. Manzel, W. Biggs III, W. W. Vale, and M. R. Montminy. 1989. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature 337:749-752. [DOI] [PubMed] [Google Scholar]
- 19.Goodison, S., S. Kenna, and S. J. Ashcroft. 1992. Control of insulin gene expression by glucose. Biochem. J. 285:563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hai, T., and T. Curran. 1991. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 88:3720-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hai, T. W., M. Horikoshi, R. G. Roeder, and M. R. Green. 1988. Analysis of the role of the transcription factor ATF in the assembly of a functional preinitiation complex. Cell 54:1043-1051. [DOI] [PubMed] [Google Scholar]
- 22.Hai, T. W., F. Liu, W. J. Coukos, and M. R. Green. 1989. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 3:2083-2090. [DOI] [PubMed] [Google Scholar]
- 23.Hamamoto, Y., Y. Tsuura, S. Fujimoto, M. Nagata, T. Takeda, E. Mukai, J. Fujita, Y. Yamada, and Y. Seino. 2000. Recovery of function and mass of endogenous beta cells in streptozotocin-induced diabetic rats treated with islet transplantation. Biochem. Biophys. Res. Commun. 287:104-109. [DOI] [PubMed] [Google Scholar]
- 24.Hellerstrom, C., I. Swenne, and A. Andersson. 1988. An islet cell replication and diabetes, p. 141-170. In P. J. Lefebvre and D. G. Pipeleers (ed.), The pathology of the endocrine pancreas in diabetes. Springer-Verlag, Heidelberg, Germany.
- 25.Hotta, M., F. Tashiro, H. Ikegami, H. Niwa, T. Ogihara, J. Yodoi, and J. Miyazaki. 1998. Pancreatic β cell-specific expression of thioredoxin, antioxidative and antiapoptotic protein prevents autoimmune and streptozotocin-induced diabetes. Exp. Med. 188:1445-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummler, E., T. J. Cole, J. A. Blendy, R. Ganss, A. Aguzzi, W. Schmid, F. Beermann, and G. Schutz. 1994. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc. Natl. Acad. Sci. USA 91:5647-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain, M. A., P. B. Daniel, and J. F. Habener. 2000. Glucagon stimulates expression of the inducible cAMP early repressor and suppresses insulin gene expression in pancreatic β-cells. Diabetes 49:1681-1690. [DOI] [PubMed] [Google Scholar]
- 28.Inada, A., Y. Yamada, Y. Someya, A. Kubota, K. Yasuda, Y. Ihara, S. Kagimoto, A. Kuroe, K. Tsuda, and Y. Seino. 1998. Transcriptional repressors are increased in pancreatic islets of type 2 diabetic rats. Biochem. Biophys. Res. Commun. 253:712-718. [DOI] [PubMed] [Google Scholar]
- 29.Inada, A., Y. Someya, Y. Yamada, Y. Ihara, A. Kubota, N. Ban, R. Watanabe, K. Tsuda, and Y. Seino. 1999. The cyclic AMP response element modulator family regulates the insulin gene transcription by interacting with transcription factor IID. J. Biol. Chem. 274:21095-21103. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki, N., T. Maekawa, T. Sudo, S. Ishii, Y. Seino, and H. Imura. 1992. c-Jun represses the human insulin promoter activity that depends on multiple cAMP response elements. Proc. Natl. Acad. Sci. USA 89:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii, S., T. Maekawa, T. Sudo, A. Sakurai, I. Yasuda, and A. Kishimoto. 1990. Structure and function of the protein CRE-BP1: heterogeneity of the protein that binds to the cAMP-responsive element. Adv. Second Messenger Phosphoprot. Res. 24:335-339. [PubMed] [Google Scholar]
- 32.Jhala, U. S., G. Canettieri, R. A. Screaton, R. N. Kulkarni, S. Krojewski, J. Reed, J. Walker, X. Lin, M. White, and M. R. Montminy. 2003. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 17:1575-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonas, J. C., A. Sharma, W. Hasenkamp, H. Ilkova, G. Patanè, R. Laybutt, S. Bonner-Weir, and G. C. Weir. 1999. Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J. Biol. Chem. 274:14112-14121. [DOI] [PubMed] [Google Scholar]
- 34.Knepel, W., J. Chafitz, and J. F. Habener. 1990. Transcriptional activation of the rat glucagon gene by the cyclic AMP-responsive element in pancreatic islet cells. Mol. Cell. Biol. 10:6799-6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueger, D. A., D. Mao, E. A. Warner, and D. R. Dowd. 1999. Functional analysis of the mouse ICER (inducible cAMP early repressor) promoter: evidence for a protein that blocks calcium responsiveness of the CAREs (cAMP autoregulatory elements). Mol. Endocrinol. 13:1207-1217. [DOI] [PubMed] [Google Scholar]
- 36.Laybutt, D. R., M. Glandt, G. Xu, Y. B. Ahn, N. Trivedi, S. Bonner-Weir, and G. C. Weir. 2003. Critical reduction in β-cell mass results in two distinct outcomes over time. J. Biol. Chem. 278:2997-3005. [DOI] [PubMed] [Google Scholar]
- 37.Laybutt, D. R., A. Sharma, D. C. Sgroi, J. Gaudet, S. Bonner-Weir, and G. C. Weir. 2002. Genetic regulation of metabolic pathways in β-cells disrupted by hyperglycemia. J. Biol. Chem. 277:10912-10921. [DOI] [PubMed] [Google Scholar]
- 38.Maekawa, T., H. Sakura, C. Kanei-Ishii, C. Sudo, T. Yoshimura, J. Fujisawa, M. Yoshida, and S. Ishii. 1989. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 8:2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao, D., E. A. Warner, S. A. Gurwitch, and D. R. Dowd. 1998. Differential regulation and transcriptional control of immediate early gene expression in forskolin-treated WEHI7.2 thymoma cells. Mol. Endocrinol. 12:492-503. [DOI] [PubMed] [Google Scholar]
- 40.Melloul, D., S. Marshak, and E. Cerasi. 2002. Regulation of insulin gene transcription. Diabetologia 45:309-326. [DOI] [PubMed] [Google Scholar]
- 41.Molina, C. A., N. S. Foulkes, E. Lalli, and P. Sassone-Corsi. 1993. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell 75:875-886. [DOI] [PubMed] [Google Scholar]
- 42.Nielson, D. A., M. Welsh, M. J. Casadaban, and D. F. Steiner. 1985. Control of insulin gene expression in pancreatic beta cells and in an insulin-producing cell line, RIN-5F cells. I. Effects of glucose and cyclic AMP on the transcription of insulin mRNA. J. Biol. Chem. 260:13585-13589. [PubMed] [Google Scholar]
- 43.Oetjen, E., T. Diedrich, A. Eggers, B. Eckert, and W. Knepel. 1994. Distinct properties of the cAMP-responsive element of the rat insulin I gene. J. Biol. Chem. 269:27036-27044. [PubMed] [Google Scholar]
- 44.Philippe, J., and M. Missotten. 1990. Functional characterization of a cAMP-responsive element of the rat insulin I gene. J. Biol. Chem. 265:1465-1469. [PubMed] [Google Scholar]
- 45.Philippe, J., E. Giordano, A. Gjinovci, and P. Meda. 1992. Cyclic adenosine monophosphate prevents the glucocorticoid-mediated inhibition of insulin gene expression in rodent islet cells. J. Clin. Investig. 90:2228-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philippe, J., I. Pacheco, and P. Meda. 1994. Insulin gene transcription is decreased rapidly by lowering glucose concentrations in rat islet cells. Diabetes 43:523-528. [DOI] [PubMed] [Google Scholar]
- 47.Powers, A. C., F. Tedeschi, K. E. Wright, J. S. Chan, and J. F. Habener. 1989. Somatostatin gene expression in pancreatic islet cells is directed by cell-specific DNA control elements and DNA-binding proteins. J. Biol. Chem. 264:10048-10056. [PubMed] [Google Scholar]
- 48.Riabowol, K., G. Draetta, L. Brizuela, D. Vandre, and D. Beach. 1989. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell 57:393-401. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg, A., F. Zindy, F. L. Deist, H. Mouly, P. Metezeau, C. Brechot, and E. Lamas. 1995. Overexpression of human cyclin A advances entry into S phase. Oncogene 10:1501-1509. [PubMed] [Google Scholar]
- 50.Ryseck, R. P., and R. Bravo. 1991. c-JUN, JUN B, and JUN D differ in their binding affinities to AP-1 and CRE consensus sequences: effect of FOS proteins. Oncogene 4:533-542. [PubMed] [Google Scholar]
- 51.Sander, M., and M. S. German. 1997. The beta cell transcription factors and development of the pancreas. J. Mol. Med. 75:327-340. [DOI] [PubMed] [Google Scholar]
- 52.Servillo, G., M. A. Della Fazia, and P. Sassone-Corsi. 1998. Transcription factor CREM coordinates the timing of hepatocyte proliferation in the regenerating liver. Genes Dev. 12:3639-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu, M., Y. Nomura, H. Suzuki, E. Ichikawa, A. Takeuchi, M. Suzuki, M. Nakamura, T. Nakajima, and K. Oda. 1998. Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp. Cell Res. 239:93-103. [DOI] [PubMed] [Google Scholar]
- 54.Walker, M. D., T. Edlund, A. M. Boulet, and W. J. Rutter. 1983. Cell-specific expression controlled by the 5′-flanking region of insulin and chymotrypsin genes. Nature 306:557-561. [DOI] [PubMed] [Google Scholar]
- 55.Welsh, M., D. A. Nielsen, A. J. MacKrell, and D. F. Steiner. 1985. Control of insulin gene expression in pancreatic beta cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J. Biol. Chem. 260:13590-13594. [PubMed] [Google Scholar]
- 56.Yam, C. H., T. K. Fung, and R. Y. C. Poon. 2000. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 59:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]