Summary
Remodeling of extracellular matrix (ECM) is a fundamental cell property that allows cells to alter their microenvironment and move through tissues. Invadopodia and podosomes are subcellular actin-rich structures that are specialized for matrix degradation and are formed by cancer and normal cells, respectively. Although initial studies focused on defining the core machinery of these two structures, recent studies have identified inputs from both growth factor and adhesion signaling as crucial for invasive activity. This Commentary will outline the current knowledge on the upstream signaling inputs to invadopodia and podosomes and their role in governing distinct stages of these invasive structures. We discuss invadopodia and podosomes as adhesion structures and highlight new data showing that invadopodia-associated adhesion rings promote the maturation of already-formed invadopodia. We present a model in which growth factor stimulation leads to phosphoinositide 3-kinase (PI3K) activity and formation of invadopodia, whereas adhesion signaling promotes exocytosis of proteinases at invadopodia.
Key words: Adhesion, Growth factor, Podosome, Invadopodia, phosphoinositide 3-kinase, PI3K, Actin cytoskeleton
Introduction
Cellular migration and invasion through tissue barriers is important for a number of physiological and pathological conditions, including immune cell transmigration and dissemination of cancer cells during metastasis. The extracellular matrix (ECM), which is assembled from proteoglycans and fibrous proteins, is a key barrier to cell invasion but also can modulate cellular behavior through direct receptor–ECM interactions. Although non-proteolytic methods of invasion through ECM have been demonstrated (Doyle et al., 2009; Provenzano et al., 2008; Wolf et al., 2003; Wolf et al., 2007; Wyckoff et al., 2006), this mode of migration is likely to be used only in areas of sparse or lightly cross-linked ECM such as loose stroma (Madsen and Sahai, 2010; Sabeh et al., 2009). Thus, cell invasion into dense matrices, such as basement membranes, requires ECM proteolysis and remodeling to clear space and create tracks along which cells can migrate more efficiently (Hotary et al., 2006; Sabeh et al., 2004; Sabeh et al., 2009). Cell-associated proteolytic activity is also likely to be important for cleavage of non-ECM targets, including latent growth factors and integrins (Black et al., 1997; Chan et al., 2012; Izumi et al., 1998; Kajita et al., 2001; Kessenbrock et al., 2010; Koshikawa et al., 2010; Lyons et al., 1990; Moss et al., 1997; Mu et al., 2002; Turk et al., 2001; Yu and Stamenkovic, 2000).
Actin-based subcellular structures termed invadopodia or podosomes are now well-characterized as a specialized machinery for ECM degradation (Linder et al., 2011; Weaver, 2006). The term invadopodium is generally used to describe structures formed in cancer cells, whereas podosome is the term used for similar structures that are involved in matrix remodeling in non-cancerous cells, including dendritic cells, macrophages, endothelial cells, vascular smooth muscle cells (VSMCs) and osteoclasts (Burns et al., 2001; Gaidano et al., 1990; Hai et al., 2002; Kanehisa et al., 1990; Marchisio et al., 1987; Miyauchi et al., 1990; Moreau et al., 2003). In osteoclasts, podosomes become organized into a belt-like structure that then forms the sealing zone to mediate bone resorption (Kanehisa et al., 1990; Miyauchi et al., 1990). Recently, the term invadosomes has been used to encompass both types of structures, as well as similar structures that are induced in normal cells by transformation with constitutively active mutant forms of the nonreceptor tyrosine kinase Src (Destaing et al., 2011; Linder et al., 2011; Saltel et al., 2011). Despite strong similarities between the structures, there are some notable differences, including how dynamic they are (Artym et al., 2011), their dependence on different signaling scaffolding proteins [e.g. NCK1 versus GRB2 (Oser et al., 2011)] and their response to upstream signaling inputs (Hoshino et al., 2012). For a more detailed description of the similarities and differences between invadopodia and podosome, as well as a comparison to focal adhesions, see Fig. 1.
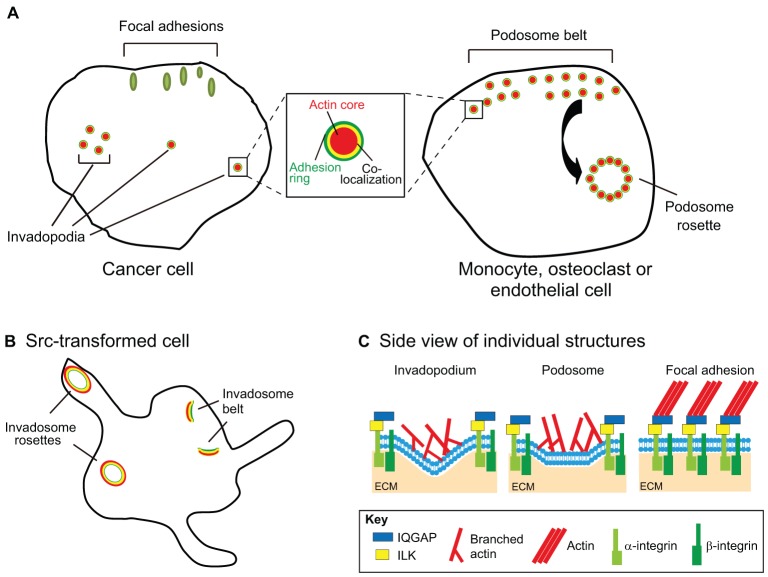
Fig. 1.
Structural features of invadopodia, podosomes and focal adhesions. (A) Comparison of invasive adhesion structures in cancer and normal cells. Focal adhesions (green ovals in figure) are streak-like structures consisting of >150 molecular components and >700 direct interactions (Zaidel-Bar and Geiger, 2010). In general, they serve as mechanical and signaling connections between the actin cytoskeleton and ECM-bound integrins. This occurs through multiple interactions between scaffolding and signaling proteins, integrins and the actin cytoskeleton. Invadopodia and podosomes (shown as circular structures in the figure) are punctate branched actin-rich structures defined, in part, by the presence of a substantial amount of actin regulatory proteins, such as the Arp2/3 complex, cortactin and N-WASp, along with TKS5, active Src kinase, tyrosine phosphorylated proteins and the proteinase MT1-MMP (Murphy and Courtneidge, 2011; Weaver, 2006). Recent proteomic studies have respectively identified ∼200 putative podosome components and ∼60 putative invadopodia components (Attanasio et al., 2011; Cervero et al., 2012). Although invadopodia-producing cells generally only form punctate actin structures, podosome puncta can assemble into higher-order structures, such as podosome rosettes and podosome belts (the double layer of podosomes at cell periphery). Podosome belts can go on to form the sealing zone in osteoclasts. Both podosomes and invadopodia contain adhesion proteins and are surrounded by adhesion rings that that typically contain vinculin and paxillin (green rings in figure). Actin filaments radiate from the inner puncta and are likely to connect to the outer adhesion ring (yellow overlap) (Luxenburg et al., 2007). Adhesion proteins have also been localized to actin puncta (Alexander et al., 2008; Bowden et al., 1999; Mueller et al., 1999). (B) Src-transformed cells also form invadopodia- and podosome-like structures termed invadosomes. Invadosomes can organize into a larger rosette that consists of giant actin rings with colocalized adhesion molecules (Brábek et al., 2004). By light microscopy, invadosome rosettes probably best resemble osteoclast podosome belts which form at the periphery (Destaing et al., 2003) or sealing zones which are highly specialized structures formed by fusion of individual podosomes (Luxenburg et al., 2007); however, it is unclear at this point how closely rosette formation by Src-transformed cells resembles the osteoclast sealing ring formation. Owing to their robust formation of invadosomes, Src-transformed cells are extremely useful as a model for studying the basic core machinery that is common to invadopodia and podosomes. However, it is likely that the presence of constitutively active Src inherently bypasses and/or alters some upstream signals that would otherwise come from growth factors and/or adhesions. (C) Side view of invasive adhesion structures. Adhesion components include integrins, IQGAP and ILK, along with typical markers such as vinculin.
Invadopodia and podosomes are protrusive structures that are associated with ECM degradation and invasive cellular migration. They are typically analyzed in two-dimensional cell culture models, which facilitate rapid assessment of ECM degradation ability. Invadopodia form and degrade matrix in multiple types of human cancer cells (Chen et al., 1994; Clark et al., 2007; Monsky et al., 1994; Seals et al., 2005) and are important for their invasive activity (Coopman et al., 1998; Schoumacher et al., 2010). On surfaces with ample space and a degradable ECM substrate in the ventral direction, podosomes in endothelial cells, VSMCs, dendritic cells and macrophages also can degrade the underlying matrix and protrude into it (Dorfleutner et al., 2008; Gawden-Bone et al., 2010; Osiak et al., 2005; Quintavalle et al., 2010; Rottiers et al., 2009; Van Goethem et al., 2011); however, invadopodia are generally thought to more aggressively degrade the matrix and protrude further into it than podosomes (Linder et al., 2011; Murphy and Courtneidge, 2011; Weaver, 2008). In three-dimensional studies, the proteolytic activity has been localized to the base of the invadopodial protrusions (Tolde et al., 2010; Wolf et al., 2007). Over time, invadopodia have also been shown to develop into larger protrusions with branched actin filaments, vesicles and other cytoskeletal elements filling most of the protrusion, and with bundled filaments at the tip (Schoumacher et al., 2010). Despite their small size [50–100 nm in diameter and 500 nm in length, as determined by electron microscopy (Enderling et al., 2008)], both invadopodia and podosomes have also recently been observed in vivo (Gligorijevic et al., 2012; Quintavalle et al., 2010; Ziel et al., 2009), and we expect further future studies to identify them at sites of tissue remodeling. Although the morphology of these structures is likely to vary somewhat depending on the three-dimensional or tissue environment, it is expected that key invadosome regulators will control invasive behavior across various settings and are good candidates for pharmacological attack.
Many studies have described the structural components comprising invadopodia (Fig. 1), but interest is now centered on the regulation of two key invadopodia stages: their formation and maturation (Fig. 2). At the heart of this interest is a deepening understanding that deregulation of signaling in cancer frequently leads to invasive behavior, of which a major component is the ability to degrade ECM. Therefore, if we can understand the key signaling inputs that regulate crucial stages in the invadopodium lifecycle, we might be able to target them and ultimately prevent tissue remodeling by cancers. In this Commentary, we describe the synergistic roles of growth factor signaling and cell–ECM adhesion on the formation and maturation of invadopodia and podosomes. We discuss recent data that implicate invadopodia as adhesive structures that respond to both chemical and physical aspects of the ECM. We also present a model in which maturation of invadopodia and podosomes occurs in stages: growth factor signaling promotes formation, whereas adhesion to the ECM promotes localized protease exocytosis, resulting in a functional, degrading structure.
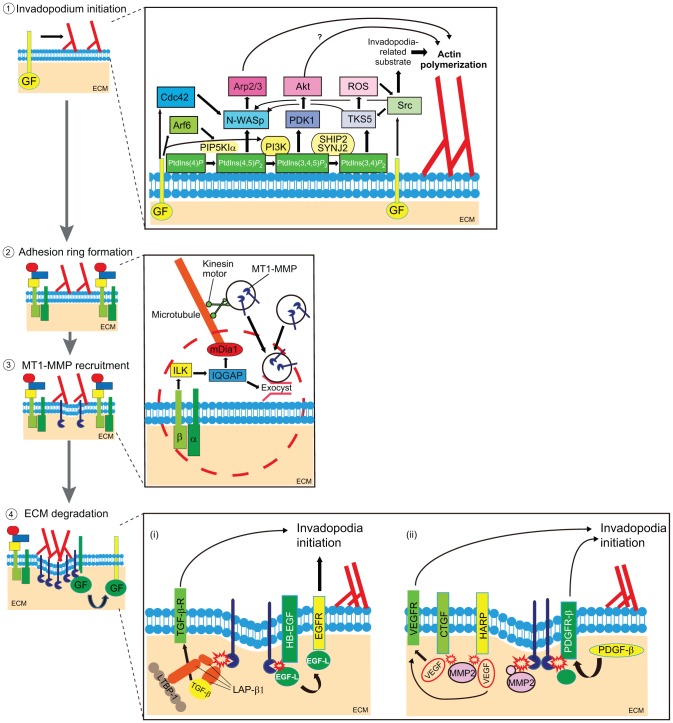
Fig. 2.
A model of invadopodia and podosome stages. (1) Invadopodia/podosome initiation. Growth factors (GF) signaling via GF receptors (yellow bars) leads to invadopodia initiation and actin polymerization (branched actin filaments shown in red). The inset on the right illustrates a proposed scheme of synergistic molecular interactions that lead to invadopodia/podosome initiation. Signals from activated lipid and protein kinases and small GTPases converge on N-WASp which activates the Arp2/3 complex to promote actin assembly. (2, 3) Adhesion ring formation and vesicle capture. The next step is the formation of the adhesion ring (indicated by molecules in the red dashed circle, especially integrins and ILK, as shown in the enlarged scheme to the right), which helps to capture vesicles (black circles) that contain proteinases, such as MT1-MMP (dark blue). The inset illustrates key molecules involved in vesicle capture at invadopodia/podosomes, including the formin mDia1, ILK, IQGAP and the exocyst complex (Branch et al., 2012; Liu et al., 2009; Lizárraga et al., 2009; Sakurai-Yageta et al., 2008). (4) ECM degradation and signaling feedback. In addition to ECM degradation, proteinase activity at invadopodia and podosomes might provide signal feedback to affect either the lifetime of existing invadosomes or formation of new invadosomes. (i) An enlarged model of potential signal feedback from proteinase activity. MT1-MMP can release growth factors (indicated by the red flash) by multiple mechanisms, including cleavage of the TGFβ-binding protein, latency-associated protein (red, LAP-β1 complex) to release active TGF-β1 (Mu et al., 2002). In addition, MT1-MMP also cleaves HB-EGF, which then releases an EGF-like domain (EGF-L) that could activate EGFR and trigger invadosome initiation (Díaz et al., 2013; Hayes et al., 2012; Koshikawa et al., 2010). (ii) MT1-MMP also cleaves other proteinases such as MMP2 (Sato et al., 1994). MMP2 is reported to disrupt vascular endothelial growth factor (VEGF)–heparin affin regulatory peptide (HARP) and VEGF–connective tissue growth factor (CTGF) angiogenic inhibitory complexes, which might release VEGF (Dean et al., 2007) and then trigger invadosome initiation (Lucas et al., 2010). Finally, MT1-MMP activation of PDGFR signaling (Lehti et al., 2005) is another potential mechanism that could promote invadosome formation (Eckert et al., 2011).
Formation and regulation of invadopodia and podosomes
Perturbation of growth factor signaling by changes in ligand availability or mutation of downstream signaling molecules is a common feature of both tumorigenesis and cancer progression (Hanahan and Weinberg, 2000). Alterations in integrins and other adhesion-related proteins are also frequent events in cancer and have been linked to cancer aggressiveness (Lahlou and Muller, 2011; Lehmann et al., 2011). At the cellular level, both growth factor and adhesion signaling can promote invasion through multiple mechanisms, including regulation of cellular motility structures and altering expression and trafficking of matrix metalloproteinases. Here, we focus on the control of the different stages of invadopodia and podosomes.
Invadopodia and podosomes have been shown to assemble in stages (Fig. 2). The first step of invadopodium formation is usually marked by the formation of actin puncta, which presumably occurs in response to activation of upstream signaling cascades (Artym et al., 2006; Oser et al., 2009; Yamaguchi et al., 2005). Cortactin is frequently used as a marker of actin puncta and its localization has been shown to occur either simultaneously (Oser et al., 2009) or minutes before (Artym et al., 2006) the arrival of the key proteinase membrane type-1 matrix metalloproteinase (MT1-MMP, also known as MMP14). Using a thin matrix preparation (50 nm thickness), it has been shown that ECM degradation occurs rapidly after MT1-MMP localization (Artym et al., 2006), whereas the degradation of a thicker ECM layer (∼1 µm thickness using standard preparations) takes place up to an hour after cortactin and MT1-MMP localize to invadopodia (Oser et al., 2009).
At podosomes, adhesions form a strong ring around actin puncta (Kaverina et al., 2003; Luxenburg et al., 2012), as visualized by immunofluorescent staining of the adhesion proteins vinculin or paxillin (Fig. 1). Live-cell imaging in VSMCs that have been stimulated to form podosomes with the protein kinase C activator phorbol myristate acetate (PMA) revealed that focal adhesions dissolve and then reorganize around newly formed actin puncta (Kaverina et al., 2003). In osteoclasts, adhesion markers arrive both before and after actin puncta formation, but the formation of a defined ring occurs only after actin polymerization (Luxenburg et al., 2012). Our recent live-cell imaging study revealed that, in invadopodia, the adhesion protein paxillin appears and forms a ring soon after actin punctum formation (Branch et al., 2012). In the same study, we found that knocking down MT1-MMP, and therefore inhibiting ECM degradation by invadopodia, does not affect adhesion ring localization (Branch et al., 2012). These data suggest that adhesions are recruited to invadopodia after actin puncta formation but before ECM degradation (Fig. 2). Additional live-cell imaging studies of both podosomes and invadopodia will be useful to compare the timing of recruitment of adhesion proteins with respect to that of other markers in the two types of structures. Super-resolution microscopy approaches, such as have been applied to the study of focal adhesions (Kanchanawong et al., 2010), might also be useful to delineate the structural relationship between multiple invadopodia core and adhesion ring markers.
Many studies assess cellular invadopodia or podosome numbers, as well as activity, using fixed microscopy assays to quantify the localization of actin-based markers in punctate structures or rosettes and the degradation of the underlying ECM. However, although fixed-cell studies are useful to assess multiple markers or the general state of the cell, live-cell imaging should ideally be used to differentiate the different stages of invadopodia and podosomes. This allows studying the regulation of invadopodia and podosome formation separately from the maturation of these structures, which is marked by proteinase recruitment and ECM degradation. In addition, a change in invadopodia or podosome numbers that has been observed in fixed cells could be due to either their altered formation or dynamics, which can only be distinguished by live imaging. Nonetheless, it is useful to compare fixed and live imaging systems within the same study, as some live-cell markers might perturb the system.
Regulation of invadopodia and podosome formation by growth factors
A number of growth factors have been shown to promote invadopodium or podosome formation and/or activity. Many factors known to produce activated or differentiated states of normal cells induce the formation of podosomes. Thus, colony stimulating factor-1 (CSF1) is a differentiation factor, and the macrophage cells resulting from that differentiation process form podosomes (Wheeler et al., 2006). The platelet product transforming growth factor β (TGF-β) (Daubon et al., 2011; Rottiers et al., 2009; Varon et al., 2006), as well as vascular endothelial growth factor (VEGF) (Osiak et al., 2005; Wang et al., 2009), activate endothelial cells and rapidly induce podosome formation in them. Finally, platelet-derived growth factor (PDGF) is a well-known inducer of the activated synthetic phenotype in VSMCs, which involves loss of contractility and acquisition of migratory characteristics (Thyberg et al., 1990; Thyberg et al., 1983; Wanjare et al., 2012) and also induces podosome formation (Quintavalle et al., 2010). In cancer cells, epidermal growth factor (EGF) is a well-described inducer of invadopodia formation (Mader et al., 2011; Yamaguchi et al., 2005). TGF-β (Mandal et al., 2008; Pignatelli et al., 2012), heparin binding (HB)-EGF (Díaz et al., 2013; Hayes et al., 2012), VEGF (Lucas et al., 2010) and hepatocyte growth factor/scatter factor (HGF) (Rajadurai et al., 2012) increase invadopodia numbers, whereas stromal cell derived factor 1α (SDF1α) has been shown to increase ECM degradation by breast cancer cells (Smith-Pearson et al., 2010). Many of these growth factor pathways converge on common signaling hubs, especially Src kinase, phosphoinositide 3-kinases (PI3Ks) and Rho family GTPases, which ultimately control invadopodia and podosomes (Linder and Aepfelbacher, 2003; Murphy and Courtneidge, 2011).
Regulation by phosphatidylinositols
At numerous cellular sites, phosphatidylinositol lipids serve as crucial activators of actin polymerization (Ueno et al., 2011; Wennström et al., 1994). PI3Ks are activated downstream of a number of extracellular signals, particularly growth factor receptor signaling (Cantley, 2002). The use of PI3K inhibitors or knockdown of class I PI3Ks was recently shown to reduce the number of invadopodia and their degradation of the ECM to a similar extent, suggesting that PI3Ks exert a specific effect on the formation of invadopodia (Hoshino et al., 2012; Yamaguchi et al., 2011). Similarly, the expression of activating mutant forms of the catalytic p110α subunit of PI3K led to a large increase in the numbers of invadopodia (Hoshino et al., 2012; Yamaguchi et al., 2011), and live-cell imaging revealed that PI3K activity controls both the formation and stability of invadopodia (Hoshino et al., 2012). Furthermore, PI3Ks are required for podosome formation downstream of protein kinase C (PKC) activation by phorbol ester (Gatesman et al., 2004; Walker et al., 2007; Xiao et al., 2013), hepatoma-derived growth factor stimulation (Kung et al., 2012) and in CSF1-induced podosome formation in macrophages (Wheeler et al., 2006). PI3K has also been implicated in podosome formation by osteoclasts (Chellaiah et al., 2001) and affects bone resorption (Shinohara et al., 2012).
Class I PI3Ks phosphorylate phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] at the D-3 position to create phosphatidylinositol (3,4,5)-trisphosphate [PtdIns(3,4,5)P3] (Fruman et al., 1998). A classical PI3K signaling pathway that functions downstream of PtdIns(3,4,5)P3 is the phosphorylation mediated by phosphoinositide-dependent protein kinase 1 (PDK1, also known as PDPK1) and activation of Akt serine/threonine kinases (Cantley, 2002). Indeed, both PDK1 and Akt have been shown to be crucial for invadopodia formation in breast cancer cells downstream of PI3K (Yamaguchi et al., 2011). However, the downstream effectors of Akt in invadopodia are unclear, and further study is required to determine the exact roles of Akt in invadopodium or podosome formation.
PtdIns(3,4,5)P3 might also serve as a precursor lipid for PtdIns(3,4)P2, which localizes to invadosomes in Src-transformed fibroblasts (Oikawa et al., 2008). To investigate the synchronized formation of invadosomes in these cells, the authors performed live imaging shortly after the removal of a drug inhibiting Src, and found that the accumulation of PtdIns(3,4)P2 at invadosome sites is an early event that precedes the recruitment of the signaling adaptor proteins GRB2 and TKS5 (also known as SH3PXD2A) (see below) and branched actin polymerization (Oikawa et al., 2008). PtdIns(3,4)P2 is formed by dephosphorylation of the 5′-inositol phosphate of PtdIns(3,4,5)P3 by inositol polyphosphate 5-phosphatases, such as SH2-domain containing phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 2 (SHIP2, also known as INPPL1) or synaptojanin-2 (SYNJ2) (Ooms et al., 2009). Synaptojanin-2 knockdown was previously shown to reduce the number of invadopodia in glioma cells (Chuang et al., 2004). We also recently found that SHIP2 overexpression in head and neck squamous cell carcinoma (HNSCC) cells can increase the number of invadopodia in a PI3K-dependent manner, suggesting that there are multiple routes through which PtdIns(3,4)P2 is formed to control invadopodia (Hoshino et al., 2012). Notably, SHIP2 overexpression has been linked to aggressive behavior in several cancers (Fuhler et al., 2012; Prasad et al., 2008; Zhou et al., 2011).
A probable effector of PtdIns(3,4)P2 at invadopodia and podosomes is the scaffold protein TKS5 (Abram et al., 2003; Crimaldi et al., 2009; Lock et al., 1998; Seals et al., 2005). TKS5 can form a complex with N-WASp (also known as WASL) and the upstream scaffolding proteins GRB2 and Nck in response to Src activity. In this way, TKS5 might promote actin polymerization at nascent invadopodia (Oikawa et al., 2008; Stylli et al., 2009). TKS5 is also important for the generation of reactive oxygen species (ROS) (Diaz et al., 2009; Gianni et al., 2009), which might potentiate tyrosine kinase activity at invadopodia and podosomes by inhibiting tyrosine phosphatases (Diaz et al., 2009; Weaver, 2009). Additional potential effectors include Akt (Klippel et al., 1997; Yamaguchi et al., 2011), TIAM1 (Ceccarelli et al., 2007) and other PtdIns(3,4)P2-binding partners with unknown functions at invadopodia. Overall, these data suggest that growth-factor-mediated activation of PI3K might affect the formation of invadopodia and podosomes through downstream effectors that regulate Src kinase signaling and actin organization (Fig. 2).
In addition to PtdIns(3,4,5)P3 and PtdIns(3,4)P2, PtdIns(4,5)P2 have also been implicated in invadopodium formation. PtdIns(4,5)P2 has been shown to surround the invadopodium protrusion and might be generated locally by phosphatidylinositol 4-phosphate 5-kinase type Iα (PIP5KIα) (Yamaguchi et al., 2010). Both PtdIns(4,5)P2 and PIP5KIα are required for ECM degradation by breast cancer cell invadopodia, but a requirement for invadopodia formation itself was not assessed in that study (Yamaguchi et al., 2010). Interestingly, the Ras family GTPase ARF6, which activates PIP5KIα downstream of EGF (Honda et al., 1999), localizes to invadopodia and is required for their activity (Hashimoto et al., 2004; Tague et al., 2004). PtdIns(4,5)P2 affects a wide range of actin regulators (Saarikangas et al., 2010), including N-WASp (Kim et al., 2000; Rohatgi et al., 2000; Rohatgi et al., 1999) and cofilin (Oser and Condeelis, 2009), that have been shown to be crucial for actin polymerization at both invadopodia and podosomes. Therefore, PtdIns(4,5)P2 might directly control actin assembly at invadopodia sites and, in addition, serve as a substrate for the generation of PtdIns(3,4,5)P3 and other phosphoinositol lipids.
Other regulators downstream of growth factor signaling
In addition to phosphatidylinositides, other key signals downstream of growth factors that control invadopodia and podosome formation include Rho GTPases, and tyrosine and serine/threonine kinases. The Rho GTPase Cdc42 is essential for actin polymerization at invadopodia and podosomes, probably owing to its ability to directly activate N-WASp and thereby promote actin nucleation by the Arp2/3 complex (Moreau et al., 2003; Nakahara et al., 2003; Rohatgi et al., 1999; Yamaguchi et al., 2005). Other Rho GTPases have also been reported to affect invadopodia formation or activity; however, these effects have been either variable or less strong than that of Cdc42 (reviewed by Linder and Aepfelbacher, 2003; Ory et al., 2008; van Helden and Hordijk, 2011). Given the core role of Cdc42 in actin polymerization at invadopodia, it seems likely that other Rho GTPases have indirect roles through modulating signaling or actin dynamics (Bravo-Cordero et al., 2011) downstream of Cdc42. Rho GTPases are likely to be influenced by phosphatidylinositide signaling, given that most of the upstream activator Rho GTPase exchange factors (GEFs) bind to phosphatidylinositides (Rossman et al., 2005).
Among the factors controlling invadopodia formation and function, Src kinase has the most links to other signaling pathways (Hoshino et al., 2012) and is thought to be both necessary and sufficient for the induction of invadopodia and podosome formation (Linder et al., 2011; Murphy and Courtneidge, 2011; Weaver, 2006). However, other protein kinases have recently been shown to control invadopodia and podosome formation, maturation or dynamics, including the Abl kinases (Smith-Pearson et al., 2010), the Abl-related gene kinase (Arg, also known as ABL2) (Mader et al., 2011), p21-activated kinase (PAK) and ERK (Ayala et al., 2008; Tague et al., 2004; Webb et al., 2005). Similar to Src, they have many substrates that could influence various stages of invadopodia and podosomes.
The two popular models for studying podosome formation are either Src-transformed cells (Fig. 1) or A7r5 VSMC cells that have been treated with phorbol ester. In both cases, podosomes form from disassembling focal adhesions (Kaverina et al., 2003; Oikawa et al., 2008), demonstrating a strong link between the two structures. Similarly, Mak and colleagues demonstrated that the expression of active PAK1 in A7r5 cells could induce a similar transition of focal adhesions to podosomes in the absence of any extraneous stimuli (Webb et al., 2005). On the basis of this idea that invadopodia and podosome structures might directly form from focal adhesions, we built theoretical network models of focal adhesions and invadopodia and used graph theory centrality analyses to identify signaling hubs that might regulate the transitions between the two structures (Hoshino et al., 2012). In an initial analysis of the results, we found that in cancer cells, PKCα expression can enhance or inhibit invadopodia formation depending on whether PI3K activity was deregulated in these cells (e.g. as can happen when there is constitutively active PIK3CA ‘hotspot’ mutants or loss of the opposing lipid kinase PTEN). We further found that in cells with overly active PI3K, PKCα functions in an important negative-feedback loop to PI3K activity (Hoshino et al., 2012). We therefore speculated that in cancer cells with deregulated PI3K activity, loss of spatial control of signaling might lead to the formation of invadopodia at non-focal adhesion sites. Because of the many potential feedback loops that control signaling and the uncertainty with regard to the stage of invadopodia formation at which most of these signaling regulators function, live imaging and systems approaches will probably be important tools to dissect how diverse kinases, Rho GTPases and other signaling regulators control invadopodia and podosomes.
Regulation of invadopodia and podosomes by ECM and adhesion proteins
Relationship between invadopodia, podosomes and focal adhesions
Podosomes, invadopodia and focal adhesions are all actin-based structures that contain ECM adhesion molecules, such as integrins and adhesion adaptor proteins. For invadopodia, the role of adhesion signaling and the adhesive ability of the invadopodium itself have been controversial, as cancer cells frequently contain both FAs and invadopodia. In fact, the presence of an adhesion ring has been suggested to be the defining difference between invadopodia and podosomes (Linder et al., 2011; Murphy and Courtneidge, 2011), with the presumption that invadopodia lack adhesion rings. However, a number of studies in different cancer cell lines have shown that adhesion-related proteins are located around or at invadopodia puncta (Alexander et al., 2008; Bowden et al., 1999; Branch et al., 2012; Mueller et al., 1999; Pignatelli et al., 2012; Spinardi et al., 2004). We recently performed a thorough analysis of the role of adhesion proteins at invadopodia and found that the adhesion adaptor proteins paxillin and vinculin as well as integrins localize to a podosome-like ring structure surrounding invadopodia of breast cancer and HNSCC cells (Branch et al., 2012). Furthermore, we find that invadopodia with adhesion rings show an increased degradation of the ECM as compared with that of invadopodia with no visible adhesion rings (Branch et al., 2012). These results suggest that invadopodia and podosomes are highly similar structures and that adhesions contribute to their functions.
Focal adhesions are streak-like adhesion structures that connect actin bundles known as stress fibers to clustered adhesion molecules including integrins (Fig. 1). Focal adhesions have long been considered to be structures without degradative activity that mediate the attachment of the cell to the ECM and serve as signaling scaffolds (Geiger and Yamada, 2011). Nonetheless, a recent study demonstrated that ECM degradation can occur underneath focal adhesions in fibrosarcoma and pancreatic cells (Wang and McNiven, 2012), in a manner dependent on many of the factors that have been implicated in invadopodia and podosome function, including MT1-MMP and Src kinase. However, these structures were determined to be separate from invadopodia, as the streak-like degradation underneath focal adhesions was distinct from the dot-like invadopodia-related ECM degradation underneath the same cell. Inhibition or knockdown of focal adhesion kinase (FAK) and p130Cas (also known as BCAR1) primarily affected focal-adhesion-related rather than invadopodia-related degradation, whereas Src inhibition abolished ECM degradation under all structures. Using a similar protocol, Machesky and colleagues also observed ECM degradation at focal adhesions that depended on FAK (Tang et al., 2013). These data are consistent with a previous study where FAK was suggested to primarily regulate invadopodia by an indirect manner in which FAK promotes the spatiotemporal localization of Src and other phosphotyrosine invadopodia components to focal adhesions (Chan et al., 2009). However, other studies have found that FAK and p130Cas directly localize to and/or promote invadopodia and podosome activity (Alexander et al., 2008; Brábek et al., 2004; Pignatelli et al., 2012; Yu et al., 2011). It seems likely that additional molecular constituents can determine the role of FAK in controlling ECM degradation at various invasive structures.
The finding that focal adhesions can degrade ECM was surprising, because focal-adhesion-mediated ECM degradation should have been evident in the many previous imaging studies. However, the authors used a thin and uncrosslinked fluorescent substrate (50 nm) (Artym et al., 2009; Artym et al., 2006), which might have allowed them to detect very low levels of degradation at focal adhesions (Wang and McNiven, 2012) that was not previously evident using the older methods with thick matrix substrates (Bowden et al., 2001). As many of the molecules present at focal adhesions are also found at invadopodia or podosomes, we speculate that a similar mechanism is used for ECM degradation in all these structures, but that additional molecular changes at invadopodia and podosomes greatly amplify their proteolytic capacity. It is unclear at the moment what those differences are, but they could include enhanced exocytosis of proteinases.
Regulation of invadopodia and podosomes by adhesion protein signaling
A number of recent studies have looked at the role of adhesion signaling in the regulation of podosome and invadopodia function. In Src-transformed cells, traction force microscopy revealed that invadosomes are mechanosensing structures, suggesting that localized mechanotransduction signaling through adhesions might control podosomes and/or invadopodia (Collin et al., 2008). Likewise, we found that ECM rigidity controls the activity, but not the formation of, invadopodia puncta (Alexander et al., 2008). In addition, several studies have shown that myosin-mediated contractility can induce the turnover of podosomes (Bhuwania et al., 2012; van Helden et al., 2008). Other studies have directly examined the role of adhesion molecules, including integrins and downstream adhesion components. In osteoclasts, podosome organization into belts (Fig. 1) and bone resorption, but not actin core formation, was reduced when combinations of β1, β2 and αV integrin were knocked out (Schmidt et al., 2011). Similarly, in Src-transformed cells, knockout of β1 integrins reduced the number of secondary rosette structures of invadosomes (see figure 1b in Destaing et al., 2010 for an explanation of rosettes). Knockdown or perturbation of downstream adhesion components, including paxillin phosphorylation, FAK, p130Cas, RhoA–ROCK signaling, and calpain, have also been shown to affect the expansion of podosome or invadosome rosettes, actin core turnover and matrix degradation (Badowski et al., 2008; Berdeaux et al., 2004; Brábek et al., 2004; Calle et al., 2006; Pan et al., 2011; Tatin et al., 2006). Taken together, these results suggest that adhesion signaling might be more crucial for podosome maturation and organization into groups than for their formation.
In cancer cells, ligation of β1 integrin increases invadopodia-associated ECM degradation (Nakahara et al., 1998; Nakahara et al., 1996). We recently demonstrated a role for RGD-binding integrins and integrin-linked kinase (ILK) in the maturation of invadopodia to degradative protrusions (Branch et al., 2012). Blocking the attachment of integrin to fibronectin, a major component of the ECM, with a soluble RGD peptide or knocking down the protein levels of ILK with small hairpin RNA led to a reduction of both the localization of adhesion ring components around invadopodia and invadopodia-mediated degradation of the ECM. Live-cell imaging specifically revealed that exocytosis of the crucial invadopodia proteinase MT1-MMP at invadopodia that had already formed was reduced under both conditions. New formation and stability of invadopodia were also affected in the ILK-knockdown cells, but not in the integrin-inhibited cells. Taken together, these data indicate that integrin-mediated cell–ECM adhesion and downstream proteins such as ILK are particularly important for the maturation of invadopodia into degradative protrusions (Fig. 2). By contrast, the cellular integrin and ECM context might be an important determinant of the response of invadopodia to integrin–ECM adhesion. Indeed, we have found that adhesion of rat bladder carcinoma cells to the matrix molecule laminin-332 via α3β1 integrin can reduce the numbers and activity of invadopodia (Liu et al., 2010), which is opposite to the effect of fibronectin–α5β1-integrin interactions in breast and HNSCC cells (Branch et al., 2012). Future live-cell imaging studies will be necessary to understand the complex role of matrix–integrin interactions in controlling invadopodia activity.
Integrin–ECM adhesion promotes membrane trafficking
The contribution of RGD-binding integrins and ILK to the localization of MT1-MMP to invadopodia (Branch et al., 2012) suggests that these proteins have a role in membrane trafficking. In fact, cell–ECM adhesions have recently emerged as an important regulator of membrane trafficking. Loss of cell–ECM attachment results in the internalization of membrane raft domains that are dependent on caveolin-1 (del Pozo et al., 2004; del Pozo et al., 2005), whereas reattachment of cells to the ECM induces the recycling of lipid rafts from endosomes to the plasma membrane (Balasubramanian et al., 2010; Balasubramanian et al., 2007). β1 integrin and ILK regulate the localization of caveolar vesicles to the plasma membrane through the ILK-binding partner IQGAP1 and the actin- and microtubule-binding protein mDia1 (Wickström et al., 2010). IQGAP1 also interacts with the exocyst complex, and this interaction is crucial for ECM degradation by breast cancer cells (Sakurai-Yageta et al., 2008). Interestingly, we have demonstrated that blocking of RGD-binding integrins or knockdown of ILK protein levels reduces IQGAP localization to invadopodia (Branch et al., 2012). Taken together, these data suggest that a common signaling pathway involving β1-integrin-mediated cell–ECM attachment, ILK, IQGAP1 and the exocyst complex regulates the delivery of lipid rafts to various cellular sites, including invadopodia (Caldieri et al., 2009; Yamaguchi et al., 2009). In cells that express MT1-MMP and other invadopodia proteinases, we speculate that their exocytosis through this pathway results in ECM degradation. Whether MT1-MMP and other invadopodia components that are delivered through exocytosis are specifically routed to invadopodia as opposed to other adhesion sites is an interesting future question.
Conclusions and perspectives
Overall, growth factor inputs appear to primarily affect the formation of invadopodia and podosomes and the early stage actin dynamics, potentially through the formation of phosphatidylinositides, including PtdIns(3,4)P2 and PtdIns(4,5)P2, and recruitment of initiation factors such as TKS5 and N-WASp. By contrast, the main role of adhesion signaling appears to be promotion of invadopodia and podosome maturation, which includes the secretion of proteinases at invadopodia to enhance degradation of ECM. Although the current evidence supports the model we propose (Fig. 2), we note that there are few studies using live-cell imaging in this area, which is the definitive way to assess control of invadopodia stages. Adhesion-based integrin signaling also intersects with growth factor signaling inputs in many cellular contexts (Azimifar et al., 2012; Levental et al., 2009) and could affect invadopodia formation through such crosstalk. In addition, it is possible that adhesion signaling could enhance formation of new invadopodia as a secondary effect of increased proteinase activity, which is known to enhance invadopodia numbers (Artym et al., 2006; Branch et al., 2012; Clark et al., 2007; Steffen et al., 2008). This could occur through the release of growth factors or ECM fragments from the ECM, or by a direct cleavage and activation of growth factors or growth factor receptors (Kessenbrock et al., 2010; Koshikawa et al., 2010; Lehti et al., 2005; Lyons et al., 1990; Mu et al., 2002; Yu and Stamenkovic, 2000). Given the complexity of adhesion and growth factor signaling inputs and the likelihood of multiple signal feedback loops (Comoglio et al., 2003; Eliceiri, 2001; Hoshino et al., 2012), we propose that a systematic-perturbation–live-imaging approach under diverse conditions will be crucial for dissecting the control of the different stages of invadopodia and podosomes by signaling inputs. Overall, this is an exciting area for future research, as invadopodia and podosomes are sites at which growth factor and adhesion inputs converge, and can be used as model systems for investigating how diverse signaling states control invasive behavior.
Acknowledgments
We thank Stefan Linder for critical reading of the manuscript.
Footnotes
Funding
The work of our laboratory is supported by the National Institutes of Health [grant numbers R01GM075126, R01CA163592, U01CA143069, U54CA113007]; and American Cancer Society [grant number RSG-09-170-01-CSM]. Deposited in PMC for release after 12 months.
References
- Abram C. L., Seals D. F., Pass I., Salinsky D., Maurer L., Roth T. M., Courtneidge S. A. (2003). The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem. 278, 16844–16851 10.1074/jbc.M300267200 [DOI] [PubMed] [Google Scholar]
- Alexander N. R., Branch K. M., Parekh A., Clark E. S., Iwueke I. C., Guelcher S. A., Weaver A. M. (2008). Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol. 18, 1295–1299 10.1016/j.cub.2008.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym V. V., Zhang Y., Seillier-Moiseiwitsch F., Yamada K. M., Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034–3043 10.1158/0008-5472.CAN-05-2177 [DOI] [PubMed] [Google Scholar]
- Artym V. V., Yamada K. M., Mueller S. C. (2009). ECM degradation assays for analyzing local cell invasion. Methods Mol. Biol. 522, 211–219 10.1007/978-1-59745-413-1_15 [DOI] [PubMed] [Google Scholar]
- Artym V. V., Matsumoto K., Mueller S. C., Yamada K. M. (2011). Dynamic membrane remodeling at invadopodia differentiates invadopodia from podosomes. Eur. J. Cell Biol. 90, 172–180 10.1016/j.ejcb.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attanasio F., Caldieri G., Giacchetti G., van Horssen R., Wieringa B., Buccione R. (2011). Novel invadopodia components revealed by differential proteomic analysis. Eur. J. Cell Biol. 90, 115–127 10.1016/j.ejcb.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Ayala I., Baldassarre M., Giacchetti G., Caldieri G., Tetè S., Luini A., Buccione R. (2008). Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J. Cell Sci. 121, 369–378 10.1242/jcs.008037 [DOI] [PubMed] [Google Scholar]
- Azimifar S. B., Böttcher R. T., Zanivan S., Grashoff C., Krüger M., Legate K. R., Mann M., Fässler R. (2012). Induction of membrane circular dorsal ruffles requires co-signalling of integrin-ILK-complex and EGF receptor. J. Cell Sci. 125, 435–448 10.1242/jcs.091652 [DOI] [PubMed] [Google Scholar]
- Badowski C., Pawlak G., Grichine A., Chabadel A., Oddou C., Jurdic P., Pfaff M., Albigès-Rizo C., Block M. R. (2008). Paxillin phosphorylation controls invadopodia/podosomes spatiotemporal organization. Mol. Biol. Cell 19, 633–645 10.1091/mbc.E06-01-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian N., Scott D. W., Castle J. D., Casanova J. E., Schwartz M. A. (2007). Arf6 and microtubules in adhesion-dependent trafficking of lipid rafts. Nat. Cell Biol. 9, 1381–1391 10.1038/ncb1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian N., Meier J. A., Scott D. W., Norambuena A., White M. A., Schwartz M. A. (2010). RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr. Biol. 20, 75–79 10.1016/j.cub.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdeaux R. L., Díaz B., Kim L., Martin G. S. (2004). Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J. Cell Biol. 166, 317–323 10.1083/jcb.200312168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuwania R., Cornfine S., Fang Z., Krüger M., Luna E. J., Linder S. (2012). Supervillin couples myosin-dependent contractility to podosomes and enables their turnover. J. Cell Sci. 125, 2300–2314 10.1242/jcs.100032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S. et al. (1997). A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–733 10.1038/385729a0 [DOI] [PubMed] [Google Scholar]
- Bowden E. T., Barth M., Thomas D., Glazer R. I., Mueller S. C. (1999). An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18, 4440–4449 10.1038/sj.onc.1202827 [DOI] [PubMed] [Google Scholar]
- Bowden E. T., Coopman P. J., Mueller S. C. (2001). Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 63, 613–627 10.1016/S0091-679X(01)63033-4 [DOI] [PubMed] [Google Scholar]
- Brábek J., Constancio S. S., Shin N. Y., Pozzi A., Weaver A. M., Hanks S. K. (2004). CAS promotes invasiveness of Src-transformed cells. Oncogene 23, 7406–7415 10.1038/sj.onc.1207965 [DOI] [PubMed] [Google Scholar]
- Branch K. M., Hoshino D., Weaver A. M. (2012). Adhesion rings surround invadopodia and promote maturation. Biol. Open 1, 711–722 10.1242/bio.20121867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero J. J., Oser M., Chen X., Eddy R., Hodgson L., Condeelis J. (2011). A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr. Biol. 21, 635–644 10.1016/j.cub.2011.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S., Thrasher A. J., Blundell M. P., Machesky L., Jones G. E. (2001). Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 98, 1142–1149 10.1182/blood.V98.4.1142 [DOI] [PubMed] [Google Scholar]
- Caldieri G., Giacchetti G., Beznoussenko G., Attanasio F., Ayala I., Buccione R. (2009). Invadopodia biogenesis is regulated by caveolin-mediated modulation of membrane cholesterol levels. J. Cell. Mol. Med. 13 8B, 1728–1740 10.1111/j.1582-4934.2008.00568.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle Y., Carragher N. O., Thrasher A. J., Jones G. E. (2006). Inhibition of calpain stabilises podosomes and impairs dendritic cell motility. J. Cell Sci. 119, 2375–2385 10.1242/jcs.02939 [DOI] [PubMed] [Google Scholar]
- Cantley L. C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655–1657 10.1126/science.296.5573.1655 [DOI] [PubMed] [Google Scholar]
- Ceccarelli D. F., Blasutig I. M., Goudreault M., Li Z., Ruston J., Pawson T., Sicheri F. (2007). Non-canonical interaction of phosphoinositides with pleckstrin homology domains of Tiam1 and ArhGAP9. J. Biol. Chem. 282, 13864–13874 10.1074/jbc.M700505200 [DOI] [PubMed] [Google Scholar]
- Cervero P., Himmel M., Krüger M., Linder S. (2012). Proteomic analysis of podosome fractions from macrophages reveals similarities to spreading initiation centres. Eur. J. Cell Biol. 91, 908–922 10.1016/j.ejcb.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Chan K. T., Cortesio C. L., Huttenlocher A. (2009). FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J. Cell Biol. 185, 357–370 10.1083/jcb.200809110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K. M., Wong H. L., Jin G., Liu B., Cao R., Cao Y., Lehti K., Tryggvason K., Zhou Z. (2012). MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev. Cell 22, 1176–1190 10.1016/j.devcel.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Chellaiah M. A., Biswas R. S., Yuen D., Alvarez U. M., Hruska K. A. (2001). Phosphatidylinositol 3,4,5-trisphosphate directs association of Src homology 2-containing signaling proteins with gelsolin. J. Biol. Chem. 276, 47434–47444 10.1074/jbc.M107494200 [DOI] [PubMed] [Google Scholar]
- Chen W. T., Lee C. C., Goldstein L., Bernier S., Liu C. H., Lin C. Y., Yeh Y., Monsky W. L., Kelly T., Dai M. et al. (1994). Membrane proteases as potential diagnostic and therapeutic targets for breast malignancy. Breast Cancer Res. Treat. 31, 217–226 10.1007/BF00666155 [DOI] [PubMed] [Google Scholar]
- Chuang Y. Y., Tran N. L., Rusk N., Nakada M., Berens M. E., Symons M. (2004). Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 64, 8271–8275 10.1158/0008-5472.CAN-04-2097 [DOI] [PubMed] [Google Scholar]
- Clark E. S., Whigham A. S., Yarbrough W. G., Weaver A. M. (2007). Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227–4235 10.1158/0008-5472.CAN-06-3928 [DOI] [PubMed] [Google Scholar]
- Collin O., Na S., Chowdhury F., Hong M., Shin M. E., Wang F., Wang N. (2008). Self-organized podosomes are dynamic mechanosensors. Curr. Biol. 18, 1288–1294 10.1016/j.cub.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoglio P. M., Boccaccio C., Trusolino L. (2003). Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 15, 565–571 10.1016/S0955-0674(03)00096-6 [DOI] [PubMed] [Google Scholar]
- Coopman P. J., Do M. T., Thompson E. W., Mueller S. C. (1998). Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin. Cancer Res. 4, 507–515 [PubMed] [Google Scholar]
- Crimaldi L., Courtneidge S. A., Gimona M. (2009). Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp. Cell Res. 315, 2581–2592 10.1016/j.yexcr.2009.06.012 [DOI] [PubMed] [Google Scholar]
- Daubon T., Buccione R., Génot E. (2011). The Aarskog-Scott syndrome protein Fgd1 regulates podosome formation and extracellular matrix remodeling in transforming growth factor β-stimulated aortic endothelial cells. Mol. Cell. Biol. 31, 4430–4441 10.1128/MCB.05474-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. A., Butler G. S., Hamma-Kourbali Y., Delbé J., Brigstock D. R., Courty J., Overall C. M. (2007). Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol. Cell. Biol. 27, 8454–8465 10.1128/MCB.00821-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M. A., Alderson N. B., Kiosses W. B., Chiang H. H., Anderson R. G., Schwartz M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839–842 10.1126/science.1092571 [DOI] [PubMed] [Google Scholar]
- del Pozo M. A., Balasubramanian N., Alderson N. B., Kiosses W. B., Grande-García A., Anderson R. G., Schwartz M. A. (2005). Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat. Cell Biol. 7, 901–908 10.1038/ncb1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Saltel F., Géminard J. C., Jurdic P., Bard F. (2003). Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol. Biol. Cell 14, 407–416 10.1091/mbc.E02-07-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Planus E., Bouvard D., Oddou C., Badowski C., Bossy V., Raducanu A., Fourcade B., Albiges-Rizo C., Block M. R. (2010). β1A integrin is a master regulator of invadosome organization and function. Mol. Biol. Cell 21, 4108–4119 10.1091/mbc.E10-07-0580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O., Block M. R., Planus E., Albiges-Rizo C. (2011). Invadosome regulation by adhesion signaling. Curr. Opin. Cell Biol. 23, 597–606 10.1016/j.ceb.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Diaz B., Shani G., Pass I., Anderson D., Quintavalle M., Courtneidge S. A. (2009). Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci. Signal. 2, ra53 10.1126/scisignal.2000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz B., Yuen A., Iizuka S., Higashiyama S., Courtneidge S. A. (2013). Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J. Cell Biol. 201, 279–292 10.1083/jcb.201209151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfleutner A., Cho Y., Vincent D., Cunnick J., Lin H., Weed S. A., Stehlik C., Flynn D. C. (2008). Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J. Cell Sci. 121, 2394–2405 10.1242/jcs.026187 [DOI] [PubMed] [Google Scholar]
- Doyle A. D., Wang F. W., Matsumoto K., Yamada K. M. (2009). One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481–490 10.1083/jcb.200810041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M. A., Lwin T. M., Chang A. T., Kim J., Danis E., Ohno-Machado L., Yang J. (2011). Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19, 372–386 10.1016/j.ccr.2011.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri B. P. (2001). Integrin and growth factor receptor crosstalk. Circ. Res. 89, 1104–1110 10.1161/hh2401.101084 [DOI] [PubMed] [Google Scholar]
- Enderling H., Alexander N. R., Clark E. S., Branch K. M., Estrada L., Crooke C., Jourquin J., Lobdell N., Zaman M. H., Guelcher S. A. et al. (2008). Dependence of invadopodia function on collagen fiber spacing and cross-linking: computational modeling and experimental evidence. Biophys. J. 95, 2203–2218 10.1529/biophysj.108.133199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D. A., Meyers R. E., Cantley L. C. (1998). Phosphoinositide kinases. Annu. Rev. Biochem. 67, 481–507 10.1146/annurev.biochem.67.1.481 [DOI] [PubMed] [Google Scholar]
- Fuhler G. M., Brooks R., Toms B., Iyer S., Gengo E. A., Park M. Y., Gumbleton M., Viernes D. R., Chisholm J. D., Kerr W. G. (2012). Therapeutic potential of SH2 domain-containing inositol-5′-phosphatase 1 (SHIP1) and SHIP2 inhibition in cancer. Mol. Med. 18, 65–75 10.2119/molmed.2011.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidano G., Bergui L., Schena M., Gaboli M., Cremona O., Marchisio P. C., Caligaris-Cappio F. (1990). Integrin distribution and cytoskeleton organization in normal and malignant monocytes. Leukemia 4, 682–687 [PubMed] [Google Scholar]
- Gatesman A., Walker V. G., Baisden J. M., Weed S. A., Flynn D. C. (2004). Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol. Cell. Biol. 24, 7578–7597 10.1128/MCB.24.17.7578-7597.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawden-Bone C., Zhou Z., King E., Prescott A., Watts C., Lucocq J. (2010). Dendritic cell podosomes are protrusive and invade the extracellular matrix using metalloproteinase MMP-14. J. Cell Sci. 123, 1427–1437 10.1242/jcs.056515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Yamada K. M. (2011). Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3, a005033 10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni D., Diaz B., Taulet N., Fowler B., Courtneidge S. A., Bokoch G. M. (2009). Novel p47(phox)-related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal. 2, ra54 10.1126/scisignal.2000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligorijevic B., Wyckoff J., Yamaguchi H., Wang Y., Roussos E. T., Condeelis J. (2012). N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J. Cell Sci. 125, 724–734 10.1242/jcs.092726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai C. M., Hahne P., Harrington E. O., Gimona M. (2002). Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp. Cell Res. 280, 64–74 10.1006/excr.2002.5592 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2000). The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Onodera Y., Hashimoto A., Tanaka M., Hamaguchi M., Yamada A., Sabe H. (2004). Requirement for Arf6 in breast cancer invasive activities. Proc. Natl. Acad. Sci. USA 101, 6647–6652 10.1073/pnas.0401753101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes K. E., Walk E. L., Ammer A. G., Kelley L. C., Martin K. H., Weed S. A. (2012). Ableson kinases negatively regulate invadopodia function and invasion in head and neck squamous cell carcinoma by inhibiting an HB-EGF autocrine loop. Oncogene 10.1038/onc.2012.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A., Nogami M., Yokozeki T., Yamazaki M., Nakamura H., Watanabe H., Kawamoto K., Nakayama K., Morris A. J., Frohman M. A. et al. (1999). Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532 10.1016/S0092-8674(00)81540-8 [DOI] [PubMed] [Google Scholar]
- Hoshino D., Jourquin J., Emmons S. W., Miller T., Goldgof M., Costello K., Tyson D. R., Brown B., Lu Y., Prasad N. K. et al. (2012). Network analysis of the focal adhesion to invadopodia transition identifies a PI3K-PKCα invasive signaling axis. Sci. Signal. 5, ra66 10.1126/scisignal.2002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary K., Li X. Y., Allen E., Stevens S. L., Weiss S. J. (2006). A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 20, 2673–2686 10.1101/gad.1451806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Hirata M., Hasuwa H., Iwamoto R., Umata T., Miyado K., Tamai Y., Kurisaki T., Sehara-Fujisawa A., Ohno S. et al. (1998). A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 17, 7260–7272 10.1093/emboj/17.24.7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita M., Itoh Y., Chiba T., Mori H., Okada A., Kinoh H., Seiki M. (2001). Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 153, 893–904 10.1083/jcb.153.5.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P., Shtengel G., Pasapera A. M., Ramko E. B., Davidson M. W., Hess H. F., Waterman C. M. (2010). Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 10.1038/nature09621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa J., Yamanaka T., Doi S., Turksen K., Heersche J. N., Aubin J. E., Takeuchi H. (1990). A band of F-actin containing podosomes is involved in bone resorption by osteoclasts. Bone 11, 287–293 10.1016/8756-3282(90)90082-A [DOI] [PubMed] [Google Scholar]
- Kaverina I., Stradal T. E., Gimona M. (2003). Podosome formation in cultured A7r5 vascular smooth muscle cells requires Arp2/3-dependent de-novo actin polymerization at discrete microdomains. J. Cell Sci. 116, 4915–4924 10.1242/jcs.00818 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. (2010). Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. S., Kakalis L. T., Abdul-Manan N., Liu G. A., Rosen M. K. (2000). Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404, 151–158 10.1038/35004513 [DOI] [PubMed] [Google Scholar]
- Klippel A., Kavanaugh W. M., Pot D., Williams L. T. (1997). A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol. Cell. Biol. 17, 338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa N., Mizushima H., Minegishi T., Iwamoto R., Mekada E., Seiki M. (2010). Membrane type 1-matrix metalloproteinase cleaves off the NH2-terminal portion of heparin-binding epidermal growth factor and converts it into a heparin-independent growth factor. Cancer Res. 70, 6093–6103 10.1158/0008-5472.CAN-10-0346 [DOI] [PubMed] [Google Scholar]
- Kung M. L., Tsai H. E., Hu T. H., Kuo H. M., Liu L. F., Chen S. C., Lin P. R., Ma Y. L., Wang E. M., Liu G. S. et al. (2012). Hepatoma-derived growth factor stimulates podosome rosettes formation in NIH/3T3 cells through the activation of phosphatidylinositol 3-kinase/Akt pathway. Biochem. Biophys. Res. Commun. 425, 169–176 10.1016/j.bbrc.2012.07.060 [DOI] [PubMed] [Google Scholar]
- Lahlou H., Muller W. J. (2011). β1-integrins signaling and mammary tumor progression in transgenic mouse models: implications for human breast cancer. Breast Cancer Res. 13, 229 10.1186/bcr2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann B. D., Bauer J. A., Chen X., Sanders M. E., Chakravarthy A. B., Shyr Y., Pietenpol J. A. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti K., Allen E., Birkedal-Hansen H., Holmbeck K., Miyake Y., Chun T. H., Weiss S. J. (2005). An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev. 19, 979–991 10.1101/gad.1294605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W. et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S., Aepfelbacher M. (2003). Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 13, 376–385 10.1016/S0962-8924(03)00128-4 [DOI] [PubMed] [Google Scholar]
- Linder S., Wiesner C., Himmel M. (2011). Degrading devices: invadosomes in proteolytic cell invasion. Annu. Rev. Cell Dev. Biol. 27, 185–211 10.1146/annurev-cellbio-092910-154216 [DOI] [PubMed] [Google Scholar]
- Liu J., Yue P., Artym V. V., Mueller S. C., Guo W. (2009). The role of the exocyst in matrix metalloproteinase secretion and actin dynamics during tumor cell invadopodia formation. Mol. Biol. Cell 20, 3763–3771 10.1091/mbc.E08-09-0967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yamashita H., Weidow B., Weaver A. M., Quaranta V. (2010). Laminin-332-beta1 integrin interactions negatively regulate invadopodia. J. Cell. Physiol. 223, 134–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizárraga F., Poincloux R., Romao M., Montagnac G., Le Dez G., Bonne I., Rigaill G., Raposo G., Chavrier P. (2009). Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 69, 2792–2800 10.1158/0008-5472.CAN-08-3709 [DOI] [PubMed] [Google Scholar]
- Lock P., Abram C. L., Gibson T., Courtneidge S. A. (1998). A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 17, 4346–4357 10.1093/emboj/17.15.4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. T., Jr, Salimath B. P., Slomiany M. G., Rosenzweig S. A. (2010). Regulation of invasive behavior by vascular endothelial growth factor is HEF1-dependent. Oncogene 29, 4449–4459 10.1038/onc.2010.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C., Geblinger D., Klein E., Anderson K., Hanein D., Geiger B., Addadi L. (2007). The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing zone assembly. PLoS ONE 2, e179 10.1371/journal.pone.0000179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxenburg C., Winograd-Katz S., Addadi L., Geiger B. (2012). Involvement of actin polymerization in podosome dynamics. J. Cell Sci. 125, 1666–1672 10.1242/jcs.075903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Gentry L. E., Purchio A. F., Moses H. L. (1990). Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J. Cell Biol. 110, 1361–1367 10.1083/jcb.110.4.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader C. C., Oser M., Magalhaes M. A., Bravo-Cordero J. J., Condeelis J., Koleske A. J., Gil-Henn H. (2011). An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 71, 1730–1741 10.1158/0008-5472.CAN-10-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen C. D., Sahai E. (2010). Cancer dissemination—lessons from leukocytes. Dev. Cell 19, 13–26 10.1016/j.devcel.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Mandal S., Johnson K. R., Wheelock M. J. (2008). TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp. Cell Res. 314, 3478–3493 10.1016/j.yexcr.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Marchisio P. C., Cirillo D., Teti A., Zambonin-Zallone A., Tarone G. (1987). Rous sarcoma virus-transformed fibroblasts and cells of monocytic origin display a peculiar dot-like organization of cytoskeletal proteins involved in microfilament-membrane interactions. Exp. Cell Res. 169, 202–214 10.1016/0014-4827(87)90238-2 [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Hruska K. A., Greenfield E. M., Duncan R., Alvarez J., Barattolo R., Colucci S., Zambonin-Zallone A., Teitelbaum S. L., Teti A. (1990). Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J. Cell Biol. 111, 2543–2552 10.1083/jcb.111.6.2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsky W. L., Lin C. Y., Aoyama A., Kelly T., Akiyama S. K., Mueller S. C., Chen W. T. (1994). A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 54, 5702–5710 [PubMed] [Google Scholar]
- Moreau V., Tatin F., Varon C., Génot E. (2003). Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol. Cell. Biol. 23, 6809–6822 10.1128/MCB.23.19.6809-6822.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M. L., Jin S. L., Milla M. E., Bickett D. M., Burkhart W., Carter H. L., Chen W. J., Clay W. C., Didsbury J. R., Hassler D. et al. (1997). Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385, 733–736 10.1038/385733a0 [DOI] [PubMed] [Google Scholar]
- Mu D., Cambier S., Fjellbirkeland L., Baron J. L., Munger J. S., Kawakatsu H., Sheppard D., Broaddus V. C., Nishimura S. L. (2002). The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 157, 493–507 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. C., Ghersi G., Akiyama S. K., Sang Q. X., Howard L., Pineiro-Sanchez M., Nakahara H., Yeh Y., Chen W. T. (1999). A novel protease-docking function of integrin at invadopodia. J. Biol. Chem. 274, 24947–24952 10.1074/jbc.274.35.24947 [DOI] [PubMed] [Google Scholar]
- Murphy D. A., Courtneidge S. A. (2011). The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol. 12, 413–426 10.1038/nrm3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H., Nomizu M., Akiyama S. K., Yamada Y., Yeh Y., Chen W. T. (1996). A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J. Biol. Chem. 271, 27221–27224 10.1074/jbc.271.44.27221 [DOI] [PubMed] [Google Scholar]
- Nakahara H., Mueller S. C., Nomizu M., Yamada Y., Yeh Y., Chen W. T. (1998). Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Biol. Chem. 273, 9–12 10.1074/jbc.273.1.9 [DOI] [PubMed] [Google Scholar]
- Nakahara H., Otani T., Sasaki T., Miura Y., Takai Y., Kogo M. (2003). Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells 8, 1019–1027 10.1111/j.1365-2443.2003.00695.x [DOI] [PubMed] [Google Scholar]
- Oikawa T., Itoh T., Takenawa T. (2008). Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol. 182, 157–169 10.1083/jcb.200801042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms L. M., Horan K. A., Rahman P., Seaton G., Gurung R., Kethesparan D. S., Mitchell C. A. (2009). The role of the inositol polyphosphate 5-phosphatases in cellular function and human disease. Biochem. J. 419, 29–49 10.1042/BJ20081673 [DOI] [PubMed] [Google Scholar]
- Ory S., Brazier H., Pawlak G., Blangy A. (2008). Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur. J. Cell Biol. 87, 469–477 10.1016/j.ejcb.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Oser M., Condeelis J. (2009). The cofilin activity cycle in lamellipodia and invadopodia. J. Cell. Biochem. 108, 1252–1262 10.1002/jcb.22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M., Yamaguchi H., Mader C. C., Bravo-Cordero J. J., Arias M., Chen X., Desmarais V., van Rheenen J., Koleske A. J., Condeelis J. (2009). Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 186, 571–587 10.1083/jcb.200812176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M., Dovas A., Cox D., Condeelis J. (2011). Nck1 and Grb2 localization patterns can distinguish invadopodia from podosomes. Eur. J. Cell Biol. 90, 181–188 10.1016/j.ejcb.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiak A. E., Zenner G., Linder S. (2005). Subconfluent endothelial cells form podosomes downstream of cytokine and RhoGTPase signaling. Exp. Cell Res. 307, 342–353 10.1016/j.yexcr.2005.03.035 [DOI] [PubMed] [Google Scholar]
- Pan Y. R., Chen C. L., Chen H. C. (2011). FAK is required for the assembly of podosome rosettes. J. Cell Biol. 195, 113–129 10.1083/jcb.201103016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli J., Tumbarello D. A., Schmidt R. P., Turner C. E. (2012). Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition. J. Cell Biol. 197, 421–437 10.1083/jcb.201108143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad N. K., Tandon M., Handa A., Moore G. E., Babbs C. F., Snyder P. W., Bose S. (2008). High expression of obesity-linked phosphatase SHIP2 in invasive breast cancer correlates with reduced disease-free survival. Tumour Biol. 29, 330–341 10.1159/000172970 [DOI] [PubMed] [Google Scholar]
- Provenzano P. P., Inman D. R., Eliceiri K. W., Trier S. M., Keely P. J. (2008). Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J. 95, 5374–5384 10.1529/biophysj.108.133116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintavalle M., Elia L., Condorelli G., Courtneidge S. A. (2010). MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J. Cell Biol. 189, 13–22 10.1083/jcb.200912096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadurai C. V., Havrylov S., Zaoui K., Vaillancourt R., Stuible M., Naujokas M., Zuo D., Tremblay M. L., Park M. (2012). Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. J. Cell Sci. 125, 2940–2953 10.1242/jcs.100834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M. W. (1999). The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231 10.1016/S0092-8674(00)80732-1 [DOI] [PubMed] [Google Scholar]
- Rohatgi R., Ho H. Y., Kirschner M. W. (2000). Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol. 150, 1299–1310 10.1083/jcb.150.6.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. (2005). GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 10.1038/nrm1587 [DOI] [PubMed] [Google Scholar]
- Rottiers P., Saltel F., Daubon T., Chaigne-Delalande B., Tridon V., Billottet C., Reuzeau E., Génot E. (2009). TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J. Cell Sci. 122, 4311–4318 10.1242/jcs.057448 [DOI] [PubMed] [Google Scholar]
- Saarikangas J., Zhao H., Lappalainen P. (2010). Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 90, 259–289 10.1152/physrev.00036.2009 [DOI] [PubMed] [Google Scholar]
- Sabeh F., Ota I., Holmbeck K., Birkedal-Hansen H., Soloway P., Balbin M., Lopez-Otin C., Shapiro S., Inada M., Krane S. et al. (2004). Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 167, 769–781 10.1083/jcb.200408028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F., Shimizu-Hirota R., Weiss S. J. (2009). Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J. Cell Biol. 185, 11–19 10.1083/jcb.200807195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai-Yageta M., Recchi C., Le Dez G., Sibarita J. B., Daviet L., Camonis J., D'Souza-Schorey C., Chavrier P. (2008). The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J. Cell Biol. 181, 985–998 10.1083/jcb.200709076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltel F., Daubon T., Juin A., Ganuza I. E., Veillat V., Génot E. (2011). Invadosomes: intriguing structures with promise. Eur. J. Cell Biol. 90, 100–107 10.1016/j.ejcb.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. (1994). A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370, 61–65 10.1038/370061a0 [DOI] [PubMed] [Google Scholar]
- Schmidt S., Nakchbandi I., Ruppert R., Kawelke N., Hess M. W., Pfaller K., Jurdic P., Fässler R., Moser M. (2011). Kindlin-3-mediated signaling from multiple integrin classes is required for osteoclast-mediated bone resorption. J. Cell Biol. 192, 883–897 10.1083/jcb.201007141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoumacher M., Goldman R. D., Louvard D., Vignjevic D. M. (2010). Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 189, 541–556 10.1083/jcb.200909113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D. F., Azucena E. F., Jr, Pass I., Tesfay L., Gordon R., Woodrow M., Resau J. H., Courtneidge S. A. (2005). The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 7, 155–165 10.1016/j.ccr.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Shinohara M., Nakamura M., Masuda H., Hirose J., Kadono Y., Iwasawa M., Nagase Y., Ueki K., Kadowaki T., Sasaki T. et al. (2012). Class IA phosphatidylinositol 3-kinase regulates osteoclastic bone resorption through protein kinase B-mediated vesicle transport. J. Bone Miner. Res. 27, 2464–2475 10.1002/jbmr.1703 [DOI] [PubMed] [Google Scholar]
- Smith-Pearson P. S., Greuber E. K., Yogalingam G., Pendergast A. M. (2010). Abl kinases are required for invadopodia formation and chemokine-induced invasion. J. Biol. Chem. 285, 40201–40211 10.1074/jbc.M110.147330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L., Rietdorf J., Nitsch L., Bono M., Tacchetti C., Way M., Marchisio P. C. (2004). A dynamic podosome-like structure of epithelial cells. Exp. Cell Res. 295, 360–374 10.1016/j.yexcr.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Steffen A., Le Dez G., Poincloux R., Recchi C., Nassoy P., Rottner K., Galli T., Chavrier P. (2008). MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr. Biol. 18, 926–931 10.1016/j.cub.2008.05.044 [DOI] [PubMed] [Google Scholar]
- Stylli S. S., Stacey T. T., Verhagen A. M., Xu S. S., Pass I., Courtneidge S. A., Lock P. (2009). Nck adaptor proteins link Tks5 to invadopodia actin regulation and ECM degradation. J. Cell Sci. 122, 2727–2740 10.1242/jcs.046680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tague S. E., Muralidharan V., D'Souza-Schorey C. (2004). ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc. Natl. Acad. Sci. USA 101, 9671–9676 10.1073/pnas.0403531101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Li A., Bi J., Veltman D. M., Zech T., Spence H. J., Yu X., Timpson P., Insall R. H., Frame M. C. et al. (2013). Loss of Scar/WAVE complex promotes N-WASP- and FAK-dependent invasion. Curr. Biol. 23, 107–117 10.1016/j.cub.2012.11.059 [DOI] [PubMed] [Google Scholar]
- Tatin F., Varon C., Génot E., Moreau V. (2006). A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J. Cell Sci. 119, 769–781 10.1242/jcs.02787 [DOI] [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. (1983). Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation 25, 156–167 10.1111/j.1432-0436.1984.tb01351.x [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hedin U., Sjölund M., Palmberg L., Bottger B. A. (1990). Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis 10, 966–990 10.1161/01.ATV.10.6.966 [DOI] [PubMed] [Google Scholar]
- Tolde O., Rösel D., Veselý P., Folk P., Brábek J. (2010). The structure of invadopodia in a complex 3D environment. Eur. J. Cell Biol. 89, 674–680 10.1016/j.ejcb.2010.04.003 [DOI] [PubMed] [Google Scholar]
- Turk B. E., Huang L. L., Piro E. T., Cantley L. C. (2001). Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol. 19, 661–667 10.1038/90273 [DOI] [PubMed] [Google Scholar]
- Ueno T., Falkenburger B. H., Pohlmeyer C., Inoue T. (2011). Triggering actin comets versus membrane ruffles: distinctive effects of phosphoinositides on actin reorganization. Sci. Signal. 4, ra87 10.1126/scisignal.2002033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goethem E., Guiet R., Balor S., Charrière G. M., Poincloux R., Labrousse A., Maridonneau-Parini I., Le Cabec V. (2011). Macrophage podosomes go 3D. Eur. J. Cell Biol. 90, 224–236 10.1016/j.ejcb.2010.07.011 [DOI] [PubMed] [Google Scholar]
- van Helden S. F., Hordijk P. L. (2011). Podosome regulation by Rho GTPases in myeloid cells. Eur. J. Cell Biol. 90, 189–197 10.1016/j.ejcb.2010.05.008 [DOI] [PubMed] [Google Scholar]
- van Helden S. F., Oud M. M., Joosten B., Peterse N., Figdor C. G., van Leeuwen F. N. (2008). PGE2-mediated podosome loss in dendritic cells is dependent on actomyosin contraction downstream of the RhoA-Rho-kinase axis. J. Cell Sci. 121, 1096–1106 10.1242/jcs.020289 [DOI] [PubMed] [Google Scholar]
- Varon C., Tatin F., Moreau V., Van Obberghen-Schilling E., Fernandez-Sauze S., Reuzeau E., Kramer I., Génot E. (2006). Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol. Cell. Biol. 26, 3582–3594 10.1128/MCB.26.9.3582-3594.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker V. G., Ammer A., Cao Z., Clump A. C., Jiang B. H., Kelley L. C., Weed S. A., Zot H., Flynn D. C. (2007). PI3K activation is required for PMA-directed activation of cSrc by AFAP-110. Am. J. Physiol. 293, C119–C132 10.1152/ajpcell.00525.2006 [DOI] [PubMed] [Google Scholar]
- Wang Y., McNiven M. A. (2012). Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J. Cell Biol. 196, 375–385 10.1083/jcb.201105153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Taba Y., Pang J., Yin G., Yan C., Berk B. C. (2009). GIT1 mediates VEGF-induced podosome formation in endothelial cells: critical role for PLCgamma. Arterioscler. Thromb. Vasc. Biol. 29, 202–208 10.1161/ATVBAHA.108.174391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanjare M., Kuo F., Gerecht S. (2012). Derivation and maturation of synthetic and contractile vascular smooth muscle cells from human pluripotent stem cells. Cardiovasc. Res. 97, 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A. M. (2006). Invadopodia: specialized cell structures for cancer invasion. Clin. Exp. Metastasis 23, 97–105 10.1007/s10585-006-9014-1 [DOI] [PubMed] [Google Scholar]
- Weaver A. M. (2008). Invadopodia. Curr. Biol. 18, R362–R364 10.1016/j.cub.2008.02.028 [DOI] [PubMed] [Google Scholar]
- Weaver A. M. (2009). Regulation of cancer invasion by reactive oxygen species and Tks family scaffold proteins. Sci. Signal. 2, pe56 10.1126/scisignal.288pe56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B. A., Eves R., Crawley S. W., Zhou S., Côté G. P., Mak A. S. (2005). PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am. J. Physiol. 289, C898–C907 10.1152/ajpcell.00095.2005 [DOI] [PubMed] [Google Scholar]
- Wennström S., Hawkins P., Cooke F., Hara K., Yonezawa K., Kasuga M., Jackson T., Claesson-Welsh L., Stephens L. (1994). Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr. Biol. 4, 385–393 10.1016/S0960-9822(00)00087-7 [DOI] [PubMed] [Google Scholar]
- Wheeler A. P., Smith S. D., Ridley A. J. (2006). CSF-1 and PI 3-kinase regulate podosome distribution and assembly in macrophages. Cell Motil. Cytoskeleton 63, 132–140 10.1002/cm.20111 [DOI] [PubMed] [Google Scholar]
- Wickström S. A., Lange A., Hess M. W., Polleux J., Spatz J. P., Krüger M., Pfaller K., Lambacher A., Bloch W., Mann M. et al. (2010). Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell 19, 574–588 10.1016/j.devcel.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Mazo I., Leung H., Engelke K., von Andrian U. H., Deryugina E. I., Strongin A. Y., Bröcker E. B., Friedl P. (2003). Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J. Cell Biol. 160, 267–277 10.1083/jcb.200209006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Wu Y. I., Liu Y., Geiger J., Tam E., Overall C., Stack M. S., Friedl P. (2007). Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol. 9, 893–904 10.1038/ncb1616 [DOI] [PubMed] [Google Scholar]
- Wyckoff J. B., Pinner S. E., Gschmeissner S., Condeelis J. S., Sahai E. (2006). ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 10.1016/j.cub.2006.05.065 [DOI] [PubMed] [Google Scholar]
- Xiao H., Bai X. H., Wang Y., Kim H., Mak A. S., Liu M. (2013). MEK/ERK pathway mediates PKC activation-induced recruitment of PKCζ and MMP-9 to podosomes. J. Cell. Physiol. 228, 416–427 10.1002/jcp.24146 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T. et al. (2005). Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 168, 441–452 10.1083/jcb.200407076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Takeo Y., Yoshida S., Kouchi Z., Nakamura Y., Fukami K. (2009). Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 69, 8594–8602 10.1158/0008-5472.CAN-09-2305 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Yoshida S., Muroi E., Kawamura M., Kouchi Z., Nakamura Y., Sakai R., Fukami K. (2010). Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Ialpha are required for invadopodia formation in human breast cancer cells. Cancer Sci. 101, 1632–1638 10.1111/j.1349-7006.2010.01574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Yoshida S., Muroi E., Yoshida N., Kawamura M., Kouchi Z., Nakamura Y., Sakai R., Fukami K. (2011). Phosphoinositide 3-kinase signaling pathway mediated by p110α regulates invadopodia formation. J. Cell Biol. 193, 1275–1288 10.1083/jcb.201009126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Stamenkovic I. (2000). Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176 [PMC free article] [PubMed] [Google Scholar]
- Yu H. G., Nam J. O., Miller N. L., Tanjoni I., Walsh C., Shi L., Kim L., Chen X. L., Tomar A., Lim S. T. et al. (2011). p190RhoGEF (Rgnef) promotes colon carcinoma tumor progression via interaction with focal adhesion kinase. Cancer Res. 71, 360–370 10.1158/0008-5472.CAN-10-2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R., Geiger B. (2010). The switchable integrin adhesome. J. Cell Sci. 123, 1385–1388 10.1242/jcs.066183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Liu Y., Tan G. (2011). Prognostic value of elevated SHIP2 expression in laryngeal squamous cell carcinoma. Arch. Med. Res. 42, 589–595 10.1016/j.arcmed.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Ziel J. W., Hagedorn E. J., Audhya A., Sherwood D. R. (2009). UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat. Cell Biol. 11, 183–189 10.1038/ncb1825 [DOI] [PMC free article] [PubMed] [Google Scholar]