Abstract
Purpose
The number of patients in Sweden treated with radical prostatectomy for localized prostate cancer has increased exponentially. The extent to which this increase reflects treatment of non-lethal disease detected through PSA screening is unknown.
Experimental design
We undertook a nationwide study of all 18,837 prostate cancer patients treated with radical prostatectomy in Sweden from 1988 to 2008 with complete follow-up through 2009. We compared cumulative incidence curves, fit Cox regression and cure models and performed a simulation study to determine changes in treatment of non-lethal cancer, in cancer-specific survival over time, and effect of lead-time due to PSA screening.
Results
The annual number of radical prostatectomies increased 25-fold during the study period. The five-year cancer-specific mortality decreased from 3.9% (95% CI 2.5 to 5.3) among patients diagnosed between 1988 and 1992 to 0.7% (95% CI 0.4–1.1) among those diagnosed between 1998 and 2002 (p for trend < 0.001). According to the cure model, the risk of not being cured declined by 13% (95% CI 12–14%) with each calendar year. The simulation study indicated that only about half of the improvement in disease-specific survival could be accounted for by lead-time.
Conclusion
Patients overdiagnosed with non-lethal prostate cancer appear to account for a substantial and growing part of the dramatic increase in radical prostatectomies in Sweden but increasing survival rates are likely also due to true reductions in the risk of disease-specific death over time. Because the magnitude of harm and costs due to overtreatment can be considerable, identification of men who likely benefit from radical prostatectomy is urgently needed.
Keywords: Overdiagnosis, prostate cancer, PSA screening, radical prostatectomy
Introduction
The techniques used to perform radical prostatectomy were refined in the 1980s (1) and soon thereafter, prostatectomy became a widespread curative treatment for localized prostate cancer (2). Concomitantly, the clinical landscape of prostate cancer was transformed following widespread PSA screening (3, 4) which dramatically increased prostate cancer incidence, with a larger proportion of cases presenting with localized disease (5, 6, 8). In Sweden, PSA testing in men aged 55 to 69 years was estimated to have increased to 56% over ten years in 2007 (9). As a corollary, the number of patients eligible for radical prostatectomy has grown. Many of these cancers have an indolent course even without therapeutic intervention (10–12). Consequently, PSA screening entails overdiagnosis of cancer that would never present clinically during the patient s lifetime (4, 5, 13).
In this context, it is timely to assess whether the growth in number of prostatectomies in Sweden has generated increasing treatment of non-lethal cancer which by definition can convey no survival benefit and for which men experience only the attendant consequences of increased morbidity and poorer quality of life associated with the treatment (14, 15). To address the hypothesis, we examine data from a nation-wide cohort comprising all prostate cancer patients in Sweden treated with prostatectomy and followed prospectively for mortality from 1988 to 2008.
Materials and Methods
The Study Cohort
We undertook a nationwide study in Sweden, including all prostate cancer patients who underwent radical prostatectomy between 1988 and 2008. The men were identified through linkage of the Swedish Cancer Register (16) with the Inpatient Register using the unique national registration number assigned to all Swedish inhabitants. Information on prostatectomy procedures was gleaned from the Inpatient Register (17) with national coverage of 100% since 1987. This register includes information on surgical procedures, discharge diagnoses as well as date of admission/discharge. Neither the cancer nor the in-patient register includes information on clinical stage, Gleason score, or other predictors of outcome. We identified 18,837 prostate cancer patients who had undergone prostatectomy between 1988 and 2008 using the Swedish Classification of Operations and Major Procedures codes: 6631, 6633, 6611 until 1995 and KEC00, KEC01, KEC10, and KEC20 from 1996 onwards.
Follow-up
Through linkage to the Swedish Death Register and the Emigration Register, the men were followed from the date of prostate cancer diagnosis until the date of death, emigration, or the end of the study period (December 31, 2009), whichever occurred first. Individuals with prostate cancer as the underlying cause were classified as having died from prostate cancer. The validity of the causes of death diagnosis among prostate cancer patients has been reported to be high (18, 19).
Statistical analysis
We plotted cumulative incidence curves for prostate cancer death, stratified by year of diagnosis to examine trends in cancer-specific survival. We graphed the distribution of age at cancer diagnosis across time to evaluate whether there was a shift towards younger ages as an indicator of increased early detection. Relative risks of prostate cancer death, were estimated using Cox proportional-hazards models.
We fitted a cure model (20, 21) that estimated the fraction of cases cured and the risk of disease-specific death among those not cured. The cure model is appropriate when the risk of disease-specific death is zero for a significant fraction of the case population. This fraction represents the cured portion of the case population. The model allows separate identification of predictors of being in the cured portion and predictors of the risk of death if not cured. We used a logistic regression model to estimate the likelihood of cure and a Cox model to estimate the survival among those not cured using the PSPCM macro in SAS (13).
To determine whether improvements in observed disease-specific survival over time were consistent with a screening effect, we performed a simulation study which replicated 100,000 prostate cancer patients who underwent radical prostatectomy. We assumed that screening was initiated after 1992, yielding a specified fraction of cases diagnosed by screening each year thereafter. We increased the fraction of screen-detected cancers from 0% in 1992 to 10% in 1996 and to 40% in 2005 based on estimates from the National Prostate Cancer Register (22).
The goal of the simulation was to compare observed and simulated disease-specific survival curves, under the assumption that screening only advances disease diagnosis with no change in life expectancy. Cases randomly assigned as screen-detected were allocated a disease-specific survival time consisting of the sum of a lead-time and a randomly generated pre-PSA survival time. The pre-PSA survival time was generated from the distribution of survival times among cases diagnosed from 1988 to1992, before screening. We used a range of mean lead times based on estimates from screening trials and simulation studies in the US and Europe (13, 23). We considered mean lead times of 5, 8, 10 and 15 years for the “screen detected cases” and assumed an exponential distribution. For each case, a time from diagnosis to other-cause death was generated from the distribution of other-cause survival among cases diagnosed from 1988 to 2008. Each man was assigned a cause of death and survival time from the minimum of the simulated disease-specific and other-cause survival times. We then graphed the simulated and observed disease-specific survival curves based on diagnoses from 1993 onwards to determine whether the addition of the lead-time associated with screening was sufficient to account for the observed improvement in survival over time.
Results
Across the study period the number of radical prostatectomies in Sweden increased with a high of 2,500 in 2005 followed by a modest decline in 2008 (Fig 1). Robotic procedures accounted for a growing proportion with almost 40% of prostatectomies conducted with the robot in 2008. The change in the number of prostatectomy procedures parallels the change in incidence of prostate cancer in Sweden, which peaked in 2004 and has been declining since (8). Of men undergoing prostatectomy, 90% were between 55 and 75 years at prostate cancer diagnosis (Table 1). The mean age at diagnosis has decreased over time, with a mean of 66.1 years in 1988, 62.5 years in 1998, and 62.4 years in 2008.
Figure 1.
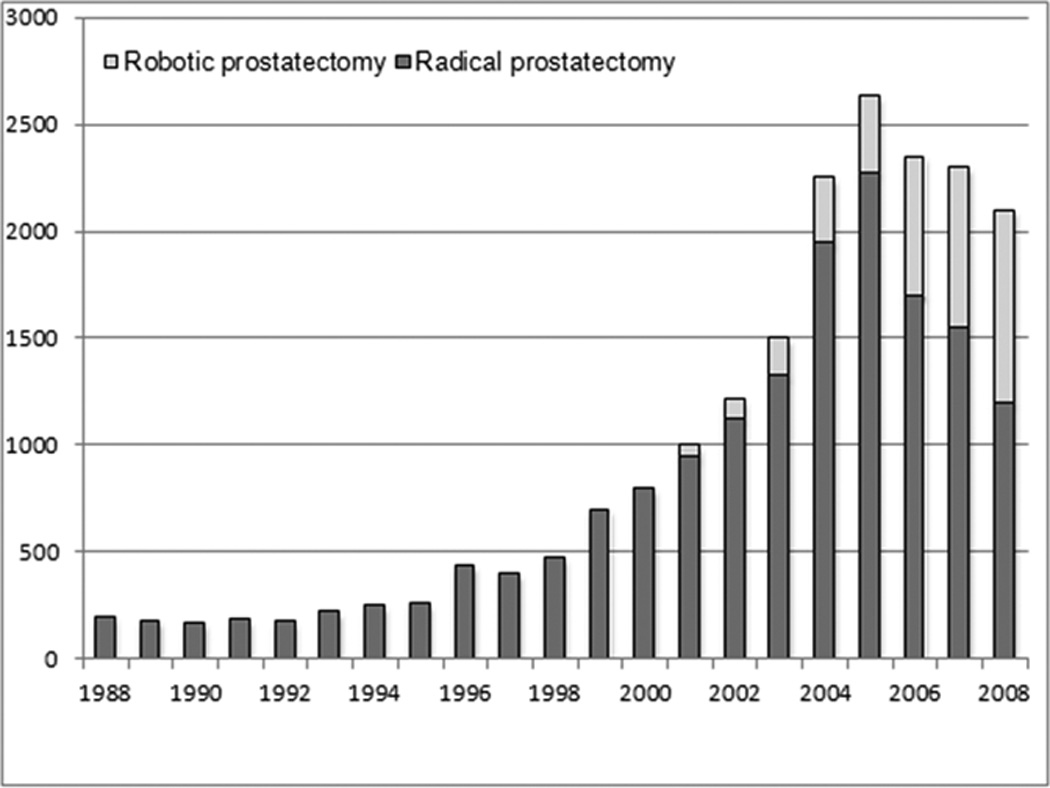
Number of radical prostatectomy procedures undertaken in Sweden during the study period, 1988 to 2008
Table 1.
The total number of radical prostatectomy procedures and deaths by calendar interval year of diagnosis and age at diagnosis, Sweden 1988 to 2008
| Age group | Year of diagnosis |
Total number |
Total number of deaths |
Number of prostate cancer deaths |
|---|---|---|---|---|
| <55 years | 1988–1992 | 62 | 26 | 14 |
| 1993–1997 | 183 | 37 | 18 | |
| 1998–2002 | 485 | 31 | 14 | |
| 2003–2007 | 1053 | 12 | 8 | |
| 55–64 years | 1988–1992 | 463 | 234 | 102 |
| 1993–1997 | 820 | 180 | 74 | |
| 1998–2002 | 2621 | 195 | 54 | |
| 2003–2007 | 6034 | 108 | 22 | |
| 65–74 years | 1988–1992 | 440 | 319 | 109 |
| 1993–1997 | 702 | 253 | 94 | |
| 1998–2002 | 1653 | 196 | 54 | |
| 2003–2007 | 4025 | 103 | 29 | |
| 75+ years | 1988–1992 | 85 | 83 | 31 |
| 1993–1997 | 73 | 69 | 31 | |
| 1998–2002 | 67 | 52 | 19 | |
| 2003–2007 | 71 | 22 | 13 | |
| Total | 18837 | 1920 | 686 | |
During a median follow-up of five years (range 0–22 years), 1920 of all 18,837 men in the cohort had died, of whom 686 (3.6 percent) died of prostate cancer and 1,234 (6.6%) from other causes. Table 1 presents the number of total and cancer-specific deaths, stratified by age at diagnosis and calendar interval of diagnosis. Figure 2 presents the cumulative incidence of prostate cancer death among men who underwent radical prostatectomy in Sweden. The five-year cancer-specific mortality decreased from 3.9% (95% CI 2.5–5.3) among patients diagnosed between 1988 and 1992 to 0.7% (95% CI 0.4–1.1) among those diagnosed between 1998 and 2002 (p for trend < 0.001). Compared with men who underwent radical prostatectomy in 1988 to 1992, those operated in 2003 to 2008 were at a 67% lower risk of dying from prostate cancer (Table 2). Compared with younger patients, men aged 65 to74 were at 62% higher and men aged 75 and older at 12.9-fold higher risk of prostate cancer death (Table 2). This trend likely reflects more restrictive use of radical prostatectomy among elderly men with low risk disease.
Figure 2.
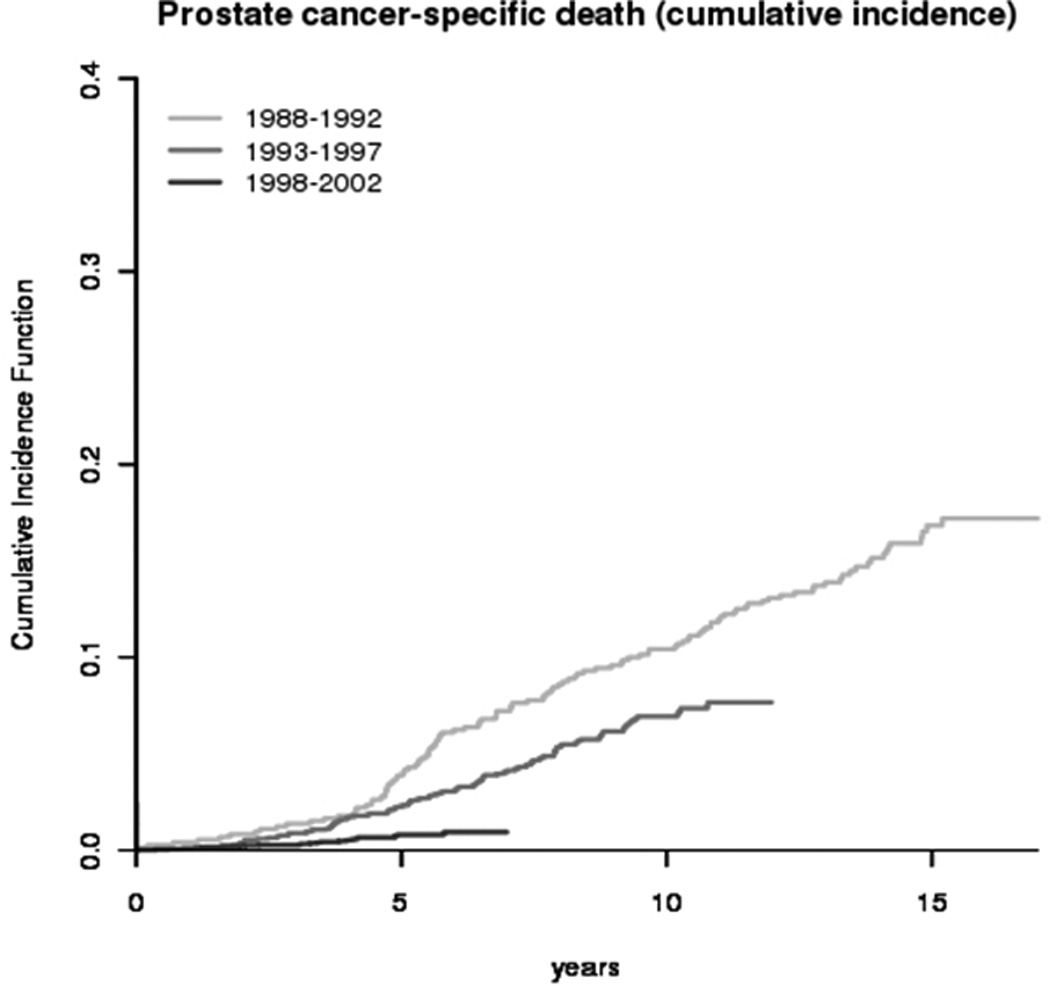
The cumulative incidence of prostate cancer death from diagnosis among men undergoing radical prostatectomy in Sweden, 1988 to 2008
Table 2.
Relative risk and 95% confidence intervals (CI) from a Cox proportional hazards model for prostate cancer death among 18,837 men who underwent radical prostatectomy in Sweden, 1988–2008
| Patient Characteristic |
Relative risk | 95% CI | P for trend |
|---|---|---|---|
| Calendar time | |||
| 1988–1992 | 1·00 | Ref | <0·001 |
| 1993–1997 | 0·75 | (0·64–0·89) | |
| 1998–2002 | 0·42 | (0·34–0·51) | |
| 2003–2008 | 0·33 | (0·25–0·42) | |
| Age group | |||
| <55 years | 1·00 | Ref | <0·001 |
| 55–64 | 0·98 | (0·75–1·28) | |
| 65–74 | 1·62 | (1·24–2·12) | |
| 75 and older | 12·94 | (9·52–17·59) |
The risk of other-cause death declined among patients diagnosed in later calendar years (figure not shown). The ten-year cumulative incidence of other-cause death was 14% (11.5%, 16.6%) among men diagnosed from 1988 to 1992, and 11.5% (9.7%, 13.3%) among men diagnosed from 1993 to 1997. This decline is particularly marked when comparing patients diagnosed before 1992 with those diagnosed thereafter; time to other-cause death does not improve noticeably after 1992. The decline in other-cause death is consistent with the trend towards younger ages at diagnosis and also with the well-documented “healthy screenee effect” (24).
The results of the cure model indicated that the likelihood of being cured by prostatectomy depends significantly on both age and year of diagnosis. Accounting for age at diagnosis, the risk of not being cured declined by 13% (95% CI 12–14%) with each calendar year. The risk of disease-specific death among those not cured, however, does not vary significantly with calendar interval.
Figure 3 presents the simulation model of the potential influence of PSA screening and lead-time bias on prostate cancer death. We plot the observed prostate-cancer specific survival, comparing the period before (<1992) and after (1992 or later) when opportunistic PSA-screening began to become wide-spread in Sweden. In addition, we plot the simulated survival in the PSA era, assuming lead-times of 5 to 15 years. The observed cancer-specific survival among men undergoing prostatectomy diagnosed before 1992 was substantially lower than among men diagnosed since PSA screening. The simulated cancer-specific survival improved after accounting for lead-time, but not to the levels of the PSA era. Even examining a lead-time of PSA screening of 15 years, the simulated survival was lower than the observed PSA-era survival. Figure 3 shows that even substantial lead times can only account for about half of the improvement in disease-specific survival.
Figure 3.
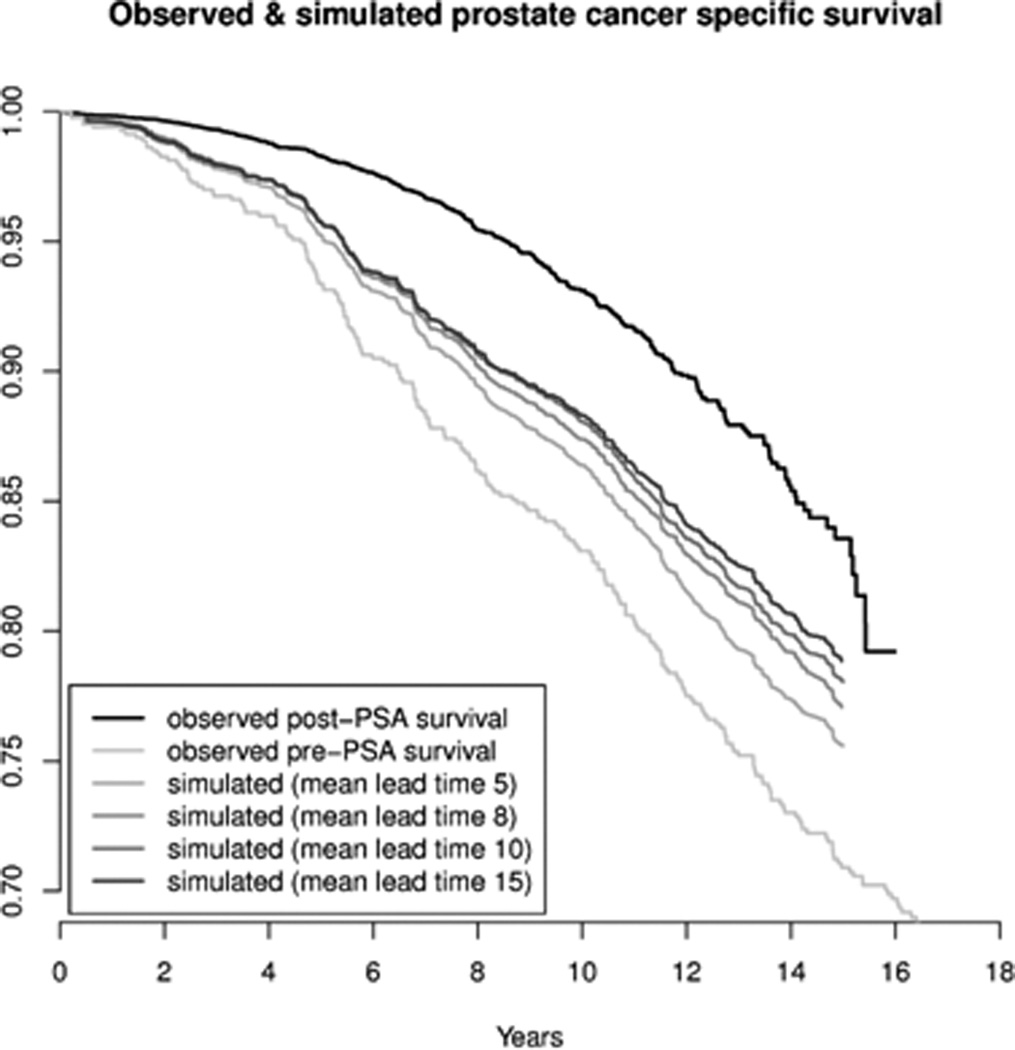
Observed and estimated prostate cancer-specific survival among men undergoing radical prostate cancer based on simulation models. The figure plots the observed prostate cancer survival among men before and after 1992, assuming introduction of PSA screening in 1993 and that 10% of cases in 1996 and 40% of cases in 2005 are detected by screening. Cases detected by screening are assigned a time to disease-specific death that is the sum of a lead-time with the specified mean and a disease-specific survival time that is generated based on the survival among cases diagnosed from 1988 to 1992. The simulated models examine mean lead times of 5, 8, 10 and 15 years.
Discussion
We set out to investigate whether trends in age at diagnosis, disease-specific and other-cause survival among men treated with radical prostatectomy in Sweden were consistent with increased diagnosis of nonlethal prostate cancers as a consequence of opportunistic PSA screening. It is now clear that even in the absence of a national policy, PSA screening in Sweden did increase over the interval of our study (9). We took advantage of healthcare registers in Sweden to identify a large, nationwide cohort of prostate cancer patients undergoing prostatectomy with virtually complete follow-up. Using several complementary analytical approaches, we found supportive evidence for our hypothesis that radical prostatectomy was increasingly used to treat non-lethal tumors. However, we could not conclusively link all of the apparent improvement in survival to overdetection and overtreatment.
Despite the fact that there was no official screening policy in Sweden during the years of our study, cases treated with prostatectomy during this time clearly exhibit features of a population in which early detection is becoming more frequent. These are: (1) a dramatic increase in disease-specific survival, (2) a decline in the average age at diagnosis and an increase in other-cause survival, and (3) a decrease in the uncured fraction over time. The results of the cure model, in particular, are consistent with the interpretation that the increase in radical prostatectomy may be largely driven by the overtreatment of patients who even without surgical intervention would probably have died from causes other than prostate cancer. We found that when fitting a cure model, the probability of cure increased dramatically with year of diagnosis whereas the survival among those not cured stayed relatively constant. This is consistent with the addition of indolent cases to the mix of diagnosed prostate cancers, since indolent cases have, by definition, no disease-specific mortality and, therefore, perfect disease-specific survival.
The findings of the simulation study are also consistent with the addition of screen-detected cases over time to the case population in the sense that they indicate that lead time associated with screening could account for about half of the observed improvement in disease-specific survival. However, we were not able to match observed increases in disease-specific survival without adding a real, post-lead-time survival improvement to the simulated survival times for early-detected cases. Note that our modeling of lead-time also allows for overdiagnosis, since cases in the simulation study can and do die of other causes within the generated lead time. Importantly, in this modeling exercise we expressly did not model any benefit of early detection or early treatment because we wanted to determine whether the apparently improved survival could be attributed solely to the addition of lead-time.
Our results are likely generalizable to other western populations with widespread PSA screening and use of prostatectomy for treatment of prostate cancer. Nevertheless, alternate interpretations of our data must be considered. Studies have suggested with more experience, surgical treatment achieves cure in a growing proportion of operated patients (25, 26). It is possible that our results could reflect improved outcomes of radical prostatectomy, or improved life expectancy due to the synergy of early detection and early treatment.
Defined as the time interval between detection through screening and the hypothetical time of clinical diagnosis, the lead-time due to PSA testing is at least 5 years (13, 23), but will vary depending on the background incidence of disease in the absence of screening and the screening protocol. The lead-time will be longer under more frequent screening and whenever a lower cutoff for biopsy referral is used because this will lead to cancer being detected earlier during the preclinical detectable phase. Even PSA-testing used for diagnostic purposes can induce a lead-time if it produces a disease diagnosis in advance of that occurring in the absence of PSA-testing. Longer lead times are associated with a greater frequency of overdiagnosis. However, our simulation results indicated that even lead-times at the high end of a plausible range could not fully explain the observed improvements in disease-specific survival given our best estimates of the prevalence of screen-detection in the Swedish population.
Overtreatment of non-lethal prostate cancer is of major concern because radical prostatectomy is associated with substantial costs and harms. Some loss of erectile function occurs in 80% of patients (14, 15, 27). In addition, up to 20% of all operated men will live with incontinence ranging from mild to severe during their remaining life-span, a condition with considerable impact on quality of life (27). Moreover, the average age at diagnosis has shifted considerably to younger men as a result of PSA screening, and thus men are living with these quality of life issues for a significantly longer time. Although radical prostatectomy might have prevented future obstructive symptoms in a few patients, such problems can usually be treated effectively with hormonal manipulation or transurethral resection. Costs to society and the health care system are considerable, and remain consistently higher following prostatectomy than with watchful management with no primary local treatment (28).
Clearly, indolent and lethal cancers need to be reliably separated allowing a much more selective use of radical prostatectomy among men who are likely to benefit. Although this is an area of much ongoing effort, validated molecular markers and signatures have yet to be implemented clinically (29). Together with the adoption of active surveillance protocols (30), this work promises to enhance our ability to identify and treat those men who will benefit the most from radical prostatectomy.
Statement of translational relevance.
Our findings have potentially profound translational relevance. It is now clear that a major surgical procedure is being used on an industrial scale for many patients who can experience only potentially severe side-effects but no survival benefit. Because the number of patients treated with radical prostatectomy is at least 2-fold higher in the US than in Sweden (after adjustment for population size), the proportion of patients with non-lethal disease is likely even larger than in our study. Consequently means to reduce overtreatment needs serious consideration. Active surveillance is becoming a more widely used alternative for low-risk cases and was recently endorsed by an NIH consensus panel as a preferred approach for managing low-risk cases. Optimal approaches for active surveillance need to be determined and patients and their clinicians need to be convinced that this provides a preferred approach in the case of newly-diagnosed, low-risk prostate cancer.
Acknowledgment
We thank David F. Penson for comments on an earlier draft of this work.
Grant Support
This work was supported by Karolinska Institutet, Distinguished Professor Award (grant # Dnr: 2368/10-221) and by Award Number U01CA157224 from the National Cancer Institute and the Centers for Disease Control.
References
- 1.Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini's plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC. Radical prostatectomy for the treatment of localized prostatic carcinoma. Urol Clin North Am. 1980;7:583–591. [PubMed] [Google Scholar]
- 3.Kvale R, Auvinen A, Adami HO, Klint A, Hernes E, Moller B, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–1887. doi: 10.1093/jnci/djm249. [DOI] [PubMed] [Google Scholar]
- 4.Adami HO. The prostate cancer pseudo-epidemic. Acta Oncol. 2010;49:298–304. doi: 10.3109/02841860903584945. [DOI] [PubMed] [Google Scholar]
- 5.Welsh H. Should I be tested for cancer? California: California University Press; 2004. [Google Scholar]
- 6.Cooperberg MR, Moul JW, Carroll PR. The changing face of prostate cancer. J Clin Oncol. 2005;23:8146–8151. doi: 10.1200/JCO.2005.02.9751. [DOI] [PubMed] [Google Scholar]
- 7.National Cancer Institute. Surveillance Epidemiology and End Results. SEER data. 1973–2008 Available from: http://seer.cancer.gov.
- 8.Bray F, Lortet-Tieulent J, Ferlay J, Forman D, Auvinen A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur J Cancer. 2010;46:3040–3052. doi: 10.1016/j.ejca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson H, Holmstrom B, Duffy SW, Stattin P. Uptake of prostate-specific antigen testing for early prostate cancer detection in Sweden. Int J Cancer. 2010;129:1881–1888. doi: 10.1002/ijc.25846. [DOI] [PubMed] [Google Scholar]
- 10.Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–248. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 11.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–2719. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 12.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Norlen BJ, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 15.Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol. 1984;23:305–313. doi: 10.3109/02841868409136026. [DOI] [PubMed] [Google Scholar]
- 17.Hansson LE, Nyren O, Hsing AW, Bergstrom R, Josefsson S, Chow WH, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 18.Godtman R, Holmberg E, Stranne J, Hugosson J. High accuracy of Swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scandinavian journal of urology and nephrology. 2011;45:226–232. doi: 10.3109/00365599.2011.559950. [DOI] [PubMed] [Google Scholar]
- 19.Fall K, Stromberg F, Rosell J, Andren O, Varenhorst E. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42:352–357. doi: 10.1080/00365590802078583. [DOI] [PubMed] [Google Scholar]
- 20.Corbiere F, Joly P. A SAS macro for parametric and semiparametric mixture cure models. Comput Methods Programs Biomed. 2007;85:173–180. doi: 10.1016/j.cmpb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Farewell VT. The use of mixture models for the analysis of survival data with long-term survivors. Biometrics. 1982;38:1041–1046. [PubMed] [Google Scholar]
- 22.Adolfsson J, Garmo H, Varenhorst E, Ahlgren G, Ahlstrand C, Andren O, et al. Clinical characteristics and primary treatment of prostate cancer in Sweden between 1996 and 2005. Scand J Urol Nephrol. 2007;41:456–477. doi: 10.1080/00365590701673625. [DOI] [PubMed] [Google Scholar]
- 23.Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, et al. Lead-time in the European Randomised Study of Screening for Prostate Cancer. Eur J Cancer. 2010;46:3102–3108. doi: 10.1016/j.ejca.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Weiss NS, Rossing MA. Healthy screened bias in epidemiologic studies of cancer incidence. Epidemiology. 1996;7:319–322. [PubMed] [Google Scholar]
- 25.Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. Journal of the National Cancer Institute. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 26.Klein EA, Bianco FJ, Serio AM, Eastham JA, Kattan MW, Pontes JE, et al. Surgeon experience is strongly associated with biochemical recurrence after radical prostatectomy for all preoperative risk categories. The Journal of urology. 2008;179:2212–2217. doi: 10.1016/j.juro.2008.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96:1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 28.Andersson SO, Andren O, Lyth J, Stark JR, Henriksson M, Adami HO, et al. Managing localized prostate cancer by radical prostatectomy or watchful waiting: Cost analysis of a randomized trial (SPCG-4) Scand J Urol Nephrol. 2011;45:177–183. doi: 10.3109/00365599.2010.545075. [DOI] [PubMed] [Google Scholar]
- 29.Sboner A, Demichelis F, Calza S, Pawitan Y, Setlur SR, Hoshida Y, et al. Molecular sampling of prostate cancer: a dilemma for predicting disease progression. BMC Med Genomics. 2010;3:8. doi: 10.1186/1755-8794-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ip SDI, Chung M, Yu WW, Balk EM, Iovin RC, Mathew P, Luongo T, Dvorak T, Lau J. An Evidence Review of Active Surveillance in Men with Localized Prostate Cancer. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [PMC free article] [PubMed] [Google Scholar]


