Abstract
Alcohol and tobacco consumption are well recognized risk factors for head and neck cancer (HNC). Evidence suggests that genetic predisposition may also play a role. Only a few epidemiologic studies, however, have considered the relation between HNC risk and family history of HNC and other cancers. We pooled individual- level data across 12 case-control studies including 8,967 HNC cases and 13,627 controls. We obtained pooled odds ratios (OR) using fixed and random effect models, and adjusting for potential confounding factors. All statistical tests were two-sided. A family history of HNC in first-degree relatives increased the risk of HNC (OR=1.7, 95% confidence interval, CI, 1.2-2.3). The risk was higher when the affected relative was a sibling (OR=2.2, 95% CI 1.6-3.1) rather than a parent (OR=1.5, 95% CI 1.1-1.8), and for more distal HNC anatomic sites (hypopharynx and larynx). The risk was also higher, or limited to, subjects exposed to tobacco. The OR rose to 7.2 (95% CI 5.5-9.5) among subjects with family history, who were alcohol and tobacco users. A weak but significant association (OR=1.1, 95% CI 1.0-1.2) emerged for family history of other tobacco-related neoplasms, particularly with laryngeal cancer (OR=1.3, 95% CI 1.1-1.5). No association was observed for family history of non-tobacco related neoplasms and the risk of HNC (OR=1.0, 95% CI 0.9-1.1). Familial factors play a role in the etiology of HNC. In both subjects with and without family history of HNC, avoidance of tobacco and alcohol exposure may be the best way to avoid HNC.
Keywords: Head and neck cancer, family history, pooled analysis, tobacco, alcohol
INTRODUCTION
There is ample variation worldwide in incidence and mortality of cancers of the oral cavity, pharynx and larynx (head and neck cancers, HNCs), with high rates being observed in some areas of Europe, North and South America (1) Also within countries, substantial changes in rates have been observed over time (2;3). These geographical and temporal variations are mainly attributable to different exposure to alcohol and tobacco, which are the major determinants of HNCs in high-resource countries (4), and together account for over 75% of cases in those areas (5;6).
In spite of well-identified lifestyle factors, there are indications that genetic susceptibility also plays a role in the development in HNCs (7-9) as for many other cancer sites (10). Familial aggregation may be an indicator of inherited susceptibility. A few epidemiologic studies considered the risk of HNC in relatives of affected individuals (11-20). The quantification of the risk remains, however, uncertain. Data are sparse and inconclusive about whether the risk varies according to head and neck subsite, age and sex of the proband or of the relative, the type of relative affected and whether a family history of non-HNCs also affects HNC risk. Very limited data are available on the combined effect of family history, and tobacco and alcohol exposure (18), (20).
The International Head and Neck Cancer Epidemiology (INHANCE) Consortium (http://inhance.iarc.fr/) was established in 2004, based on the collaboration of research groups leading large, molecular epidemiology studies of head and neck cancer that are on-going or have been recently completed. The consortium was established with the primary goal to address research questions that are difficult to answer in individual studies, many of which involve 500 to 1000 cases and a comparable number of controls. The INHANCE database thus provides a unique opportunity to investigate the role of family history on risk of HNCs. The goal of the present study was to investigate the association between various aspects of family history of HNC and other cancers and the risk of HNC, also in combination with alcohol and tobacco use.
MATERIAL AND METHODS
In the version 1.0 of the INHANCE pooled data, 15 individual case-control studies, comprising 10,302 head and neck cancer cases and 15,329 controls were included, of which 12 studies (9025 cases and 13739 controls) had information about the family history of cancer (21-30). Subjects with missing data on age, sex, or race/ethnicity, and cases with missing information on the site of origin of their cancer were excluded (58 cases and 102 controls), thus the data for this analysis included 8,967 head and neck cancer cases and 13,627 controls.
Characteristics of the individual studies included in the consortium are provided in Table 1 and in a previous article (4). Most were hospital-based case-control studies; all studies frequency matched the controls to cases (i.e., no individual matching) on age, sex and additional factors. Interviews in all studies were conducted face-to-face. Questionnaires were collected from all the individual studies to assess the comparability of the data and wording of interview questions. Data from individual studies were received at IARC with personal identifiers removed. Each data item was checked for illogical or missing values. Queries were sent to investigators and inconsistencies were resolved.
Table 1.
Summary of individual studies in INHANCE pooled data v1.0, by region and study period
| Study Location | Recruitment period |
Cases |
Controls2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Source | Participation rate |
Age eligibility |
Number | Source | Participation rate |
Number | ||
| Europe | ||||||||
| Milan, Italy | 1984-1989 | Hospital | 95%3 | <80 | 416 | Hospital - unhealthy | 95%3 | 1531 |
| Aviano, Italy | 1984-1989 | Hospital | >95%3 | >18 | 482 | Hospital - unhealthy | 95%3 | 855 |
| Italy (Aviano, Milan, Latina)4 | 1990-1999 | Hospital | >95% | 18-80 | 1058 | Hospital - unhealthy | 95% | 2579 |
| Switzerland | 1991-1997 | Hospital | 95% | <80 | 516 | Hospital - unhealthy | 95% | 883 |
| Central Europe (Banska Bystrica, Bucharest, Budapest, Lodz, Moscow)4 |
1998-2003 | Hospital | 96% | ≥15 | 762 | Hospital - unhealthy | 97% | 907 |
| North America | ||||||||
| North Carolina | 1994-1997 | Hospital | 88% | >17 | 180 | Hospital – unhealthy | 86% | 202 |
| Tampa, FL | 1994-2003 | Hospital | 98% | ≥18 | 207 | Cancer screening clinic - healthy | 90% | 897 |
| Los Angeles, CA | 1999-2004 | Cancer registry | 49% | 18-65 | 417 | Neighborhood | 67.5% | 1005 |
| Houston, TX | 2001-2006 | Hospital | 95% | ≥18 | 829 | Hospital visitors | >80% | 865 |
| South/Central America | ||||||||
| Puerto Rico | 1992-1995 | Cancer registry | 71% | 21-79 | 350 | Residential records | 83% | 521 |
| South America (Buenos Aires, Havana, Goiãnia, Pelotas, Porto Alegre, Rio de Janeiro, São Paolo) 5 | 2000-2003 | Hospital | 95% | 15-79 | 2191 | Hospital - unhealthy | 86% | 1706 |
| International | ||||||||
| International (Italy, Spain, Ireland, Poland, Canada, Australia, Cuba, India, Sudan) 4 |
1992-1997 | Hospital | 88.7 | na | 1559 | Hospital/Community | 87.3 | 1676 |
Representative publication in which study methods are available.
All studies frequency matched controls to cases, minimally on age and sex. Additional frequency matching factors included center (Italy, Central Europe, South America and International multicenter studies), ethnicity (Tampa, Los Angeles studies), neighborhood (Los Angeles study).
Participation rate not formally assessed, estimated response rate reported.
Multicenter study
Cases were subjects with invasive tumors of the oral cavity (lip, tongue, gum, floor of mouth and hard palate; ICD10 codes C00.3-C00.9, C02.0-C02.3, C03, C04, C05.0, C06), oropharynx (base of tongue, lingual tonsil, soft palate, uvula, tonsil and oropharynx; ICD10 codes C01, C02,4, C05.1, C05.2, C09 and C10), hypopharynx (pyriform sinus and hypopharynx; ICD10 codes C12 and C13) oral cavity or pharynx not otherwise specified (NOS including overlapping sites (ICD10 codes C02.8, C02.9, C05.8, C05.9, C14), larynx (glottis, supraglottis, subglottis; ICD10 codes C32), or head and neck cancer unspecified. Cancers of the external lip (ICD10 codes C00.1-C00.2) salivary gland (ICD10 codes C07-C08) or nasopharynx (C11) were excluded due to the different etiologic pattern (31).(32) In the overall dataset there was a total of 2332 oral cases, 2922 pharyngeal cases (668 hypopharynx and 2254 oropharynx), 835 unspecified oral/pharynx cases, 2572 laryngeal cases and 306 overlapping head and neck cases.
Three studies restricted case eligibility to squamous cell carcinomas (SCC) (North Carolina, Tampa, & Houston). For other studies which provided the ICD-O-2 or ICD-O-3 histological coding for each tumor (Switzerland, Central Europe, Los Angeles, Puerto Rico, South America and all but four centers in the International multicenter study), SCC are defined as histologies 805-808 and malign behavior code (/3). For the Milan, Aviano, Italy multicenter studies and four centers in the International multicenter study (Bangalore, Madras, and Trivandrum in India and Khartoum in Sudan), no data were available on histological type. Of the 6,307 head and neck cancer cases for which histological information was available, 6,069 were squamous cell carcinomas (96.2%). In this analysis, we included all available cases.
Three categories of cancers in family members were considered:
- Head and neck cancers including only cancers with topography previously described in the methods section of this paper.
- Other tobacco related cancers (33) (lung (C34), nasopharynx (C11), nasal cavity (C30), paranasal sinuses (C31), esophagus (C15), stomach (C16), pancreas (C25), liver (C22), kidney (body and pelvis) (C64), urinary bladder (C67), uterine cervix (C53) and bone marrow (myeloid leukemia, C92).
- All other cancers.
We used the definitions of smoking of cigarettes, pipes and cigars and alcohol drinking categories adopted in a previous paper, where a detailed description on the method used for pooling data on smoking and alcohol across different studies is also provided (4a)..Information on smokeless tobacco use was collected in some studies only, as its prevalence of use was negligible in several geographic areas.
Information on family history included the number of brothers and sisters, the number of first degree relatives (parent, siblings and children) with a history of cancer, the site of the cancer and the type of affected relative. For the study from Milan (21), information was available only for a history of HNCs, and not for the number or type of relatives affected, while for Aviano (22) the information available was if a parent and/or siblings were affected, but not the sex of the relative. The definition of HNC for first degree relatives included malignant neoplasm with ICD9 codes 161 (larynx) and 140-149 (lip, oral cavity and pharynx), except ICD codes 140.0, 140.1 and 140.9 (lip, vermillion border) and 147 (nasopharynx). We considered a subject to have a family history for a given cancer site if the subject reported at least one affected first degree relative.
Statistical Analysis
For subjects with missing education level (326 cases and 252 controls), we applied multiple imputation (5 imputations) with the PROC MI procedure in SAS. We used the logistic regression model (34a) to predict education level with age, sex, race/ethnicity, study, and case/control status within each geographic region.
We assessed the associations between head and neck cancer and family history of cancer by estimating odds ratios (OR) and 95% confidence intervals (CI). First, we obtained study specific estimates by means of unconditional logistic regression models (35). The models included age, sex, education, race/ethnicity and packyears of cigarette smoking (continuous), years of cigar smoking (continuous), years of pipe smoking (continuous), smokeless tobacco (snuff or chewing tobacco) use (ever/never), number of alcoholic drinks per day, and number of brothers and sisters. Pooled ORs were estimated with fixed effects unconditional logistic regression models, adjusted for all the factors mentioned before and study centre.
The heterogeneity between studies was estimated by a likelihood ratio test comparing a model that included the product terms between each study with the variable of interest and a model without the product terms. When significant heterogeneity between studies was detected, the pooled OR was also computed by including the study-specific estimates in a two-stage random effect logistic regression model with the maximum likelihood estimator. When data were analyzed in strata of covariates, interaction tests between strata were performed
RESULTS
Table 2 gives the characteristics of cases and controls according to age, sex and other selected covariates. There was a high predominance of male cases (81%), and cases were more frequently and heavily exposed to tobacco and alcohol. There were more Latin Americans among cases and less non Hispanic whites, and cases tended to be less educated than controls.
Table 2.
Demographic characteristics of 8967 cases and 13627 controls included in the pooled analysis
| Demographic characteristics |
Cases | (%) | Controls | (%) |
|---|---|---|---|---|
| Age | ||||
| <40 | 323 | 3.6 | 892 | 6.6 |
| 40-<45 | 533 | 5.9 | 1039 | 7.6 |
| 45-<50 | 1012 | 11.3 | 1587 | 11.7 |
| 50-<55 | 1415 | 15.8 | 2181 | 16.0 |
| 55-<60 | 1665 | 18.6 | 2353 | 17.3 |
| 60-<65 | 1480 | 16.5 | 2088 | 15.3 |
| 65-<70 | 1243 | 13.9 | 1734 | 12.7 |
| 70-<75 | 837 | 9.3 | 1232 | 9.0 |
| >=75 | 459 | 5.1 | 521 | 3.8 |
| Sex | ||||
| Men | 7248 | 80.8 | 9794 | 71.9 |
| Women | 1719 | 19.2 | 3833 | 28.1 |
| Χ2 test | <0.0001b | |||
| Race/Ethnicity | 1 | |||
| Non hispanic white | 5560 | 62.0 | 10332 | 75.8 |
| Black | 341 | 3.8 | 454 | 3.3 |
| Hispanic/Latino | 141 | 1.6 | 329 | 2.4 |
| Asian/Pacific islanders | 616 | 6.9 | 654 | 4.8 |
| Latin Americans | 2191 | 24.4 | 1706 | 12.5 |
| Others | 118 | 1.3 | 152 | 1.1 |
| Χ2 test | <0.0001 | |||
| Education | ||||
| None | 446 | 5.0 | 304 | 2.2 |
| <Junior high school | 4383 | 48.9 | 6240 | 45.8 |
| Some high school | 1126 | 12.6 | 1521 | 11.2 |
| High school graduate | 1022 | 11.4 | 1502 | 11.0 |
| Vocational, some college | 831 | 9.3 | 1758 | 12.9 |
| >=College | 833 | 9.3 | 2051 | 15.1 |
| Missing | 326 | 3.6 | 251 | 1.8 |
| Χ2 test | <0.0001 | |||
| Tobacco smoking | ||||
| Never# | 913 | 10.2 | 5298 | 38.9 |
| ever | 8051 | 89.8 | 8320 | 61.1 |
| Missing | 3 | 9 | ||
| Χ2 test | <0.0001 | |||
| Packyears | ||||
| non cigarette smokers | 1322 | 14.9 | 5489 | 40.8 |
| 1-10 py | 632 | 7.1 | 2032 | 15.1 |
| 11-20 py | 910 | 10.3 | 1607 | 11.9 |
| 21-30 py | 1270 | 14.3 | 1358 | 10.1 |
| 31-40 py | 1366 | 15.4 | 1084 | 8.1 |
| 41-50 py | 1072 | 12.1 | 722 | 5.4 |
| >50 py | 2300 | 25.9 | 1183 | 8.8 |
| Missing | 95 | 152 | ||
| Χ2 test | <0.0001 | |||
| Alcohol drinking | ||||
| never | 1435 | 16.0 | 3653 | 26.8 |
| ever | 7521 | 84.0 | 9967 | 73.2 |
| Missing | 11 | 7 | ||
| Χ2 test | <0.0001 | |||
| Frequency of drinking | 1 | |||
| never drinker | 1435 | 16.0 | 3653 | 26.8 |
| <1drink per day | 1286 | 14.4 | 3000 | 22.0 |
| 1-2 drinks per day | 1459 | 16.3 | 2920 | 21.4 |
| 3-4 drinks per day | 1030 | 11.5 | 1560 | 11.5 |
| 5-6 drinks per day | 807 | 9.0 | 784 | 5.8 |
| >=7 drinks per day | 2597 | 29.0 | 1343 | 9.9 |
| Missing | 353 | 3.9 | 367 | 2.7 |
| Χ2 test | <0.0001 | |||
| HNC subtype | ||||
| Oral cavity | 2332 | 26.0 | ||
| Oroharynx | 668 | 7.4 | ||
| Hypopharynx | 2254 | 25.1 | ||
| Oral/Pharynx NOS | 835 | 9.3 | ||
| Larynx | 2572 | 28.7 | ||
| Overlapping HNC | 306 | 3.4 |
Never tobacco users were subjects that never used cigarette, pipe, cigar, snuff or chew.
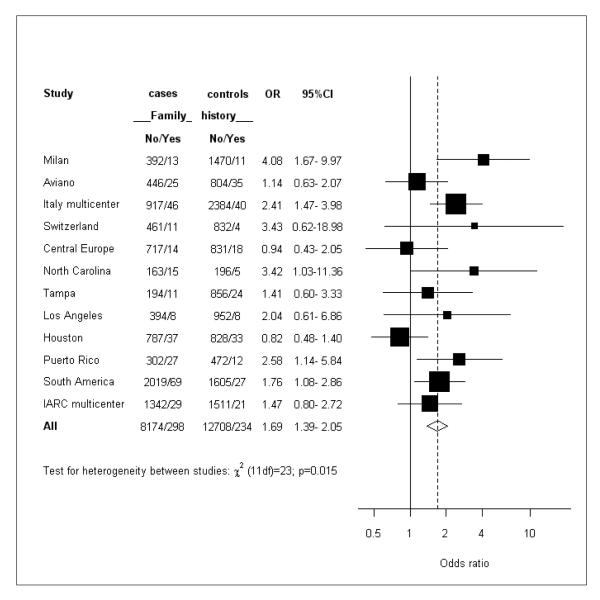
Table 3 presents the ORs of HNC according to selected aspects of family history of HNC. A family history of HNC was reported by 305 (3.6%) cases and 238 (1.8%) controls, and significantly increased the risk of HNCs (OR=1.68, 95% CI 1.23-2.29). Only 18 cases (0.23%) and 10 controls (0.09%) reported two or more first degree relatives with HNCs and the corresponding OR was 2.65 (95% CI 1.13-6.22). Although the OR varied slightly across strata of sex and age, all point estimates were above unity, and no clear pattern emerged. The OR was 1.53 for probands with cancer at the oral cavity, 1.55 for oropharynx, 2.28 for hypopharynx, 1.82 for oral cavity/pharynx not otherwise specified and 2.07 for laryngeal cancer. Subjects with an affected sibling (OR=2.23, 95% CI 1.61-3.08) had a significantly (p=0.036) higher risk than subjects with an affected parent (OR=1.45, 95% CI 1.14-1.84). The risk was somewhat higher for probands aged 50 years or more (OR=1.83, 95% CI 1.47-2.26) than for younger ones (OR=1.38, 95% CI 0.87-2.18), although the difference was not statistically significant.. Study-specific estimates were significantly heterogeneous for the overall effect of family history and in a few strata. The random effect estimates, however, did not differ substantially from the fixed effect ones, although the CIs were wider. Under the random effect model the OR for family history of HNC was 1.68 (95% 1.23-2.29). When recalculated excluding each study at a time, the pooled OR ranged between 1.57 (95% CI 1.19-2.06, excluded study: Milan) and 1.83 (95% CI 1.42-2.36, excluded study: Houston). Grouping the studies by location did not explain the observed heterogeneity (data not shown). The results did not materially change when the analysis was restricted to the cases for which the histology was known to be squamous cell carcinoma.
Table 3.
Family history of head and neck (oral cavity/pharynx/larynx) cancers (HNC) in first degree relatives and risk of HNC according to selected probands’ or relatives’ characteristics
| Family history of HNC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | ||||||||
| Cases | Controls | Cases | Controls | OR# | (95% CI) | p for heterogeneity§ |
OR* | 95%CI | |
| All subjects | 8134 | 12741 | 305 | 238 | 1.75 | (1.44, 2.12) | 0.02 | 1.68 | (1.23, 2.29) |
| No of affected relative ~ | 0.17 | ||||||||
| 1 | 7742 | 11271 | 274 | 217 | 1.62 | (1.32, 1.98) | |||
| ≥2 | 18 | 10 | 2.65 | (1.13, 6.22) | |||||
| Probands’sex | |||||||||
| Male | 6552 | 9098 | 249 | 172 | 1.73 | (1.39, 2.15) | 0.07 | ||
| Female | 1582 | 3643 | 56 | 66 | 1.70 | (1.10, 2.61) | 0.06 | ||
| Probands’ age | |||||||||
| <50 years | 1751 | 3308 | 45 | 48 | 1.38 | (0.87, 2.18) | 0.04 | 1.43 | (0.63, 3.21) |
| ≥50 years | 6383 | 9433 | 260 | 190 | 1.83 | (1.47, 2.26) | 0.07 | ||
| Probands’ cancer site | |||||||||
| Oral cavity | 2085 | 12741 | 66 | 238 | 1.53 | (1.11, 2.11) | 0.11 | ||
| Oropharynx | 2069 | 12741 | 80 | 238 | 1.55 | (1.16, 2.07) | 0.03 | 1.72 | (1.09, 2.70) |
| Hypopharynx | 603 | 11230 | 28 | 217 | 2.28 | (1.46, 3.54) | 0.69 | ||
| Oral cavity/pharynx NOS | 743 | 12741 | 22 | 238 | 1.82 | (1.13, 2.92) | 0.08 | ||
| Larynx | 2357 | 10758 | 107 | 205 | 2.07 | (1.57, 2.73) | 0.15 | ||
| Overlapping | 277 | 3116 | 2 | 48 | |||||
| Type of affected relative | |||||||||
| Parents | 8134 | 12741 | 178 | 157 | 1.45 | (1.14, 1.84) | 0.04 | 1.37 | (0.96, 1.94) |
| siblings | 121 | 72 | 2.23 | (1.61, 3.08) | 0.03 | 2.06 | (1.23, 3.45) | ||
| Sex of affected relative | |||||||||
| Male | 8134 | 12741 | 189 | 126 | 1.98 | (1.54, 2.55) | 0.20 | ||
| Female | 85 | 70 | 1.40 | (0.98,2.02) | 0.22 | ||||
Odds ratio for family history (yes vs no) were adjusted on age (categorical), sex race, education level ,centers, packyear, drinks per day (continuous) number of sisters, number of brothers, duration of pipe use and duration of cigar use, chew status and snuff status
Between studies
Random effect estimates
Does not include Milan
Figure 1 shows the study specific estimates. The point estimates ranged between 0.82 and 4.08. Only in two out of 12 studies the OR was below unity (Central Europe and Houston).
Figure 1.
Study specific estimates of the odds ratio (OR) of head and neck cancer (HNC) in subjects with a family history of HNC in first degree relatives. Squares = study-specific odds ratios; size of the square = the weight given to this study (inverse of the variance of the log odds ratio) when estimating the summary odds ratio; horizontal lines = study-specific confidence intervals (CIs); diamond = summary estimate combining the study-specific estimates with a random-effects model; solid vertical line = odds ratio of 1; dashed vertical line = summary odds ratio.
Table 4 shows the ORs for family history of HNC in strata of tobacco and alcohol consumption. Light tobacco users where defined as subjects who smoked ≤20 tobacco-years (combination of packyear of cigarettes and packyear of cigars and pipe in cigarette equivalent) or subjects who snuffed tobacco only. Heavy tobacco users were subjects who smoked >20 tobacco-years or subjects who ever chewed tobacco. The ORs were 0.86 (95% CI 0.48-1.55) in never tobacco users, 3.45 (95% CI 2.19-5.43) in light tobacco users and 1.72 (95% CI 1.32-2.23) in heavy tobacco users. For never, light (≤3 drinks of alcoholic beverages per day) and heavy (>3 drinks per day) alcohol users the OR were 1.49, 1.63 and 2.02, respectively.
Table 4.
Family history of Head and Neck (oral cavity/pharynx/larynx) cancers (HNC) in first degree relatives and risk of HNC
| Family history of head and neck cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | 95% CI | |||||||
| Cases | Controls | Cases | Controls | OR | p for heterogeneity§ | OR* | 95%CI | ||
| Tobacco consumption a | |||||||||
| Never tobacco users# | 818 | 4803 | 15 | 89 | 0.86 | (0.48, 1.55) | 0.60 | ||
| Light tobacco users# | 1291 | 3204 | 53 | 38 | 3.45 | (2.19, 5.43) | 0.36 | ||
| Heavy tobacco users# | 5308 | 3888 | 223 | 93 | 1.72 | (1.32, 2.23) | 0.03 | 1.52 | (1.10, 2.10) |
| Alcohol consumption b | |||||||||
| Never drinkers~ | 1367 | 3480 | 40 | 61 | 1.49 | (0.95, 2.36) | 0.08 | ||
| Light drinkers~ | 2125 | 5090 | 60 | 83 | 1.63 | (1.11, 2.38) | 0.24 | ||
| Heavy drinkers~ | 3925 | 3340 | 191 | 76 | 2.02 | (1.50, 2.72) | 0.03 | 1.84 | (1.28, 2.65) |
Between studies
Random effect estimates
Light tobacco users where defined as subjects who smoked ≤20 tobacco-years (combination of packyear of cigarettes and packyear of cigars and pipe in cigarette equivalent) or subjects who snuffed tobacco only. Heavy tobacco users were subjects who smoked >20 tobacco-years or subjects who ever chewed tobacco.
adjusted on age (categorical) , sex, race, education level, centers, number of drinks per day, number of sisters, number of brothers (continuous)
Light drinkers were defined as subjects who drank ≤3 drinks of alcoholic beverages per day, and heavy drinkers >3 drinks per day.
adjusted on age (categorical) , sex, race, education level, centers, packyear (continuous), duration of pipe use (continuous), duration of cigar use (continuous), number of sisters, number of brothers, chew status and snuff status
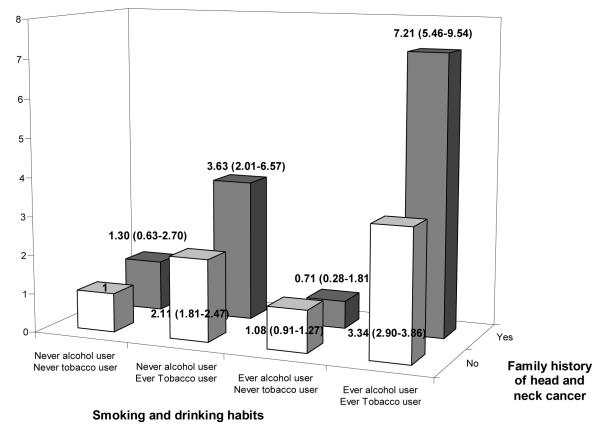
Figure 2 shows the OR of HNC for various combinations of family history of HNC (No/Yes) and tobacco and alcohol consumption (Never/Ever) relative to subjects with no family history, and were never users of tobacco and alcohol. Among . never users of alcohol and tobacco there were 419 cases and 1958 controls without a family history of HNC (reference category) and 10 cases and 37 controls with family history of HNC. Having a first degree relative with HNC changed the OR from 1 to 1.30 in never tobacco and alcohol users, from 2.11 to 3.63 in users of tobacco only, from 1.08 to 0.71 in users of alcohol only and from 3.34 to 7.21 in alcohol and tobacco users(p-for interaction <0.0001).
Figure 2.
Interaction between smoking and drinking habits and family history of head and neck cancer
The OR for family history of (non HNC) tobacco related cancers and for other cancers are presented in Table 5. A weak association was observed for family history of tobacco related cancers (OR=1.11, 95% CI 1.01-1.23). The association was stronger - or limited to - probands with laryngeal cancer (OR=1.27, 95% CI 1.09-1.47). When family history of lung cancer only was considered, the ORs were 1.21 (95% CI 1.04-1.40) for all HNCs, and 1.37 (95% CI 1.10-1.71) for laryngeal cancer (data not shown). No association emerged for family history of other cancers (OR=0.98, 95% CI 0.89-1.08).
Table 5.
Family history of (non HNC) smoking related1 and other cancers in first degree and risk of Head and Neck cancers (HNC)
| Family history of HNC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | * | |||||||
| Cases | Controls | Cases | Controls | OR# | 95% CI | p for heterogeneity§ | OR* | 95%CI | |
| Family history of (non HNC) smoking-related cancer1 | |||||||||
| All subjects | 6973 | 10067 | 1061 | 1431 | 1.11 | (1.01, 1.23) | 0.36 | ||
| Probands’ age | |||||||||
| <50 years | 1559 | 2652 | 183 | 273 | 1.17 | (0.93, 1.47) | 0.77 | ||
| 50≥ years | 5414 | 7415 | 878 | 1158 | 1.10 | (0.98, 1.22) | 0.30 | ||
| Proband’ cancer site | |||||||||
| Oral cavity | 1876 | 10067 | 232 | 1431 | 1.10 | (0.92, 1.30) | 0.94 | ||
| Oropharynx | 1806 | 10067 | 309 | 1431 | 1.15 | (0.99, 1.34) | 0.56 | ||
| Hypopharynx | 537 | 8631 | 67 | 1335 | 0.98 | (0.73, 1.31) | 0.16 | ||
| Oral cavity/pharynx NOS | 629 | 10067 | 72 | 1431 | 0.93 | (0.71, 1.22) | 0.21 | ||
| Larynx | 1853 | 8149 | 374 | 1333 | 1.27 | (1.09, 1.47) | 0.2 | ||
| Overlapping | 272 | 2902 | 7 | 262 | |||||
|
| |||||||||
| Family history of other cancer | |||||||||
| All subjects | 6813 | 9632 | 1221 | 1866 | 0.98 | (0.89, 1.08) | 0.18 | ||
| Probands’ age | |||||||||
| <50 years | 1517 | 2513 | 225 | 412 | 0.92 | (0.75, 1.14) | 0.32 | ||
| 50≥ years | 5296 | 7119 | 996 | 1454 | 0.98 | (0.88, 1.10) | 0.02 | 0.91 | (0.72, 1.15) |
| Proband’ cancer site | |||||||||
| Oral cavity | 1822 | 9632 | 286 | 1866 | 0.93 | (0.79, 1.10) | 0.21 | ||
| Oropharynx | 1728 | 9632 | 387 | 1866 | 0.93 | (0.81, 1.08) | 0.54 | ||
| Hypopharynx | 548 | 8259 | 56 | 1707 | 0.82 | (0.59, 1.13) | 0.09 | ||
| Oral cavity/pharynx NOS | 589 | 9632 | 112 | 1866 | 0.96 | (0.75, 1.22) | 0.94 | ||
| Larynx | 1859 | 7810 | 368 | 1672 | 1.10 | (0.95, 1.29) | 0.39 | ||
| Overlapping | 267 | 2811 | 12 | 353 | |||||
Odds ratio for family history yes vs no, adjusted on age (categorical), sex race, education level ,center, packyears of cigarettes, drinks per day (continuous) number of sister, number of brothers, duration of pipe use and duration of cigar use, chew status, snuff status.
Between studies
Random effect estimates
Includes cancers of the lung, nasopharynx, nasal cavity, paranasal sinuses, esophagus, stomach, pancreas, liver, kidney (body and pelvis), urinary bladder, uterine cervix and bone marrow (myeloid leukemia)
DISCUSSION
In this pooled analysis of case-control studies, a family history of HNCs in first degree relatives increased the risk of HNCs, and the association appeared stronger for distal HNC (hypopharynx and larynx). The risk was significantly higher if the affected relative was a sibling, rather than a parent. The increase in risk was more marked – or limited to – subjects who had ever used tobacco. The association of HNC risk with family history of other smoking related cancers was significant, but weak, and no association emerged for all other cancers.
An elevated risk of HNC in subjects with a history of cancer at the same site has been reported in other studies, some of which are included in this pooled analysis. Five case control studies from the United States (US) (12;17), Puerto Rico (18), Brazil (14), and Italy and Switzerland (20) investigated the risk of HNCs (or of some subsite combination) in subjects with a family history of HNCs in first degree relatives, relative to those without, reporting ORs ranging between 1.2 and 3.8. In three record linkage studies from Utah (11), Norway (16) and Sweden (19), the relatives of HNC cases had standardized incidence ratios (SIR) of developing HNC that ranged between 1.4 and 8.0. In a study from the Netherlands, parents and siblings of HNC cases had a 3.5 times higher risk of developing HNC than the relatives of proband’s spouses (13). In a Canadian study with a similar design the relative risk of developing HNC in first degree relatives of HNC cases was 3.8, as compared to relatives of spouses, and 7.9 when the index case had multiple primary cancers (14), (15).
Although the overall evidence suggests that having an affected first degree relative increases the risk of HNCs, the point estimates vary widely across studies. This is not surprising, since only a small proportion of subjects had a family history of HNC, and estimates were often based on small numbers.
Familial aggregation may indicate that inheritable genetic factors play a role in HNC risk, but may also reflect a tendency of relatives to have similar alcohol and tobacco behaviors. Several genetic polymorphisms in genes involved in the metabolism of carcinogens, DNA repair, or in several other processes have been associated to HNC risk, although results were not always consistent (36;37).. On the other hand, genetic variants in the alcohol metabolism genes ADH1B and ADH7 were shown to be associated with head and neck cancer risk in 3 independent populations (38). Given that the differential ability to metabolize carcinogens matters only when exposure occurs, it is also possible that the familial risk reflects both a higher genetic susceptibility to HNC together with an aggregation of exposures.
In this analysis, there was significant heterogeneity between studies, which may be related to different genetic susceptibility in various populations, but also to variable exposure to major non-genetic risk factors for HNC. Given the different characteristics of the various populations, including variable exposure to alcohol, tobacco and other environmental factors, and the different methods used, a degree of heterogeneity across studies is to be expected. For example, in the Houston study, controls were recruited from hospital visitors, and individuals with a family history of HNC may have been more willing to participate. Moreover, the Houston study had the highest proportion of never smokers among the included studies. These facts may explain a lower estimate of the effect of family history.
It is also possible that the prevalence of susceptibility genes varies in different populations, as has indeed been observed for some polymorphic genes linked to the metabolism of carcinogens (39). With only a few exceptions, however, all study estimates were above unity, and sensitivity analysis showed that results were not strongly driven by any single study. The two studies for which the point estimate was below one were from central Europe and Houston (Texas, US) and did not identify a clear geographic or ethnic pattern. In fact, the location of the studies did not explain the heterogeneity between studies. Despite a certain degree of heterogeneity between studies, however, it is reassuring that the results were similar when fixed or random effect models were used. In many of the studies included in this analysis, family history of cancer was self-reported, in the absence of any other verification. Moreover, all the studies included in this pooled analysis had a case-control design, and were thus prone to reporting bias. It is possible that cases were more sensitized to the issue, and a differential recall of cancers in the family occurred. On the other hand, in the Tampa study, controls were recruited through a screening center, and may have been more health conscious than the general population.
The three record linkage studies that investigated the issue found risks above unity: In the Swedish Family Cancer Database the standardized incidence ratio (SIR) of HNC was 1.4 (95% CI 0.98-2.0) in offspring of subjects with HNC in a record linkage study based on the Norwegian Cancer Registry the SIR of HNCs below age 45 years was 1.9 (95% 0.9-3-5) in those with a family history of lung or HNC, and in a study based on the Utah Population Database, the SIR of oral and laryngeal cancer were, respectively, 1.8 (95% CI 0.5-4.0) and 8.0 (95% CI 2.1-17.9) in subjects with a family history of cancers at the same sites. Notwithstanding the difficulties in comparing these results, based on different aggregations of cancer sites and age groups, to our data, there is no indication that studies not relying on self-reported family history or on a case-control design showed a systematic tendency towards estimating lower risks.
For many cancer sites the risk is increased in those with a family history of cancer at that site (40), and it is not surprising if this is true for HNCs too. In our study, the OR was higher when the proband had a more distal HNC cancer, i.e. at the hypopharynx or larynx. The few other studies available also suggest that familial risk may be stronger for distal HNC. A case-control study from the US on 1,114 cases of oral and pharyngeal cancer (12) found odds ratios (OR) of 1.2 (95% confidence interval, CI, 0.7-2.3) and 1.6 (0.7-3.8) in subjects reporting family history of oral/pharyngeal and laryngeal/esophageal cancer, respectively. In the Utah population database (11), the SIR of laryngeal cancer in subjects with a family history of laryngeal cancer (SIR=8.0) was higher than that of oral/pharyngeal cancer associated with a family history of cancer at the same sites (SIR=1.8). In the case-control study (956 cases and 2362 controls) of oral and pharyngeal cancer from Italy and Switzerland included in this pooled analysis, the multivariate ORs were 2.6 for a family history of oral cancer, and 3.8 for family history of laryngeal cancer (20).
In this pooled analysis, the OR was slightly but significantly higher when the affected relative was a sibling, rather than a parent. In a study conducted in the Netherlands (13) on a retrospective cohort of first degree relatives of patients with HNC and of the patients’ spouses, the OR of HNC was 1.9 (95% CI 0.9-3.8) for parents and 14.6 (3.1-69) for siblings. Similarly, in a case-control study conducted in Brazil (14) on 754 cases of HNCs the OR was 2.5 (95% CI 1.0-6.0) for a family history of HNC in the father and 8.6 (95% CI 2.7-27) if the affected relative was a sibling. In a case-control study from Puerto Rico (18), based on 342 oral/pharyngeal cases and included in the present collaborative analysis, the OR was 2.0 for history of esophageal or HNC in a parent and 2.7 in a sibling. In a study from Italy and Switzerland (20) the OR of oral/pharyngeal cancer in subjects with a parent or a sibling with HNC were, respectively, 2.6 and 3.4. Taken together, the epidemiologic evidence consistently indicates that the risk is higher if the affected relative is a sibling. This may also explain the slightly lower SIR estimated in the Swedish Family Cancer Database (19), where only parents and offspring were considered, as compared to other studies. The higher risk associated with a history of HNC in a sibling suggests a recessive mode of inheritance of HNC susceptibility. An alternative explanation may be that siblings share more environmental exposures than children do with their parents.
When we investigated the effect of family history in combination with alcohol and tobacco use, the risk associated with family history appeared stronger in – or limited to - subjects exposed to tobacco. The joint effect of these three factors has been considered in two case-control studies (18), (20), both included in the present pooled analysis. In the study from Puerto Rico (18), the addition of family history increased the risk at least two-fold in all combinations of tobacco and alcohol use, but the risks appeared somewhat higher in subjects more heavily exposed. In the case-control from Italy and Switzerland, the risk appeared stronger for subjects more heavily exposed to tobacco (20). In those two studies, however, the limited sample size did not allow for setting the reference category to non-alcohol and non-tobacco users. Even in this combined reanalysis the standard errors of the estimates were large, given the rarity of family history of HNC and of cases of HNC not exposed to alcohol and tobacco. Thus, this subgroup analysis should be considered with caution. An interpretation of this result is that what is inherited is the susceptibility to the damage caused by tobacco. In the absence of tobacco exposure, subjects inheriting the predisposition do not show an increased risk of HNC. If this were true, the best way of avoiding HNC would be avoiding alcohol and, most of all, tobacco exposure, particularly in subjects with a family history of the disease. Information on the tobacco and alcohol exposure of the relatives would provide further insight on this issue. Unfortunately, these data were not available in the studies considered.
An Italian study calculated that, even in smokers who had stopped smoking around 50 years of age, the excess risk of HNC by age 75 years (as compared to never smoking) was approximately halved in comparison with continuing smoking (41). Thus, stopping smoking is an effective measure to reduce HNC cancer risk. For some cancer sites, the risk associated with family history is higher at younger ages (40). In our study the point estimate was higher in older subjects, although the interaction of family history of HNC with age was not significant. It is possible that a differential contribution of the various studies to the age-specific groups may have obscured a relation with age. On the other hand, if familial susceptibility to HNC is mediated by the exposure to tobacco, which generally starts in the late second decade of life, it is conceivable that the age-distribution of familial HNC differs from that of other cancers. We found a small but significant increased risk of HNC, and particularly laryngeal cancer, in subjects with a family history of tobacco-related cancers. It is conceivable that an inherited susceptibility to the damage of tobacco affects the risk of all or most tobacco-related neoplasms, though, as for HNC, familial aggregation may reflect similar attitudes towards tobacco consumption.
The percent attributable risk (PAR) to family history is low in the overall dataset, i.e. on the order of 1 to 2%, even for more distal sites or when the affected relative is a sibling. However, this does not necessarily imply that the influence of susceptibility is low assuming that high risk alleles show high frequency and low penetrance (10). Furthermore, the PAR is population-dependent and hence influenced by the population mix considered in this pooled analysis. .
Acknowledgements
This work was supported by a grant from the US National Institutes of Health, National Cancer Institute (R03 CA113157). The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The individual studies were funded by the following grants: Milan study: Italian Association for Research on Cancer (AIRC); Aviano and Italy Multicenter studies: Italian Association for Research on Cancer (AIRC), Italian League Against Cancer, Italian Ministry of Research; Swiss study: Swiss League against Cancer and the Swiss Research against Cancer/Oncosuisse (KFS-700 and OCS-1633); Central Europe study: World Cancer Research Fund and the European Commission’s INCO-COPERNICUS Program (Contract No. IC15-CT98-0332); North Carolina study: NIH grant R01CA61188 and in part by a grant from the National Institute of Environmental Health Sciences (P30ES010126); Tampa study: US NIH P01CA068384 and K07CA104231; Los Angeles study: National Institute of Health (P50CA90388, R01DA11386, R03CA77954, T32CA09142, U01CA96134, and R21ES011667) as well as the Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center; Houston study: US NIH R01ES11740 and R01CA100264; South America study: FONCYT (Fondo para la Investigacion Cientifica y Tecnologica) Argentina, IMIM (Barcelona), Fundação de Amparo á Pesquisa no Estado de São Paulo (FAPESP) No 01/01768-2, European Commission (IC18-CT97-0222); IARC Multicenter study: Fondo de Investigaciones Sanitarias (FIS) of the Spanish Government (FIS 97/0024, FIS 97/0662, and BAE 01/5013), International Union Against Cancer (UICC), Yamagiwa-Yoshida Memorial International Cancer Study Grant;
Reference List
- (1).Ferlay J, Bray F, Pisani P, Parkin DM. Cancer Incidence, Mortality and Prevalence Worldwide. IARC Press; Lyon: 2004. GLOBOCAN 2002. IARC CancerBase No.5. [Google Scholar]
- (2).La Vecchia C, Lucchini F, Negri E, Levi F. Trends in oral cancer mortality in Europe. Oral Oncol. 2004 Apr;40(4):433–9. doi: 10.1016/j.oraloncology.2003.09.013. [DOI] [PubMed] [Google Scholar]
- (3).Bosetti C, Garavello W, Levi F, Lucchini F, Negri E, LaVecchia C. Trends in laryngeal cancer mortality in Int. Europe J Cancer. 2006 Aug 1;119(3):673–81. doi: 10.1002/ijc.21855. [DOI] [PubMed] [Google Scholar]
- (4).Hashibe M, Brennan P, Benhamou S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Wunsch-Filho V, Franceschi S, Hayes RB, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007 May 16;99(10):777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- (5).Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF., Jr Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988 Jun 1;48(11):3282–7. [PubMed] [Google Scholar]
- (6).Negri E, Franceschi S, Tavani A. Attributable risk for oral cancer in northern Italy. Cancer Epidemiol Biomarkers Prev. 1993 May;2(3):189–93. [PubMed] [Google Scholar]
- (7).Harty LC, Caporaso NE, Hayes RB, Winn DM, Bravo-Otero E, Blot WJ, Kleinman DV, Brown LM, Armenian HK, Fraumeni JF, Jr., Shields PG. Alcohol dehydrogenase 3 genotype and risk of oral cavity and pharyngeal cancers. J Natl Cancer Inst. 1997 Nov 19;89(22):1698–705. doi: 10.1093/jnci/89.22.1698. [DOI] [PubMed] [Google Scholar]
- (8).De Andrade M, Amos CI, Foulkes WD. Segregation analysis of squamous cell carcinoma of the head and neck: evidence for a major gene determining risk. Ann Hum Genet. 1998 Nov;62(Pt 6):505–10. doi: 10.1046/j.1469-1809.1998.6260505.x. [DOI] [PubMed] [Google Scholar]
- (9).Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000 Feb;9(2):185–91. [PubMed] [Google Scholar]
- (10).Peto J, Houlston RS. Genetics and the common cancers. Eur J Cancer. 2001 Oct;37(Suppl 8):S88–S96. doi: 10.1016/s0959-8049(01)00255-6. [DOI] [PubMed] [Google Scholar]
- (11).Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994 Nov 2;86(21):1600–8. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- (12).Goldstein AM, Blot WJ, Greenberg RS, Schoenberg JB, Austin DF, Preston-Martin S, Winn DM, Bernstein L, McLaughlin JK, Fraumeni JF., Jr Familial risk in oral and pharyngeal cancer. Eur J Cancer B Oral Oncol. 1994 Sep;30B(5):319–22. doi: 10.1016/0964-1955(94)90032-9. [DOI] [PubMed] [Google Scholar]
- (13).Copper MP, Jovanovic A, Nauta JJ, Braakhuis BJ, de Vries N, van der Waal I, Snow GB. Role of genetic factors in the etiology of squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1995 Feb;121(2):157–60. doi: 10.1001/archotol.1995.01890020019005. [DOI] [PubMed] [Google Scholar]
- (14).Foulkes WD, Brunet JS, Kowalski LP, Narod SA, Franco EL. Family history of cancer is a risk factor for squamous cell carcinoma of the head and neck in Brazil: a case-control study. Int J Cancer. 1995 Dec 11;63(6):769–73. doi: 10.1002/ijc.2910630603. [DOI] [PubMed] [Google Scholar]
- (15).Foulkes WD, Brunet JS, Sieh W, Black MJ, Shenouda G, Narod SA. Familial risks of squamous cell carcinoma of the head and neck: retrospective case-control study. BMJ. 1996 Sep 21;313(7059):716–21. doi: 10.1136/bmj.313.7059.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mork J, Moller B, Glattre E. Familial risk in head and neck squamous cell carcinoma diagnosed before the age of 45: a population-based study. Oral Oncol. 1999 Jul;35(4):360–7. doi: 10.1016/s1368-8375(98)00069-4. [DOI] [PubMed] [Google Scholar]
- (17).Yu GP, Zhang ZF, Hsu TC, Spitz MR, Schantz SP. Family history of cancer, mutagen sensitivity, and increased risk of head and neck cancer. Cancer Lett. 1999 Nov 1;146(1):93–101. doi: 10.1016/s0304-3835(99)00249-9. [DOI] [PubMed] [Google Scholar]
- (18).Brown LM, Gridley G, Diehl SR, Winn DM, Harty LC, Otero EB, Fraumeni JF, Jr., Hayes RB. Family cancer history and susceptibility to oral carcinoma in Puerto Rico. Cancer. 2001 Oct 15;92(8):2102–8. doi: 10.1002/1097-0142(20011015)92:8<2102::aid-cncr1551>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- (19).Li X, Hemminki K. Familial upper aerodigestive tract cancers: incidence trends, familial clustering and subsequent cancers. Oral Oncol. 2003 Apr;39(3):232–9. doi: 10.1016/s1368-8375(02)00091-x. [DOI] [PubMed] [Google Scholar]
- (20).Garavello W, Foschi R, Talamini R, La Vecchia C, Rossi M, Dal Maso L, Tavani A, Levi F, Barzan L, Ramazzotti V, Franceschi S, Negri E. Family history and the risk of oral and pharyngeal cancer. Int J Cancer. 2007 doi: 10.1002/ijc.23199. In Press. [DOI] [PubMed] [Google Scholar]
- (21).Franceschi S, Talamini R, Barra S, Baron AE, Negri E, Bidoli E, Serraino D, La Vecchia C. Smoking and drinking in relation to cancers of the oral cavity, pharynx, larynx, and esophagus in northern Italy. Cancer Res. 1990 Oct 15;50(20):6502–7. [PubMed] [Google Scholar]
- (22).Baron AE, Franceschi S, Barra S, Talamini R, La Vecchia C. A comparison of the joint effects of alcohol and smoking on the risk of cancer across sites in the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993 Nov;2(6):519–23. [PubMed] [Google Scholar]
- (23).Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, La Vecchia C. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003 Oct;12(10):1091–4. [PubMed] [Google Scholar]
- (24).Levi F, Pasche C, Lucchini F, Franceschi S, Monnier P. Food groups and risk of oral and pharyngeal cancer. Int J Cancer. 1998 Aug 31;77(5):705–9. doi: 10.1002/(sici)1097-0215(19980831)77:5<705::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- (25).Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Janout V, Fabianova E, Bencko V, Moullan N, Chabrier A, Hung R, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):696–703. doi: 10.1158/1055-9965.EPI-05-0710. [DOI] [PubMed] [Google Scholar]
- (26).Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002 Jul;23(7):1229–34. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- (27).Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao J, Cao W, Cozen W, Mack TM, Zhang ZF. Polymorphism of Xeroderma Pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer. 2006 Feb 1;118(3):714–20. doi: 10.1002/ijc.21413. [DOI] [PubMed] [Google Scholar]
- (28).Zhang Z, Shi Q, Liu Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of methionine synthase and methionine synthase reductase and risk of squamous cell carcinoma of the head and neck: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2005 May;14(5):1188–93. doi: 10.1158/1055-9965.EPI-04-0501. [DOI] [PubMed] [Google Scholar]
- (29).Hayes RB, Bravo-Otero E, Kleinman DV, Brown LM, Fraumeni JF, Jr., Harty LC, Winn DM. Tobacco and alcohol use and oral cancer in Puerto Rico. Cancer Causes Control. 1999 Feb;10(1):27–33. doi: 10.1023/a:1008876115797. [DOI] [PubMed] [Google Scholar]
- (30).Herrero R, Castellsague X, Pawlita M, Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B, Pintos J, Fernandez L, Idris A, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003 Dec 3;95(23):1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- (31).Sun EC, Curtis R, Melbye M, Goedert JJ. Salivary gland cancer in the United States. Cancer Epidemiol Biomarkers Prev. 1999 Dec;8(12):1095–100. [PubMed] [Google Scholar]
- (32).Yu MC, Yuan JM. Nasopharyngeal cancer. In: Schottenfeld David, Fraumeni Joseph.F., editors. Cancer Epidemiology and Prevention. 3rd edition ed Oxford University Press; Oxford: 2006. [Google Scholar]
- (33).IARC working group on the Evaluation of Carcinogenic Risks to Humans . Tobacco smoke and involuntary smoking. IARC Press; Lyon: 2004. [PMC free article] [PubMed] [Google Scholar]
- (34).Rubin D. Multiple imputation for nonresponse in survey. John Wiley and Sons, Inc.; New York: 1987. [Google Scholar]
- (35).Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case- control studies. IARC Sci Publ. 1980;(32):5–338. [PubMed] [Google Scholar]
- (36).Hashibe M, Brennan P, Strange RC, Bhisey R, Cascorbi I, Lazarus P, Oude Ophuis MB, Benhamou S, Foulkes WD, Katoh T, Coutelle C, Romkes M, et al. Meta- and pooled analyses of GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes and risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2003 Dec;12(12):1509–17. [PubMed] [Google Scholar]
- (37).Hung RJ, van der HO, Tavtigian SV, Brennan P, Boffetta P, Hashibe M. Perspectives on the molecular epidemiology of aerodigestive tract cancers. Mutat Res. 2005 Dec;30;592(1-2):102–18. doi: 10.1016/j.mrfmmm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- (38).Hashibe M, McKay JD, Curado MP, Oliveria JC, Koifman S, Koifman R, Zaridze D, Shangina O, Wunsch-Filho V, Eluf-Neto J, Levi JE, Matos E, et al. Multiple ADH genes are associated with upper aerodigestive cancers. Advanced online publication. (2008 ed) 2008 May 25; doi: 10.1038/ng.151. [DOI] [PubMed] [Google Scholar]
- (39).Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, Boffetta P, Bouchardy C, Breskvar K, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001 Dec;10(12):1239–48. [PubMed] [Google Scholar]
- (40).Houlston RS, Peto J. The search for low-penetrance cancer susceptibility alleles. Oncogene. 2004 Aug 23;23(38):6471–6. doi: 10.1038/sj.onc.1207951. [DOI] [PubMed] [Google Scholar]
- (41).Bosetti C, Gallus S, Peto R, Negri E, Talamini R, Tavani A, Franceschi S, La VC. Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers. Am J Epidemiol. 2008 Feb 15;167(4):468–73. doi: 10.1093/aje/kwm318. [DOI] [PubMed] [Google Scholar]