Abstract
A key deficit in alcohol dependence is disrupted prefrontal function leading to excessive alcohol seeking, but the molecular events underlying the emergence of addictive responses remain unknown. Here we show by convergent transcriptome analysis that the pyramidal neurons of the infralimbic cortex are particularly vulnerable for the long-term effects of chronic intermittent ethanol intoxication. These neurons exhibit a pronounced deficit in metabotropic glutamate receptor subtype 2 (mGluR2). Also, alcohol-dependent rats do not respond to mGluR2/3 agonist treatment with reducing extracellular glutamate levels in the nucleus accumbens. Together these data imply a loss of autoreceptor feedback control. Alcohol-dependent rats show escalation of ethanol seeking, which was abolished by restoring mGluR2 expression in the infralimbic cortex via viral-mediated gene transfer. Human anterior cingulate cortex from alcoholic patients shows a significant reduction in mGluR2 transcripts compared to control subjects, suggesting that mGluR2 loss in the rodent and human corticoaccumbal neurocircuitry may be a major consequence of alcohol dependence and a key pathophysiological mechanism mediating increased propensity to relapse. Normalization of mGluR2 function within this brain circuit may be of therapeutic value.
Introduction
The molecular and neuroanatomical substrates underlying substance use disorders including alcohol dependence remain poorly understood. Imbalances in glutamate neurotransmission and homeostasis are considered to play a central role for the increased propensity to relapse in addicted individuals (Everitt and Robbins, 2005; Kalivas, 2009; Spanagel, 2009). In particular, the glutamatergic corticoaccumbal pathway plays an essential role for reinstating drug-seeking behavior in animal models of relapse (Kalivas, 2009). It has been shown that lesions or inactivation of the medial prefrontal cortex (mPFC) or nucleus accumbens prevent reinstatement of drug seeking following extinction, while activation of either structure stimulates drug seeking (Cornish and Kalivas, 2000; Capriles et al., 2003; McFarland et al., 2004). Supporting this notion, human functional magnetic resonance imaging identified a positive correlation between cue reactivity, craving, and activity in prefrontocortical regions in addicted patients (Wilson et al., 2004; Schacht et al., 2013). A dysregulation of central glutamate levels in these areas during withdrawal and protracted abstinence was recently reported as well (Hermann et al., 2012a,b). Despite these findings on the role of the mPFC–accumbal pathway in relapse, relatively little is known about the molecular and cellular neuroadaptations within this circuit that result in susceptibility to relapse.
Here we set out to elucidate alcohol-induced dysregulation of mPFC function in rats with a history of alcohol dependence, i.e., by exposure to daily cycles of intermittent alcohol vapor intoxication and withdrawal, a paradigm that produces high intoxication with brain alcohol levels above 200 mg/dl and induces behavioral and molecular changes relevant for the pathophysiology of alcoholism in both rats and mice (Rogers et al., 1979; Roberts et al., 2000; Rimondini et al., 2002, 2003, 2008; Becker and Lopez, 2004; O'Dell et al., 2004; Hansson et al., 2008; Sommer et al., 2008; Melendez et al., 2012). Animals derived from this procedure are termed “postdependent” to emphasize the fact that neuroadaptations induced through a history of alcohol dependence remain even in the absence of continued ethanol intoxication. This phenomenon has been consistently demonstrated for a long-lasting behavioral sensitivity to stress and altered amygdala (Amy) gene expression (Funk et al., 2006; Heilig and Koob, 2007; Sommer et al., 2008; Vendruscolo et al., 2012). In this sense, postdependent animals may model the increased propensity to relapse in abstinent alcoholic patients (Björk et al., 2010; Heilig et al., 2010). We used a multilayered search strategy that started with an unbiased transcriptome screening of multiple brain regions and converged on a distinct neuronal population that exhibits a profound metabotropic glutamate receptor subtype 2 (mGluR2) deficit. This receptor belongs to the Class II metabotropic glutamate receptors (mGluR2/3) that are key to regulating glutamatergic neurotransmission in brain regions mediating drug seeking and incentive motivation, including the mPFC–accumbal pathway (Ohishi et al., 1993; Olive, 2009). mGluR2/3 negatively modulate glutamate transmission as autoreceptors by inhibiting glutamate release and by reducing neuronal excitability at the postsynaptic level (Ferraguti and Shigemoto, 2006). Dysregulation of mGluR2/3 function within the mPFC–accumbal pathway has been found after withdrawal from chronic exposure to cocaine, nicotine, and opioids (Liechti and Markou, 2007; Moussawi et al., 2009; Olive, 2009). Here we found that the mGluR2 autoreceptor function is specifically disrupted after a history of alcohol dependence, which allowed us to develop a rescue strategy for restoring behavioral control in alcohol-dependent rats by focal mGluR2 overexpression.
Materials and Methods
Animal husbandry.
Male Wistar rats, initial weight 220–250 g, were used (Charles River), housed four per cage under a 12 h light/dark cycle with ad libitum access to food and water. All behavioral testing was performed during the dark phase, 5 d per week. All experiments were conducted in accordance with the ethical guidelines for the care and use of laboratory animals and were approved by the local animal care committee (Regierungspraesidium Karlsruhe, Karlsruhe, Germany). Five batches of animals were uniformly treated with either intermittent alcohol vapor or air exposure: Batch 1, n = 10 per group for microarray and n = 8 per group for in situ hybridization; Batch 2, n = 8 per group for laser-capture microscopy (LCM) study; Batch 3, n = 8 per group for microdialysis; Batches 4 and 5, n = 8 and 16 per group for operant self-administration experiments, respectively.
Ethanol exposure.
Rats were weight-matched, assigned into the two experimental groups, and exposed to either ethanol vapor or normal air using a rodent alcohol inhalation system as described previously (Rimondini et al., 2002). Briefly, pumps (Knauer) delivered alcohol into electrically heated stainless-steel coils (60°C) connected to an airflow of 18 L/min into glass and steel chambers (1 × 1 × 1 m). For the next 7 weeks rats were exposed to five cycles of 14 h of ethanol vapor per week (0:00 A.M. to 2:00 P.M.) separated by daily 10 h periods of withdrawal. Twice per week, blood (∼20 μl) was sampled from the lateral tail vein, and blood alcohol concentrations were determined using an AM1 Analox system (Analox Instruments). After the last exposure cycle, rats remained abstinent for 2–3 weeks before further entering further experiments (3 weeks for gene expression and microdialysis analysis, 2 weeks for resumption of operant training).
Measurement of ethanol withdrawal signs.
Using a withdrawal rating scale according to Macey et al. (1996), alcohol withdrawal signs including irritability to touch (vocalization), body tremors, tail rigidity, and ventromedial limb retraction were weekly scored, 6 h after ethanol vapor was turned off. Each sign was assigned a score of 0–2, based on the following severity scale: 0, no sign; 1, moderate; 2, severe. The sum of the four observation scores (0 to 8) was used as a quantitative measure of withdrawal severity. For these behavioral observations, animals were individually transferred from their home cages to a quiet observation room to avoid extraneous stimulation, and animals were observed in a blind fashion.
Rat brain tissue samples and microarray experiment.
Three weeks after the last exposure cycle, postdependent (alcohol exposed, n = 10) and control (air exposed, n = 10) animals were killed during the first 4 h of the light cycle by decapitation, and brains were frozen in −40°C isopentane and kept at −80°C. Bilateral samples were obtained under a magnifying lens using anatomical landmarks (Paxinos and Watson, 1998). Amygdala, nucleus accumbens, and medial prefrontal cortex including Cg1 + 2, prelimbic cortex, and infralimbic cortex) according to Paxinos and Watson (1998) were prepared as described previously (Arlinde et al., 2004). Briefly, amygdala was prepared from a 2 mm-thick-coronal slice, taken in a Kopf brain slicer by placing the rostral blade on the caudal edge of the optic chiasm. For preparation of cingulate cortex and accumbens, the rostral blade was placed 4 mm rostral to this landmark, and a second 2 mm coronal slice was obtained. Cortical tissue was dissected out with a scalpel, while amygdala and accumbens tissues were obtained using a punch (2 mm diameter). Samples were stored at −80°C until RNA was prepared.
Total RNA was extracted with Trizol reagent (Invitrogen) followed by an RNeasy (Qiagen) column-based cleanup step according to the manufacturer's instructions. All RNA samples showed A260/280 absorption ratios between 1.9 and 2.1. RNA integrity was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies), and only material without signs of degradation was used.
Microarray target preparation was done for individual samples and hybridization to RAE230A arrays, staining, washing, and scanning of the chips were performed according to the manufacturer's technical manual (Affymetrix). The Microarray Analysis Suite 5.0 (MAS5)-produced CEL files were inspected for regional hybridization bias and quality control parameters as described previously (Reimers et al., 2005). Forty-eight microarrays (mPFC, 9 and 9; accumbens, 7 and 7; amygdala, 7 and 9, postdependent and control rats, respectively) passed quality control. The MAS5 recognized ∼60% of the 15 800 probe sets on the RAE230A array as present in our samples. Robust multichip average expression values were obtained and tested for differential gene expression using Welch's two-sample t test, assuming unequal variances at a p < 0.05 threshold. The microarray CEL files were imported into gene set enrichment analysis (GSEA) software, available at www.broadinstitute.org/gsea/, and gene set enrichment analysis was performed against gene sets for glutamatergic and GABAergic neurons described by Sugino et al. (2006) (Table 1).
Table 1.
Gene sets for glutamatergic and GABAergic neurons
| Glutamatergic genes | GABAergic genes | |
|---|---|---|
| Adora1 | Kpna1 | Abat |
| Ak3l1 | Lmo4 | Capza1 |
| Ap1gbp1 | Lmo7 | Cds2 |
| Arpc5 | Mast3 | Cygb |
| Arpp21 | Neurod2 | Gad1 |
| Baiap2 | Nphp1 | Gad2 |
| Cpd | Nrgn | Grik1 |
| Crip2 | Nupl1 | Kcnc1 |
| Crym | Ppp3ca | Klhl13 |
| Dgat2 | Ptk2 | Ltbp3 |
| Dusp14 | Ptk2b | Map3k1 |
| Egr4 | Rap2b | Paip2 |
| Ensa | Rin1 | Pcaf |
| Ets2 | Srr | Pcp4l1 |
| Fhl2 | St3gal5 | Pdxk |
| Galntl1 | Stx1a | Ppp3cb |
| Gpm6b | Synpo | Ptprm |
| Gria2 | Tesc | Rpp25 |
| Hebp1 | Tjp1 | Slc32a1 |
| Igfbp6 | Tyro3 | Slc6a1 |
| Itpka | Zfp179 | Socs5 |
| Klf10 | Zfp238 | Sv2a |
| Klhl2 | Txnl1 | |
Gene sets were taken from Sugino et al. (2006) who found extremely divergent expression profiles from GABA-ergic interneurons and glutamatergic pyramidal neurons. p values for top candidates ranged from a maximum of 1.5 × 10−11 to 1.8 × 10−27 (GABAergic versus glutamatergic population). Only genes that had rat homologes and were present on the Affymetrics arrays were used. Significant genes in the microarray experiment from mPFC are in bold letters.
Human brain tissue.
Human brain tissue samples were obtained from the New South Wales Tissue Resource Centre at the University of Sydney, Australia (http://www.pathology.usyd.edu.au/trc.htm). Tissue from 30 male subjects of European descent consisting of 15 chronic alcoholics and 15 control cases was used for this study. Subject affiliation to the alcoholics or control group was confirmed postmortem using the Diagnostic Instrument for Brain Studies–Revised, which is consistent with the criteria of the Diagnostic and Statistical Manual for Mental Disorders, fourth edition (DSM-IV) (American Psychiatric Association, 1994). All alcoholics had consumed >80 g of ethanol per day, whereas the control cases had an average daily consumption of <20 g. To reduce the number of confounding factors, we tried to not include any subjects where the cause of death was suicide, the postmortem interval was >40 h, or blood alcohol or significant amounts of psychiatric medication (concentration >1.0 mg/L) was detected at the autopsy whenever possible. For each subject, we analyzed tissue samples from the anterior cingulate cortex.
RNA extraction and analysis was done as described previously (Sommer et al., 2010). RNA from brain tissue was isolated using Trizol according to manufacturer's protocol (Invitrogen). RNA samples underwent a cleanup step using the RNeasy Mini Kit (Qiagen) and were then treated with RQ1 RNase-free DNase (Promega) following manufacturer's instructions, to eliminate DNA contamination. All RNA samples had acceptable 260/280 ratios (1.8–2.1). RNA samples were then analyzed with an Agilent 2100 Bioanalyzer and the RNA integrity number. RNA (100 ng) was used for cDNA synthesis using reverse transcription reagents according to the manufacturer's protocol (Applied Biosystems). For the quantitative real-time (qRT)-PCR method, see below, Quantitative RT-PCR from micropunched, amplified, and human tissue. In addition to GAPDH, we used ALUSX as a second endogenous control. Results were similar for both reference genes.
Stereotaxic injections.
For stereotaxic injections of the retrograde tracer (n = 8 per group), rats were anesthetized (isofluran) and placed in a Kopf stereotaxic instrument, and 300 nl of rhodamine-labeled fluorescent latex microspheres (Lumafluor) were delivered to the nucleus accumbens shell at 70 nl/min using a WPI microinjectionpump through a 33 gauge beveled needle. The stereotaxic coordinates for the injections (Wistar rats, 500 g) were +1.8 mm AP, +0.9 mm ML, and −7.5 mm DV relative to bregma. Following surgery, rats were single housed for 2 d. After a 7 d recovery period, rats were euthanized for tissue collection as described below.
For lentiviral injections, rats received 600 nl of either Lenti-control or Lenti-mGluR2 to bilaterally into the infralimbic cortex at 70 nl/min. The stereotaxic coordinates for the injections (500 g Wistar rats) were +3.2 mm AP, ±0.52 mm ML, and −5.1 mm DV relative to bregma.
Gene expression analysis of infralimbic projection neurons via qRT-PCR.
Rats recovered for 1 week following stereotaxic tracer delivery. For perfusions, rats were anesthetized (ketamin/xylazin, 100/5 mg/kg, i.p.) and transcardially perfused with ice-cold 50 ml PBS followed by 80 ml 0.5% paraformaldehyde (PFA) containing 20% sucrose. After perfusions, brains were removed and flash frozen in −40°C isopentanol and stored at −80°C up to 72 h before sectioning.
Frozen brains were cut into 12-μm-thick coronal sections with a cryostat. Sections were mounted on PALM membrane slides and kept at −80°C and process at the same day. Just before LCM, slides were thawed to −25°C; rapidly trimmed of tissue tech; dehydrated with 75% EtOH (30s), 95% EtOH (30s), 100% EtOH (30s), and xylene (1 min); and then air-dried for 5 min and immediately used for LCM.
LCM was performed using a Zeiss PALM laser system. Tracer-labeled cells were identified using a CY3 advanced filter cube (excitation, bandpass 546/12; emission, bandpass 575–640). The laser focus followed a circular trajectory of 8–10 μm in diameter to cut out and separate tracer positive cells from the adjacent tissue, following a final slightly subfocal laser pulse to catapult the cell into an LCM cup. Laser-targeted cells were bonded to adhesive LCM caps by aiming the laser beam at the thin plastic sheet in the cap directly above the target cell. Per animal, ∼70–100 cells were collected.
RNA was extracted with the RNeasy Micro kit for microdissected cryosections. All steps were performed according to the manufactures recommendations. A speed vac (Vacufuge 2015727; Eppendorf) was used to dry down the eluted RNA to 3 μl for the further amplification step. Total RNA was amplified using the TargetAmp 2-Round aRNA Amplification Kit 2.0 (Epicenter) according to manufacturer's recommendations. We typically obtained 2–5 μg of amplified RNA after the second amplification round. Amplifications were performed from six exposed and seven control tracer cell RNA extractions.
Quantitative RT-PCR from micropunched, amplified, and human tissue.
RNA (100 ng total) was reverse transcribed using the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems) following the manufacturer's protocol. Samples were assayed in triplicate in a total reaction volume of 20 μl using Power SYBR Green PCR Master Mix (Applied Biosystems) on an Applied Biosystems 7900 HT RT-PCR System (40 cycles of 95°C for 15 s and 60°C for 1 min). A melting profile was recorded at the end of each PCR to check for aberrant fragment amplifications. Primers for each target were designed toward the 3′ end of the coding sequence by considering exon–exon junctions when possible, based on the National Center for Biotechnology Information reference sequence database. Amplicons were designed with 95–110 bp length and melting temperatures >75°C to be able to distinguish between amplicons and primer-dimer formations in the melting analysis. For primer sequences, see Table 2. The Applied Biosystems SDS 2.2.2 software was used to analyze the SYBR Green fluorescence intensity and to calculate the theoretical cycle number when a defined fluorescence threshold was passed (Ct values). Relative quantification was done according to the 2-ΔΔCT method, whereby Actin β (Actb) was used as internal normalizer for rat tissue and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for the human tissue. The 2-ΔΔCt method is defined such that a cycle (Ct) is the cycle at which there is a significant detectable increase in fluorescence; the ΔCt value is calculated by subtracting the Ct value for the endogenous control from the Ct value for the mRNA of interest. The ΔΔCt value is calculated by subtracting the ΔCt value of the control sample from the ΔCt of the experimental sample. For graphical interpretation, the ΔΔCt values were transformed (−x); thus downregulated genes show ΔΔCt values <0 and upregulated genes show ΔΔCt values >0. The −ΔΔCt values were compared by an unpaired t test for each gene (p < 0.05 considered significant). Actb and GAPDH Ct values were not different between groups. Statistical testing was done by t test on the ΔΔCT values. The software and ΔΔCT method was used to determine statistical significance. Melting curves for all primers used in this study exhibited single fluorescence change peaks at the appropriate melting temperatures. This indicates the absence of primer-dimer formation.
Table 2.
Primer sequences used of QRT-PCR
| Species | Name | RefSeq ID | Forward primer | Reverse primer |
|---|---|---|---|---|
| Rat | Actb | NM_031144.2 | AGCCATGTACGTAGCCATCCA | TCTCCGGAGTCCATCACAATG |
| Rat | Crym | NM_053955 | CATCTGGCAAGTGAGCAGAA | GGGACTAGGCCTCCTTTGAT |
| Rat | Egr1 | NM_012551.2 | CAGGAGTGATGAACGCAAGA | GGGATGGGTAGGAAGAGAGG |
| Rat | Egr2 | NM_053633 | TCCGAGTTCTGAACCTTTGG | GGACACTTGCAACACCACTG |
| Rat | Egr4 | NM_019137.1 | CACAGAGCAGGCGATACCTT | ACATCCCCAGCTTGACTCTG |
| Rat | Grm2 | NM_001105711.1 | GTGAGGTGGTGGACTCAACA | CGTGGATGAGGGTCTATGCT |
| Rat | Gria3 | NM_032990.2 | ACTGAAAACGTGGCTGCTTC | GAAAGGTCATTGCACCATCA |
| Rat | Nr4a1 | NM_024388.1 | CCTCATTCCAGAAGATGGACA | TGAGCTGGGAGGGATAAGAG |
| Rat | Nr4a3 | NM_017352 | TACGGAGTCCGCACCTGCGA | CGACGTCTCTTGTCTACCGGGC |
| Rat | Slc1a3 | NM_019225.1 | TGGGCCTGCCCACGGATGA | CCCGGCCCCGAGGGAG |
| Human | Gapdh | NM_002046.4 | CATGAGAAGTATGACAACAGCCT | AGTCCTTCCACGATACCAAAGT |
| Human | Grm2 | NM_001130063.1 | CGCCGCCTCTACAAGGACT | GGCCAATACCATCACCAAAG |
In situ hybridization.
Riboprobes and in situ hybridizations were performed as described previously (Hansson et al., 2008). In a parallel batch of animals to the microarray, postdependent (alcohol exposed, n = 8) and control (air exposed, n = 8) animals were killed by decapitation during the first 4 h of the light phase, and brains were frozen in −40°C isopentane and kept at −80°C. Coronal brain sections (10 μm) were cryosectioned at forebrain bregma levels +3.0 mm and +2.0 mm. The rat-specific riboprobes for all genes were generated based on the gene reference sequence in the PubMed database (http://www.ncbi.nlm.nih.gov/Entrez): Egr-1, position 1384 to 1851 bp on rat cDNA (gene reference number, NM_012551.1); mGluR2, position 1327 to 1620 bp on rat cDNA (gene reference number, XM_343470.1); mGluR3, position 314 to 662 bp on rat cDNA (gene reference number, XM_342626.1); NMDA receptor 2a, position 434 to 876 bp on rat cDNA (gene reference number, RATNMDA2A); NMDA receptor 2b, position 205 to 591 bp on rat cDNA (gene reference number, NM_012574.1).
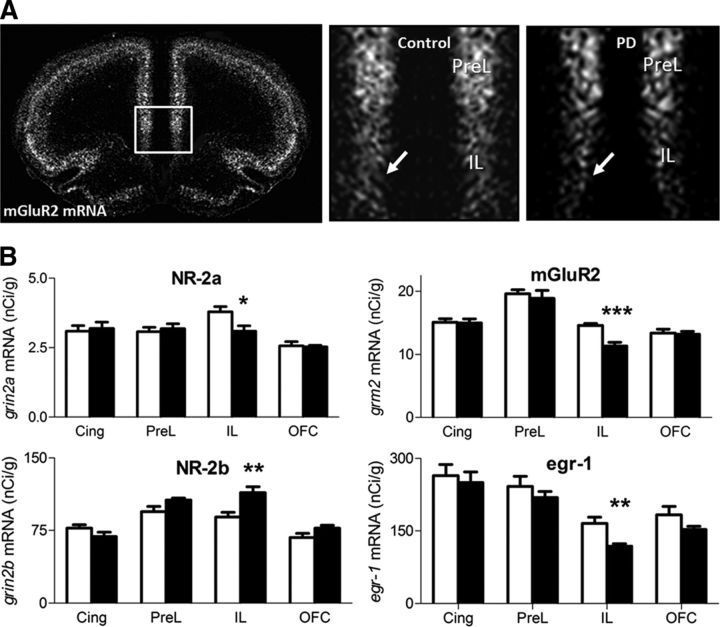
Phosphor imager-generated (Fujifilm Bio-Imaging Analyzer Systems) digital images were analyzed using MCID Image Analysis Software (Imaging Research). Regions of interest were defined by anatomical landmarks as described in the atlas of Paxinos and Watson (1998) and illustrated in Figure 2. Based on the known radioactivity in the 14C standards, image values were converted to nanocuries per gram.
Figure 2.
Prefrontal in situ hybridization point to the infralimbic cortex as major site of neuroadaptations. A, Dark-field microphotographs from autoradiograms of in situ hybridization of Grm2 from control and postdependent (PD) rats on coronal sections, +2.2 mm relative to bregma level. Enlarged images for both groups on the right. Arrows indicate signal in the infralimbic region. B, Quantification of in situ expression levels (nanocuries per gram, mean ± SEM) in postdependent (black bars) versus control rats (white bars) for selected candidate genes significantly altered. Nr-2a, Nr-2b, Egr1, and Grm2 mRNAs are robustly altered within the infralimbic cortex, but unaffected in cingulate, prelimbic, or orbitofrontal cortex. *p < 0.05; **p < 0.01; ***p < 0.001 comparing postdependent versus control (Student's t test). Cing, Cingulate cortex; PreL, prelimbic cortex; IL, infralimbic cortex; OFC, orbitofrontal cortex.
Microdialysis and assay of microdialysate glutamate levels.
Three weeks after ethanol exposure, rats weighed 450–550 g for surgery and were housed in groups of four before and individually after surgery. Rats were anesthetized (isofluran, 1.5–2%) and placed in a stereotaxic frame (Kopf Instruments). CMA11 guide cannula (20 gauge, 14 mm; CMA Microdialysis) were unilaterally implanted 2.0 mm above the nucleus accumbens shell (+1.6 mm AP, ±0.8 mm ML, and 5.6 mm DV). Coordinates were based on bregma, midline, and dura, respectively (Paxinos and Watson, 1998). Cannulas were anchored with three stainless-steel screws and dental acrylic. Animals were allowed to recover from surgery for 1 week.
Microdialysis experiments were conducted in conscious, freely moving rats, 3 weeks after last ethanol vapor exposure. Dialysis probes (CMA11 2 mm; CMA Microdialysis) with 2 mm active membrane were introduced into the guide cannula 12 h before the beginning of the dialysis experiments to minimize damage-induced release of neurotransmitters and metabolites. Each animal participated in one only microdialysis experiment. Samples were collected every 15 min at a flow rate of 1.5 μl/min. After 3 baseline samples, rats were injected with a saline solution as a control. Thirty minutes later rats were injected intraperitoneally with 3 mg/kg mGluR2/3 agonist LY379268 ((1R,4R,5S,6R)-4-amino-2-oxabicyclo[3.1.0]hexane-4,6-dicarboxylic acid), and sampling continued for the remaining time of the experiment.
Eight microliters of ortho-pthaldialdehyde/N-isobutyryl-l-cysteine solution (from Calbiochem and Fluka, respectively) were added to 20 μl microdialysate or standard volume. After three times mixing and a reaction time of 3 min, 14 μl were injected (CTC PAL autosampler; Axel Semrau) onto a HPLC column (Waters Xbridge C18 3.5 μm 10/2.1 mm guard cartridge and Waters Xbridge C18 3.5 μm 100/2.1 mm). The mobile phase consisted of 50 mm Na2HPO4, 1 mm Na-EDTA, 20% methanol, pH 6.5, with phosphoric acid. Flow rate was set to 0.3 ml/min (Rheos flux pump; Axel Semrau). Between every single injection, the system was flushed with 20 μl acetonitrile. Glutamate was measured via a fluorescence detector (L-7480; Merck). The system was calibrated by standard solutions of glutamate containing 10 pmol/10 μl per injection. Glutamate was identified by its retention time and peak height with an external standard method using chromatography software (Chrom Perfect; Justice Laboratory Software).
Immunohistochemistry.
Rats were killed by transcardial perfusion with 0.9% saline (w/v) followed by 4% PFA (w/v) in 1× PBS. Brains were postfixed in 4% buffered PFA at 4°C for 12 h, dehydrated in 1× PBS-sucrose (10%) solution for 3–7 d, and flash frozen at −80°C. Sections (14 μm) were cut with a cryostat, cycled with an ImmunoPen, washed one time for 5 min (200 μl of 0.01 m PBS, pH 7.4 on the sections), and air dried. Sections were incubated with primary antibody in diluted 0.01 m PBS, pH 7.4, plus 0.3% Triton X-100 at 4°C overnight, followed by appropriate secondary antibodies (diluted with 0.01 m PBS, pH 7.4, plus 0.03% Triton X-100) for 1 h at room temperature. All antibodies were tested for optimal dilution, the absence of cross-reactivity, and nonspecific staining. To detect the enhanced GFP, we used rabbit eGFP, diluted 1:500 (Invitrogen), as primary antibody and donkey-anti-rabbit Alexa 488, diluted 1:600 (Invitrogen), as secondary antibody. For visualization of the mGluR2 we used a mouse-mGluR2, diluted 1:500 (Santa Cruz Biotechnology), as primary antibody and the donkey-anti-mouse 594, diluted 1:800 (Invitrogen), as secondary antibody.
Operant alcohol self-administration apparatus.
All alcohol-seeking experiments were performed in operant chambers (MED Associates) enclosed in ventilated sound-attenuating cubicles. The chambers were equipped with a response lever on each side panel of the chamber. Responses at the appropriate lever activated a syringe pump that delivered a ∼30 μl drop of fluid into a liquid receptacle next to it. A light stimulus (house light) was mounted above the right response lever of the self-administration chamber. An IBM-compatible computer controlled the delivery of fluids, presentation of stimuli, and data recording.
The reinstatement protocol used in the present report is the one that was used by Ciccocioppo et al. (2002) with a slight modification, i.e., a syringe pump delivered a ∼30 μl drop of fluid into a liquid receptacle as opposed to 100 μl drop of fluid in the Ciccocioppo protocol. This modification markedly increased responding for alcohol (approximately fivefold), which allowed us to better monitor animals' motivation to receive alcohol.
Alcohol self-administration training.
All animal training and testing sessions were performed during the dark phase of their light cycle. Animals were trained to self-administer 10% (v/v) ethanol in daily 30 min sessions using a fixed-ratio 1 (FR1) schedule using Samson's sucrose-fading procedure (Tolliver et al., 1988). During the first 3 d of training, animals were kept fluid deprived for 20 h per day. Responses at the left lever were reinforced by the delivery of 0.2% (w/v) saccharin solution. For the next 3 d, animals underwent the same procedure without fluid deprivation. Following acquisition of saccharin-reinforced responding, rats were trained to self-administer ethanol. During the next three sessions, responses at the left lever resulted in the delivery of 0.03 ml of 5% (v/v) ethanol plus 0.2% saccharin solution. Responses at the left lever were recorded but had no programmed consequences. Thereafter, the concentration of ethanol was increased first to 8% and then to 10% v/v, and the concentration of saccharin was decreased until saccharin was eliminated completely from the drinking solution.
Conditioning phase.
The purpose of the conditioning phase was to train the animals to associate the availability of ethanol with the presence of specific discriminative stimuli. This phase started after the completion of the saccharin-fading procedure. Discriminative stimuli predicting ethanol (10%) availability were presented during each subsequent daily 30 min session. An orange flavor extract served as the cue stimulus for ethanol. This olfactory stimulus was generated by depositing six drops of an orange extract into the bedding of the operant chamber before each session. In addition, each lever press resulting in ethanol delivery was accompanied by a 5 s blinking conditioned light stimulus (CS). The 5 s period served as a “time-out,” during which responses were recorded but not reinforced. At the end of each session, the bedding of the chamber was changed, and trays were thoroughly cleaned. The animals received a total of 10 ethanol conditioning sessions. Throughout the conditioning phase, responses at the right lever were recorded but not reinforced (inactive lever). After the final conditioning phase, rats were sorted into two balanced experimental groups of which one was exposed to alcohol vapor (resulting in the postdependent group) and the other received normal air (control group).
Conditioning and extinction phase in postdependent rats.
Following a 2 week abstinence phase, all animals were reconditioned to self-administer 10% ethanol in 10 daily conditioning sessions. After completing the reconditioning phase, rats were subjected to daily 30 min extinction sessions for 12 consecutive days, which in total were sufficient to reach the extinction criterion of <10 lever responses per session. Extinction sessions began by extending the levers without presenting olfactory discriminative stimuli. Responses at the previously active lever activated the syringe pump, without resulting in the delivery of ethanol or the presentation of response-contingent cues (stimulus light).
Reinstatement testing.
For reinstatement, animals were divided into two groups per condition (control and postdependent) on the basis of their performance during the last four retraining sessions. After the last extinction trial, animals received bilateral stereotaxic injections in the infralimbic cortex (for details, see above, Stereotaxic injections). Reinstatement began 7 d after the final extinction session. In these tests, rats were exposed to the same conditions as during the conditioning phase, except that the ethanol was not available. Sessions were initiated by the extension of both ethanol-associated and inactive levers and the presentation of the discriminative stimulus predicting ethanol. Responses at the ethanol-associated lever were followed by the activation of the syringe pump without any ethanol delivery and the presentation of the CS (light).
Generation of Lenti-mGluR2 vector.
The mGluR2 cDNA was amplified using the IMAGp998E1215366Q clone as a template (Imagenes). After purification, the cDNA was cloned into the pCDH-MCS-T2A-copGFP vector (BioCat). The vector containing the mGluR2 insert was purified, sequenced, tested in cell culture, and finally used for lentiviral production. Active lentiviral particles were produced by System Biosciences.
Statistics.
Microarray, PCR and in situ hybridization data were compared by t test. Data from the microdialysis experiment were analyzed using two-way repeated-measures ANOVA. Behavioral experiments were analyzed by two-way ANOVA or t test where appropriate. Post hoc testing was done with Fisher LSD test. The withdrawal scores were compared using a Mann–Whitney test. Statistical significance was set at a p < 0.05. Statistica 10.0 software for Windows was used (StatSoft).
Results
Gene expression analysis in ethanol responsive brain regions point to the infralimbic region
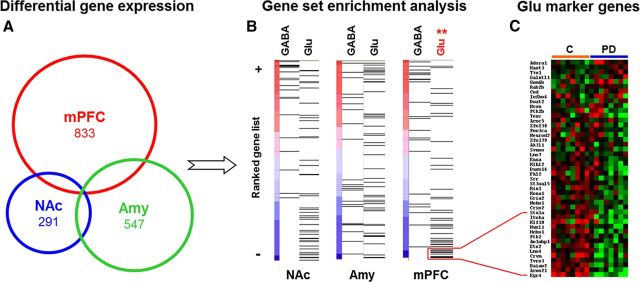
We started with an unbiased transcriptome analysis to determine potential targets of alcohol-induced neuroadaptations, classified the affected cell types in the region, and identified candidate genes for further experiments. Microarray-based transcriptome analysis revealed that chronic intermittent alcohol exposure had long-term effects on gene expression in three brain regions implicated in drug dependence, namely, mPFC, nucleus accumbens, and amygdala (Koob and Volkow, 2010) (Fig. 1A). We used GSEA (Subramanian et al., 2005) to test the hypothesis of functionally related postdependent neuroadaptations in GABAergic or glutamatergic neurons. For this purpose, we used two marker gene sets described previously as extremely divergent between GABAergic and glutamatergic neurons (Sugino et al., 2006). Results indicate a highly significant enrichment of downregulated glutamatergic marker genes (p < 0.01) in the mPFC of postdependent rats (Fig. 1B,C; Table 1). We selected a number of candidate genes for corroborative analysis by quantitative PCR (Table 3). Among the confirmed candidates was Grm2, the gene coding for mGluR2, which was robustly downregulated in the mPFC of postdependent rats compared to controls. We next used in situ hybridization to address the question whether or not a specific subregion of the mPFC is preferentially affected in postdependent rats. Several genes derived from the transcriptome study, i.e., members of the activity-dependent Egr-family (Egr1 and Egr2) and glutamate receptors (Nr-2a, Nr-2b, Grm2) showed significant downregulation only in the infralimbic cortex, with the most profound effect again the gene for mGluR2 (Fig. 2A,B). In contrast, the expression of the pharmacologically highly similar mGluR3 was not altered in this region (mean nanocuries per grams ± SEM; infralimbic cortex, control, 40.80 ± 2.13; postdependent, 41.59 ± 2.19; not significant; prelimbic cortex, control, 51.49 ± 1.75; postdependent, 53.06 ± 1.09; not significant). Together, these findings suggest that the infralimbic cortex is a hot spot within the mPFC for alcohol dependence-induced alterations.
Figure 1.
Expression analysis from three brain regions of postdependent (PD) rats and controls showing a distinct downregulation of glutamatergic marker genes in mPFC. Samples from mPFC, nucleus accumbens (NAc), and Amy of postdependent rats and controls were processed on Affymetrix GeneChip arrays. A, Venn diagram showing the number of significant differently expressed genes in each region. B, GSEA shows significant downregulation of an a priori defined set of glutamatergic marker genes in the mPFC, but not in the other regions. Each line corresponds to a gene of the respective set and is positioned according to its ranked effect size among all analyzed genes on the microarray (for gene sets, see Table 1). NAc-GABA, Normalized enrichments score, 1.4159; nominal p value, 0.0819; false discovery rate (FDR) q value, 0.1082; NAc-GLU, normalized enrichments score, −1.2078; nominal p value, 0.2218; FDR q value, 0.2596; Amy-GABA, normalized enrichments score, −0.6877; nominal p value, 0.8511; FDR q value, 0.8632; Amy-GLU, normalized enrichments score, 0.9564; nominal p value, 0.4991; FDR q value, 0.5629; mPFC-GABA, normalized enrichments score, 1.4622; nominal p value, 0.0489; FDR q value, 0.1259; mPFC-GLU, normalized enrichments score, −1.6227; nominal p value, 0.0; FDR q value, 0.0021. **p < 0.01 (corrected). C, Heat map showing the expression of the glutamatergic marker genes postdependent and control rats. Thirteen of 45 genes of the set are significantly downregulated in postdependent rats (p < 0.05). Red shows higher and green shows lower expression compared to the mean of all samples.
Table 3.
QRT-PCR validation of selected candidate genes from micropunched tissue and IL projection neurons cells
| Gene | Gene title | Microarray bulk |
qRT-PCR bulk |
qRT-PCR IL neurons |
|||
|---|---|---|---|---|---|---|---|
| p | FC | p | FC | p | FC | ||
| Egr1 | Early growth response 1 | 0.0231 | −0.2524 | 0.0137 | −0.5738 | 0.196 | −1.294 |
| Egr2 | Early growth response 2 | 0.0084 | −0.7276 | 0.0123 | −0.8669 | 0.006 | −9.844 |
| Egr4 | Early growth response 4 | 0.0006 | −0.5907 | 0.0077 | −0.7916 | 0.041 | −1.203 |
| Gria3 | Ionotrophic glutamate receptor 3 | 0.0356 | −0.3008 | 0.0368 | −0.3686 | 0.683 | −0.316 |
| Grm2 | Metabotropic glutamate receptor 2 | 0.0044 | −0.2747 | 0.0102 | −0.5101 | 0.004 | −2.402 |
| Crym | Mu-crystallin homolog | 0.0065 | −0.2962 | 0.0292 | −0.6153 | 0.161 | −0.897 |
| Nr4a1 | Nuclear receptor subfamily 4, group A, member 1 | 0.0074 | −0.2802 | 0.0063 | −0.7108 | 0.001 | −2.402 |
| Nr4a3 | Nuclear receptor subfamily 4, group A, member 3 | 0.0061 | −0.3224 | 0.0005 | −1.2155 | Nondetectable | |
| Slc1a3 | Glial high affinity glutamate transporter | 0.7954 | −0.0163 | Not assessed | Nondetectable | ||
Validation of selected genes determined to be differentially expressed in the mPFC exposed group (n = 9) versus control (n = 9) by microarray analysis or in the IL of the exposed group (n = 5) versus control (n = 5) is shown. Bold values are confirmed qRT-PCR data from microarray results. FC, fold change.
Infralimbic–accumbal glutamatergic projection neurons are highly sensitive to alcohol dependence-induced neuroadaptations
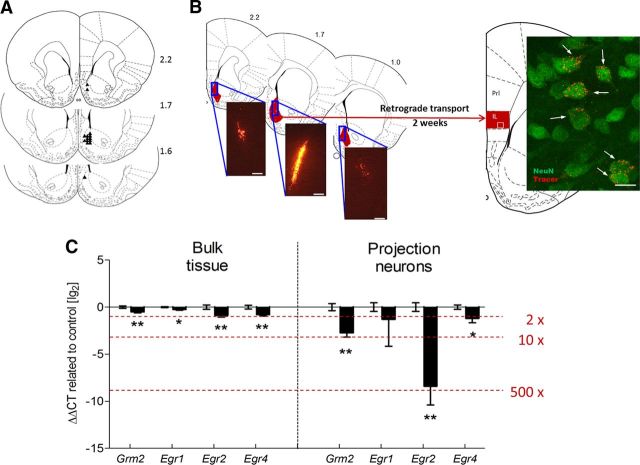
Together, these experiments lead to the conclusion that glutamatergic neurons in the infralimbic cortex are highly sensitive to alcohol-induced neuroplasticity. To identify the specific neurocircuitry involved, we used a strategy that allows labeling pyramidal neurons within the infralimbic cortex via their projections to the nucleus accumbens shell subregion. We performed retrograde tracing by infusing rhodamine-labeled fluorescent latex microspheres into the nucleus accumbens shell (Katz and Iarovici, 1990; Reynolds and Zahm, 2005), isolated the labeled cell population (∼70–100 cells) within the infralimbic cortex through LCM, and extracted the RNA for expression analysis (Fig. 3A,B).
Figure 3.
Robust downregulation of Grm2 transcripts in rat infralimbic accumbens shell projection neurons lead to blunted response to Grm2 agonist treatment in postdependent (PD) rats. A, Locations of the 33 gauge injection cannula tips for the injections the retrograde tracer into the nucleus accumbens shell are represented by small black triangles. The cannula placements for the nucleus accumbens shell were verified within the region from +1.6 to +2.2 mm. B, Distribution of retrograde tracer within the nucleus accumbens shell (range, +2.2 to +1.0 mm relative to bregma). Fluorescent cells were clearly visible in sections from +1.9 to +2.5 mm relative to bregma in the infralimbic and the dorsal peduncular cortex. Insert shows a representative confocal microscope image. Arrows indicate retrograde tracer-positive cells, colabeled with the neuronal marker NeuN. Scale bars: left, 100 μm; right, 10 μm. C, Downregulated genes in glutamatergic projection neurons in the infralimbic cortex compared to the micropunched mPFC (bulk tissue) from the microarray study. The graph represents the delta delta cycle threshold ± SEM on a logarithmic scale of selected mRNAs, expressing the change in cycle thresholds from treatment to controls compared back to an endogenous control. In addition, Crym, a marker gene of glutamatergic pyramidal neurons according to the GENSAT mouse brain atlas (www.gensat.org), was highly expressed in all samples (quantitative PCR cycle threshold of ∼14), whereas Slc1a3, the gene for the glial glutamate transporter, was not detectable (cycle threshold, >39), indicating that we indeed succeeded in collecting a highly purified glutamatergic neuronal population. *p < 0.05; **p < 0.01. PreL, Prelimbic cortex; IL, infralimbic cortex.
We tested eight candidate genes from the mPFC microarray experiment (Table 3). Among these, Grm2 as well as the Egr-family genes Egr2 and Egr4 were identified as significantly downregulated in the infralimbic cortex neurons of the postdependent group. Expression differences detected within the purified neuronal population were markedly enhanced compared to those in the analysis performed on tissue homogenates. Expression of Grm2 and Egr2 was ∼10-fold and ∼500-fold, respectively, altered in enriched infralimbic projection neuron populations from postdependent rats (Fig. 3C), although these differences were less than twofold when applying the same PCR analysis to tissue homogenates. The experiment reveals the extent to which major dysregulation can be disguised in heterogeneous samples and emphasizes the importance of studying well-characterized cell populations in the brain. In conclusion, we demonstrate that infralimbic–accumbal glutamatergic projection neurons are highly sensitive to alcohol dependence-induced neuroadaptations, and identify mGluR2 receptor downregulation in this pathway as a candidate mechanism for observed behavioral deficits.
Functional consequences of mGluR2 reduction
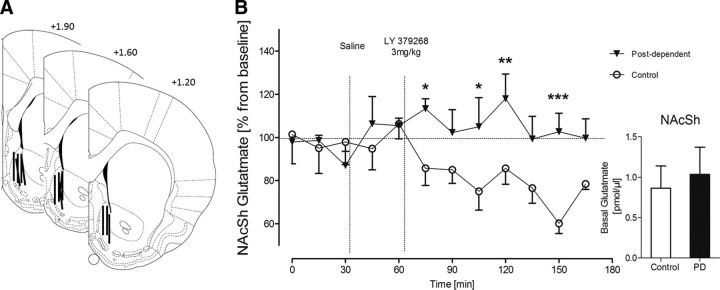
mGluR2 function in the corticoaccumbal pathway was assessed by in vivo microdialysis. We measured extracellular glutamate levels in the nucleus accumbens shell of freely moving rats (Fig. 4A). Given its role as a presynaptic autoreceptor, stimulation of mGluR2 is expected to downregulate glutamate release, resulting in reduced glutamate overflow in the dialysate. Accordingly, systemic administration of the mGluR2/3 agonist LY379268 (3 mg/kg, i.p.) resulted in a robust and sustained decrease of extracellular glutamate levels in the nucleus accumbens shell of control rats. In contrast, no such effect was seen in postdependent rats (Fig. 4B). Basal glutamate levels were not different between postdependent and control rats (Fig. 4B). These data are consistent with the interpretation that the downregulation of Grm2 expression could lead to a lack of mGluR2 autoreceptor function at the terminals of the infralimbic projection neurons. Such a deficit would impact on activity-dependent glutamatergic neurotransmission in the corticostriatal pathway and presumably also on behavioral output.
Figure 4.
Glutamate microdialysis in postdependent (PD) versus control animals shows blunted response to mGluR2 agonist treatment in postdependent rats. A, The active membranes of the microdialysis probes are represented by black lines and were verified within the nucleus accumbens shell from +1.9 to +1.2 anterior to bregma. B, Nucleus accumbens shell glutamate levels after intraperitoneal application of 3 mg/kg mGluR2/3 agonist LY379268. Control animals show decrease of extracellular glutamate levels, whereas postdependent rats show a blunted response to the agonist treatment, indicating a downregulation of mGluR2. Inset, Basal glutamate levels (two-way ANOVA; main effect of ethanol dependence history, F(1,11) = 6.672, p < 0.05; main effect of time, F(11,121) = 1.521, not significant; significant interaction of ethanol dependence history by time, F(11,121) = 2.331, p < 0.05). *p < 0.05; **p < 0.01; ***p < 0.001 (Fisher LSD post hoc test). NAcSh, Nucleus accumbens shell.
Restoration of mGluR2 attenuates excessive cue-induced alcohol seeking
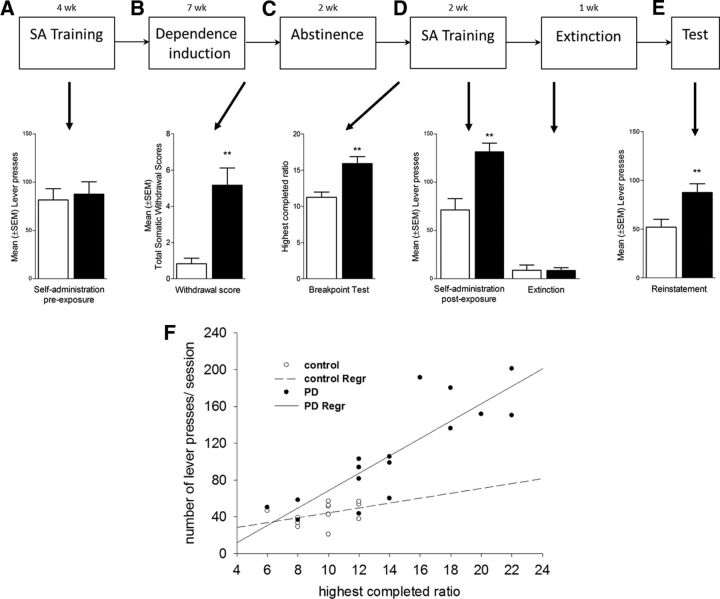
We next examined the role of mGluR2 receptors in infralimbic neurons projecting to the nucleus accumbens shell for cue-induced reinstatement of alcohol-seeking behavior, an established animal model of relapse (Epstein et al., 2006; Sanchis-Segura and Spanagel, 2006). First, rats were trained to self-administer alcohol before alcohol vapor exposure (Fig. 5A–E). After the last exposure cycle, postdependent rats showed clear signs of withdrawal (approximately five of a maximal eight points from a global withdrawal score) (Macey et al., 1996), whereas this rating for control rats was close to zero (p < 0.004, Mann–Whitney; Fig. 5B). After 2 weeks of recovery, all rats were retrained to self-administer ethanol until stable response rates were achieved once more. Control rats regained self-administration rates that were similar to their preexposure rates (90% of preexposure). In contrast, postdependent rats rapidly escalated their self-administration rates by >155% (Fig. 5A,D). Motivation to obtain alcohol was further assessed by a progressive ratio reinforcement schedule (Hodos, 1961). Postdependent rats showed a significantly higher break point for alcohol self-administration than controls (t(29) = 3.09, p < 0.01; Fig. 5C), a significantly steeper slope of the correlation between response rates during ethanol self-administration under an FR1 schedule, and progressive ratio breakpoints (correlation equations test, p < 0.05; Fig. 5F). This shows that escalated alcohol self-administration in postdependent rats is associated with an increased motivation to obtain the drug reinforcer, a key characteristic of addictive behavior (Deroche-Gamonet et al., 2004). These data are consistent with a recent report that also showed increased motivation to obtain ethanol following a history of experimenter imposed alcohol dependence (Kufahl et al., 2011; Vendruscolo et al., 2012).
Figure 5.
Diagram illustrating the experimental procedure of the postdependent (PD) cue-induced reinstatement model. A, Animals underwent alcohol self-administration under a fixed ratio FR1 schedule until they reached stable lever presses accompanied by discrete cues predicting alcohol availability (CS+). Control and postdependent rats do not differ in this phase. After initial training, half of the animals (resulting in the postdependent group) were alcohol vapor exposed for 7 weeks. B, At the end of the 7 week exposure, withdrawal signs were scored 8 h after last intoxication. Following 2 weeks of abstinence, rats were retrained to self-administer alcohol. C, D, Postdependent rats show higher self-administration (D) and also higher motivation (C) for alcohol in a progressive ratio test. Two-way ANOVA showed a significant main effects of ethanol dependence history (F(1,24) = 8.91, p < 0.01) and significant interactions of ethanol dependence history by self-administration condition (F(1,92) = 6.08, p < 0.05). Extinction training was not different between groups (postdependent vs control groups, t(30) = 0.243, not significant). E, Presentation of the CS+ elicits in significant reinstatement in control and postdependent rats with significant higher levels in the postdependent group (control, 42.1 ± 3.4 vs postdependent, 84.4 ± 13.2; t(13) = −2.896, p < 0.01). F, Linear regression analysis of mean lever presses and performance in the progressive ratio test of postdependent and control rats. Both groups show a significant deviation from zero (postdependent, p = 0.004; control, p = 0.009). Furthermore, postdependent rats show a significantly steeper slope of the correlation between response rates during ethanol self-administration under an FR1 schedule, and progressive ratio breakpoints. Error bars indicate SEM.
Alcohol associated cues are potent triggers of relapse in alcoholic patients. This pathological behavior is typically modeled in the reinstatement procedure (Epstein et al., 2006; Sanchis-Segura and Spanagel, 2006). Following stable lever responding accompanied by discrete cues predicting alcohol availability (CS+), postdependent and control rats underwent extinction (Fig. 5D) followed by a cue-induced reinstatement test. Postdependent rats displayed significantly higher reinstatement of alcohol seeking than control rats (p < 0.01; Fig. 5E). To directly assess the role of mGluR2 in mPFC for cue-induced reinstatement of alcohol seeking, we generated two lentiviral vectors expressing either the mGluR2 receptor together with eGFP or eGFP alone (lenti-mGluR2 and lenti-control, respectively; Fig. 6A). Following alcohol/cue training, all rats went through extinction training, resulting in fewer than 10 responses (Fig. 6F). After completion of extinction training, rats were bilaterally injected with the respective lentiviral constructs into the infralimbic cortex (Fig. 6B–F), allowed to recover, and examined for cue-induced reinstatement of alcohol seeking. Notably, immunohistochemistry confirmed the coexpression of the lenti-mGluR2 construct for all studied animals exclusively in the infralimbic cortex and in their neuronal projection target, the nucleus accumbens shell, EGFP-positive axon terminals were clearly visible (Fig. 6D,E). Presentation of the ethanol-associated cues resulted in significant resumption of operant responding in animals receiving the control lentiviral construct (paired t test; control, t(7) = 4.157, p < 0.01; postdependent, t(7) = 4.211, p < 0.01; Fig. 6F), with a highly increased mean (±SEM) number of responses in postdependent (87.6 ± 8.9) compared to control (51.9 ± 8.1) rats. Lenti-mGluR2 did not significantly alter drug-seeking behavior in controls. However, lenti-mGluR2 showed significant reduction in postdependent animals, such that their lever-pressing behavior declined for ∼40% to control levels. These effects were confirmed by two-way ANOVA with significant main effects of ethanol dependence history (F(1,24) = 9.947, p < 0.01) and virus treatment (F(1,24) = 7.932, p < 0.01), but no significant interaction (F(1,43) = 2.032, p = 0.163). We did not find time-dependent spontaneous recovery of lever pressing when reexposing the animals to the operant chamber after the 1 week time delay between the last extinction trial followed by sham operation and the reinstatement test (extinction, 8.9 ± 1.3; spontaneous recovery, 10.9 ± 2.5, not significant). We also did not find evidence for behavioral abnormalities (for example, weight loss, agitation, and self-injury) in any experimental group. Lenti-control and lenti-mGluR2 rats did not differ in locomotor activity or their responding for natural rewards under the same reinforcement schedule used for ethanol self-administration (Fig. 7A–C), demonstrating that effects of mGluR2 overexpression on reduced ethanol-seeking behavior were not secondary to alterations in task performance.
Figure 6.
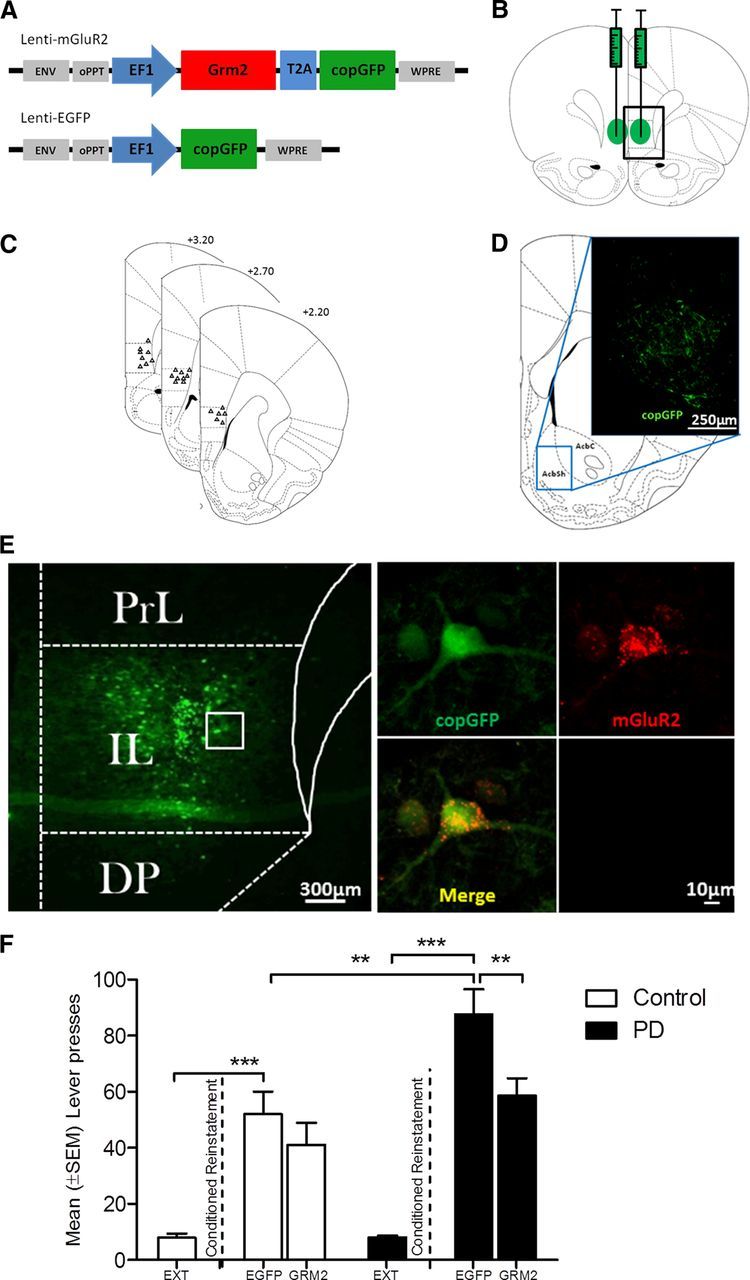
Conditioned reinstatement of drug-seeking behavior attenuates only with lenti-mGluR2 bilateral viral injections. A, Schematic representation of the lentiviral expression plasmids used for the production of Lenti-mGluR2 and Lenti-EGFP. cPPT, central polypurine tract; copGFP, copepod Pontellina plumata GFP; WPRE, Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element. B, Illustration of bilateral Lenti-mGluR2 and Lenti-EGFP injection sites. Green circles represent the spread area of the virus. C, Locations of the 33 gauge injection cannula tips for the lentiviral injection into the infralimbic cortex are represented by small black triangles, respectively. The cannula placements were verified within the infralimbic cortex from +3.2 to +2.2 anterior to bregma. D, Schematic representation of the infralimbic projection site. The inset shows the nucleus accumbens shell region with its EGFP positive axons originating from the injection site at 7 d after lentiviral infection. E, Left, Site of Lenti-mGluR2 delivery adapted from Paxinos and Watson's (1998) rat brain atlas. Right, Representative microscope image of a virally infected cell in the infralimbic cortex showing mGluR2, EGFP, merged and secondary antibody negative control. EGFP and mGluR2 expression was assessed using immunohistochemistry. PrL, Prelimbic cortex; IL, infralimbic cortex; DP, dorsal peduncular cortex. F, Presentation of the CS+ elicits in significant reinstatement in both control and PD rats with lenti-EGFP. Lenti-mGluR2 significantly attenuates drug-seeking behavior only in postdependent (PD) rats down to the level of the control group. *p < 0.05; **p < 0.01; ***p < 0.001. For detailed statistics, see Results. Error bars indicate SEM.
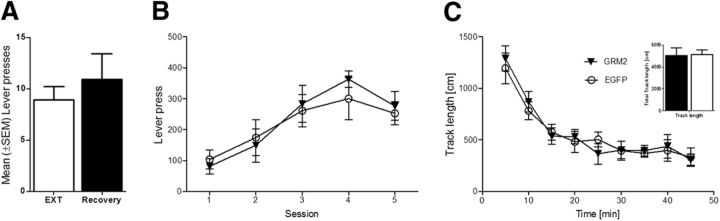
Figure 7.
Behavioral observations for the delayed reinstatement and between Lenti-mGluR2- and Lenti-EGFP-injected animals in responding for natural rewards and in locomotor activity. A, We did not find time-dependent spontaneous recovery of lever pressing when reexposing the animals to the operant chamber after the 1 week time delay between last extinction trial followed by sham operation and reinstatement test. No differences were observed between Lenti-mGluR2- and Lenti-EGFP-injected animals in responding for natural rewards and in locomotor activity. B, Both groups were exposed to five 30 min operant sessions to self-administered sweetened condensed milk under an FR1 schedule. Lenti-mGluR2 and Lenti-EGFP lever-pressing behavior did not differ. C, Lenti-mGluR2 and Lenti-EGFP rats show equal locomotion when exposed to a 45 min open-field session. Inset, Total track length completed in the 45 min session. Error bars indicate SEM.
Downregulated GRM2 in human alcoholics
To translate these animal findings to humans, we determined GRM2 expression in postmortem brain tissue samples from alcohol-dependent patients and controls matched for age and postmortem interval (Sheedy et al., 2008).
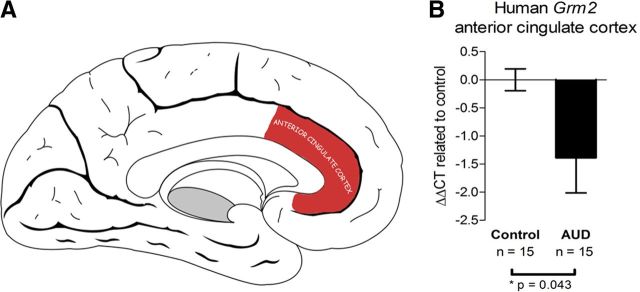
A human brain region that is anatomically and functionally related to the rodent mPFC is the anterior cingulated cortex (Uylings et al., 2003). However, it has to be pointed out that a one-to-one relationship between human and rodent prefrontocortical regions does not exist and functional elements of rodent distinctions including of the infralimbic cortex can be found in various areas of the enlarged human prefrontocortical volume. Within the anterior cingulate cortex, we found a significant, 2.6-fold decrease in GRM2 transcript levels in alcoholics compared to controls (Fig. 8A,B).
Figure 8.
Downregulation of Grm2 transcript in the human anterior cingulated cortex. A, Schematic representation of the anterior cingulate cortex of the human brain on a sagittal section (adapted from Standring, 2008). B, RT-qPCR showing Grm2 downregulation in the human anterior cingulate cortex of patients classified with alcohol use disorder compared to respective controls. *p < 0.05 (Student's t test). Error bars indicate SEM. AUD, alcohol use disorder.
Discussion
The data presented here provide a fundamentally new insight into the molecular basis by which a prolonged history of alcohol dependence causes a substantial and long-lasting reorganization of the medial prefrontal cortex. To the best of our knowledge, the present data from gene expression and functional studies constitute strong experimental evidence of anatomical and molecular pathway-specific plasticity in the mPFC as a sequel to alcohol dependence and establish a key pathophysiological mechanism for the increased propensity to relapse. In particular, we discovered a locally restricted but profound molecular pathology, namely, the infralimbic cortex-specific expression deficit of mGluR2, as a critical component for excessive alcohol seeking in postdependent rats, and that restoring this receptor function is sufficient for regaining control over this addictive behavior.
The infralimbic cortex shows a unique pattern of alcohol dependence-induced alterations, as evidenced by the regional-specific downregulation of transcription factors Egr1 and Egr2 known to be involved in neuronal plasticity, as well as on the glutamate receptor genes Grin2a, Grin2b, and Grm2. Importantly, the downregulation of Grm2 and Egr2 is much more pronounced in purified infralimbic–accumbens shell projection neurons, ∼10-fold and ∼500-fold, respectively, highlighting these genes within this cell population as functionally relevant for the pathological process. Notably, the Grm2 promoter contains transcription factor binding sites for the Egr-family as well as a unique Egr2 binding site (according to the DECODE database, http://www.sabiosciences.com/chipqpcrsearch.php?app=TFBS), which provide a potential substrate for regulation of Grm2 expression by Egr2. On the other hand, mGluR2 may regulate Egr2 expression, as suggested by experiments in Grm2 knock-out mice that show a lack of Egr2 activation following drug application (Moreno et al., 2011). Whether or not the downregulation of these genes is functionally related is yet unknown, but both seem to be involved in dependence-related plasticity of glutamatergic neurons, mGluR2 directly at the level of the synapse, and Egr2 via stimulus-transcription coupling.
Importantly, we found a lack of mGluR2 receptor function in the terminal fields of the infralimbic projections, which became evident as an inability of these neurons to modulate nucleus accumbens shell glutamate levels in response to receptor stimulation with an mGluR2/3 agonist. This effect is consistent with the pronounced reduction in mGluR2 expression—with no change in mGluR3 expression—in the infralimbic cortex of postdependent rats. However, a recent study found no differences in mGluR2/3 functional activity within the mPFC in postdependent rats after 4 weeks of repeated cycles of vapor exposure (Kufahl et al., 2011). How can this discrepancy been explained? We demonstrated previously that in the alcohol vapor exposure procedure, a temporal threshold for induction of escalation of alcohol consumption and concomitant neuroplastic changes occur (Rimondini et al., 2003). Hence, postdependent rats that were exposed to alcohol vapor for 7 weeks displayed a marked increase in alcohol self-administration, whereas postdependent rats exposed for shorter periods (2 and 4 weeks) did not show such an escalation (Rimondini et al., 2003). Here we report that postdependent rats exposed to alcohol vapor for 7 weeks show an augmented reinstatement response of alcohol-seeking behavior and that this dependence-like phenotype is directly linked to an mGluR2 deficit in the infralimbic cortex. In the study by Kufahl et al. (2011), rats were exposed to alcohol vapor for 4 weeks and did not differ either in their reinstatement response nor in mGluR2/3 functional activity from controls, which supports the definition of a temporal threshold for induction of escalation in alcohol consumption and alcohol seeking and the herewith associated neuroplastic changes. Together, a careful, cell type-specific investigation of the group II mGluRs shows a highly restricted mGluR2 downregulation in sparsely distributed glutamatergic neurons located in the ventral part of the mPFC, the infralimbic region.
With our viral rescue experiment, we could further show that mGluR2 receptors in infralimbic neurons are necessary for the control exerted by this region on alcohol seeking. Consequently, infralimbic neurons in postdependent animals are capable of eliciting a sufficient glutamate response to drug cues, but in the absence of feedback provided by mGluR2 receptors, the information transmitted by this signal cannot be properly processed, thereby disrupting adequate behavioral control. On the other hand, adding extra mGluR2 autoreceptors to normal infralimbic neurons does not seem to disrupt glutamatergic signaling and behavioral output in a task controlled by this brain structure. This concept is further supported by electrophysiological evidence from long-term cocaine-exposed rats., Using in vivo stimulation from the prefrontal cortex of long-term cocaine-exposed rats revealed an mGluR2/3 deficit in the nucleus accumbens (Moussawi et al., 2011). In another model of cocaine-induced addiction-like behavior, there was a lack in mGluR2/3-mediated long-term depression in mPFC neurons that was associated with a strong downregulation of mGluR2/3 receptors (Kasanetz et al., 2012). Thus, both alcohol and cocaine dependence are associated with medioprefrontal mGluR2 deficits that may lead to an inflexible state of the brain. Although we did not provide electrophysiological evidence, our study substantially extends the findings from the cocaine models by demonstrating that an addiction-like behavior, here excessive alcohol seeking, can be rescued through restoring mGluR2 levels in the mPFC.
Impairments in executive control over behavior are known risk factors for drug addiction (Everitt and Robbins, 2005). Alcohol-dependent patients have severe deficits in many aspects of prefrontocortical functions encompassing emotion, cognition, and behavior, whereby medial subdivisions of the prefrontal cortex are of particular interest here because of their role in motivation, control of emotions, salience attribution, and decision making (Goldstein and Volkow, 2011). These functions have been established not only in humans but also in rodents (Uylings et al., 2003). Typical behaviors seen in patients with damage to the ventromedial PFC are social inappropriateness, impulsivity, and poor judgment (Bechara et al., 1994). Enduring medioprefrontal gray matter losses were found in alcoholic patients and are associated with severe functional deficits in the ability to control reward-predicting stimuli (Duka et al., 2011). Interestingly, these deficits increase with the number of detoxifications experienced by the patients, which resonates with previous observations in experimental animals that the number of withdrawals, rather than the mere level of intoxication, is important for the occurrence of long-lasting behavioral and neural symptoms, i.e., a postdependent state (Roberts et al., 2000; Stephens et al., 2005; Sommer et al., 2008; Heilig et al., 2010). A previous fMRI study in alcoholics found increased mPFC activation in response to alcohol cues, which was positively correlated with relapse risk (Grüsser et al., 2004). In experimental animals, cue presentation of conditioned stimuli predicting a drug reward results in a significant increase in glutamate levels in the nucleus accumbens (Hotsenpiller et al., 2001). Most likely, this input derives from prefrontal areas, given that the mPFC–accumbal glutamatergic pathway is necessary for reinstating drug-seeking behavior. Likewise, our observed deficit in mGluR2 autoreceptor function within the infralimbic cortex of postdependent rats may lead to increased accumbal glutamate levels after cue presentation, with subsequent excessive drug-seeking behavior.
Importantly, we also find a reduction in GRM2 expression in the anterior cingulate cortex from alcohol-dependent patients, which suggests that the deficits found in our animal model may be a feature in alcoholism in at least some patients. It remains to be clarified whether or not the reduced GRM2 expression found in the present sample is functionally linked to the progressive reduction in prefrontal neuronal density, which was seen in a previous study on postmortem brain tissue from alcoholics (Miguel-Hidalgo et al., 2006). However, the reduction in GRM2 expression and number of neurons may together lead to an absolute deficit of mGluR2 receptors in the mPFC of alcoholics. This may have important implications for the development of treatments for relapse prevention because absolute deficits cannot be efficiently targeted by agonist treatment. Indeed, this may be one of the reasons for the relatively narrow therapeutic window for reducing alcohol seeking in experimental animals by mGluR2/3 agonists (Kufahl et al., 2011). Thus, instead of focusing on the development of more specific mGluR2 ligands, novel therapeutic strategies should attempt to overcome the blockade of mGluR2 expression. Focal virus-mediated gene therapy, although potentially feasible (Kaplitt et al., 2007), is unlikely to be applied for the treatment of addictions. Alternatively, pharmacological approaches targeting key proteins involved in glutamate homeostasis, such as glutamate transporters or mGluRs, could potentially be effective treatments in relapse prevention. In conclusion, the present study illustrates the feasibility of a structured discovery strategy, that starting with an unbiased screening over progressively narrowing experimental approaches allows identifying a specific pathological mechanism and can point toward new directions for therapeutic development.
Footnotes
This work was supported by the Bundesministerium für Bildung und Forschung within the frameworks of NGFN Plus (FKZ 01GS08151, 01GS08152, and 01GS08155; see www.ngfn-alkohol.de, Spanagel et al., 2010) and ERA-Net TRANSALC (FKZ 01EW1112), the European Commission FP-6 Integrated Project IMAGEN (PL037286), the Deutsche Forschungsgemeinschaft (Center Grant SFB636; project Grant HA 6102/1-1 to A.C.H.; Reinhart-Koselleck Award SP 383/5-1 to R.S.), and the Intramural Research Program of the NIAAA (M.H.). We thank Elisabeth Röbel and Fernando Leonardi-Essmann for assistance in laboratory experiments.
The authors declare no competing financial interests.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arlinde C, Sommer W, Björk K, Reimers M, Hyytiä P, Kiianmaa K, Heilig M. A cluster of differentially expressed signal transduction genes identified by microarray analysis in a rat genetic model of alcoholism. Pharmacogenomics J. 2004;4:208–218. doi: 10.1038/sj.tpj.6500243. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Björk K, Hansson AC, Sommer WH. Genetic variation and brain gene expression in rodent models of alcoholism implications for medication development. Int Rev Neurobiol. 2010;91:129–171. doi: 10.1016/S0074-7742(10)91005-2. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Duka T, Trick L, Nikolaou K, Gray MA, Kempton MJ, Williams H, Williams SC, Critchley HD, Stephens DN. Unique brain areas associated with abstinence control are damaged in multiply detoxified alcoholics. Biol Psychiatry. 2011;70:545–552. doi: 10.1016/j.biopsych.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. Anatomy: descriptive and surgical. 1918 [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. others. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur J Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012a;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Hermann D, Frischknecht U, Heinrich M, Hoerst M, Vollmert C, Vollstädt-Klein S, Tunc-Skarka N, Kiefer F, Mann K, Ende G. MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol. 2012b;17:659–667. doi: 10.1111/j.1369-1600.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.59. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Katz LC, Iarovici DM. Green fluorescent latex microspheres: a new retrograde tracer. Neuroscience. 1990;34:511–520. doi: 10.1016/0306-4522(90)90159-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Martin-Fardon R, Weiss F. Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR(2/3) agonist LY379268 and increased functional activity of mGluR(2/3) in rats with a history of ethanol dependence. Neuropsychopharmacology. 2011;36:1–12. doi: 10.1038/npp.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol. 2007;74:1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol. 2012;17:351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Reimers M, Heilig M, Sommer WH. Gene discovery in neuropharmacological and behavioral studies using Affymetrix microarray data. Methods. 2005;37:219–228. doi: 10.1016/j.ymeth.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall'Olio R, Heilig M. Long-lasting tolerance to alcohol following a history of dependence. Addict Biol. 2008;13:26–30. doi: 10.1111/j.1369-1600.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian Brain Bank: a critical investment with a high return! Cell Tissue Bank. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Lidström J, Sun H, Passer D, Eskay R, Parker SCJ, Witt SH, Zimmermann US, Nieratschker V, Rietschel M, Margulies EH, Palkovits M, Laucht M, Heilig M. Human NPY promoter variation rs16147:T>C as a moderator of prefrontal NPY gene expression and negative affect. Human Mut. 2010;31:E1594–E1608. doi: 10.1002/humu.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Bartsch D, Brors B, Dahmen N, Deussing J, Eils R, Ende G, Gallinat J, Gebicke-Haerter P, Heinz A, Kiefer F, Jäger W, Mann K, Matthäus F, Nöthen M, Rietschel M, Sartorius A, Schütz G, Sommer WH, Sprengel R, et al. An integrated genome research network for studying the genetics of alcohol addiction. Addict Biol. 2010;15:369–379. doi: 10.1111/j.1369-1600.2010.00276.x. [DOI] [PubMed] [Google Scholar]
- Standring S. Gray's anatomy: the anatomical basis of clinical practice. Ed 40. New York: Churchill-Livingstone/Elsevier; 2008. [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH. Ethanol preference following the sucrose-fading initiation procedure. Alcohol. 1988;5:9–13. doi: 10.1016/0741-8329(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]