Abstract
Objective
Calcium oxalate monohydrate crystals (COM) stimulate OPN and MCP-1 expression in renal tubular cells. Reactive oxygen species (ROS) play an important but not yet fully defined role in expression of inflammatory genes such as MCP-1 and OPN. Recently, it has been proposed that ROS serve as intracellular signals. NADPH oxidase is the major source of superoxide in many tissues including kidney. We tested the hypothesis that COM exposure of a renal epithelial cell line, NRK 52E, will upregulate NADPH oxidase subunit p47phox, enhance superoxide production and increase MCP-1 and OPN mRNA levels. The latter will be attenuated by diphenileneiodium chloride (DPI), an inhibitor for NADPH Oxidase.
Materials and Methods
Confluent cultures of NRK52E cells were exposed to COM (66.7μg/cm2) with or without pre treatment with DPI (10×10−6M) under serum free conditions. The conditioned medium was collected and total cellular RNA isolated from the cells, and subjected to enzyme-linked immunosorbent assay (ELISA) and real time PCR. Production of ROS was estimated by dihydroethidium (DHE) staining using fluorescence microscope. Immunohistochemistry and real time PCR were used to analyze p47phox in NRK52E cells.
Results
COM treated NRK52E cells showed enhanced expression of p47phox and production of superoxide. COM-induced production of MCP-1 and OPN was significantly reduced following treatment with DPI.
Conclusions
While the generation of a large amount of ROS may play a major role in tissue injury or death, the regulated generation of low concentration of ROS, possibly by NADPH oxidase, may represent a second messenger system for generation of COM induced MCP-1 and OPN in the renal tubules.
Introduction
Kidney stone formation is a common urological disorder with a lifetime risk in the U.S. of nearly 13% in men and 7% in women,1 and the numbers of stone formers are increasing. Over the last 21 years the incidence of stone formation increased nearly 3-fold in Germany (0.54 to 1.47%), with a resulting rise in prevalence from 4.0 to 4.7% for the population as a whole. Among men age 50-64 years, the prevalence was even higher at 9.7%, and over 40% were recurrent stone formers.2 In the United States, the economic impact was recently estimated at $5.3 billion per year, with the majority attributed to direct medical costs.3 The pathogenesis of stones is still poorly understood. Results of tissue culture and animal model studies have led us to conclude that stone formation is a harmful outcome of crystallization and crystal cell interaction within the kidneys.4
Since calcium oxalate (CaOx) is the major constituent of the majority of kidney stones we have been investigating cell/CaOx crystal interaction. Calcium oxalate monohydrate (COM) crystals stimulate renal tubular epithelial cells to produce monocyte chemoattractant protein-1 (MCP-1) as well as osteopontin (OPN). Both these molecules are involved in crystal induced inflammation in various organs including kidneys. Osteopontin also plays a key role in biomineralization, including kidney stone formation where it modulates crystal nucleation, growth, aggregation and retention within the renal tubules.5, 6 Although we have shown that renal epithelial exposure to COM crystals stimulate OPN and MCP-1 expression, the second messenger systems for upregulation of these genes remain unclear. Recently it has been proposed that oxygen radicals may serve as a second messenger system for activation of many genes.7 The generation of large amounts of free radicals play a major role in tissue injury, regulated generation of low concentrations of oxygen radicals, may however, represent a second messenger system of generation of cytokines involved in tissue injury and repair in general.
Attention is currently focused on NADPH oxidase as a critical determinant of the redox state of the kidney.8 NADPH oxidase is a significant source of ROS particularly in the presence of Angiotensin II.9 NADPH oxidase consists of 6 subunits, the two transmembrane units, p22phox and gp91phox; and four cytosolic units, p47phox, p67phox, p40phox and the small GTPase rac1 or rac2. The two transmembrane units, gp91phox and p22phox associate with a flavin to make cytochrome b558. The gp91phox unit is the core catalytic component for the electron transfer activity while p22phox has regulatory and stabilizing functions. Cytosolic units translocate to the membrane and assemble with the cytochrome to activate the enzyme. Several homologues of gp91phox have been recognized. The NADPH oxidase enzyme transfers electrons to molecular O2 via the flavin containing subunit. Gp91phox and the homologues Nox1 and Nox4 have been identified as the electron transferring subunit. Nox4 with 39% sequence identity to gp91phox, often called renal oxidase or renox, has high expression in various segments of the renal tubules and high constitutive activity.7 We hypothesize that NADPH oxidase is involved in the generation of free radicals which can represent a second messenger system for induction of MCP-1 and OPN in renal epithelial cells exposed to COM crystals.10-12 We therefore investigated the expression of the cytosolic unit p47phox in NRK52E cells exposed to COM crystals in the presence and absence of diphenileneiodium chloride (DPI), an inhibitor of NADPH oxidase. We expected that exposure to COM crystals would lead to increased superoxide production, upregulation of p47phox and increased production of MCP-1 and OPN. DPI treatment would also lead to a decrease in the production of superoxide, MCP-1 and OPN without significantly affecting the expression of p47phox since DPI is not the inhibitor of subunit production but of the flavin dependent membrane bound cytochrome.13
We used NRK52E cells, a normal rat kidney cell line, for our studies. The NRK52E cell line has already been used to investigate various steps involved in renal interstitial inflammation and fibrosis including redox modulation. We have previously shown that NRK52E cells constitutively express MCP-1 and exposure of the cells to high concentrations of Ox and CaOx crystals increases MCP-1 expression.10, 11 In addition we have shown that antioxidant treatment reduces MCP-1 expression. This cell line has also been used to study the expression of osteopontin.
Materials And Methods
Cell culture
A normal rat kidney epithelial-derived cell line, NRK52E, was obtained from American Type Culture Collection (CRL-1571; Manassas, VA). They were maintained as continuously growing monolayers (confluent condition) in 75 cm2 Falcon T-flask (Fisher, Atlanta, GA) in culture in 1:1 ratio Dulbecco’s modified essential medium nutrient mixture and F-12 (DMEM/F-12, Gibco BRL, Grand Island, NY) containing 4% fetal calf serum, 15 mmol/L HEPES, 20 mmol/L sodium bicarbonate, 0.5 mmol/L sodium pyruvate, 17.5 mmol/L glucose, streptomycin and penicillin at 37°C in a 5% CO2 air atmosphere incubator. Under these conditions, cells achieved confluence, they were washed with serum and sodium pyruvate free DMEM/F-12 media and incubated for 24 hours, and then exposed to COM (66.7 μg/cm2) with or without pre-incubation with the inhibitor: Diphenileneiodium chloride (DPI, 10−6M) for 1 hour. The cells were incubated for 6 hours for isolation of mRNA and culture medium was collected for 24 hours for protein assay (ELISA) for OPN and MCP-1 in culture mediums. For immunofluorescence studies of p47phox, cells were incubated under the same condition with COM for 24 hours.
Control cultures were untreated cells. The duration of cell exposure and concentration of COM crystals to which they were exposed was selected based on the results of earlier studies.10-12 COM crystals at a concentration of 66.7 μg/cm2 induce MCP-1 production without significant injury as indicated by absence of significant release of LDH into the medium.
Reverse transcriptase for real time PCR
The transcription of GAPDH (glyceraldehyde3-phosphate dehydrogenase), MCP-1, OPN and p47phox mRNA in NRK52E in 75 cm2 Falcon T-flask was determined using real time PCR. Total RNA was isolated from treated and untreated cells using TRIZOL Reagent (Gibco BRL, Grand Island, NY) according to the manufacture’s protocol. Two μg of total RNA was reverse transcribed to complementary DNA (cDNA). In brief, 50 μl reactions contained 3 μl of 100 mmol/L MgCl, 1.25 μl of RNase inhibitor, 5 μl of 10× PCR buffer, 10 μl of 10mM dNTP mix, 1.3 μl of Oligo d(T), 1.5 μl. of reverse transcriptase (all above materials come from Gibco BRL, Grand Island, NY). This mixture was incubated 60 min at 37°C, and then heating the reaction mixture to 94°C for 5 min stopped the reaction.
Primers for real time PCR and Real-time quantitative PCR
Primers were designed using Primer Express software (PE Applied Biosystems, Foster City, CA), according to the cDNA sequences of GAPDH (Accession No. NM_017008) as follows: 5′-TGCCAAGTATGATGACATCAAGAA-3′ (forward primer, bases 780-803) and 5′-AGCCCAGGATGCCCTTTAGT-3′ (reverse primer, bases 831-850), OPN (Accession No. M14656) as follows: 5′-TGAGACTGGCAGTGGTTTGC-3′ (forward primer, bases 81-100) and 5′-ACAGAGGGCCACTTTCACC-3′ (reverse primer, bases 125-143), MCP-1 (Accession No. M57441) as follows: 5′-CAGATCTCTCTTCCTCCACCACTAT-3′ (forward primer, bases 17-41) and 5′-CAGGCAGCAACTGTGAACAAC-3′ (reverse primer, bases 69-89), and p47phox (Accession No. AF260779) as follows: 5′ -GGGACTGCCCATGAAGATCTC-3′ (forward primer, bases 248-268) and 5′-TCATCGGGCCGCACTTT-3′ (reverse primer, bases 297-313). Relative quantity of MCP-1, OPN and p47phox mRNA stimulated with COM for 24 hours was normalized to GAPDH mRNA. The OPN mRNA value was determined by dividing by the GAPDH mRNA value (mean ± SD, *P<0.05 vs. control) in five different samples.
All PCR reactions were performed using an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems). For each PCR run, a master mix was prepared:1×SYBR PCR buffer, 3mM MgCl2, 200 μM dATP, dCTP and dGTP, 400 μM dUTP, 300μM primer set for MCP-1, OPN, p47phox and GAPDH, and 1.25 units of AmpliTaq Gold DNA polymerase. Five μl of diluted (1:20) cDNA was added to 45 μl of the PCR master mix. After an initial 10 min denaturation at 95° C, the thermal cycling comprised 40 cycles of denaturation at 95 °C for 15 sec, and annealing and extension at 60 °C for 1 min.
OPN and MCP-1 measurements by enzyme linked-immunosorbent assay (ELISA)
Cells were maintained as continuously growing monolayers (confluent condition) in 12 well culture dish (SUMILON®, SUMITOMO BAKELITE, Japan) in the same condition of mRNA isolation which was mentioned before. Under these conditions, they were washed with serum and sodium pyruvate free DMEM/F-12 media and then exposed to COM for 24 hours in the same medium (10 ml). The content of MCP-1 and OPN in the culture supernatants was determined by ELISA using ELISA kit for rat OPN (IBL, Gunnma, Japan) or MCP-1 (rat MCP-1 ELISA Kit, BIOSOURCE, CA) according to the manufacture’s instructions repeated. Protein concentration was determined using BCA Protein Assay Kit (PIERCE, Rockford, IL) for normalizing. Data are presented by mean ± SD in which values was determined in ten different samples.
Superoxide detection
We used staining with Dihydroethidium (DHE, Sigma, St Louis, MO), which is specific for Superoxide detection. NRK 52E cell were cultured in 12 well culture dish (SUMILON®, SUMITOMO BAKELITE, Japan) and then treated with COM for 12 hours with or without pre-incubated with Diphenileneiodium chloride (DPI, 10−6M) for 1 hour at 37°C in a humidified chamber. After washing with PBS for 30 minutes at 37°C while protected from light, superoxide levels were determined by DHE (10 μM in PBS) using a confocal microscope (LSM 5 PASCAL, Zeiss, Germany) and Luzex® chemiluminescence’s detection system (NIRECO, Japan). DHE is oxidized on reaction with Superoxide to ethidium bromide, which binds to DNA in the nucleus and fluoresces red. A 534 nm He-Ne laser combined with 560 nm long pass filter was used to detect ethidium bromide.
Immunofluorescence for p47phox
Immunofluorescence was assessed using treated and untreated NRK52E cells fixed in 4% paraformaldehyde. Cells were incubated primary antibodies (goat polyclonal p47phox antibody, Santa Cruz Biotechnology Inc., [1:500 dilution]) in PBS for 24 hours at 4°C. The cells were washed and incubated with a second antibody (chicken anti-goat IgG fluorescein conjugated antibody, CHEMICON INTERNATIONAL, [1:500 dilution,] for 30 minutes at room temperature. Samples were washed with PBS and then were examined with a confocal microscope (LSM 5 PASCAL, Zeiss, Germany) and a Luzex® chemiluminescence detection system (NIRECO, Japan).
Statistical analysis
We used one-way analysis of variance to test for overall differences among the groups, followed by Fisher’s modified least significant difference to compare the separate groups (StatView, SAS Institute Inc., NC, USA). P values less than 0.05 denoted the presence of statistically significant difference. Real time PCR, ELISA and LDH assay were done for 5 different samples (N=10).
Results
Superoxide production in COM treated cells
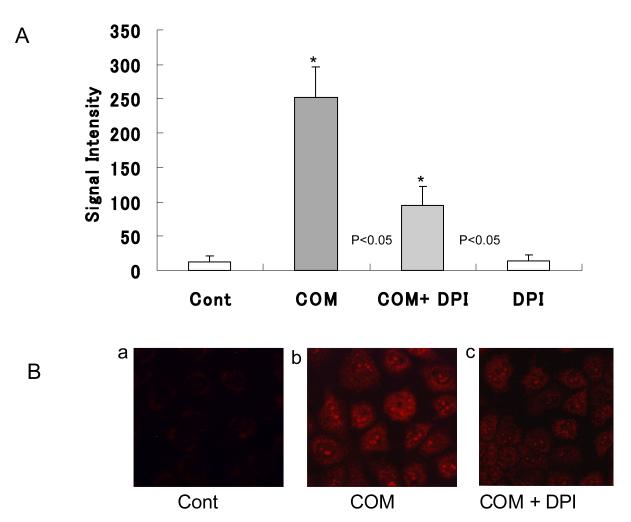
Low intensity fluorescence was observed in the untreated control cells indicating that the cells normally produce small amounts of superoxide. The superoxide production was significantly increased, 21 fold compared to control (Fig. 1B-b) by cells cultured with COM crystals for 6 hours. Pretreatment with DPI significantly reduced the increase in superoxide production to only 7.9 fold compared to controls (Fig. 1 B-C). As expected, DPI itself had no significant effect on the production of superoxide (Fig. 1A).
Figure 1.
NRK 52E cell were treated with COM (66.7 μg/cm2) for 12 hours with or without pre-incubated with Diphenileneiodium chloride (DPI, 10−6M) for 1 hour. Superoxide levels were determined by Dihydroethidium (10 μM) in Fig 1 B-a, b, c with Luzex® system (Fig 1 A). COM induced superoxide was suppressed by DPI in NRK 52E. (*p<0.05 vs. Cont, N=10)
Detection of p47phox mRNA transcriptions and p47phox protein synthesis
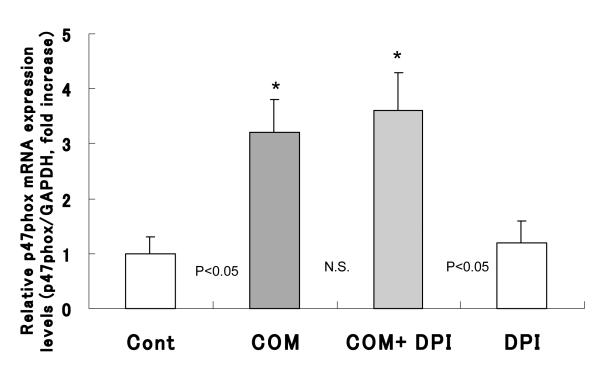
The p47phox mRNA was significantly increased, 3.2 fold, in cells cultured with COM crystals for 6 hours compared to controls not exposed to the crystals (Fig. 2). Pretreatment of cells with DPI had no effect on p47phox mRNA levels. As expected, there was no effect on the expression of p47phox by exposure to DPI alone (Fig. 2).
Figure 2.
NRK 52E cell were treated with COM (66.7 mg/cm2) for 6 hours with or without pre-incubated with Diphenileneiodium chloride (DPI, 10-6M) for 1 hour. p47phox mRNA was clearly increased compared to control. Pretreatment with DPI had no effect for the amount of p47phox mRNA level. DPI its self has also no effect for the production of p47phox (*p<0.05 vs. Cont, N=10)
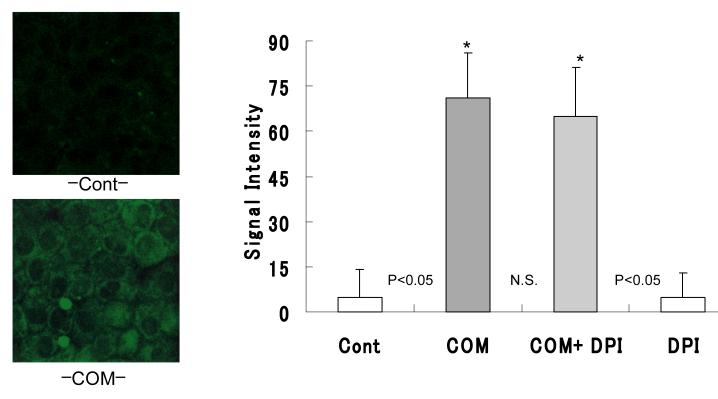
There was a significant increase in the production of p47phox by cells cultured with COM crystals for 24 hours (Fig. 3) compared to the controls not exposed to COM crystals. Pretreatment with DPI had no effect on the amount of p47phox produced by exposure to COM crystals. As expected, DPI itself had no effect on the production of p47phox (Fig. 3).
Figure 3.
NRK 52E cell were treated with COM (66.7 mg/cm2) for 24 hours with or without pre-incubated with Diphenileneiodium chloride (DPI, 10-6M) for 1 hour. Chemiluminescence of p47phox levels were determined by Luzex® system. p47phox protein production were clearly increased compared to control. Pretreatment with DPI had no effect for the amount of p47phox. DPI itself has no effect for the production of Superoxide. (*p<0.05 vs. Cont, N=10)
Detection of OPN mRNA transcriptions and OPN protein synthesis
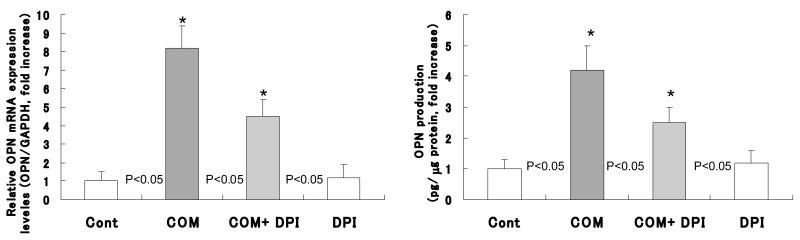
Exposure to COM crystals produced significant increases in the expression of OPN mRNA (Fig. 4, left) as well as production of OPN protein (Fig. 4, right) by the cells exposed to the COM crystals compared to cells not exposed to the crystals. Pretreatment with DPI resulted in significant reduction of expression of OPN mRNA (Fig. 4, left) and production of OPN protein (Fig. 4, right). The expression of OPN mRNA decreased from 8.2 fold increase by cells exposed to only the COM crystals, to 4.2 folds increase by the cells exposed to COM crystals after DPI pretreatment. Similarly OPN protein production decreased from 4.5 fold increase by cells exposed to COM crystals only to 2.5 folds increase by cells pretreated with DPI and then exposed to COM crystals.
Figure 4.
Exposure to COM caused a significant increase in the expression of OPN mRNA as well as production of OPN protein. DPI could reduce the amount of expression of OPN mRNA and OPN protein.
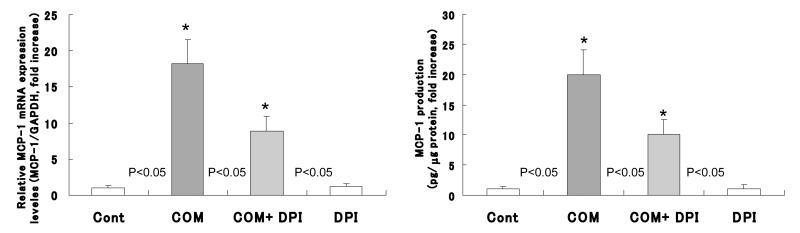
Detection of MCP-1 mRNA transcriptions and MCP-1 protein synthesis
Exposure of epithelial cells to COM crystals caused a significant increase in expression of MCP-1 mRNA (Fig. 5, left) as well as the production of MCP-1 protein (Fig. 5, right) compared to the cells not exposed to the crystals. DPI pretreatment caused significant reduction in expression of MCP-1 mRNA (Fig. 5, left) as well as production of MCP-1 protein (Fig. 4, right). The MCP-1 mRNA expression decreased from 18.2 fold increase by cells exposed to COM alone to 8.8 folds increase by the cells exposed to COM crystals after DPI pretreatment when compared to controls. Similarly MCP-1 protein production decreased from 20 fold increase by cells exposed to COM only to 10 fold increase by cells pretreated with DPI and then exposed to COM crystals.
Figure 5.
Exposure to COM caused a significant increase in the expression of MCP-1 mRNA as well as production of MCP-1 protein. DPI could reduce the amount of expression of MCP-1 mRNA and MCP-1 protein.
Discussion
Nephrolithiasis is a pathological biomineralization process in which crystals nucleate and grow within the kidneys. Nucleation, growth, aggregation and retention of crystals within the kidneys is modulated by a number of macromolecules including OPN.14 Animal model and cell culture studies have shown that synthesis and secretion of OPN is increased by renal epithelial cells exposed to high levels of oxalate and CaOx crystals.5, 6, 15 Interestingly, renal epithelial cells are also injured by exposure to high Ox and CaOx crystals and injury is associated with development of oxidative stress and the production of reactive oxygen species.16-19 Administration of angiotensin receptor-1 blocker (ARB) to hyperoxaluric rats results in reduction of oxidative stress, CaOx crystal deposition and expression of OPN synthesis and production in the kidneys suggesting involvement of rennin angiotensin system involvement in coax nephrolithiasis.20
Experimentally induced CaOx crystal deposition in the kidneys is also associated with localized inflammation as evidenced by migration of monocytes and macrophages to the site.4 Exposure of renal epithelial cells in culture to high Ox and CaOx crystals also leads to the production MCP-1 indicative of inflammatory process.10-12 Confluent cultures of NRK52E cells were exposed to CaOx at a concentration of 250 μg/ml (66.7 μg/cm2). They were exposed for 1,3,6,12,24 and 48 hours for isolation of mRNA and 24 hours for ELISA to determine the secretion of protein into the culture medium. We also investigated the effect of free radical scavenger, catalase on the crystal induced expression of MCP-1 mRNA and protein. Exposure of NRK52E cells to the crystals resulted in increased expression of MCP-1 mRNA and production of the chemoattractant.10, 11 Treatment with catalase significantly reduced increased expression of both MCP-1 mRNA and protein, which indicates the involvement of reactive oxygen species in upregulation of MCP-1 production.
ROS are generally considered cytotoxic and can damage a variety of macromolecules such as DNA, lipids and proteins.21 There is however, compelling evidence that under stress, ROS initiated signals activate protective and repair mechanisms. Furthermore redox signaling is also considered a part of normal metabolism in non-stressed cells.
ROS activate signaling molecules such as protein kinase C (PKC), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), and transcription factors such as NF-κB and activated protein-1 (AP-1). Activation of these molecules leads to upregulation of genes and proteins such as MCP-1, OPN, fibronectin and TGF-β1. Recently it has been shown that CaOx crystals selectively activate p38 MAPK signal transduction pathways.22, 23 In addition p38 MAPK is essential for re-initiation of the induced DNA synthesis. Oxalate exposure also causes modest activation of JNK as determined by c-Jun phosphorylation. Apparently, the renal epithelial response to CaOx oxalate involves signal transduction via MAP kinases, similar to the cellular responses to many other challenges. Cytosolic phospholipase A2 (cPLA2) is released upon the activation of MAP kinases and translocated to the cell membrane. cPLA2 preferentially hydrolyses arachidonoyl phospholipids generating a number of byproducts including arachidonic acid and lysophospholipids. Exposure of MDCK cells to oxalate produced a time and concentration dependent increase in cPLA2 activity.17 Inhibition of cPLA2 activity blocked ceramide production, and the oxalate-induced upregulation of Egr-1, c-jun and c-myc genes. Exposure of MDCK cells to oxalate also increased the generation of ceramide, another signaling lipid, most probably through the activation of neutral sphingomyelinase.
A likely source of oxalate induced superoxide production is membrane-associated NADPH oxidase, a major source of ROS in the kidneys,8 particularly in the presence of Angiotensin II.9 Angiotensin II is implicated in causing oxidative stress by stimulating membrane bound NAD(P)H oxidase leading to increased generation of superoxide.24 A significant reduction in hyperoxaluria-induced production of renal lipid peroxides after administration of AT 1 receptor blockers,20, 25 or ACE inhibitors,26 was seen in hyperoxaluric rats. In addition these treatments produced a reduction in TGF-β expression in the kidneys. TGF-β has been shown to participate in ROS production through the activation of NADPH oxidase. Similarly Ox-induced generation of ROS in LLC-PK1 cells was associated with increased production of TGF-β1 which was significantly reduced by treatment with neutralizing TGF-β antibodies.27 The catalytic core of NADPH oxidase is gp91phox that contains two hemes in the N-terminal transmembrane region and NADPH-binding and FAD-binding domains in the C-terminal cytoplasmic region. Electrons are transported from NADPH via FAD and two hemes to molecular oxygen. The gp95phox is complexed with p22phox to form a flavocytochrome b558 complex, and constituitively generates superoxide. DPI targets flavin containing domain and is a commonly used inhibitor of NADPH oxidase activities. DPI is suggested to attenuate oxidase activity by with drawing an electron from the oxidase, causing adduct formation with FAD and inhibiting superoxide formation. We have shown earlier that oxalate-induced injury of NRK52E cells was significantly reduced in the presence of DPI .28 Prior incubation with10−6M DPI, same concentration as used in this study, resulted in a reduction of oxalate as well as CaOx and brushite crystal induced LDH release, and production of H2O2 and 8-isoprostane. In addition DPI reduced both CaOx and brushite crystal induced gene expression and production of MCP-1. Our previous study also demonstrated that DPI and catalse, a free radical scavenger, caused similar reductions in LDH release and production of H2O2 and 8-isoprostane as well as MCP-1 gene expression and protein production.
Cell culture as well as animal model studies discussed above, have shown that high levels of Ox and high concentrations of CaOx crystals are injurious to renal epithelial cells, which develop oxidative stress and produce ROS. Antioxidant treatments lead to reduction in ROS production and protect the cells from Ox/CaOx crystal induced injury. Reduction in ROS production and injury by DPI treatment, as well as ACE inhibitors, suggest an involvement of NADPH oxidase in the Ox induced cell injury. However, no study has been undertaken to show actual changes in the enzyme or its activity. Results presented here are the first to show that exposure to 66.7 μg/cm2 CaOx crystals results in increased expression of p47phox, a NADPH oxidase subunit. Upregulated p47phox expression was associated with a significant increase in the production of superoxide as well as MCP-1 and OPN without noticeable signs of injury. As we mentioned earlier, NADPH oxidase consists of a number of subunits, cytosolic as well membrane associated. Cytosolic units not only translocate to the membrane but also assemble with the cytochrome to activate the enzyme. In this study cytosolic p47phox was upregulated but exposure to DPI inhibited its assembly with the cytochrome resulting in reduced enzyme activity and superoxide production as well as MCP-1 and OPN synthesis and production. To our knowledge this is first study to show upregulation of a NADPH oxidase subunit by exposure to the CaOx crystals. The results suggest that the calcium oxalate crystal-induced ROS production and MCP-1 and OPN synthesis occurs through activation of NADPH oxidase and that activation of NADPH oxidase may be one of the crucial mechanisms responsible for oxalate-induced oxidative stress in renal tubular epithelial cells.
A number of current studies have shown that kidneys of stone patients are under oxidative stress and produce reactive oxygen species which are cytotoxic. We have been involved in investigating the production of reactive oxygen species and their role in calcium oxalate nephrolithiasis. Results of the present study show that reactive oxygen species may actually be playing a significant role as second messengers for expression of molecules such as osteopontin and monocyte chemoattractant protein-1. The pathways involved may prove important in developing therapies for the recurrent stone formers.
References
- 1.Stamatelou KK, Francis ME, Jones CA, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Internal. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Hesse A, Brändle E, Wilbert D, Köhrmann K-U, Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. European Urol. 2003;44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 3.Saigal CS, Joyce G, Timilsina AR. Direct and indirect costs of nephrolithiasis in an employed population: Opportunity for disease management? Kidney Int. 2005;68:1808–1814. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 4.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue culture studies. Clinical and Experimental Nephrology. 2004;8:75–88. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 5.Kleinman JG, Wesson JA, Hughes J. Osteopontin and calcium stone formation. Nephron Physiol. 2004;98:43–47. doi: 10.1159/000080263. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens. 2006;15:374–380. doi: 10.1097/01.mnh.0000232877.12599.f4. [DOI] [PubMed] [Google Scholar]
- 7.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 8.Shiose A, Kuroda J, Tsutuya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-oroducing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 9.Hanna IR, Taniyama Y, Szocs K, Rocic P, Griendling KK. NAD(P)H Oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxidants & Redox Signaling. 2002;4:899–913. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 10.Umekawa T, Chegini N, Khan SR. Oxalate ions and calcium oxalate crystals stimulate MCP-1 expression by renal epithelial cells. Kidney Int. 2002;61:105–12. doi: 10.1046/j.1523-1755.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 11.Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant. 2003;18:664–669. doi: 10.1093/ndt/gfg140. [DOI] [PubMed] [Google Scholar]
- 12.Umekawa T, Byer K, Uemura H, Khan SR. Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant. 2005;20:870–878. doi: 10.1093/ndt/gfh750. [DOI] [PubMed] [Google Scholar]
- 13.Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: Does it more than simply attract monocytes? Nephrol Dial Transplant. 2002;17:2043–2047. doi: 10.1093/ndt/17.12.2043. [DOI] [PubMed] [Google Scholar]
- 14.Khan SR, Kok DJ. Modulators of Urinary Stone Formation. Frontiers in Bioscience. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 15.Khan SR, Johnson JM, Peck AB, Cornelius JG, Glenton PA. Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol. 2002;168:1173–1181. doi: 10.1016/S0022-5347(05)64621-6. [DOI] [PubMed] [Google Scholar]
- 16.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–57. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 17.Jonassen JA, Cao LC, Honeyman T, Scheid CR. Mechanisms mediating oxalate-induced alterations in renal cell functions. Crt Rev in Eukar Gene Expr. 2003;13:55–72. doi: 10.1615/critreveukaryotgeneexpr.v13.i1.50. [DOI] [PubMed] [Google Scholar]
- 18.Khand FD, Gordge MP, Robertson WG, Noronha-Dutra AA, Hothersall JS. Mitochondrial superoxide production during oxalate mediated oxidatve stress in renal epithelial cells. Free Radic Biol and Med 2002. 2002;32:1339–1350. doi: 10.1016/s0891-5849(02)00846-8. [DOI] [PubMed] [Google Scholar]
- 19.Meimaridou E, Lobos E, Hothersall JS. Renal oxidative vulnerability due to changes in mitochondrial-glutothione and energy homeostasis in a rat model of calcium oxalate urolithiasis. Am J Physiol Renal Physiol. 2006;291:731–740. doi: 10.1152/ajprenal.00024.2006. [DOI] [PubMed] [Google Scholar]
- 20.Umekawa T, Hatanaka Y, Kurita T, Khan SR. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in the rat kidneys. J Am Soc Nephrol. 2004;15:635–644. doi: 10.1097/01.asn.0000113321.49771.2d. [DOI] [PubMed] [Google Scholar]
- 21.Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling system, the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8:445–467. doi: 10.1007/s10522-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi LS, Koul S, Sekhon A, Bhandari A, Menon M, Koul HK. Oxalate selectively activates the p38 mitoge-activated protein kinase and c-Jun N-terminal kinase signal transduction pathway in renal epithelial cells. J Biol Chemistry. 2002;277:13321–13330. doi: 10.1074/jbc.M108203200. [DOI] [PubMed] [Google Scholar]
- 23.Koul HK, Menon M, Chaturvedi LS, Koul S, Sekhon A, Bhandari A, Huang M. COM crystals activate the p38 mitoge-activated protein kinase (MAPK) signal transduction pathway in renal epithelial cells. J Biol Chemistry. 2002;277:36845–36852. doi: 10.1074/jbc.M200832200. [DOI] [PubMed] [Google Scholar]
- 24.James EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Intl. 1998;54:775–784. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- 25.Toblli JE, Ferder L, Stella I, de Cavanagh MVE, Angerosa M, Inserra F. Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol. 2002;168:1550–1555. doi: 10.1016/S0022-5347(05)64519-3. [DOI] [PubMed] [Google Scholar]
- 26.Toblli JE, Ferder L, Stella I, Angerosa M, Inserra F. Protective role of enalapril for chronic tubulointerstitial lesions of hyperoxaluria. J Urol. 2001;166:275–280. [PubMed] [Google Scholar]
- 27.Rashid T, Menon M, Thamilselvan S. Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: effect of antioxidants. Am J Nephrol. 2004;24:557–568. doi: 10.1159/000082043. [DOI] [PubMed] [Google Scholar]
- 28.Umekawa T, Byer K, Uemura H, Khan SR. Diphenyleneiodium (DPI) reduces oxalate ions and calcium oxalate monohydrate and brushite crystal-induced up-regulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant. 2005;20:870–878. doi: 10.1093/ndt/gfh750. 2005. [DOI] [PubMed] [Google Scholar]