Summary
The identification of endothelium-derived relaxing factor as nitric oxide dramatically altered the course of vascular biology, as well as other biomedical disciplines. The ubiquity of this natural product of cell metabolism and the complexity of its biochemistry provide a rich source of molecular mediators of phenotype in health and disease.
When Furchgott and Zawadzki first demonstrated in 1980 that the endothelium released a substance that relaxed blood vessels in response to muscarinic agonists (1), the field of vascular biology underwent a seismic change. The chemical nature of this effector, denoted at that time endothelium-derived relaxing factor (EDRF), was unknown. Over the next several years, certain properties of EDRF were reported that moved the field closer to understanding its likely structure. Patterns of vasorelaxation were similar to those of direct nitrovasodilators, except that these agents exerted their effects independent of the endothelium. In 1977, Murad’s group had shown that nitrovasodilators and nitric oxide activate guanylyl cyclase to effect smooth muscle relaxation and vasodilation (2). Vasorelaxation by EDRF, like that by direct nitrovasodilators, was also shown to be temporally preceded by activation of guanylyl cyclase and cyclic guanosine monophosphate (cGMP) accumulation (3,4). Vasorelaxation by EDRF, like that by direct nitrovasodilators, was inhibited by hemoglobin and myoglobin (5,6). In addition and importantly, Ignarro and colleagues in their classic article utilized the shift in the Soret peak of the absorption spectrum of deoxyhemoglobin from 433 nm to 406 nm as evidence for the formation of nitrosyl-hemoglobin (7). This key observation definitively demonstrated that EDRF is, indeed, nitric oxide (NO.), a conclusion that was independently reached by Moncada’s group by its unique chemiluminescent product with ozone (8). Owing to this essential observation, the senior author of the classic article (7), Louis Ignarro, received the Nobel Prize in Physiology or Medicine in 1998 together with Robert Furchgott and Ferid Murad.
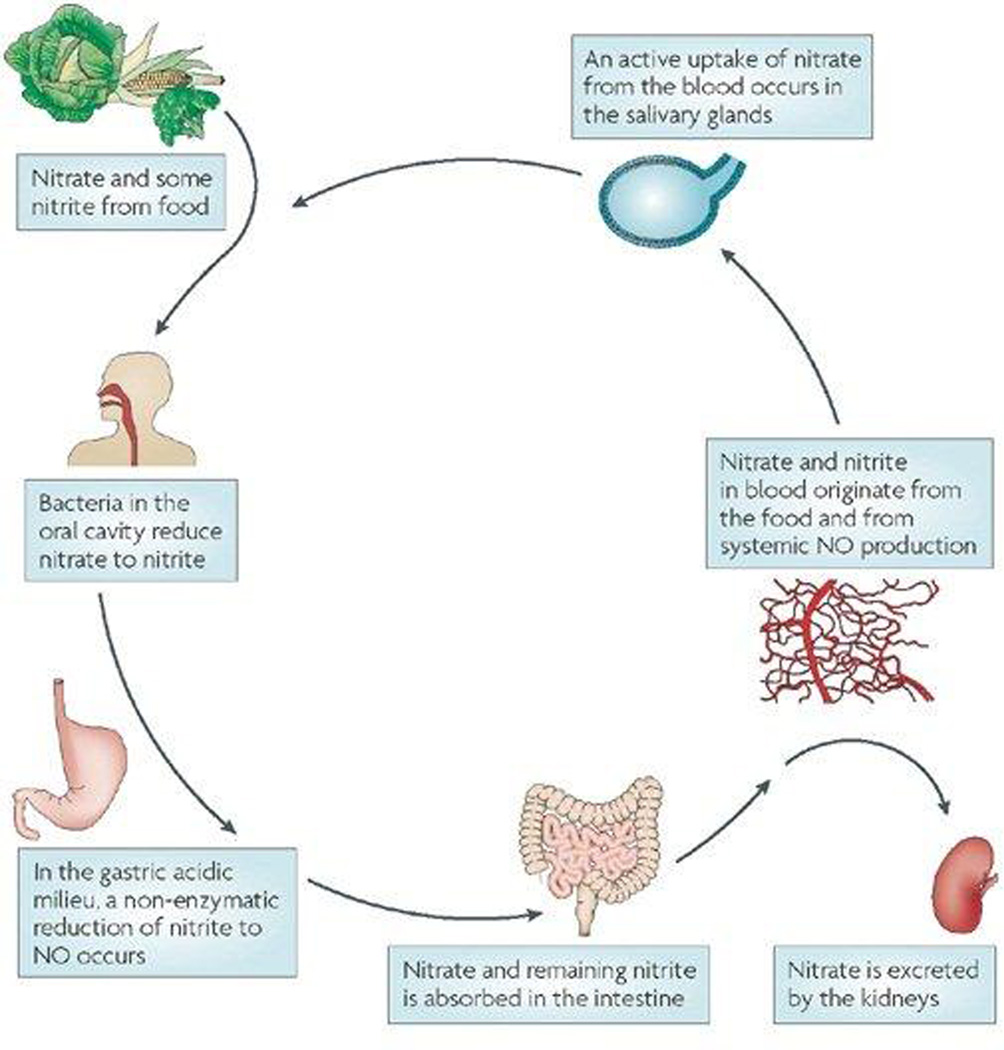
The discovery that EDRF is nitric oxide ushered in a new era of investigation in biomedicine. The ubiquity and dazzling pluripotentiality of this heterodiatomic molecule was rapidly demonstrated as literature reports of its many actions grew exponentially over time. Nitric oxide is synthesized by the family of nitric oxide synthases from L-arginine, yielding citrulline as a co-product. Nitric oxide is a free radical, but one that is not as reactive as other biological free radicals, diffusing on average micron distances before engaging in a reaction with another molecule. The structural simplicity of this compound betrays an astonishing complexity of reactions that result from its unique biochemistry (9). One example of this biochemical complexity includes the range of redox states of nitrogen oxides and their physiological relationship to nitric oxide itself. Specifically, nitrite and nitrate are higher oxidation states of nitric oxide that were previously believed to be stable oxidation end-products of the redox sequence. Recent work, however, suggests that these oxidized species can be recycled in vivo in a process that involves oral microbiota and foodstuffs. Oral bacteria can reduce dietary nitrate to nitrite, which, upon entering the acidic milieu of the stomach, undergoes nonenzymatic reductive conversion to nitric oxide. In parallel, nitrate and nitrite in blood that originates from the diet and from systemic production and oxidation of nitric oxide can be taken up actively by the salivary glands, whereupon it enters the nitrate reductase-rich bacterial milieu of the oral cavity facilitating the cycle (Figure 1) (10).
Figure 1. The entero-salivary circulation of nitrogen oxides.
Nitrate in the diet is reduced by oral bacteria to nitrite, which is converted to nitric oxide upon entering the acidic environment of the stomach. Nitrate that enters the small intestine is absorbed into the bloodstream where it joins with circulating nitrate and nitrite derived from the oxidative metabolism of endogenous nitric oxide. This circulating pool of nitrate and nitrite is taken up by the salivary glands and secreted into the oral salivary pool. Excess nitrate is excreted by the kidney. [Reproduced with permission from MacMillan Publishers Ltd: (reference 10)].
A second example of this biochemical complexity are the multiple pathways within the complex metabolism of nitric oxide by which S-nitrosothiols form, including S-nitrosoproteins. S-Nitrosothiols comprise an ever-increasing pool of species that stabilize nitric oxide minimizing its oxidative inactivation and promoting its many biological actions. Multiple lines of evidence also suggest that S-nitrosothiols may, in many instances, be the direct proximate mediator of EDRF-like effects (11–13). In addition and importantly, S-nitrosylation of the proteome is a unique form of post-translational modification that can have significant consequences for protein function and cell phenotype. S-Nitrosothiols can form by the reaction of the thiol moiety with dinitrogen trioxide (N2O3), peroxynitrite anion (ONOO−), or nitrosonium ion (NO+), or by trans-S-nitrosylation, as illustrated in Figure 2 (14). Furthermore, S-nitrosothiols can also form in the acidic environment of the stomach from nitrite (acidified nitrite) and thiols, or mediated via hemoglobin in blood. This and other biochemical pathways parallel the wide-ranging cellular and physiological effects of nitric oxide, leading to its designation as the Molecule of the Year by the journal Science in 1992.
Figure 2. Nitric oxide metabolism and S-nitrosothiol formation.
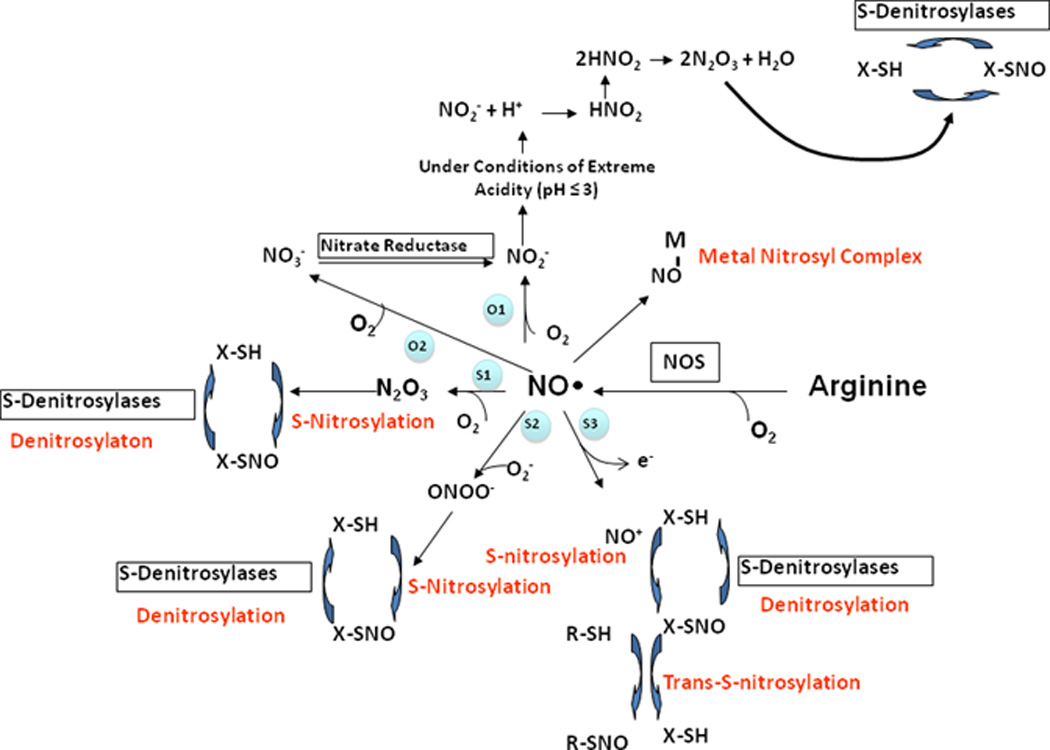
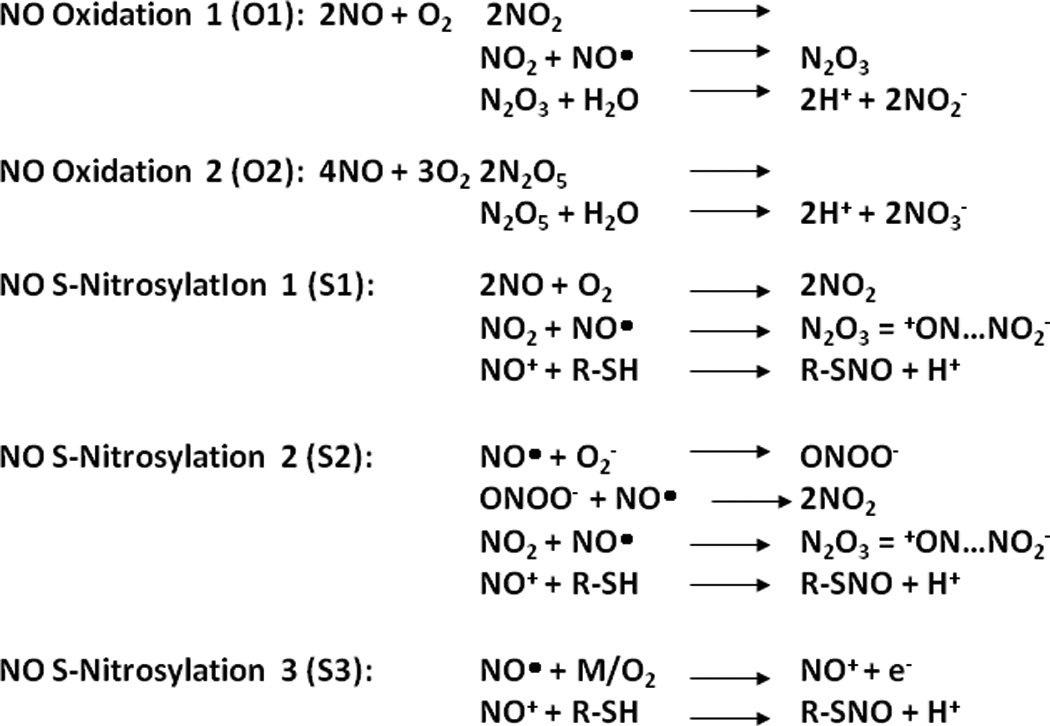
(A) Nitric oxide (NO.) is synthesized by the nitric oxide synthases (NOS), and can undergo oxidation to nitrite and nitrate, react with superoxide anion (.O2) to form peroxynitrite (ONOO−), or bind to transition metals (NO-M). Three different mechanisms of trans-S-nitrosylation are indicated, as well as denitrosylation steps. Detailed reaction mechanisms for key reactions highlighted in blue are provided in (B). [Reproduced with agreement by Mary Ann Liebert Publisher, Inc., which does not require formal permission to obtain approval for personal reuses (reference 14).]
Given the structural simplicity of nitric oxide and its ubiquity, it is reasonable to ask why it took so long to recognize that it is a normal product of eukaryotic cell metabolism. One response to this question is that the biochemistry is highly versatile, complicating detection of the biologically active species. In addition, the steady-state concentrations of nitric oxide are low in vivo compared with those of its higher oxidation states, and the available assays have been insensitive, limiting its detection. However, this seems an unlikely explanation because reasonable analytical methods for detecting nitric oxide have been available for decades. Hermann first demonstrated the interaction of nitric oxide with (a component of ) blood in 1865 (15), which was highlighted by the formation of a bright red color. This color change was not unlike that caused by the reaction of carbon monoxide with blood (later recognized as the formation of carboxyhemoglobin), and probably led to the erroneous diagnosis of carbon monoxide poisoning in cases of overwhelming sepsis caused by nitrifying organisms reported as early as 1925 (16). Spectrophotometric detection of nitrosyl-hemoglobin was first reported in 1925 by Anson and Mirsky (17), and this technique was subsequently used to demonstrate the presence of nitric oxide in the urine of a patient with hematuria and bacteriuria in 1955 (18). With the advent of electron paramagnetic resonance spectroscopy and its application to biological systems, nitrosyl-hemoglobin was demonstrated in the blood of mice and rabbits exposed to inhaled nitric oxide by monitoring the appearance of the hyperfine triplet at g ~ 2.0 (19). This study focused on the interaction of nitric oxide with hemoglobin as a potential marker of the in vivo toxicity of the inhaled gas. Interestingly, in one figure in that paper, the kinetics of the formation and decay of nitrosyl-hemoglobin are plotted following exposure to 10.6 ppm of nitric oxide. In the steady-state, ~0.13% of total hemoglobin is converted to nitrosylhemoglobin. With discontinuation of the inhaled NO, the nitrosylhemoglobin decayed but persisted at detectable levels of ~0.01% out to 30 minutes or 81.8 half-lives (20), well beyond what one would predict if there were not some other endogenous source of nitric oxide to maintain the new steady-state. The authors commented on this unexpected finding, stating that “…even after 30 minutes [nitrosylhemoglobin] was still present.” Thus, these and other data hinted at the existence of nitric oxide or a nitric oxide-like species as a product of normal mammalian metabolism. Yet, it would take another 12 years before the endogenous production of nitric oxide was definitively proven in this ‘classic’ paper in the journal, ushering in a very broad field in biomedicine that continues to be a rich source of discovery.
Acknowledgement
The author wishes to thank Stephanie Tribuna for expert technical assistance.
Support: NIH Grants HL061795, HL048743, HL107192, and HL108630
Footnotes
Disclosures: None
References
- 1.Furchgott RF, Zawadzki JF. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3’,5’-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Protein Phosphor Res. 1982;8:409–419. [PubMed] [Google Scholar]
- 4.Rapoport RM, Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983;52:352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- 5.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- 6.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation of rabbit aorta by certain ferrous hemoproteins. J Pharmacol Exp Ther. 1985;232:679–685. [PubMed] [Google Scholar]
- 7.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 8.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 9.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 11.Loscalzo J. N-Acetylcysteine potentiates inhibition of platelet aggregation by nitroglycerin. J Clin Invest. 1985;76:703–708. doi: 10.1172/JCI112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Nat’l Acad Sci USA. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Nat’l Acad Sci USA. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maron BA, Tang SS, Loscalzo J. S-Nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antiox Redox Signal. 2013;18:270–287. doi: 10.1089/ars.2012.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermann L. Ueber die Wirkungen des Stickstoffoxydgasses auf das Blut. Arch Anat Physiol Wissenschaf Med. 1865:469–481. [Google Scholar]
- 16.Banham HAL, Haldane JS, Savage T. The presence post-mortem of nitric-oxide-haemoglobin—its clinical and medicolegal significance. Brit Med J. 1925;2:187–189. [Google Scholar]
- 17.Anson ML, Mirsky AE. On the combination of nitric oxide with haemoglobin. J Physiol. 1925;60:100–102. doi: 10.1113/jphysiol.1925.sp002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron DN, Rose CFM. Nitric oxide haemoglobin in urine due to contamination. Brit Med J. 1955;1:398. doi: 10.1136/bmj.1.4910.398-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda H, Kusumoto S, Nakajima T. Nitrosyl-hemoglobin formation in the blood of animals exposed to nitric oxide. Arch Environ Health. 1975;30:453–456. doi: 10.1080/00039896.1975.10666749. [DOI] [PubMed] [Google Scholar]
- 20.Hon YY, Sun H, Dejam A, Gladwin MT. Characterization of erythrocytic uptake and release and disposition pathways of nitrite, nitrate, methemoglobin, and iron-nitrosyl hemoglobin in the human circulation. Drug Metab Dispos. 2010;38:1707–1713. doi: 10.1124/dmd.110.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]