Abstract
A concern about emerging swine diseases led to a pilot study to determine the feasibility of an active surveillance system referred to as the Ontario Swine Veterinary-based Surveillance System (OSVS). The OSVS recorded the incidence of various syndromes and investigated potential outbreaks. However, validation of the disease patterns observed was needed. The objective of this study was to compare the disease patterns observed in the OSVS system with submission data obtained from a regional diagnostic laboratory — the Animal Health Laboratory (AHL). Higher rates of submission were reported to the OSVS compared with AHL records. However, OSVS and AHL data captured similar trends of disease. The OSVS data captured potential outbreaks that were not reflected in the laboratory data. Validation of active and passive syndromic surveillance data is necessary, and efforts should be made to integrate these types of data sources.
Résumé
Comparaison des tendances des maladies chez la population porcine de l’Ontario à l’aide d’une surveillance active effectuée par les praticiens et d’une surveillance passive basée sur les laboratoires (2007–2009). Des inquiétudes à propos de l’émergence de maladies porcines ont donné lieu à une étude pilote pour déterminer la faisabilité d’un système de surveillance active appelé système de surveillance des vétérinaires porcins de l’Ontario (Ontario swine veterinary-based surveillance system [OSVS]). L’OSVS a enregistré l’incidence de divers syndromes et a fait enquête sur les flambées potentielles. Cependant, la validation des tendances observées pour les maladies était requise. Cette étude avait pour objectif de comparer les tendances des maladies observées dans le système OSVS avec les données soumises obtenues d’un laboratoire de diagnostic régional — l’Animal Health Laboratory (AHL). Des taux de soumission supérieurs ont été signalés pour l’OSVS comparativement aux dossiers de l’AHL. Cependant, les données de l’OSVS et de l’AHL ont reflété des tendances semblables pour les maladies. Les données de l’OSVS reflétaient des flambées potentielles qui n’étaient pas signalées dans les données de laboratoire. La validation des données de surveillance syndromique active et passive sont nécessaires et des efforts devraient être déployés pour intégrer ces types de sources de données.
(Traduit par Isabelle Vallières)
Introduction
Syndromic surveillance relies on detection of clinical features or laboratory findings that are usually apparent before a definitive disease diagnosis is possible, allowing the mobilization of rapid response to reduce morbidity and mortality (1,2). Syndromic surveillance systems often involve the use of non-traditional data sources, obtained actively or passively. Animal health active surveillance often relies on the regular or periodic collection of reports or the submission of samples from veterinary practitioners or farmers. Practicing veterinarians play an important role in detecting initial cases of novel infectious diseases or changes in the incidence of specific syndromes (e.g., respiratory disease or mortality) that may provide the first warning of a disease outbreak. This type of early warning could allow for more rapid and efficient collection of diagnostic samples and implementation of disease control strategies (3). In contrast, passive surveillance leaves the responsibility to health care givers to report any health events of concern, or uses existing data from pharmacy sales, abattoirs, or submissions of cases to laboratories before any diagnoses have been performed, resulting in the investigator having no control over the data collection (4,5).
The need for an early-warning disease surveillance program for swine in Ontario was evident based on disease events that occurred from 2004 to 2006. Swine disease outbreaks associated with a triple-reassortant human-avian-swine influenza H3N2 virus, new strains of porcine reproductive and respiratory syndrome virus (PRRSv), and porcine circovirus associated disease (PCVAD) were reported in Ontario from 2004 to 2006 (6–9). These outbreaks led to the decision to conduct a pilot study, starting in the summer of 2007, to determine the feasibility of an active veterinary-based syndromic surveillance system referred to as the Ontario Swine Veterinary-based Surveillance System (OSVS). The results of this evaluation have been described elsewhere (10). The OSVS project also recorded the occurrence/incidence of various syndromes among farms serviced by 5 major swine veterinary practices in Ontario. Increased rates of disease in space, time, and space-time were also evaluated to identify potential disease outbreaks.
Syndromic surveillance should integrate data from other sources because this increases the sensitivity and specificity of any disease outbreak identified by a specific source (11–13). In this study we compared the OSVS data with data obtained from pre-diagnostic clinical histories obtained from the swine submissions to the Animal Health Laboratory (AHL) at the University of Guelph. The AHL is the primary location for swine practitioners in Ontario to submit case material for diagnostic and routine monitoring services. Consequently, data from both sources (OSVS and AHL) should be comparable to each other.
Passive laboratory surveillance often suffers from under-reporting that may underestimate the real disease status of a population and may not be the fastest approach to detect emerging diseases. Also, passive surveillance may be influenced by the disease awareness and willingness of people reporting suspect cases (14). In contrast, active veterinary-based surveillance may be an inexpensive manner to obtain valuable surveillance data from a large proportion of farms during a short period of time. However, the success of this type of surveillance depends on the compliance of participants, the timeliness of submission of reports, and the data quality obtained from the data providers (5,10).
Unlike human disease surveillance, there are no published studies in veterinary medicine that have compared the information on disease status from sydnromic surveillance data based on active veterinary-based surveillance and from passive laboratory-based surveillance data over several years. The main objectives of this study were to: i) compare the total number of submissions obtained actively from veterinarians participating in the OSVS system and passive data obtained through case histories that accompanied their laboratory submissions (OSVS and all AHL data), and ii) compare the total number of submissions of passive data obtained from OSVS members to all passive data submitted to the AHL (subset of AHL data from OSVS members and all AHL data). These comparisons should provide insight into whether active surveillance provides value or redundant information to a syndromic surveillance system.
Materials and methods
Description of data sources
The rates of submission from July 1, 2007 to June 30, 2009, were compared among: i) submissions made to the OSVS program (OSVS); ii) a subset of data obtained from pre-diagnostic clinical histories obtained from submissions made to the AHL from OSVS members (OSVS subset of AHL data); and iii) data from pre-diagnostic clinical histories obtained from all the swine submissions made to the AHL (all AHL data). A submission refers to a report or sample(s) sent from a production unit on a specific date to any of these data sources. A submission can involve multiple animals, and a production unit can make multiple submissions over time.
The laboratory data were obtained from the database of files submitted to the AHL. For each case, the AHL data included the date of submission, name of the clinic and practitioner submitting the sample, clinical history of the case and the following categories for body systems and parameters affected: respiratory, digestive, reproductive, integument/senses, endocrine, musculoskeletal, abortions, urinary, multisystemic, nervous, hemopoietic, mammary, sudden death, and abnormal production. The classification of body systems and parameters affected reported in the AHL submission files was checked for completeness/accuracy by reading the clinical history of each case. The information was completed by the OSVS project coordinator (Rocio Amezcua) in cases in which the clinical history was reported, but the options for body systems or parameters affected were not recorded. Additional clinical signs including off-feed, fever, poor response to treatment, increase in mortality, increase in morbidity, and poor growth rate were recorded from the clinical histories. The AHL subset from OSVS submissions made by participating veterinarians was also completed using this process.
Seven practitioners from 5 major swine clinics, in different areas of Ontario, agreed to record and transmit information to the OSVS pilot project on a weekly basis (10). However, 2 practitioners and 1 practice only started providing data in fall 2007. All remaining clinicians and practices provided data from summer 2007 to spring 2009. A total of 2222 swine farms were enumerated in Ontario during the 2006 Agricultural Census. Of these, 1226 farms (55.17%) were clients of the participating clinics (10). An update on the evaluation of the OSVS project showed that 791 farms were visited by the 7 practitioners, resulting in a general coverage of the OSVS program of 35.6% of the total farms in Ontario. The OSVS data collection form was created based on the classification of syndromes listed on the submission form of the AHL. The OSVS data included disease records identified by practitioners participating in the program during their farm visits/calls. The farm visits/calls were summarized according to the body system and production parameters affected. Epidemiological information related to the date when the farm visit/call was performed, the farm code and location, and name of practitioners were obtained. The possible body systems and/or production parameters affected included: respiratory, digestive, reproductive, nervous, musculoskeletal, integument/senses, urinary-genital, increase in mortality, increase in morbidity, poor growth rate, poor farrowing rate, poor conception rate, and reduced litter size. The AHL and OSVS forms were designed so that practitioners could check boxes depending on the body systems and/or parameters affected.
Statistical analyses
Descriptive statistics
The total number of submissions made to each data source was calculated and graphed by season. In addition, the counts and proportion of submissions by the total number of submissions (disease and routine monitoring including submission with no clinical history for the AHL data) for each data source were calculated. Only the categories of body systems affected that were most commonly reported were considered for further analyses.
Comparison of rates among data sources
A multivariable model was used to compare the submission rates of the most commonly reported body systems affected among OSVS data, all AHL data, and a subset of AHL data from OSVS members. The effect of time measured as season-year (e.g., summer-2007) and 3 different body system submissions (i.e., respiratory, digestive, and reproductive) were evaluated in this model. Interaction terms among time, body system, and data source were also examined. The rate data were fitted using a negative binomial model, instead of Poisson, if the over-dispersion term, alpha, indicated an improved fit using the negative binomial model. The offset of the negative binomial model was the total number of submissions (disease and routine monitoring) made to each data stream for each season-year period observed (15). The AHL data submissions that did not have a clinical history, but were recorded as being a disease or routine monitoring were included in this denominator.
The model building process included the evaluation of the contribution of subsets of predictors (source, system, time, and interactions among these variables) in the model by using the likelihood ratio test. Nested models with and without the different predictors were compared. If the likelihood ratio test between a simpler and the full model was significant (P < 0.05) the variable/interaction term was included in the model. Anscombe and deviance residuals were estimated from the final negative binomial model to determine if the model fit the data and to identify outliers that would require further investigation. Predicted rates by source and system were calculated and graphed by time, and contrasts were performed to determine differences among sources, systems, and season-year from the final negative binomial models (15). The statistical analyses were performed using STATA 10MP (Stata Corp., College Station, Texas, USA). All tests performed were two-tailed with a statistical significance level of 5%.
Results
Descriptive statistics
The total number of submissions by source, from July 2007 to June 2009, included: OSVS = 3424 submissions; OSVS subset of AHL data = 2333 and all AHL data = 6917.
The proportion of submissions by body system affected, based on all submissions by source, are summarized in Table 1.
Table 1.
Proportion of submissions by body system affected based on all swine submissions made to the OSVS, AHL and the subset of AHL from OSVS participating members from summer 2007 to spring 2009 in Ontario
| All submissions (disease, routine monitoring, and submissions with no clinical history) | OSVS (n = 3424) | Subset of AHL from OSVS (n = 2333) | AHL all (n = 6917) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Number | %a | Number | % | Number | % | |
| Respiratory | 648 | 18.93 | 282 | 12.10 | 573 | 8.29 |
| Reproductive | 241 | 7.04 | 87 | 3.73 | 172 | 2.49 |
| Digestive | 541 | 15.80 | 169 | 7.24 | 412 | 5.96 |
| Integument | 138 | 4.03 | 21 | 0.90 | 54 | 0.78 |
| Musculoskeletal | 239 | 6.98 | 51 | 2.19 | 88 | 1.27 |
| Nervous | 140 | 4.09 | 20 | 0.86 | 45 | 0.65 |
| Other body systems or production parameters recorded | 1726 | 50.40 | 482 | 20.66 | 1036 | 14.98 |
Proportions add up to more than 100% because in some cases, more than one body system or production parameter affected were recorded.
OSVS — Ontario Swine Veterinary-based Surveillance System, AHL — Animal Health Laboratory.
The proportion of disease and routine submissions by source are summarized as follows:
For the OSVS program 1425 (41.6%) records were related to disease and 1999 (58.4%) were related to routine monitoring. In 483 routine monitoring records, a disease was reported. Therefore a total of 1908 disease reports were obtained.
In the OSVS subset of AHL data, 651 (27.9%) records had a clinical history of disease; and 725 (31.1%) disease records did not have a clinical history. A total of 957 (41.0%) records reported no clinical signs because these submissions were related to monitoring of different diseases or management issues.
Based on all AHL data, 1407 (20.3%) records had a clinical history of disease, 3302 (47.7%) disease records did not include a clinical history. A total of 2208 (31.9%) disease records were related to monitoring of different diseases or management issues.
The most commonly reported systems affected in all data sources were: respiratory, digestive, and reproductive (Table 1), which were, therefore, included for further analyses. These systems were reported in 41.7% of all submissions made to the OSVS; 23% of all submissions made to the subset of AHL data for OSVS participants, and in 16.7% of all submissions of all AHL data (Table 1).
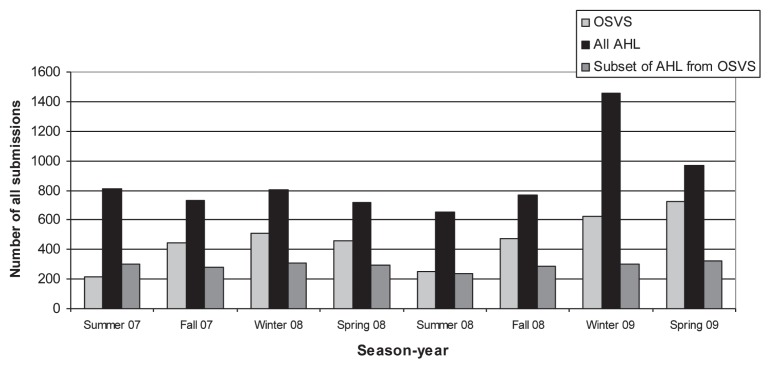
The total rate of submissions by source and season-year are presented in Figure 1. The total number of submissions made to the AHL increased from an average of 747.6 submissions per season from summer 2007 to fall 2008, to an average of 1215 submissions in winter and spring 2009. Similarly, the total rate of submissions made to the OSVS increased from an average of 392 submissions per season from summer 2007 to fall 2008, to an average of 674.5 submissions in the winter and spring of 2009, while the total of submissions to the AHL by the OSVS participating veterinarians remained relatively constant ranging from 285 to 311 during the same time periods.
Figure 1.
Total number of all submissions from summer 2007 to spring 2009 in Ontario. Submissions included disease and routine monitoring and disease with no clinical history for the AHL. OSVS — Ontario Swine Veterinary-based Surveillance System, AHL — Animal Health Laboratory.
Negative binomial model
The final model included source, season-year, system, and the interaction of source*season-year and system*season-year (Table 2). The interaction between system*source was not significant (likelihood ratio χ2 = 7.3, P = 0.102) and was not included in the final model. The likelihood ratio test for alpha was highly significant (P < 0.001), indicating that over-dispersion was present and the negative binomial model would fit the data better than the Poisson model. Normal distribution of Anscombe residuals showed that the data fit the model and no unusual observations were observed when plotting the deviance residuals (15).
Table 2.
Negative binomial model comparing all the swine submission (disease, routine monitoring including disease submissions with no clinical history for AHL) rates of the most commonly reported body systems affected among OSVS data, all AHL data, and the subset of AHL from OSVS participating members from summer 2007 to spring 2009 in Ontario
| IRRd | 95% CIe | P-value | |
|---|---|---|---|
| Sourcea | |||
| OSVS subset of AHL | 0.66 | 0.46–0.94 | 0.02 |
| All AHL | 0.54 | 0.39–0.76 | < 0.001 |
| Systemb | |||
| Digestive | 0.62 | 0.45–0.84 | 0.002 |
| Reproductive | 0.30 | 0.21–0.43 | < 0.001 |
| Season-yearc | |||
| Fall — 2007 | 1.15 | 0.78–1.69 | 0.48 |
| Winter — 2008 | 1.34 | 0.91–1.97 | 0.14 |
| Spring — 2008 | 0.86 | 0.57–1.29 | 0.46 |
| Summer — 2008 | 0.68 | 0.44–1.05 | 0.08 |
| Fall — 2008 | 0.74 | 0.48–1.13 | 0.16 |
| Winter — 2009 | 0.47 | 0.31–0.71 | < 0.001 |
| Spring — 2009 | 0.52 | 0.35–0.77 | 0.001 |
| Source*Season-year | |||
| OSVS subset of AHL*Fall — 2007 | 0.78 | 0.48–1.27 | 0.32 |
| OSVS subset of AHL*Winter — 2008 | 0.70 | 0.43–1.14 | 0.15 |
| OSVS subset of AHL*Spring — 2008 | 0.59 | 0.34–1.01 | 0.05 |
| OSVS subset of AHL*Summer — 2008 | 0.47 | 0.27–0.83 | 0.01 |
| OSVS subset of AHL*Fall — 2008 | 0.73 | 0.43–1.24 | 0.24 |
| OSVS subset of AHL*Winter — 2009 | 1.17 | 0.70–1.96 | 0.53 |
| OSVS subset of AHL*Spring — 2009 | 1.44 | 0.87–2.38 | 0.16 |
| All AHL*Fall — 2007 | 0.87 | 0.56–1.34 | 0.54 |
| All AHL*Winter — 2008 | 0.70 | 0.46–1.08 | 0.11 |
| All AHL*Spring — 2008 | 0.61 | 0.38–0.98 | 0.04 |
| All AHL*Summer — 2008 | 0.49 | 0.30–0.80 | 0.004 |
| All AHL*Fall — 2008 | 0.74 | 0.47–1.19 | 0.22 |
| All AHL*Winter — 2009 | 0.51 | 0.32–0.82 | 0.005 |
| All AHL*Spring — 2009 | 0.99 | 0.63–1.57 | 0.97 |
| System*Season-year | |||
| Digestive*Fall — 2007 | 0.89 | 0.58–1.37 | 0.61 |
| Digestive*Winter — 2008 | 0.96 | 0.63–1.46 | 0.86 |
| Digestive*Spring — 2008 | 1.18 | 0.75–1.84 | 0.46 |
| Digestive*Summer — 2008 | 2.40 | 1.50–3.84 | < 0.001 |
| Digestive*Fall — 2008 | 1.33 | 0.85–2.08 | 0.21 |
| Digestive*Winter — 2009 | 1.80 | 1.16–2.79 | 0.008 |
| Digestive*Spring — 2009 | 1.02 | 0.66–1.59 | 0.91 |
| Reproductive*Fall — 2007 | 1.52 | 0.95–2.43 | 0.08 |
| Reproductive*Winter — 2008 | 1.08 | 0.67–1.74 | 0.74 |
| Reproductive*Spring — 2008 | 0.66 | 0.38–1.16 | 0.15 |
| Reproductive*Summer — 2008 | 1.37 | 0.77–2.45 | 0.27 |
| Reproductive*Fall — 2008 | 0.90 | 0.53–1.55 | 0.71 |
| Reproductive*Winter — 2009 | 1.11 | 0.66–1.88 | 0.68 |
| Reproductive*Spring — 2009 | 0.90 | 0.54–1.50 | 0.68 |
OSVS — Ontario Swine Veterinary-based Surveillance System, AHL — Animal Health Laboratory.
Referent — OSVS.
Referent — respiratory.
Referent — summer 2007.
Incident rate ratio.
95% confidence interval (CI).
Contrasts were performed after fitting the model to determine the difference among the 3 different sources (OSVS, OSVS subset of AHL, and all AHL data) among season-year periods while holding the disease category constant. Briefly, the OSVS data had a significantly higher rate of total submissions compared to all AHL data for all periods: fall 2007, winter 2008, spring 2008, summer 2008, fall 2008, winter 2009, and spring 2009 (P < 0.001; Table 3). A higher rate of submissions was observed for OSVS compared to the subset of AHL data from OSVS participating veterinarians for fall 2007, winter 2008, spring 2008, summer 2008, and fall 2008 (P < 0.001; Table 3). Submissions in winter and spring of 2009 were higher than in summer of 2007 but not significantly different. The subset of AHL from OSVS participants had a higher submission rate compared with all AHL data in winter 2009, and spring 2009 (Table 3).
Table 3.
Contrasts of rates between season-year period of all swine submissions from three data sources (OSVS, all AHL, and the subset of AHL from OSVS participating members) from summer 2007 to spring 2009 in Ontario
| OSVS versus all AHL | Data sources OSVS versus subset of AHL from OSVS | Subset of AHL from OSVS versus all AHL | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Season-year period | IRR | P-value | IRR | P-value | IRR | P-value |
| Fall — 2007 | 2.09 | < 0.001 | 1.95 | < 0.001 | 1.07 | 0.67 |
| Winter — 2008 | 2.59 | < 0.001 | 2.16 | < 0.001 | 1.20 | 0.27 |
| Spring — 2008 | 2.98 | < 0.001 | 2.58 | < 0.001 | 1.15 | 0.50 |
| Summer — 2008 | 3.70 | < 0.001 | <.21 | < 0.001 | 1.15 | 0.5< |
| Fall — 2008 | 2.45 | < 0.001 | 2.10 | < 0.001 | 1.17 | 0.4< |
| Winter — 2009 | 3.54 | < 0.001 | 1.<0 | 0.16 | 2.7< | < 0.001 |
| Spring — 2009 | 1.84 | < 0.001 | 1.05 | 0.75 | 1.74 | 0.00< |
OSVS — Ontario Swine Veterinary-based Surveillance System, AHL — Animal Health Laboratory, Negative binomial model included all submissions as the offset (disease and routine monitoring and disease with no clinical history for AHL) from each data source.
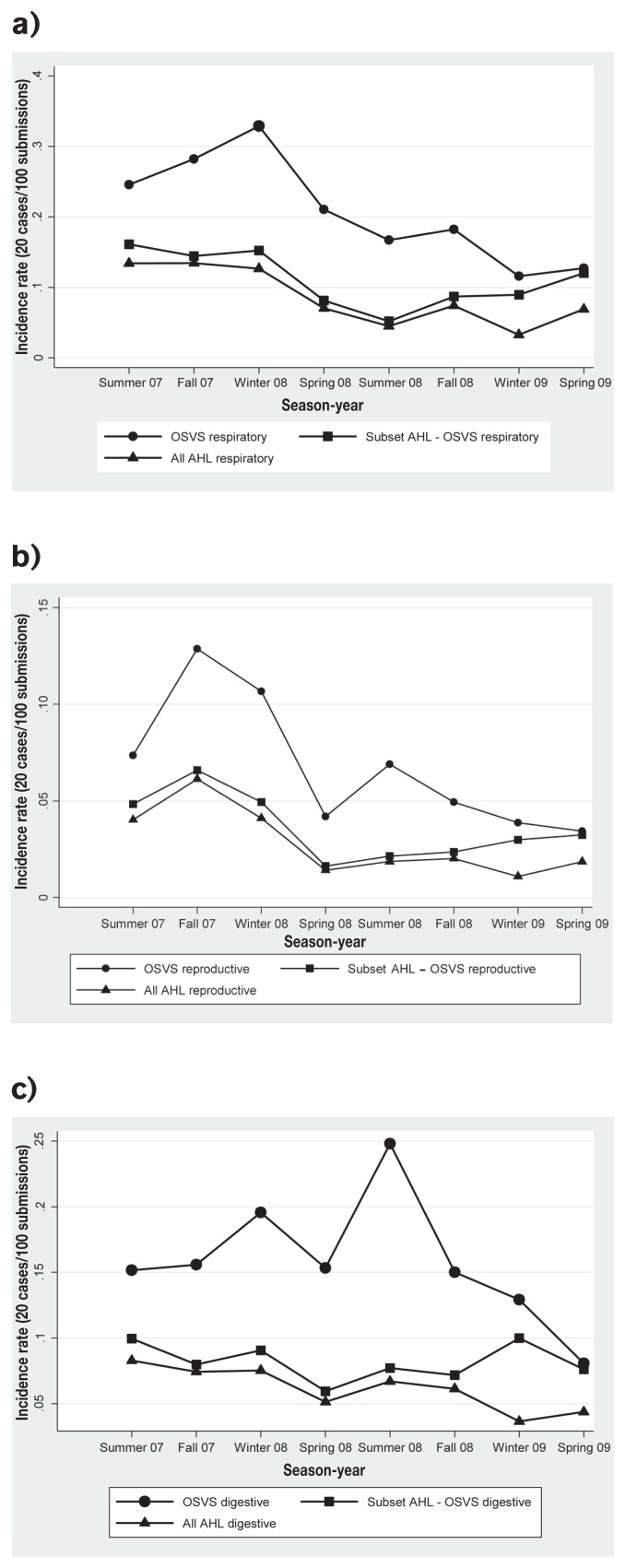
Contrasts of the model were performed to determine the difference in rates of reporting diseases associated with respiratory, digestive, and reproductive within systems among season-year periods by source. Results are summarized for respiratory, digestive, and reproductive submissions in Tables 4a, 4b, and 4c, respectively. In general, the respiratory and reproductive submissions of the OSVS had significantly lower rates at the end of the study period, in the winter and spring of 2009 compared with the summer of 2007 (Table 4a; Figure 2a). However, for the subset of AHL data from OSVS participants, and for all AHL data, significantly lower rates of respiratory and reproductive submissions were observed from spring 2008 to the end of the study period compared with summer 2007 (Tables 4a and 4c; Figures 2a and 2b).
Table 4.
Contrasts of a) respiratory; b) reproductive, and c) digestive rates between season-year periods of all swine submissions from three data sources (OSVS, all AHL and the subset of AHL from OSVS participating members) from summer 2007 to spring 2009 in Ontario
| OSVS | Data sources subset of AHL from OSVS | All AHL | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Season-year perioda | IRR | P-value | IRR | P-value | IRR | P-value |
| a) Fall — 2007 | 1.14 | 0.48 | 0.89 | 0.60 | 1.00 | 0.98 |
| Winter — 2008 | 1.33 | 0.14 | 0.94 | 0.78 | 0.94 | 0.75 |
| Spring — 2008 | 0.86 | 0.46 | 0.50 | 0.004 | 0.52 | 0.001 |
| Summer — 2008 | 0.68 | 0.08 | 0.32 | < 0.001 | 0.33 | < 0.001 |
| Fall — 2008 | 0.74 | 0.11 | 0.54 | 0.008 | 0.55 | 0.003 |
| Winter — 2009 | 0.47 | < 0.001 | 0.55 | 0.01 | 0.24 | < 0.001 |
| Spring — 2009 | 0.52 | 0.001 | 0.74 | 0.18 | 0.51 | 0.001 |
| b) Fall — 2007 | 1.74 | 0.02 | 1.36 | 0.22 | 1.52 | 0.05 |
| Winter — 2008 | 1.45 | 0.12 | 1.02 | 0.93 | 1.02 | 0.92 |
| Spring — 2008 | 0.57 | 0.04 | 0.33 | < 0.001 | 0.35 | < 0.001 |
| Summer — 2008 | 0.93 | 0.81 | 0.44 | 0.01 | 0.46 | 0.005 |
| Fall — 2008 | 0.67 | 0.13 | 0.48 | 0.01 | 0.50 | 0.008 |
| Winter — 2009 | 0.52 | 0.013 | 0.62 | 0.09 | 0.27 | < 0.001 |
| Spring — 2009 | 0.46 | 0.003 | 0.67 | 0.15 | 0.46 | 0.002 |
| c) Fall — 2007 | 1.02 | 0.89 | 0.80 | 0.35 | 0.89 | 0.59 |
| Winter — 2008 | 1.29 | 0.21 | 0.90 | 0.68 | 0.90 | 0.63 |
| Spring — 2008 | 1.01 | 0.95 | 0.59 | 0.04 | 0.62 | 0.03 |
| Summer — 2008 | 1.63 | 0.02 | 0.77 | 0.33 | 0.81 | 0.31 |
| Fall — 2008 | 0.98 | 0.96 | 0.72 | 0.19 | 0.74 | 0.15 |
| Winter — 2009 | 0.85 | 0.46 | 1.01 | 0.99 | 0.44 | < 0.001 |
| Spring — 2009 | 0.53 | 0.003 | 0.76 | 0.26 | 0.53 | 0.002 |
OSVS — Ontario Swine Veterinary-based Surveillance System, AHL — Animal Health Laboratory; Negative binomial model included all submissions as the offset (disease and routine monitoring and disease with no clinical history for AHL) from each data source.
Referent — summer 2007.
Figure 2.
Predicted rates of swine a) respiratory, b) reproductive, and c) digestive submissions including all data from three data sources (OSVS, all AHL and the subset of AHL from OSVS participating members) from summer 2007 to spring 2009 in Ontario. ● OSVS predictive rates, ▲ All AHL predictive rates, ■ Subset OSVS-AHL predictive rates. OSVS — Ontario Swine Veterinary-based Surveillance System; AHL — Animal Health Laboratory.
The OSVS had significantly higher rates of digestive submissions in the summer of 2008 and of reproductive submission in the fall of 2007 compared to the summer of 2007, but significantly lower rates were observed at the end of the study period compared to the summer of 2007 for both body systems (Tables 4b and 4c; Figures 2b and 2c). For the subset of AHL data from OSVS members and all AHL data, significantly lower rates of digestive submissions were observed in spring 2008 compared with summer 2007. All AHL data also had significantly lower rates in spring 2008 and in winter and spring 2009 compared with the summer of 2007 (Table 4c; Figure 2c).
Discussion
No single data source can capture all the information required for syndromic surveillance. Despite the challenge of integrating data from different formats and sources over time; this should be considered in any surveillance system because different data sources can complement each other (16,17). In this study, we compared the rate of submissions recorded by practitioners in an active veterinary-based syndromic surveillance system with the rates of pre-diagnostic submissions obtained passively from the clinical history reported to a laboratory-based syndromic surveillance system. The latter was subdivided into submissions made by all veterinary practitioners and a subset of laboratory data from veterinarians participating in the active surveillance system. The offsets were set as the total number of submissions in the process of assessing whether active surveillance provided any additional insight into disease occurrence in Ontario. Similar trends of disease patterns were observed in both active and passive surveillance systems; however, the active veterinary-based surveillance system appeared to capture potential outbreaks of endemic diseases that were not reflected in the passive laboratory-based submission. We also noted that practitioners participating in an active veterinary-based surveillance system were more likely to record information in the laboratory forms. It might be worth considering using a group of sentinel practices with good compliance and response practices for quantitative surveillance of laboratory data. Although both active and passive data sources can be affected by practice and/or laboratory activities, efforts should be placed on integrating these types of data sources to improve the sensitivity of a surveillance system.
The most common disease reports in all data sources were associated with respiratory problems, followed by digestive and reproductive problems. Diseases of concern in Ontario since the outbreaks of PRRS, PCVAD, and swine influenza in 2005 were mainly associated with respiratory presentations. The PCVAD diagnoses were often associated with enterocolitis in finisher pigs, but affected pigs usually had a rasping cough (18). A wide spectrum of infectious agents associated with respiratory problems were observed concomitant with PCV-2 infection (6,19–21). Abortions were commonly seen during PRRS outbreaks (7). A decrease in the rates of respiratory submissions, based on syndromic results from all submission records of all data sources from 2007 to 2009 was observed, which agreed with AHL-based diagnostic reports that showed a decline in PCVAD diagnoses in 2007 and further decline in 2008 compared with 2005 and 2006 (22). These data may reflect the increase in use of PCV-2 vaccines in Ontario (22). In addition, the decrease in the rates of reproductive disease submissions observed in our data may be consistent with a decline in the cases of PRRS reflected in the number of laboratory test reports from the AHL. According to the AHL, the annual PRRSv average percent-positive tests have declined from 51% in 2004 to 45% in 2005, 33% in 2006, and 27% in 2007, and declined further in 2008 compared to 2005 (23).
Many digestive system problems such as E. coli post-weaning diarrhea, coccidiosis, and rotavirus infection are commonly seen on Ontario farms but are not commonly identified through traditional passive surveillance involving a diagnostic laboratory because of their endemic nature. Interestingly, although peaks of digestive system problems were only observed in the OSVS data in 2008 compared with AHL data, an AHL-based diagnostic report of 17 submissions related to enteric coccidiosis due to Isosopora suis was observed in 2008 (24). Nine of the 17 cases reported to the AHL came from nursery pigs, and only 1 listed coccidiosis as a potential rule-out (24). The majority of submissions consisted of fresh and formalin-fixed tissues collected by the referring veterinarian. Five submissions included 2 to 6 live pigs delivered to the AHL for complete diagnostic examination. A total of 49 submissions associated with digestive system problems were reported to the OSVS in the summer of 2008, and 5 were tentatively diagnosed by the practitioners as potential coccidiosis and/or E. coli problems in nursery pigs. However, only 1 of these reports resulted in a submission to the AHL. This might reflect potential under-reporting of gastrointestinal disease cases to the AHL. Enteric coccidiosis infection is a well-recognized cause of diarrhea in suckling pigs but is not commonly associated with nursery pigs. One advantage of active surveillance is the possibility of identifying known diseases that may be having an unusual presentation. It may also be helpful in identifying new strains of familiar pathogens, based on increased rates of disease or emerging infections with a similar disease presentation to an endemic infection.
The rates of submission made to the OSVS for all systems in both models were several times higher throughout the study compared with the syndromic laboratory data. A disadvantage of laboratory data is the potential for under-reporting and therefore failure to provide reliable information on the actual disease status of a population (5,25,26). Reports show that many factors may influence the sensitivity of passive surveillance, such as the probability of infected animals showing clinical signs, the disease awareness of persons responsible for reporting, and the sensitivities of persons responsible for reporting (26). Among these factors, disease awareness of the person responsible for reporting plays a pivotal role in influencing the sensitivity of passive surveillance (14). However, better compliance with the OSVS was observed from 2007 to 2008 compared to 2009, possibly because interest in participating in disease surveillance lessened the more removed participants were from the severe disease outbreaks that occurred in 2005 (27,28).
Usually morbidity and mortality are the 2 main categories of events for which rates are calculated in which the denominator is the number of animal-time units at risk (15). The negative binomial model reflected the rates of disease relative to overall veterinary practitioner visits. Our analyses showed that a significant interaction of data stream with time in the model captured peaks of submission rates in the OSVS data that were not reflected in the laboratory-based submission data. These peaks might have reflected potential outbreaks of endemic diseases not reported to the AHL (e.g., OSVS digestive submissions in the Summer of 2008).
Disease awareness implies not only the knowledge about disease characteristics and the vigilance of persons with reporting duties like farmers and veterinarians but also includes the willingness of those persons to report cases (26). In this study, passive surveillance was influenced by the willingness of practitioners to record data accurately. In all AHL data, almost 50% of submissions were not accompanied by a record of the clinical history of the case, compared with 31.1% in the subset of AHL records from OSVS participating practitioners. In general, higher numerical submission rates were observed throughout the study in the subset of AHL data from OSVS participants compared to all AHL data. The average proportion of submissions made to the AHL by the OSVS participants was about 50% for all body systems. This suggests that a small number of practitioners who volunteered to participate in the OSVS project were more likely to record information on the laboratory form than other practitioners submitting their cases to the laboratory. Consequently, it may be worth considering using a group of sentinel practices with good compliance for quantitative surveillance of laboratory data. In addition, the results of the OSVS evaluation with respect to data quality demonstrated that participating practitioners did not have problems completing the portion of the form asking for the body system or production parameter affected, when they conducted a disease visit during the pilot project (96% and 74.4%, respectively) (10). However, in many cases where a clinical history was reported in the AHL data, the body systems or parameters affected were not recorded and had to be completed by the OSVS project coordinator. In addition, laboratory data were often found to lack important epidemiological information. The AHL data usually did not contain information regarding the names and location of the farms and this missing information compromises the implementation of space-time methods to identify disease clusters. An update on the evaluation of the OSVS data showed that during this study period 3732 submissions were recorded and only 149 did not have information on the location of the farm. From a total of 791 farms visited, 99.6% were geo-located. This information allowed the OSVS project to identify various epidemiologically significant space, time, and space-time disease clusters during the study period.
Active veterinary-based surveillance provided value to a passive laboratory-based disease surveillance system in Ontario, especially when outbreaks of endemic diseases were present. Active veterinary-based surveillance also provides a better sense of disease in Ontario’s swine herds. This supports the value of maintaining both systems to improve the overall sensitivity of a single surveillance system. Further studies should investigate the changes in the rates of submission of active and passive veterinary surveillance systems using different subsets of data such as the total only-disease visits/submissions.
Acknowledgments
We thank the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) and the Food Safety Initiative of the Canadian Food Safety & Quality Program of the Agriculture Policy Framework for their financial and technical support. We also thank the Animal Health Laboratory, Laboratory Services Division, University of Guelph, for providing the data associated with the swine submissions made to the AHL. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Glickman LT, Moore GE, Glickman NW, Caldanaro RJ, Aucoin D, Lewis HB. Purdue university-Banfield national companion animal surveillance program for emerging and zoonotic diseases. Vector-Borne Zoonotic Dis. 2006;6:14–23. doi: 10.1089/vbz.2006.6.14. [DOI] [PubMed] [Google Scholar]
- 2.Henning KJ, Mostashari F. Syndromic surveillance. Issues Sci Tech. 2005;21:10–10. [Google Scholar]
- 3.McIntyre LH, Davies PR, Alexander G, et al. VetPAD-veterinary practitioner aided disease surveillance system. Proc of the 10th International Symposia on Veterinary Epidemiology and Economics (ISVEE). Surveillance session; 2003. p. 335. [Google Scholar]
- 4.Akhtar S, White F. Animal disease surveillance: Prospects for development in Pakistan. Revue Scien Tech (International Office of Epizootics) 2003;22:977–987. doi: 10.20506/rst.22.3.1449. [DOI] [PubMed] [Google Scholar]
- 5.Shephard RW. PhD dissertation. Faculty of Veterinary Science, University of Sydney; Sydney, Australia: 2006. The development of a syndromic surveillance system for the extensive beef cattle producing regions of Australia. [Google Scholar]
- 6.Gagnon CA, Tremblay D, Tijssen P, Venne MH, Houde A, Elahi SM. The emergence of porcine circovirus 2b genotype (PCV-2b) in swine in Canada. Can Vet J. 2007;48:811–819. [PMC free article] [PubMed] [Google Scholar]
- 7.Carman S, McEwen B, Josephson G, Fairles J. PRRSV outbreak in south western Ontario. Animal Health Laboratory (AHL) Newsletter. 2005 Mar;9:1–6. [Google Scholar]
- 8.Carman S, McEwen B, DeLay J, et al. Porcine circovirus-2 associated disease in swine in Ontario (2004 to 2005) Can Vet J. 2006;47:761–762. [PMC free article] [PubMed] [Google Scholar]
- 9.Karasin AI, Carman S, Olsen CW. Identification of human H1N2 and human-swine reassortant H1N2 and H1N1 influenza A viruses among pigs in Ontario, Canada (2003 to 2005) J Clin Microbiol. 2006;44:1123–1126. doi: 10.1128/JCM.44.3.1123-1126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amezcua R, Pearl DL, Friendship RM, McNab WB. Evaluation of a veterinary-based syndromic surveillance system implemented for swine. Can J Vet Res. 2010;74:241–251. [PMC free article] [PubMed] [Google Scholar]
- 11.Henning KJ. What is syndromic surveillance? Centres for Disease Control and Prevention (CDC) MMWR morbidity and mortality weekly report. 2004;53(Suppl):5–11. [Google Scholar]
- 12.Lombardo JS, Burkom H, Pavlin J. ESSENCE II and the framework for evaluating syndromic surveillance systems. Centres for Disease Control and Prevention (CDC) MMWR morbidity and mortality weekly report. 2004;53(Suppl):159–165. [PubMed] [Google Scholar]
- 13.Mandl KD, Overhage JM, Wagner MM, et al. Implementing syndromic surveillance: A practical guide informed by the early experience. J Am Med Inform Assoc JAMIA. 2004;11:141–150. doi: 10.1197/jamia.M1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadorn DC, Haracic SS, Stark KDC. Comparative assessment of passive surveillance disease-free and endemic situation: Example of Brucella melitensis surveillance in Switzerland and in Bosnia and Herzegovina. BMC Vet Res. 2008;4:52. doi: 10.1186/1746-6148-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiology Research. Charlottetown, Prince Edward Island, Canada: AVC Inc; 2003. Modeling count and rate data; pp. 391–407. [Google Scholar]
- 16.Clothier HJ, Fielding JE, Kelly HA. An evaluation of the Australian sentinel practice research network (ASPEN) surveillance for influenza-like illness. Comm Dis Intel. 2005;29:231–247. [PubMed] [Google Scholar]
- 17.Barnouin J, Bridges VE, De Groot BD, et al. Detecting emerging diseases in farm animals through clinical observations (Perspective) Emerg Infect Dis. 2006;12:204–210. doi: 10.3201/eid1202.050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dreumel T, Josephson G, Lusis P. Animal Health Laboratory (AHL) Newsletter. 9,3. University of Guelph Laboratory Services; Mar, 2005. Porcine circovirus type 2-associated conditions in pigs; p. 22. [Google Scholar]
- 19.Carman S, Cai HY, DeLay J, et al. The emergence of a new strain of porcine circovirus-2 in Ontario and Quebec swine and its association with severe porcine circovirus associated disease — 2004–2006. Can J Vet Res. 2008;72:259–268. [PMC free article] [PubMed] [Google Scholar]
- 20.Quintana J, Segalés J, Rosell C, et al. Clinical and pathological observations on pigs with post-weaning multi-systemic wasting syndrome. Vet Rec. 2001;149:357–361. doi: 10.1136/vr.149.12.357. [DOI] [PubMed] [Google Scholar]
- 21.Carman S, McEwen B, DeLay J, Cai H, Fairles J. Animal Health Laboratory (AHL) Newsletter. 10,2. University of Guelph Laboratory Services; Jun, 2006. [Last accessed May 7, 2013]. Porcine circovirus type 2-associated disease diagnoses continue into 2006; p. 14. Available from: http://guelphlabservices.com/files/AHL/AHL%20Newsletters/2006/AHLNewsletter%20June%202006.pdf. [Google Scholar]
- 22.Carman S, McEwen B, DeLay J, Cai H, Fairles J, van Dreumel T. Animal Health Laboratory (AHL) Newsletter. 12,3. University of Guelph Laboratory Services; Sep, 2008. [Last accessed May 7, 2013]. Porcine circovirus 2-associated disease diagnoses decline in 2007 and 2008; p. 22. Available from: http://guelphlabservices.com/files/AHL/AHL%20Newsletters/2008/AHLNewsletter%20September%202008.pdf. [Google Scholar]
- 23.Carman S, McEwen B, Fairles J. Animal Health Laboratory (AHL) Newsletter. 12,4. University of Guelph Laboratory Services; Dec, 2008. [Last accessed May 7, 2013]. Fewer PRRSV-positive cases identified in 2008; p. 33. Available from: http://www.guelphlabservices.com.asp1-13.dfw1-2.websitetestlink.com/files/AHL/AHL%20Newsletters/2008/AHLNewsletter%20December%202008.pdf. [Google Scholar]
- 24.Spinato MT, Peregrine AS. Animal Health Laboratory (AHL) Newsletter. 13,1. University of Guelph Laboratory Services; Mar, 2009. [Last accessed May 7, 2013]. Coccidiosis in weaned pigs; p. 5. Available from: http://www.guelphlabservices.com.asp1-13.dfw1-2.websitetestlink.com/files/AHL/AHL%20Newsletters/2009/AHLNewsletter%20March%202009.pdf. [Google Scholar]
- 25.Richard JL, Vidondo B, Mausezahl M. A 5 year comparison of performance of sentinel and mandatory notification surveillance systems for measles in Switzerland. Eur J Epidemiol. 2008;23:55–65. doi: 10.1007/s10654-007-9187-1. [DOI] [PubMed] [Google Scholar]
- 26.Martin PA, Cameron AR, Greiner M. Demonstrating freedom from disease using multiple complex data sources I: A new methodology based on scenario trees. Prev Vet Med. 2007;79:71–97. doi: 10.1016/j.prevetmed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Delay J, McEwen B, Carman S, van Dreumel T, Fairles J. Animal Health Laboratory (AHL) Newsletter. Vol. 9. University of Guelph Laboratory Services; Sep, 2005. Porcine circovirus type 2-associated disease is increasing; p. 22. [Google Scholar]
- 28.Carman S, McEwen B, Josephson G, Fairles J. Animal Health Laboratory (AHL) Newsletter. Vol. 9. University of Guelph Laboratory Services; Mar, 2005. PRRSV outbreak in south western Ontario; p. 31. [Google Scholar]