Abstract
To investigate the role of the kinase zeta-associated protein of 70 kDa (ZAP-70) in T cells, we generated mice expressing a ZAP-70 mutant whose catalytic activity can be selectively blocked by a small molecule inhibitor. Conventional naïve, effector and memory T cells were dependent on ZAP-70 kinase activity for their activation, demonstrating a non-redundant role for ZAP-70 in TCR-induced signals. In contrast, ZAP-70 catalytic activity was not required for activation of the GTPase Rap1 and inside-out signals that promote integrin adhesion. This ZAP-70 kinase-independent pathway is sufficient for regulatory T (TREG) cell suppressive activity, which was unperturbed by ZAP-70 catalytic inhibition. Our results implicate ZAP-70 as an attractive therapeutic target.
Introduction
Following T cell receptor (TCR) engagement by peptide-bound major histocompatibility complex (MHC) molecules, the immunoreceptor tyrosine-based activation motifs (ITAMs) within the CD3 and ζ chains are phosphorylated by the Src family kinase (SFK) Lck. The tyrosine kinase ζ-associated protein of 70 kDa (ZAP-70) is recruited to, and associates with, dually phosphorylated ITAMs, where it is phosphorylated by Lck and becomes catalytically active1. Subsequently, active ZAP-70 (http://www.signaling-gateway.org/molecule/query?afcsid=A002396) phosphorylates at least two substrates, the adaptor proteins linker for activated T cells (LAT) and SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76), which in turn facilitate the assembly of molecular complexes important for the activation of downstream signals2,3.
The critical role of ZAP-70 in TCR signal transduction was demonstrated by the defects in thymic development resulting from ZAP-70 deficiency. In mice, ZAP-70 expression is required for thymic development to progress beyond the CD4+CD8+ double positive (DP) stage4,5, whereas ZAP-70 deficient human patients suffer from a form of severe combined immunodeficiency (SCID) characterized by a lack of peripheral CD8+ T cells and the presence of peripheral CD4+ T cells that fail to induce signals downstream of the TCR6,7.
The use of knockout mice has proven to be a powerful approach to study the role of many tyrosine kinases in TCR signal transduction and T cell biology. However, due to the paucity of normal mature T cells in ZAP-70 deficient mice and humans, it has been difficult to determine the requirements for ZAP-70 in primary peripheral T cell responses. Additionally, mouse knockouts are limited by genetic compensation and redundancy of functions. With inducible deletions, the uncertainty of the time at which loss of protein expression occurs and the level at which effective loss of function occurs diminishes their value for examining rapid effects on loss of function. Finally, such loss-of-function studies are not reversible, short of gene transfer.
Small-molecule inhibitors can be used to study the temporal requirements of a kinase’s catalytic activity for TCR signaling and activation as well as potential therapies. However, specificity is a major limitation in the use of kinase inhibitors. A cell permeable, highly specific inhibitor of ZAP-70 has not yet been reported. To address this, we generated a ZAP-70 mutant that retains catalytic activity, yet can be inhibited by an analog of the small molecule kinase inhibitor 4-amino-1-tert-butyl-3-(p-methylphenyl) pyrazolo [3,4-d] pyrimidine (PP1), which does not inhibit wild-type (WT) kinases by virtue of a conserved bulky ‘gatekeeper’ residue across the kinome8. Specificity for the inhibitor in this system is encoded genetically, thus allowing for specific inhibition of the engineered mutant, but not WT ZAP-70. The analog-sensitive ZAP-70 protein, ZAP-70(AS), contains a mutation of the ‘gatekeeper’ residue methionine 414 to alanine within the ATP-binding domain, which allows it to accommodate a bulkier analog of PP1, 3-MB-PP19. The genetic control of inhibitor sensitivity also allows the use of mixtures of T cell populations with differing ZAP-70 inhibitor sensitivity to assess the role of the same kinase in different cellular contexts. Together, these features make the analog-sensitive system a powerful tool for determining the role of ZAP-70 catalytic activity in T cells.
To explore the requirements for ZAP-70 activity by mature murine T cells, we generated mice expressing a Zap70(AS) transgene. The analog-sensitive system allowed us to investigate the circumstances under which T cells require ZAP-70 activity for activation through the TCR. Secondly, this system enabled us to study the role of ZAP-70 activity in the activation of naturally occurring CD4+CD25+ regulatory T (TREG) cell function. Thirdly, the use of a catalytic inhibitor allowed us to uncover the biological importance of a non-catalytic function of ZAP-70 in TREG cells.
Results
Generation of ZAP-70 Analog-Sensitive mice
To analyze the requirements for ZAP-70 catalytic activity by mature murine T cells, we generated transgenic mice that express a bacterial artificial chromosome (BAC) transgene containing the murine Zap70 locus bearing the gatekeeper residue mutation M413A. Methionine 413 in murine ZAP-70 is homologous to the human ZAP-70 M414 gatekeeper residue. We generated two independent founder ZAP-70 M413A transgenic strains and crossed them with ZAP-70 null mice4 to generate ZAP-70 M413A transgene-expressing Zap70−/− mice. We used a BAC transgene strategy rather than a knock-in strategy for two reasons. First, genetic changes within the endogenous Zap70 locus, by knock-in as well as spontaneous mutation, have resulted in decreased ZAP-70 protein expression and T cell lymphopenia10,11. Secondly, the ZAP-70(AS) mutant kinase is hypomorphic, with catalytic activity approximately one third that of WT ZAP-709. We reasoned that increased expression of the ZAP-70(AS) protein might be needed to rescue T cell development. Indeed, one founder strain, which expressed 3.5-fold less ZAP-70 protein compared to WT T cells (Supplementary Fig. 1a), had few peripheral CD4+ and CD8+ T cells (Supplementary Fig. 1b). The second founder strain expressed 10-fold more ZAP-70 protein compared to WT (Supplementary Fig. 2a), but had similar frequencies and total numbers of thymocyte subsets compared to WT mice (Supplementary Fig. 2b,c). Based on these data, we decided to continue our studies with this strain, hereafter referred to as Zap70(AS).
Phenotypic analysis of the thymi from Zap70(AS) mice revealed that expression of the analog-sensitive ZAP-70 mutant was sufficient to support the development of CD4 single positive (SP) and CD8 SP thymocytes, overcoming the developmental blockade in Zap70−/− mice at the DP stage (Supplementary Fig. 2b,c). Further analysis of peripheral T cells showed that the percentages and total numbers of CD4+ and CD8+ T cells in the spleens of Zap70(AS) mice were comparable to WT and Zap70+/− mice (Supplementary Fig. 2b,d). Within the peripheral T cell population, the frequencies of T cells with a naïve CD44loCD62Lhi versus effector-memory CD44hiCD62Llo phenotype were more comparable between Zap70(AS) and Zap70+/− mice (Supplementary Fig. 2e and data not shown). Based on the phenotypic similarities between Zap70(AS) mice with Zap70+/− heterozygous mice, we used Zap70+/− T cells as the control cell type in our subsequent studies.
ZAP-70 catalytic inhibition impairs T cell activation
We examined the circumstances under which ZAP-70 catalytic activity is required during different stages of a T cell response: initial activation, effector function, and the memory response. The best-known biochemical role for ZAP-70 involves the phosphorylation of its substrates LAT and SLP-76, which nucleate a complex of signaling molecules, leading to the phosphorylation and activation of phospholipase C-γ (PLCγ) and subsequent second messenger pathways, such as the IP3-calcium flux pathway and the diacylglycerol-MAP kinase pathway. Stimulation of Zap70(AS) or control Zap70+/− CD4+ T cells by cross-linking cell surface-bound anti-CD3ε monoclonal antibody (mAb) in the presence of vehicle (DMSO) resulted in a robust increase in the cytoplasmic free Ca2+ concentration [Ca2+]i (Fig. 1a and Supplementary Fig. 3a). However, simultaneous addition of the ZAP-70(AS)-specific inhibitor 3-MB-PP1 with anti-CD3ε mAbs resulted in a dose-dependent reduction in the [Ca2+]i. Inhibition of [Ca2+]i increase by 3-MB-PP1 could be bypassed with the addition of ionomycin (data not shown). In the presence of high concentrations of 3-MB-PP1 (e.g. 10 µM 3-MB-PP1), there was a small inhibitory effect on the [Ca2+]i increase in Zap70+/− T cells or B cells, however this effect did not reach the same magnitude of inhibition as seen with Zap70(AS) T cells, or with Zap70+/− T cells in other assays. We have also never reached an IC50 dose for cells expressing WT ZAP-70. Thus, we conclude that 3-MB-PP1 is specific for ZAP-70(AS), but not for WT ZAP-70 or other WT kinases known to be required for calcium increases (i.e., SFK, Syk, and Tec family kinases).
Figure 1.
T cell activation is dependent on ZAP-70 catalytic activity. (a) Flow cytometric analysis of Fluo-3 loaded Zap70+/− and Zap70(AS) CD4+ cells. For T cells, the arrows indicate 1) the simultaneous addition of 3-MB-PP1 and anti-CD3ε mAb and 2) the addition of crosslinking antibodies. For B cells, the arrow indicates the addition of anti-IgM plus the indicated doses of 3-MB-PP1. (b) Flow cytometric analysis of phospho-ERK staining in Zap70+/− (top) and Zap70(AS) (bottom) CD4+ T cells. Cells were stimulated with biotinylated anti-CD3ε and anti-CD4 plus streptavidin, in the presence of vehicle only (blue line), 5 µM (orange line), 10 µM (green line) and 10 µM 3MB-PP1 plus PMA (thin black line). Unstimulated cells are indicated by the filled gray histogram. (c) % of Zap70+/− (gray bars) and Zap70(AS) (black bars) T cells positive for CD69 after stimulation with plate-bound anti-CD3ε and anti-CD28 for 16 hours in the presence of 3-MB-PP1. Concentration of 3-MB-PP1 indicated on the x-axis. Percentages are normalized to vehicle-treated cells. (d) Immunoblot analysis of whole cell lysates from purified Zap70+/− and Zap70(AS) CD4+ T cells. Cells were left unstimulated, or stimulated with anti-CD3ε and crosslinking antibodies in the presence of vehicle, 5 µM, or 10 µM 3-MB-PP1 for 2 minutes. Blots probed with antibodies specific for the indicated epitopes. Data are representative of at least 3 independent experiments.
The use of a small molecule inhibitor allows for temporal control of the inhibition of ZAP-70 catalytic activity. Consistent with previous experiments in Jurkat cells9, addition of 3-MB-PP1 to Zap70(AS) T cells at the peak of the TCR-induced calcium response resulted in a rapid decrease in [Ca2+]i within 30 seconds (Supplementary Fig. 3b). Thus, continuous ZAP-70 catalytic activity is required to sustain increases in [Ca2+]i.
Alternatively, we asked if the effects of 3-MB-PP1 were reversible. To test this, we stimulated Zap70(AS) T cells in the presence of 3-MB-PP1, which resulted in the inhibition of [Ca2+]i (Supplementary Fig. 3a). However, when the Zap70(AS) T cells were washed with fresh media, [Ca2+]i increase was rapidly observed (Supplementary Fig. 3c). These results show that the chemical-genetic system is not only specific, but can be rapidly induced and reversed.
TCR-dependent MAP kinase activation is similarly dependent on ZAP-70 catalytic activity. TCR stimulation in the presence of vehicle alone induced a robust phospho-ERK increase in both Zap70+/− and Zap70(AS) CD4+ cells (Fig. 1b). 3-MB-PP1 was effective at inhibiting phospho-ERK levels in Zap70(AS), but not Zap70+/−, CD4+ T cells in a dose-dependent manner (Fig. 1b). Furthermore, stimulation of ERK phosphorylation with the phorbol ester PMA was not impaired in the presence of 3-MB-PP1, consistent with a role for 3-MB-PP1 in specifically inhibiting the upstream kinase activity of ZAP-70 and not other downstream kinases in the Ras-MAPK pathway. TCR stimulation in the presence of 3-MB-PP1 for 16 hours also had a striking inhibitory effect on the Ras-MAPK-dependent upregulation of CD69 on Zap70(AS), but not Zap70+/− CD4+ T cells (Fig. 1c).
Immunoblotting of whole cell lysates from peripheral CD4+ T cells from Zap70+/− and Zap70(AS) mice (Fig. 1d) showed that phosphorylation of the activation loop tyrosine of Lck was not inhibited in the presence of 3-MB-PP1, consistent with a role for Lck upstream of ZAP-70 (Fig. 1d, top). As expected, phosphorylation of molecules downstream of ZAP-70, such as LAT Tyr132 and ERK was impaired in the presence of 3-MB-PP1 (Figure 1d, bottom). Interestingly, two tyrosines within ZAP-70 itself (Tyr319 and Tyr493) were still phosphorylated in the presence of 3-MB-PP1, consistent with our previous results9, presumably since phorphorylation of these sites is mediated by Lck (Fig. 1d, middle). Together, these results suggest that the activation of early events in mature naive T cells requires ZAP-70 catalytic function.
T cell proliferation requires ZAP-70 catalytic activity
We hypothesized that T cell proliferation would also be highly dependent on ZAP-70 catalytic activity. In response to anti-CD3ε-CD28 stimulation in the presence of vehicle alone, both Zap70(AS) and Zap70+/− CD4+ T cells underwent similar amounts of proliferation, as measured by [3H]-thymidine uptake on Day 3 (Fig. 2a). However, Zap70(AS) T cell proliferation was roughly halved in the presence of 5 µM 3-MB-PP1, and nearly completely inhibited at a dose of 10 µM. The decreased sensitivity of the proliferative response (IC50 of ~5 µM), compared to the CD69 induction (IC50 of ~2 µM), also indicates that different assays of T cell function may have different sensitivities to inhibition. For that reason, in nearly all our subsequent assays, we used a high dose of 3-MB-PP1 to ensure maximal Zap70(AS) inhibition while minimizing off target effects, as assessed via WT genetic controls.
Figure 2.
Proliferation by CD4+ T cells requires ZAP-70 catalytic activity. (a) Purified polyclonal CD4+ T cells from Zap70+/− (gray bars) and Zap70(AS) (black bars) were stimulated with plate-bound anti-CD3ε and anti-CD28 mAbs for 72 hours in the presence of vehicle or 3-MB-PP1; concentrations indicated below the x-axis. Data are presented as the mean 3H thymidine uptake from triplicate cultures. Error bars indicate the standard deviation of the mean. (b) CFSE loaded Zap70+/− (filled gray histogram) and Zap70(AS) (black line) OT-II T cells were stimulated with irradiated antigen presenting cells and 1 µM OVA323–339 peptide for 72 hours. The concentration of 3-MB-PP1 present in each culture is indicated above each plot.
We observed similar inhibitory results by CFSE (carboxyfluorescein diacetate succinimidyl ester) dilution in cultures of Zap70+/− and Zap70(AS) OT-II TCR transgenic CD4+ T cells stimulated with ovalbumin323–339 peptide in the presence of antigen-presenting cells (APC) (Fig. 2b). Thus, ZAP-70 kinase activity is required to drive anti-CD3 antibody- or antigen-induced proliferative responses by CD4+ T cells.
Effector T cell responses require ZAP-70 kinase activity
We next examined whether ZAP-70 catalytic activity is required at the time of a secondary TCR stimulation for the execution of effector T cell function. The ZAP-70 analog-sensitive system allowed us, for the first time, to investigate specifically whether ZAP-70 catalytic activity is required for the production of effector cytokines and cytolytic activity.
To determine whether effector CD4+ cells require ZAP-70 activity to produce effector cytokines, we cultured Zap70+/− and Zap70(AS) OT-II transgenic cells under TH1 or TH2 polarizing conditions in the absence of 3-MB-PP1. Primed effector cells were then re-stimulated in the presence of vehicle alone or graded concentrations of 3-MB-PP1 (Fig. 3a). In the presence of vehicle alone, Zap70(AS) TH1 and TH2 effectors could produce IFNγ and IL-4, respectively. However, in the presence of 10 µM 3-MB-PP1, the frequencies of Zap70(AS) IFNγ+ TH1 cells and IL-4+ Th2 cells were reduced at least 10-fold. Stimulation of effector cells with ionomycin and PMA, which bypass proximal TCR signaling events, was not sensitive to 3-MB-PP1 (Fig. 3a, right). These results suggest that previously activated CD4+ T cells are dependent on ZAP-70 catalytic activity when stimulated via their TCR for the production of effector cytokines.
Figure 3.
Execution of effector T cell functions requires ZAP-70 catalytic activity. (a) Flow cytometric analysis of intracellular cytokine staining for IFNγ and IL-4 by effector TH1 and TH2 Zap70+/− and Zap70(AS) OT-II cells, respectively. Numbers within the dot plots indicate the percentage of cells within each quadrant. The concentrations of 3-MB-PP1 present in each culture are indicated above the dot plots. (b) Lysis of allogeneic 51Cr labeled P815 target cells by Zap70+/− and Zap70(AS) CTLs. Cell assay cultures containing effector and target cells were supplemented with vehicle alone (gray line), 10 µM 3-MB-PP1 (black), or CsA (dashed black line). (c) Alloreactive Zap70+/− (gray bars) and Zap70(AS) (black bars) CTLs, generated as in (b) were stimulated by P815 cells in the presence of 3-MB-PP1 (top) or CsA (bottom). Numbers on the x-axis indicate the final concentration of 3-MB-PP1 (µM) or CsA (µg/ml). Data are representative of 3 experiments.
To address whether ZAP-70 activity is required for CD8+ T cell effector function, we generated C57BL/6 (H-2b) Zap70+/− and Zap70(AS) cytotoxic T lymphocytes (CTLs) in mixed lymphocyte reactions with allogeneic stimulator cells (DBA/2, H-2d). After 5 days, the primed Zap70+/− and Zap70(AS) effector cells were co-cultured with 51Cr labeled P815 mastocytoma target cells (H-2d) to assess cytolytic effector function (Fig. 3b). In contrast to Zap70+/− control cells (Fig. 3b, top), Zap70(AS) CTLs were severely impaired in their ability to lyse target cells in the presence of 10 µM 3-MB-PP1 (Fig. 3b, bottom). These results suggest that cytolytic activity by primed CTLs is highly dependent on ZAP-70 activity. In contrast, addition of the calcineurin inhibitor cyclosporine A (CsA), failed to inhibit CTL activity by either Zap70+/− or Zap70(AS) T cells.
We next asked whether cytokine production by alloreactive CD8+ cells is also sensitive to the inhibition of ZAP-70 activity. Similar to Zap70(AS) CD4+ effectors, Zap70(AS) CD8+ effectors were defective in producing TNF in the presence of 3-MB-PP1 (Fig. 3c, top). By comparison, CsA was also effective at inhibiting the production of TNF by both Zap70+/− and Zap70(AS) alloreactive CD8+ T cells (Fig. 3c, bottom). In addition, IFNγ production by Zap70(AS) CD8+ effectors could also be potently inhibited in the presence of 3-MB-PP1, while CsA inhibited IFNγ production by both Zap70+/− and Zap70(AS) cells (Supplementary Fig. 4). Thus, in this experimental system, the TCR-dependent signals required for cytolytic activity are dependent on ZAP-70 kinase activity, but independent of calcineurin activity, whereas effector cytokine production is dependent on the activities of both ZAP-70 and calcineurin.
Memory CD8+ T cell responses require ZAP-70 activity
To examine whether memory T cell cytokine responses require ZAP-70 activity, we generated CD8+ memory T cells by infecting Zap70+/− and Zap70(AS) mice with lymphocytic choriomeningitis virus (LCMV). After >50 days post-infection, T cells were re-stimulated ex vivo in the presence or absence of 3-MB-PP1. Both Zap70+/− and Zap70(AS) T cells produced IFNγ and TNF following stimulation with LCMV peptides GP33–41 (Fig. 4a) and NP396–404 (Fig. 4b) in the presence of vehicle alone. Strikingly, addition of 3-MB-PP1 resulted in a dose-dependent reduction in the frequencies of IFNγ+TNF+Zap70(AS) cells (Fig. 4c,d), demonstrating that, similar to recently activated CD8+ effector cells, memory CD8+ T cells are highly dependent on ZAP-70 catalytic activity for effector cytokine production.
Figure 4.
CD8 memory responses are dependent on ZAP-70 catalytic activity. (a,b) Flow cytometric analysis of LCMV (a) GP33–41-specific and (b) NP396–404-specific IFNγ and TNFα production by Zap70+/− and Zap70(AS) memory CD8+ cells. Contour plots are gated on CD8+ CD44high memory cells. The concentrations of 3-MB-PP1 present are indicated above the plots. (c, d) Graphs display the percentage of IFNγ+ TNFα+ cells at each concentration of 3-MB-PP1 tested. Percentages are of CD8+ CD44high memory Zap70+/− (black line) and Zap70(AS) (gray line) cells. Error bars indicate the standard deviation from the mean; n=3. Data are representative of 3 experiments.
Suppression by TREG does not require ZAP-70 activity
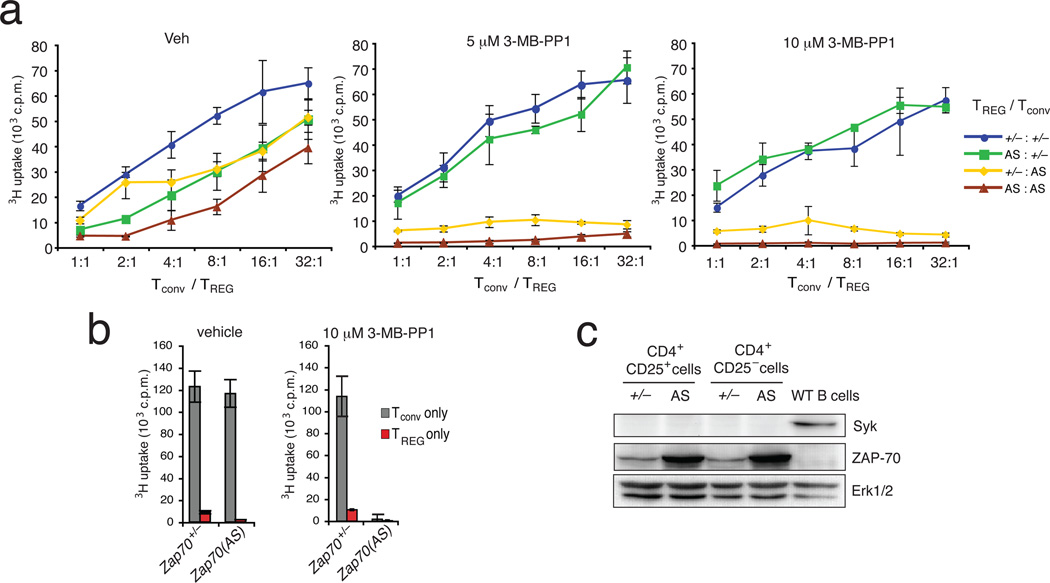
Our results suggest that conventional T cells require ZAP-70 activity for activation of naive, effector and memory cells. However, it remained unclear whether naturally-occurring CD4+CD25+ TREG cells also require the activity of ZAP-70 to exert their suppressive activity. One advantage of the ZAP-70(AS) system is the ability to determine the requirement for ZAP-70 activity in a single type of T cell, within a complex culture of multiple T cell subsets. Specifically, by co-culturing Zap70+/− CD4+ CD25− conventional T cells (Tconv) with Zap70(AS) TREG, we could address the requirements for ZAP-70 activity by TREG cells. Previous studies have suggested that TREG cells require stimulation through their TCR to activate their suppressive function(s) in vitro12,13. Based on these reports, we hypothesized that TREG cells also require the catalytic activity of ZAP-70 to suppress the proliferation of Tconv cells. Thus, we cultured Tconv and TREG cells from Zap70+/− and Zap70(AS) mice in all combinations, and at different ratios of Tconv:TREG (Fig. 5a). In the presence of vehicle alone, we observed that TREG cells from either Zap70+/− or Zap70(AS) mice suppressed the proliferation of either Zap70+/− or Zap70(AS) Tconv cells (Fig. 5a, left). By adding 3-MB-PP1 to the culture, we predicted the suppressive activity of Zap70(AS) TREG cells would be impaired, allowing for uninhibited proliferation of the Zap70+/− Tconv population. However, Zap70(AS) TREG cells suppressed the proliferation of Zap70+/− Tconv cells (Fig. 5a, middle and right), even in the presence of concentrations of 3-MB-PP1 that severely inhibited the proliferation of Zap70(AS) Tconv cells (Fig. 5b). Similar results were observed in a CFSE-based suppression assay (Supplementary Fig. 5). Contrary to our initial hypothesis, these results suggest that in vitro TREG cell-mediated suppression does not require the catalytic activity of ZAP-70. This led us to ask whether the activity of ZAP-70 is redundant in TREG cells, for instance, due to expression of the related kinase Syk. However, neither Tconv cells nor TREG cells from Zap70+/− and Zap70(AS) mice expressed detectable Syk protein by immunoblot (Fig. 5c). These results indicate that the capacity of Zap70(AS) TREG cells to suppress Tconv proliferation in a ZAP-70 kinase-independent manner is not due to redundant function provided by Syk.
Figure 5.
ZAP-70 catalytic activity is dispensable for TREG cell suppressive activity in vitro. (a) In vitro suppression assays used CD4+CD25− Tconv cells from both Zap70+/− and Zap70(AS) mice, together with irradiated antigen-presenting cells, anti-CD3ε mAbs, and titrated numbers of Zap70+/− or Zap70(AS) CD4+CD25+ TREG cells. Cultures were set up in the presence of vehicle alone, 5 µM, or 10 µM 3-MB-PP1. Data are presented as the average 3H thymidine uptake in triplicate cultures. Error bars indicate the standard deviation of the mean. (b) Bar graphs show the 3H thymidine uptake of Tconv or TREG cells alone in the presence of vehicle alone or 3-MB-PP1. (c) Immunoblots of whole cell lysates from purified Zap70+/− and Zap70(AS) Tconv and TREG cells were probed for expression of Syk, ZAP-70, and ERK1-ERK2. Data in all panels are representative of at least 3 experiments.
Activation of Rap1 is independent of ZAP-70 activity
To determine how TREG cells might suppress Tconv cell proliferation independently of ZAP-70 kinase activity, we asked whether there are TCR-induced signal transduction pathways that are unperturbed in the presence of a ZAP-70 catalytic inhibitor. We first analyzed the calcium responses of Zap70+/− and Zap70(AS) CD4+CD25+ TREG cells previously expanded in vitro14. In the absence of inhibitor, the calcium response was markedly reduced, but detectable in CD25+ TREG cells compared to CD25− cells (Fig. 6a), consistent with previous reports of hypo-responsive TCR signaling in TREG cells15,16. Addition of 3-MB-PP1 potently inhibited the [Ca2+]i increase in Zap70(AS) Tconv and TREG cells, indicating that ZAP-70 catalytic activity is required even for the reduced TCR-stimulated [Ca2+]i increases in TREG cells. In response to TCR stimulation alone, we also observed reduced, but detectable ERK phosphorylation in Foxp3+ TREG cells, compared to Foxp3− cells (Fig. 6b). Again, the levels of phospho-ERK were inhibited to basal levels following TCR stimulation in the presence of 3-MB-PP1 in both Foxp3− and Foxp3+ Zap70(AS) cells. Together, these results suggest that ZAP-70 catalytic activity is required for TCR-induced [Ca2+]i increases and ERK responses in TREG cells, but not for their suppressive function.
Figure 6.
TCR-induced activation of Rap1 and adhesion to ICAM-1 are ZAP-70 kinase-independent. (a) CD4+CD25− Tconv and CD4+CD25+ TREG cells were loaded with Fluo-3 and Fura Red for analysis of intracellular calcium levels by flow cytometry. Cells were stimulated in the presence of vehicle alone (red) or 3-MB-PP1 (blue). Arrow “1” indicates the addition of biotinylated anti-CD3ε and anti-CD4, arrow “2” indicates the addition of streptavidin, and arrow “3” indicates the addition of ionomycin. Tconv were >99% Foxp3− and TREG cells were >98% Foxp3+. (b) Splenocytes were stimulated ex vivo by crosslinking anti-CD3 mAbs and stained for phospho-ERK. Histograms are gated on Foxp3-negative or Foxp3-positive CD4+ cells. Cells were either unstimulated (filled gray histograms) or stimulated in the presence of vehicle alone (red), 5 µM 3-MB-PP1 (green), 10 µM 3-MB-PP1 (blue), or 10 µM 3-MB-PP1 plus PMA (gray line). (c) CrkII was immunoprecipitated (IP) from Zap70(AS) thymocyte lysates. Cells were stimulated by crosslinking anti-CD3 mAbs for 2 minutes. Immunoprecipitated proteins were immunoblotted (IB) for ZAP-70 and CrkII (top). Whole cell lysates were also immunoblotted for the presence of ZAP-70 and CrkII (bottom). (d) Pull-down assay for Rap1-GTP in Zap70+/− and Zap70(AS) thymocytes. Cells were left unstimulated, or stimulated with crosslinked anti-CD3ε mAb for 2 minutes. The concentrations of 3-MB-PP1 are indicated. Total Rap1 levels in whole cell lysates are shown below. (e) CD4+ T cells from Zap70+/− and Zap70(AS) were stimulated with anti-CD3ε for 10 minutes in wells coated with recombinant ICAM-1. Cells were then washed and counted by flow cytometry. Data in all panels are representative of 3 independent experiments.
ZAP-70 itself is phosphorylated normally on Tyr319 and Tyr493 in the presence of a ZAP-70 catalytic inhibitor (Fig. 1d). These results raised the possibility that ZAP-70 could act as a scaffold protein in the absence of its catalytic activity, via interaction between phosphorylated tyrosines within its Interdomain B region and other SH2 domain-containing proteins. One candidate that fits this model is the adaptor protein CT10 regulator of kinase (Crk) II, which has been reported to bind phosphorylated Tyr315 following TCR stimulation17. Previous work also showed that Crk adaptor proteins associate with the guanine nucleotide exchange factor (GEF) C3G18,19. C3G can act as a GEF for the GTPase Rap1, which potentiates the inside-out signaling pathway to the integrin LFA-1, resulting in increased affinity of LFA-1 for its ligand, ICAM-1. Thus, it is possible that even in the absence of catalytic function, phosphorylated ZAP-70 is able to form a complex with CrkII and C3G, leading to enhanced LFA-1-mediated cellular adhesion.
To test this model, we asked whether ZAP-70 can co-immunoprecipitate with CrkII, but also whether this association was dependent on ZAP-70’s catalytic activity. Indeed, ZAP-70(AS) protein co-immunoprecipitated with CrkII in a TCR-inducible manner, but this interaction was unperturbed in the presence of 3-MB-PP1 (Fig. 6c). To test an additional signal in the inside-out pathway, we assayed for Rap1 activation in Zap70+/− and Zap70(AS) CD4+ T cells using a Rap1-GTP pull-down assay. Following TCR stimulation in the presence of vehicle alone, Rap1 activation was induced in both Zap70+/− and Zap70(AS) T cells. In the presence of 3-MB-PP1, activation of Rap1-GTP was still induced in Zap70(AS) T cells (Fig. 6d). These results reveal that ZAP-70 catalytic activity is not required for TCR-induced activation of Rap1. Consistent with these results, TCR-induced adhesion to plate-bound ICAM-1 by CD4+ T cells was reduced by PP2, an inhibitor of Src family kinases, but not by 3-MB-PP1 (Fig. 6e), implying that TCR-induced inside-out signaling regulating cellular adhesion is dependent on Src family kinase (Lck, Fyn) activity, but not ZAP-70 kinase activity.
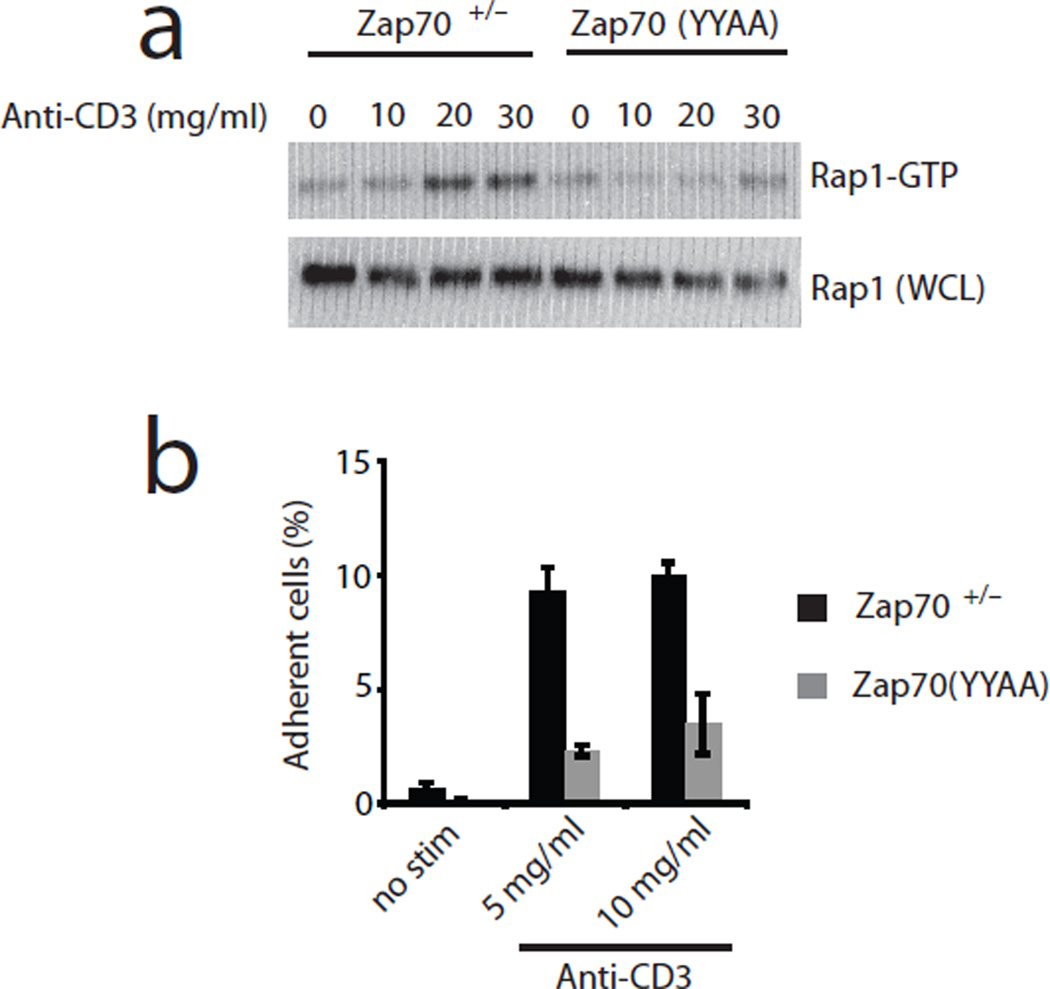
We next asked whether mutation of tyrosine residues 315 and 319 within ZAP-70 would disrupt TCR-induced activation of Rap1. We previously generated knock-in mice bearing mutations of both ZAP-70 Tyr315 and Tyr319 to alanine (Zap70(YYAA))10. Indeed, Rap1-GTP induction in Zap70(YYAA) T cells was reduced compared to WT (Fig. 7a), suggesting that Tyr315 and Tyr319 are required for TCR-induced Rap1 activation. Similarly, Zap70(YYAA) T cells were also impaired in their TCR-induced adhesion to ICAM-1 (Fig. 7b). Together, these results imply a role for ZAP-70 Tyr315 and Tyr319 in inside-out signaling to integrin activation and cellular adhesion.
Figure 7.
TCR-induced activation of Rap1 and adhesion to ICAM-1 are dependent on ZAP-70 adapter function. (a) Rap1 pulldown assay from Zap70+/− and Zap70(YYAA) thymocytes. Cells were stimulated with the indicated doses of anti-CD3ε for 2 minutes in the absence of 3-MB-PP1. (b) TCR-induced ICAM-1 adhesion by Zap70+/− and Zap70(YYAA) T cells. Peripheral CD4+ T cells were stimulated as in Fig. 6d with the indicated doses of anti-CD3ε. Data are representative of 3 independent experiments.
From these results we conclude that a ZAP-70 kinase-independent scaffold function can contribute to activation of integrin-mediated adhesion to ICAM-1. Our model proposes that Zap70(AS) TREG cells are able to activate their suppressive function(s), even when ZAP-70 catalytic activity is inhibited, by maintaining close proximity to target Tconv cells through enhanced ICAM-1 adhesion. Consistent with this model, TREG cells bearing the Zap70(YYAA) mutations, which impair Rap1 activation and ICAM-1 adhesion, also display defective suppressive activity in vitro (Supplementary Fig. 6 and reference10). Also consistent with this model, IL-2 stimulation of Zap70(AS) TREG cells results in STAT5 phosphorylation, even in the absence of ZAP-70 catalytic function (Supplementary Fig. 7). These data describe a novel, kinase-independent role for ZAP-70 in integrin activation and TREG cell activity.
Discussion
The analog-sensitive system allowed us, for the first time, to determine when ZAP-70 catalytic activity is required for the activation of mature, primary murine T cells. Our results demonstrate that initial T cell activation, proliferation, effector function, and memory responses by conventional CD4+ and CD8+ T cells are all highly dependent on ZAP-70 catalytic activity. These data are consistent with previous work which demonstrated failed TCR signal transduction in ZAP-70 deficient Jurkat cell lines and similar impairments in peripheral CD4 T cells of ZAP-70 deficient SCID patients6.
However, we did not anticipate that the in vitro suppressive activity of TREG cells would not require ZAP-70 cataytic activity. We show that TCR-induced association of ZAP-70 with CrkII, activation the GTPase Rap1 and adhesion to ICAM-1 occurs even in the presence of a ZAP-70 catalytic inhibitor. These results are in agreement with previous reports showing associations between ZAP-70 Tyr315, CrkII, and C3G17,20. This model implies a role for ZAP-70 as a protein scaffold, independent of its catalytic function. While the proposed scaffold function of ZAP-70 appears not to be sufficient for the activation and effector function of conventional T cells, the same might not be true for TREG cell function. We propose that Zap70(AS) TREG cells, even in the presence of 3-MB-PP1, have TCR-induced phosphorylation of ZAP-70 on Tyr315, followed by the association of this phosphorylated residue with CrkII-C3G, thus facilitating the activation of Rap1, resulting in increased LFA-1 adhesion to ICAM-1 expressed on APCs. Enhanced adhesion by Zap70(AS) TREG cells potentially enhances their close proximity to APCs and target Tconv cells, where they can utilize multiple mechanisms of suppression; i.e., via CTLA-4, competition for IL-2, suppression of co-stimulatory molecule expression on dendritic cells, or metabolic disruption of Tconv proliferation through the production of adenosine via the ectoenzymes CD39 and CD7321,22. Consistent with this model, Zap70(AS) TREG cells do not require ZAP-70 catalytic function to respond to IL-2. These results are compatible with recent computational modeling studies, which implicates the distance between TREG cells and Tconv cells as an important factor in the competition between these cells for IL-223. Additionally, studies of CD18-deficient murine TREG cells as well as LFA-1 deficient TREG cells from human leukocyte adhesion deficiency type1 (LAD-1) patients, provide evidence that LFA-1 on TREG cells is important for their suppressive function24,25. Moreover, TREG cells expressing a constitutively active Rap1 mutant transgene display enhanced suppressive activity26.
A prediction from our model is that TREG cells bearing ZAP-70 mutations at residues Tyr315 and Tyr319 would have impaired ZAP-70 kinase-independent scaffold function, leading to impaired adhesion and TREG cell-mediated suppression. Indeed, we show here (and in reference 10) that TREG cells from Zap70(YYAA) knock-in mice have impaired suppressive activity. While Zap70(YYAA) T cells do have impairments in downstream signaling, such as calcium increases and ERK phosphorylation, the SKG mutation, a different hypomorphic allele of ZAP-70, results in even greater impairments in downstream TCR signaling, yet has little impact on TREG suppressive activity10. These results provide strong evidence for an important non-catalytic function of ZAP-70 in TREG cell function. We conclude that, at least in the context of ZAP-70 catalytic inhibition (Zap70(AS) TREG cells in the presence of 3-MB-PP1), ZAP-70 scaffold-mediated activation of integrin adhesion is sufficient to enable TREG cell suppression in vitro. It remains to be tested whether ZAP-70 catalytic activity is required for TREG cell function in vivo.
Previously published reports show that TREG cells require TCR stimulation to activate their suppressive activity12,13. Recent data also show that TREG cells lacking Lck expression are defective in suppression27. Our data are not necessarily incompatible with these studies. Rather, we conclude that in vitro TREG cell suppression requires both TCR and ZAP-70, but more specifically, the scaffold function of ZAP-70. An intriguing implication for this finding is the possibility that a catalytic inhibitor of ZAP-70 might be utilized to dampen the response of pathogenic conventional T cells in settings of autoimmunity or allograft rejection, while not rendering the suppressive activity of TREG cells deficient.
Our results show that the activation of naïve, effector, and memory T cells requires ZAP-70 catalytic activity. These data imply that it could be possible to use a catalytic inhibitor of ZAP-70 to both prevent a pathogenic T cell response, as in the setting of allogeneic transplantation, and also dampen previously established pathogenic T cell responses, as in the setting of autoimmunity. Furthermore, a catalytic inhibitor of ZAP-70 might have advantages over existing inhibitors, such as the calcineurin inhibitor CsA. Treatment with a ZAP-70 catalytic inhibitor was capable of attenuating both cytotoxicity and effector cytokine production by CTL, while treatment with CsA was only effective in impairing cytokine production.
The efficacy of a ZAP-70 inhibitor in the treatment of mouse models of autoimmunity remains to be tested; however the half-life of 3-MB-PP1 in vivo appears too short to achieve long-term blood concentrations approaching 10 µM to perform such studies at this time. Characterizations of structural analogs of 3-MB-PP1 that have more suitable pharmacokinetics for long term in vivo use are currently under investigation.
This study describes the first model system to study the role of ZAP-70 catalytic activity in the activation of mature, primary murine T cells. Chemical-genetic approaches may prove to be powerful techniques to define the temporal, as well as catalytic-dependent versus -independent functions of other tyrosine kinases involved in TCR signaling.
Methods
Generation of Zap70(AS) mice
The Zap70(AS) transgenic mice were generated by the UCSF Transgenic and Targeted Mutagenesis Core Facility. A bacterial artificial chromosome (BAC) RP23-6M17 containing the region of chromosome 1 encoding the Zap70 locus was purchased from BACPAC Resources at the Children’s Hospital Oakland Resource Institute (Oakland, CA). Mutation of murine ZAP-70 M413 residue (homologous to human M414) to alanine was engineered by bacterial recombineering. The BAC containing the Zap70(AS) mutation was injected into fertilized Zap70+/− embryos. Mice are genotyped with the following PCR primers:
Transgenic allele: 5’ TTCCTCTCTAACCCGGGAGT 3’; 5’ TCCCGCCATCTCCGC 3’
Wild-type allele: 5’ TTCCTCTCTAACCCGGGAGT 3’; 5’ TCCCGCCATCTCCAT 3’
Knockout allele: 5’ GCACATATGCACTGTCCCTGGTCTA 3’ 5’ TGGCTACCCGTGATATTGCTGAAGA 3’
Mice
Mice used for these studies were 6–10 weeks of age and housed in the specific pathogen-free facility at UCSF and were treated according to protocols approved by university animal care ethics and veterinary committees in accordance with NIH guidelines. Zap70+/− and Zap70(AS) strains were crossed to OT-II TCR transgenic mice. Antigen presenting cells (APCs) were harvested from TCR α-chain deficient mice (Jackson Laboratory). DBA/2 mice were purchased from Taconic. Knock-in mice expressing the Zap70(YYAA) mutation were described previously10.
Inhibitors
The synthesis of 3-methylbenzyl-Pyrazolopyrimidine (3-MB-PP1) has been described previously8. For CD8+ effector T cell assays, Cyclosporine A (Calbiochem) was added at the indicated concentrations.
Flow Cytometry
Cells were stained as indicated and analyzed on FACSCalibur (Becton Dickinson) or CyAn ADP (Beckman Coulter) cytometers. Antibodies against CD4 and CD8 were purchased from the UCSF Antibody Core Facility. Antibodies against IFNγ, TNFα, IL-4, CD25, CD44, CD69, CD62L were purchased from BD Biosciences. Anti-Foxp3 antibodies were purchased from eBioscience.
Calcium flux/phospho-ERK assays
T cells were stimulated for calcium flux and phospho-ERK analysis with soluble biotinylated anti-CD3ε (10 µg/ml) and anti-CD4 (2 µg/ml) mAbs, followed by streptavidin crosslinking. Alternatively, cells were stimulated with anti-CD3ε and crosslinking anti-Armenian Hamster Abs (Jackson Immunoresearch). For calcium analysis, splenocytes were loaded with Fluo-3 (2 µg/ml) and Fura Red (4 µg/ml) (Invitrogen). mAbs against B220, DX5, CD11b, MHC II, Gr-1, Ter119 (BD Pharmingen), and CD8 were used for dump gates.
For phospho-Erk staining, cells were fixed, permeablized with ice-cold 90% methanol and stained with anti-phospho ERK (Cell Signaling). As a positive control, cells were stimulated with 200 µg/ml PMA (Sigma).
Proliferation assays
For [3H] thymidine uptake assays, T cells were stimulated with plate-bound anti-CD3ε (2 µg/ml) and anti-CD28 (2 µg/ml) mAbs, for 72 hours. For the final 6 hours of culture, 1 µCi [3H]-thymidine (GE Healthcare) was added. For CFSE-based proliferation assays, purified T cells were loaded with 5 µM CFSE (Invitrogen) and then stimulated with APCs plus 1 µM OVA323–339 peptide (New England Peptide).
Chromium release assay
Alloreactive CD8+ T cells were generated in a mixed lymphocyte reaction between 25 × 106 Zap70+/− or Zap70(AS) splenocytes and 25 × 106 irradiated DBA/2 splenocytes (1000 rad). On day 5, effectors were titrated and cultured in triplicate with 5 × 103 [51Cr] labeled P815 target cells for 4 hours. P815 cells were loaded with 50 µCi [51Cr]-sodium chromate (Perkin Elmer). Specific lysis was calculated as the percentage of lysis minus the spontaneous release (no effector T cells present), divided by total lysis (hypotonic lysis followed by freeze/thaw).
Th1/Th2 effector cytokine staining
Zap70+/− and Zap70(AS) OT-II cells were stimulated with 1 µM OVA323–339 peptide and irradiated APCs. Th1 polarizing conditions contained, 10 U/ml rhIL-2 (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Dr. Maurice Gately, Hoffmann - La Roche Inc.), 10 ng/ml rIL-12 (Peprotech) and anti-IL-4 (25 µg/ml). Th2 polarizing conditions contained 10 U/ml rhIL-2, 50 ng/ml rIL-4 and anti-IL-12 (UCSF Antibody Hybridoma Core Facility). Effector cells were re-stimulated with plate-bound anti-CD3ε (0.5 µg/ml) for 6 hours, with 10 µg/ml Brefeldin A (Epicentre) present for the final 4 hours.
LCMV infection
Zap70(AS) and Zap70+/− mice were infected with 1 × 105 PFU LCMV (Armstrong) by intraperitoneal injection. After >50 days, splenocytes were harvested and cultured with 0.2 µg/ml NP396–404 peptide (sequence: FQPQNGQFI) or GP33–41 peptide (sequence: KAVYNFATM) for 6 hours in the presence of Brefeldin A and titrated doses of 3-MB-PP1 or vehicle. Cells were fixed and permeabilized using Cytofix and Cytoperm buffers (BD biosciences) according to the manufacturer’s instructions, and stained for IFNγ and TNFα.
Western blot/Immunoprecipitation
Cells were lysed in 2x SDS sample buffer and analyzed by SDS-PAGE and western blot as previously described9. Blots were probed for ZAP-70 (clone 2F3.2), Syk (clone 5F5), and ERK1 and ERK2 (Santa Cruz). For analysis of TCR signaling, antibodies against p-Erk, p-Src Tyr416, and p-ZAP-70 Tyr319 and Tyr493 were from Cell Signaling. Antibodies against p-LAT Tyr132 were from Biosource.
For immunoprecipitation studies, anti-CD3 stimulated thymocytes were lysed in a 1% NP-40 lysis buffer. Lysates were incubated for 2 hours at 4°C with protein A-sepharose beads pre-incubated with polyclonal antisera against CrkII (Santa Cruz).
Rap1 pull-down assay
For each condition, 30 × 106 thymocytes were stimulated with soluble anti-CD3ε (10 µg/ml) and crosslinking anti-hamster Abs (50 µg/ml) (Jackson Immunoresearch) for 2 minutes. Rap1 pull-down assays were done according to the manufacturer’s instructions (Thermo Scientific).
Suppression assays
CD4+CD25− Tconv and CD4+CD25+ TREG cells were enriched using regulatory T cell enrichment kits from Miltenyi or Stem Cell Technologies according to the manufacturers’ protocols. For [3H] thymidine-based assays, 1 × 105 Tconv plus 1 × 105 irradiated APCs were co-cultured with TREG cells for 72 hours in triplicate wells, with 1 µCi [3H] thymidine present for the final 12 hours. Also present was anti-CD3ε mAb at a final concentration of 1 µg/ml, and titrated doses of 3-MB-PP1 or vehicle (DMSO).
ICAM-1 binding assay
Purified CD4+ cells (1 × 106) were stimulated with soluble anti-CD3ε and crosslinking anti-hamster Abs in 96 well plates coated with 3 µg/ml recombinant mouse ICAM-1-Fc (R&D Systems). Wells were washed three times, and plate-bound cells were removed with cell dissociation buffer (Gibco). Plate-bound cells were counted by FACS. The percentage of cells bound was calculated as [(number of live CD4+ cells bound to the plate) ÷ (input number of cells per well)] × 100.
Supplementary Material
Acknowledgements
We would like to thank Al Roque for excellent assistance in animal husbandry, Cliff MacArthur for cell sorting, and Jie Wei for generating the Zap70(AS) BAC transgene construct. This work was supported in part by grants from the NIH, RC2AR058947 (to A.W. from NIAMS), and F32AR056174 (to L.H. from NIAMS).
Footnotes
Author Contributions
B.B.A.Y. performed most of the experiments and wrote the paper. S.E.L. performed the initial characterization of the Zap70(AS) mice. N.K. designed the strategy for generating the Zap70(AS) mice. D.A.C. Assisted with the ICAM-1 adhesion assays and calcium flux assays. L.H. helped with the Zap70(YYAA) TREG cell suppression assay. K.M.S and C.Z. provided advice and synthesized the inhibitor 3-MB-PP1. A.W. directed the project.
References
- 1.Au-Yeung BB, et al. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 2.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 3.Horejsi V, Zhang W, Schraven B. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat Rev Immunol. 2004;4:603–616. doi: 10.1038/nri1414. [DOI] [PubMed] [Google Scholar]
- 4.Kadlecek TA, et al. Differential requirements for ZAP-70 in TCR signaling and T cell development. J Immunol. 1998;161:4688–4694. [PubMed] [Google Scholar]
- 5.Negishi I, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 6.Chan AC, et al. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 7.Elder ME, et al. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 8.Bishop AC, et al. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- 9.Levin SE, Zhang C, Kadlecek TA, Shokat KM, Weiss A. Inhibition of ZAP-70 kinase activity via an analog-sensitive allele blocks T cell receptor and CD28 superagonist signaling. J Biol Chem. 2008;283:15419–15430. doi: 10.1074/jbc.M709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009 doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka S, et al. Graded Attenuation of TCR Signaling Elicits Distinct Autoimmune Diseases by Altering Thymic T Cell Selection and Regulatory T Cell Function. J Immunol. 2010;185:2295–2305. doi: 10.4049/jimmunol.1000848. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 13.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 16.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 17.Gelkop S, Gish GD, Babichev Y, Pawson T, Isakov N. T cell activation-induced CrkII binding to the Zap70 protein tyrosine kinase is mediated by Lck-dependent phosphorylation of Zap70 tyrosine 315. J Immunol. 2005;175:8123–8132. doi: 10.4049/jimmunol.175.12.8123. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, et al. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. J Biol Chem. 2003;278:23978–23983. doi: 10.1074/jbc.M212671200. [DOI] [PubMed] [Google Scholar]
- 19.Gelkop S, Isakov N. T cell activation stimulates the association of enzymatically active tyrosine-phosphorylated ZAP-70 with the Crk adapter proteins. J Biol Chem. 1999;274:21519–21527. doi: 10.1074/jbc.274.31.21519. [DOI] [PubMed] [Google Scholar]
- 20.Sasahara Y, et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell. 2002;10:1269–1281. doi: 10.1016/s1097-2765(02)00728-1. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 22.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Busse D, et al. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A. 2010;107:3058–3063. doi: 10.1073/pnas.0812851107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marski M, Kandula S, Turner JR, Abraham C. CD18 is required for optimal development and function of CD4+CD25+ T regulatory cells. J Immunol. 2005;175:7889–7897. doi: 10.4049/jimmunol.175.12.7889. [DOI] [PubMed] [Google Scholar]
- 25.Tran DQ, et al. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–2938. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, et al. Rap1-GTP is a negative regulator of Th cell function and promotes the generation of CD4+CD103+ regulatory T cells in vivo. J Immunol. 2005;175:3133–3139. doi: 10.4049/jimmunol.175.5.3133. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, et al. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PLoS One. 2009;4:e6580. doi: 10.1371/journal.pone.0006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.