Abstract
Expansion of the pancreatic endocrine cell population occurs during both embryonic development and during post-natal pancreatic growth and regeneration. Mechanisms of the expansion of endocrine cells during embryonic development are not completely understood, and no clear mechanistic link has been established between growth of the embryonic endocrine pancreas and the islet cell replication that occurs in an adult animal. We found that transforming growth factor-beta (TGF-β) superfamily signaling, which has been implicated in many developmental processes, plays a key role in regulating pancreatic endocrine maturation and development. Specifically, the intracellular mediators of TGF-β signaling, smad2 and smad3, along with their inhibitor smad7, appear to mediate this process. Smad2, smad3 and smad7 were all broadly expressed throughout the early embryonic pancreatic epithelium. However, during later stages of development, smad2 and smad3 became strongly localized to the nuclei of the endocrine positive cells, whereas the inhibitory smad7 became absent in the endocrine component. Genetic inactivation of smad2 and smad3 led to a significant expansion of the embryonic endocrine compartment, whereas genetic inactivation of smad7 led to a significant decrease in the endocrine compartment. In vitro antisense studies further corroborated these results and supported the possibility that interplay between the inhibitory smad7 and the intracellular mediators smad2/3 is a control point for pancreatic endocrine development. These results should provide a better understanding of the key control mechanisms for β-cell development.
Keywords: Pancreas, TGF-β, Smads, Embryonic
Introduction
The pancreas is an endodermally derived organ consisting of two morphologically and functionally distinct tissues, the exocrine and endocrine pancreas. During embryonic growth, the pancreas goes through three stages of development. The first is a relatively undifferentiated stage where pancreatic morphogenesis is initiated with endodermal evagination. The second stage involves epithelial branching morphogenesis, which also includes the delamination of differentiating islet progenitors from the basement membrane. The final stage begins with the formation of acinar cells at the apices of the ductal structures. During these stages, the endocrine compartment undergoes amplification during two distinct waves of differentiation. The primary wave (pre-E13.5), followed by the secondary wave of differentiation from the ducts (E13.5–E16.5)(Edlund, 2001; Prasadan et al., 2002). Specific factors governing this endocrine expansion are a subject of active study.
We have previously investigated the role of TGF-β isoform signaling in early pancreatic organogenesis, demonstrating that the developing pancreas expresses TGF-beta type II receptor (TBR-II) and type I receptor. TBR-II localizes to ducts at later stages of pancreatic development (Crisera et al., 1999). Blocking TGF-β signaling in the embryonic pancreas, using a transgenic mouse expressing a dominant-negative TGF-β-type-II-receptor (DNTβRII) led to an increased number of endocrine cells arising from the embryonic ducts. The enhanced endocrine expansion was most prominent at E16.5, which corresponded to the normal peak of secondary wave endocrine differentiation (Tulachan et al., 2007). Whether adult pancreatic ducts can recapitulate the embryonic mode of development to give rise to new β-cells remains hotly debated (Bonner-Weir et al., 2008; El-Gohary et al., 2012; Furuyama et al., 2011; Inada et al., 2008; Kopp et al., 2011; Solar et al., 2009).
In vivo studies of the role of TGF-β ligands in development have been difficult since mice deficient in TGF-β2 and TGF-β3 developed severe embryonic developmental defects and 100% embryonic lethality (Kaartinen et al., 1995; Sanford et al., 1997); therefore, the role of TGF-β2 and TGF-β3 has been difficult to assess in vivo. Furthermore, disrupting the TGF-β1 gene leads to severe multifocal inflammatory diseases, thus confounding analyses of different tissues (Shull et al., 1992). Using a DNTβRII transgene avoided some of the problems associated with deletion of TGFβ ligands, and shed some light on a possible role for TGF-β signaling in regulating pancreatic endocrine expansion and maturation during development (Bottinger et al., 1997; Tulachan et al., 2007).
Canonical TGF-β signaling involves ligand binding to the TGF-β-receptor-type-II, a serine/threonine kinase receptor. Subsequently the type II receptor recruits and phosphorylates the type I receptors, which in turn activate downstream smad2/3 transcription factors that mediate TGF-β-regulated gene expression (Massague, 1998; Shi and Massague, 2003). Smad6 and smad7 are the inhibitory smads (Park, 2005; Yan et al., 2009). Smad6 is thought to inhibit specifically those smads that are downstream of BMP signaling, i.e. smads 1, 5, and 8 (Massague and Gomis, 2006; Park, 2005), whereas smad7 seems to be more globally active against all receptor-activated smads (smads 1, 5, 8, plus smad2 and smad3). Thus, smad7 is the only inhibitory smad that inhibits smad2 and smad3, which are the TGF-beta and activin signaling smads (Park, 2005; Yan et al., 2009).
To investigate in more depth the mechanism of enhanced endocrine expansion in the DNTβRII mice, we specifically targeted the intracellular mediators of TGF-β signaling, smad2 and smad3, by analyzing smad2 conditional (Smad2fx/fx)(Ju et al., 2006) and smad3 global mutant (exon2 deletion) mice (Datto et al., 1999). Smad2fx/fx mice were crossed with a pdx1-cre-ERT mouse to create tamoxifen-inducible smad2 conditional mutants for the pancreas. Ablation of smad2 or smad3 led to a significant increase in endocrine cell numbers at E18. Furthermore, we generated a smad7fx/fx knock-in mouse, conditionally deleting smad7 in the pancreas by crossing it with pdx1-cre-ERT mice. We saw a severely diminished number of hormone+ cells at E18.
These results implicate an important role for smad2 and smad3 in the expansion of the pancreatic endocrine compartment, and implicate smad7 as a key regulator of this expansion, likely through suppression of smads 2 and 3. These results should provide a better understanding of the key control mechanisms of β-cell development, and may provide a mechanistic link between developmental neogenesis of pancreatic endocrine cells and pancreatic islet cell replication.
Materials and Methods
Transgenic animals and genotyping
All the animal experiments were performed in accordance with guidelines established by the University of Pittsburgh Institutional Animal Care and Use Committees. Smad3-exon 2 null mutant mice were obtained from Jackson Laboratories (stock 003451), originally made by Luis Parada UT Southwestern. Transgenic mice expressing Smad2fx/fx were generous gifts from Dr. Erwin P. Bottinger, Mt. Sinai School of Medicine. All transgenic mice were crossed with Pdxcre-ER™ (Gu et al., 2002) (Mouse Models of Human Cancers Consortium, MMHCC). Non-pancreatic tissues from the embryos and adult mice were used for genotyping with the Extract-N-Amp PCR mix (Sigma, St. Louis, MO) kit and PCR probe that is specific for the transgene. Pancreata were isolated by micro-dissection from the transgenic embryos, as well as from the littermate controls.
Tamoxifen Injection
The cre-ER™/LoxP system, tamoxifen (Sigma, St Louis, MO) was dissolved at 20mg/ml in corn oil (Sigma) and was administered into adult mice intraperitoneally. For embryonic studies, pregnant females received a single dose 2mg per 40g body weight intraperitoneally at E10.5 and embryonic pancreases were subsequently harvested at different time points. The pancreas were then harvested and fixed in 4% paraformaldehyde overnight in 4°C then placed in 30% sucrose overnight in 4°C for cryoprotection. Tissues were then embedded in Tissue-Tech O.C.T compound and then frozen for sectioning.
Histology and Immunohistochemistry
Harvested tissues were fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose overnight at 4°C, then embedded in Tissue-Tech O.C.T compound and frozen in −20°C. 6–8 μm-thick frozen sections were cut at −23°C in a cryostat and mounted on gelatin-coated glass microscope slides (Superfrost Plus, Fisherbrand). For immunostaining, optimal dilutions and controls were used for each antibody used. Insulin guinea pig anti-swine 1:500 (Dako, Carpinteria, CA), glucagon rabbit monoclonal 1:2000 (Linco), amylase rabbit anti-human 1:400 (Sigma, St. Louis, MO), smad7 rabbit polyclonal IgG 1:50 (Santa Cruz biotech, CA), PDX-1 rabbit polyclonal 1:1400 (generous gift from Prof. Chris Wright, Vanderbilt University Medical School, Nashville, TN), PDX-1 goat polyclonal 1:1000 (Abcam), Dolichos biflorus agglutinin FITC conjugated (DBA), which binds to lectins present on ductal cells, 1:50 (Vector Laboratories, CA), anti-bromo-deoxyuridine (BrdU) rat monoclonal antibody 1:400 (Abcam), anti-bromo-deoxyuridine rat mono-cloncal antibody 1:100 (Novus Biologicals). Primary antibodies were incubated for 2 hour at room temperature or at 4°C overnight.
Biotinylated Vectastain ABC kit or AMCA/CY3/FITC fluorescent conjugated donkey secondary antibodies were used for 1.5 hour at room temperature. Immunoperoxidase was detected by DAB kit (Dako, Carpintaria, CA) or AEC (Sigma, St. Louis, MO) and fluorescently labeled samples were imaged using a fluorescent microscope. Tissue sections were viewed on an upright Axio Imager Z1 microscope. Images were captured with the AxioCam MRc5 and processed using AxioVs40 V4.8.2.0 software or with an inverted Olympus Fluoview 1000 confocal microscope to confocally image the tissue sections. The pictures were generated by overlay of the colors followed by merging of all color channels into one.
Bromo-deoxyuridine (BrdU) incorporation and cell counting
Pregnant mice were injected with BrdU (Sigma, St. Louis, MO) 200mg/Kg intraperitoneally 4 hours before harvesting the embryos The pancreas is then fixed in 4% paraformaldehyde overnight in 4°C then placed in 30% sucrose overnight in 4°C. Tissues were then embedded in Tissue-Tech O.C.T compound and then frozen for sectioning. Antigen retrieval was done on the slides by treating with sodium citrate (10mM, pH 6.0), heating it in the microwave at low power for 15 min and then subsequently treating it with 2M HCl for 35 minutes, followed by overnight incubation with primary antibodies. To quantify number of BrdU positive cells in embryonic wild-type and transgenic pancreases, the whole frozen pancreas was sectioned 6–8 μm-thick at −23°C in a cryostat and mounted on gelatin-coated glass microscope slides (Superfrost Plus, Fisherbrand), 8 sections/slide. A total of 7 embryos were analyzed from 2 different litters for E12.5 CD1, a total of 7 embryos were analyzed from 2 different litters for E14.5 CD1 and a total of 7 embryos were analyzed from 1 litter for E16.5 CD1. The entire embryonic pancreas was sectioned (100 sections total over 15 slides) and evenly distributed 6um sections throughout the organ were selected for analysis and quantification. The total number of smad7 positive cells that were BrdU positive divided by total number of smad7 positive cells using the image tracing software (Stereoinvestigator, Microbrightfield) to count and tag individual BrdU positive/smad7 positive cells using dapi. Using the Stereoinvestigator program, individual BrdU positive cells were tagged and linked to Neurolucida software (MicroBrightField, Inc.) to quantify number of cells.
Morpholino antisense organ culture experiment
Dorsal pancreatic rudiments of four E11.5 CD1 mouse embryos were grown in hanging drop organ culture containing either control scrambled morpholino or Smad2, Smad3, Smad7 morpholino. The morpholino antisense targeting specific genes were custom designed at www.gene-tools.com. A novel peptide based aqueous Endo-Porter system (www.gene-tools.com) was used according to manufacture’s instructions for cytosolic delivery. A drop consisting of 50μl of control media with 20μM morpholino antisense was placed on a 35mm Petri dish. Pancreas rudiment was then placed inside the drop and inverted so that the drop hangs. The bottom of the Petri dish was filled partially with media to keep the environment moist. The drop was replaced every day and the cultures were maintained at 37°C and 5% CO2. The explants were harvested at day 3 or day 7 for histological analysis and downstream applications.
Morphometric analysis and cell counting
Time-pregnant transgenic mice, Pdxcre-ER™; Smad7fx/fx, Pdxcre-ER™; Smad2fx/fx, Pdxcre-ER™; Smad2fx/fx Smad3 exon2 were administered a single dose of tamoxifen intraperitoneally, 2mg per 40g body weight, at E10.5 and harvested at E18.5. Littermates that were cre negative were used as controls. A total of 18 embryos were analyzed from 6 different litters. The entire embryonic pancreas was sectioned (100 sections total over 20 slides) and evenly distributed 6um sections throughout the organ were selected (every 5th slide, 4 slides total, were chosen for analysis and quantification). Twenty five sections were counted (5 sections on each slide). Total number of insulin and glucagon positive cells was counted with dapi, using the image tracing software (Stereoinvestigator, Microbrightfield) to count and tag individual insulin positive and glucagon positive cells. Using the Stereoinvestigator program, individual endocrine positive cells were tagged and linked to Neurolucida software (MicroBrightField, Inc.) to quantify number of cells.
The embryonic pancreas for time pregnant smad3 exon2 global knockout female mice were harvested at E18.5, with heterozygote smad3 exon2 being used as controls. Similarly, a total of 15 embryos were analyzed from 7 different litters. The entire embryonic pancreas was sectioned (100 sections total over 20 slides) and evenly distributed 6um sections throughout the organ were selected (every 5th slide, 4 slides total, were chosen for analysis and quantification). Twenty five sections were counted (5 sections on each slide). Total insulin and glucagon area was determined using Image J software.
Results
Phospho-smad2 and phospho-smad3 expression pattern in the developing mouse pancreas
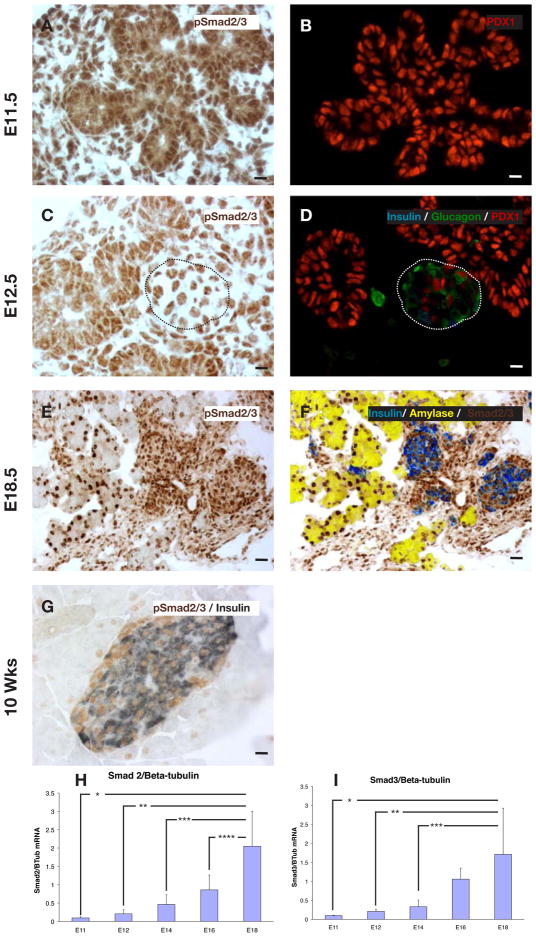
Previous studies have shown that smads 2 and 3 are present in the developing pancreas (Brorson et al., 2001), though the distribution of the activated phosphorylated forms (p-smads) has not been studied. We found broad expression of p-smad2/3 in the embryonic mouse pancreas, including expression in the mesenchyme and epithelium at embryonic day 11 (E11) (Fig. 1A,B). Of note, both nuclear and cytoplasmic staining was detected in the epithelium at E11, E12, and E18 (Fig. 1 and Fig. S1A–D). Interestingly, by E12.5 p-smad2/3 was found to then localize specifically in the nucleus of newly formed glucagon+ and insulin+ cells (Fig. 1C–F). This nuclear localization is persistent, and prominent in the E18.5 and adult 10week old mouse pancreas (Fig. 1E–G). This nuclear localization suggested a stronger activity of the p-smads in these endocrine-committed, hormone-positive cells, and such hormone-positive cells in the early embryonic pancreas are known to rarely be proliferative (Jensen et al., 2000). Semi-quantitative RT-PCR (QPCR) analysis of smad and TGFβ ligand expression at serial gestational ages revealed that smads 2, 3, and 4 as well as TGFβ1,2 and 3 all showed a rise toward the end of gestation, consistent with a possible role in mediating the maturation of endocrine cells (Fig. 1H,I and Fig. S1E–H). We also stained with antibodies specific for either p-smad2 or p-smad3. These antibodies appear to have a weaker affinity for their target, and no clear signal was detectable until E15 (Fig. S1A–D). At that time, and even more so at E18, both of these individual p-smads were found to be localized to the ductal and periductal/endocrine region of the central portion of the pancreas. Thus, it seems plausible that the staining we see with a common p-smad2/3 antibody represents a mix of both p-smads, rather than predominantly one or the other p-smad.
Figure 1.
Ontogeny of phospho-smad2/3 (p-smad2/3) expression in the embryonic pancreas. (A,B) p-smad2/3 is expressed fairly diffusely in both epithelium and mesenchyme early in pancreatic development (E11.5). (C,D) However, by E12.5 the cells that have committed to become endocrine, as evidenced by staining for hormones (here, insulin and glucagon), have focal nuclear localization (dotted lines outline endocrine cluster). (E,F) This nuclear-specific pattern persisted at E18.5. (G) 10 week old adult pancreas, p-smad2/3 is strongly localized to the nuclei of all islet cells. (H) QPCR ontogeny for smad2 at different stages of embryonic development (n=5, * p<0.002, **p<0.003, ***p=0.007, ****p=0.03, ±S.D). (I) QPCR ontogeny for smad3 at different stages of embryonic development (n=5, *p<0.02, **p<0.03, ***p<0.04, ±S.D). Scale bar (A–D, G) is 10μm, (E,F) is 20μm.
In vitro inhibition of embryonic pancreas phospho-smad2/3
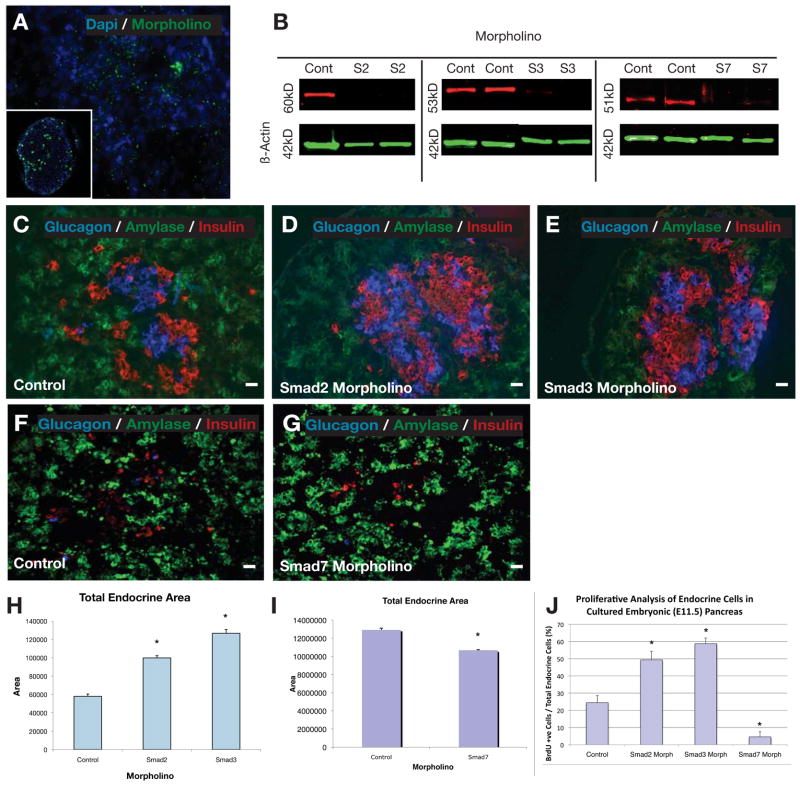
In order to determine a possible role for activated (phosphorylated) smad2/3 in the pancreas, we first treated E11 embryonic pancreas in a hanging drop culture system with smad2 or smad3 morpholino antisense with an endoporter system for 5-days, similar to previous studies (Prasadan et al., 2011)(Figure 2). Control fluorescent morpholino antisense was found to penetrate into the explants and into individual cells (Figure 2A). Secondarily, Western blotting and immunostaining against the smad proteins revealed significant knock-down in these explants after treatment with morpholinos (Figure 2B and Figure S2A–D). Morpholinos against either smad2 or smad3 were used, but not in combination due to apparent toxicity of the higher total concentration of morpholinos. Here, with inhibition of either smad2 or smad3, significantly enhanced numbers of insulin+ and glucagon+ cells were seen after the 5-days in culture (Figure 2C–E). Overall, the number of endocrine cells (only insulin+ and glucagon+ cells were counted) increased 1.7-fold with smad2 antisense treatment, and doubled with smad3 antisense treatment (Figure 2H, n=4, p<0.001). The relative distribution between insulin+ and glucagon+ cells did not change significantly over the 7-day culture period, remaining in the range of 50–65% insulin cells (data not shown). In addition, more than double the percentage of endocrine cells were BrdU+ after treatment with smad2 or smad3 antisense (Figure 2J n=3, p<0.005, Figure S2E–G). This result suggests that inhibition of smad2/3 may be a normal, endogenous mechanism to allow for proliferation of endocrine hormone-positive cells, further suggesting that the increased numbers of endocrine cells was due to enhanced proliferation. Furthermore, QPCR analysis revealed a significant upregulation of the endocrine progenitor markers, neuroD and ngn3, consistent with the development of an enhanced number of endocrine cells (Fig. S2K,L). This result suggests that inhibition of smad2/3 may be an endogenous mechanism for allowing proliferation of endocrine hormone-positive cells.
Figure 2.
Morpholino culture experiments with E11.5 pancreas. (A) Wholemount E11.5 pancreas with FITC-tagged morpholino in culture showing penetration of explant by morpholinos, with the inset demonstrating a histologic section of the cultured E11.5 pancreas with FITC-tagged morpholino within the pancreatic epithelium. (B) Western blot showing the effective knockdown of smad2, smad3 and smad7 with morpholino treatment compared to control. (C & F) Control morpholino of an E11.5 pancreas harvested and placed in culture for 5days revealing baseline expression of insulin, glucagon and amylase. (D) When smad2 morpholino was added to an E11.5 pancreas and cultured for 5days, a significant increase in the endocrine compartment was observed. (E) This significant expansion in the endocrine compartment was even more pronounced when smad3 mopholino was added to the E11.5 pancreatic culture. (G) The addition of smad7 morpholino to the cultured E11.5 pancreas led to a significant decrease in the endocrine compartment. (H,I) Quantitative analysis of the total endocrine area with smad2, smad3 and smad7 morpholino compared to control (n=4, p<0.001, ±SEM). (J) Quantitative proliferative analysis of the percentage of endocrine cells in cultured E11.5 pancreas that were BrdU+ after adding smad2 or smad3 or smad7 morpholino (n=3, p<0.005, ±SEM). Scale bar: 20μm.
To try to understand the mechanism by which there was greater formation of endocrine cells, we harvested tissues after only 36 hours of culture and saw a similar increase (2–3 fold) in the number of pax6+/glucagon+ double-positive cells in smad2 antisense and smad3 antisense treated explants (Fig. S2M–O). This early increase suggests that smad2 and smad3 normally act to suppress recruitment of new progenitors in addition to affecting endocrine cell maturation. The presence of these smads throughout the early (E11–E12) epithelium (Figure 1A) would also be consistent with this role. Furthermore, by day 3 of culture, we saw a large number of BrdU+/Nkx2.2+ cells, suggesting that the endocrine progenitors continued to expand inappropriately due to blocked smad2/3 (data not shown), again particularly pronounced with smad3 antisense treatment.
In vivo smad2/3 ablation
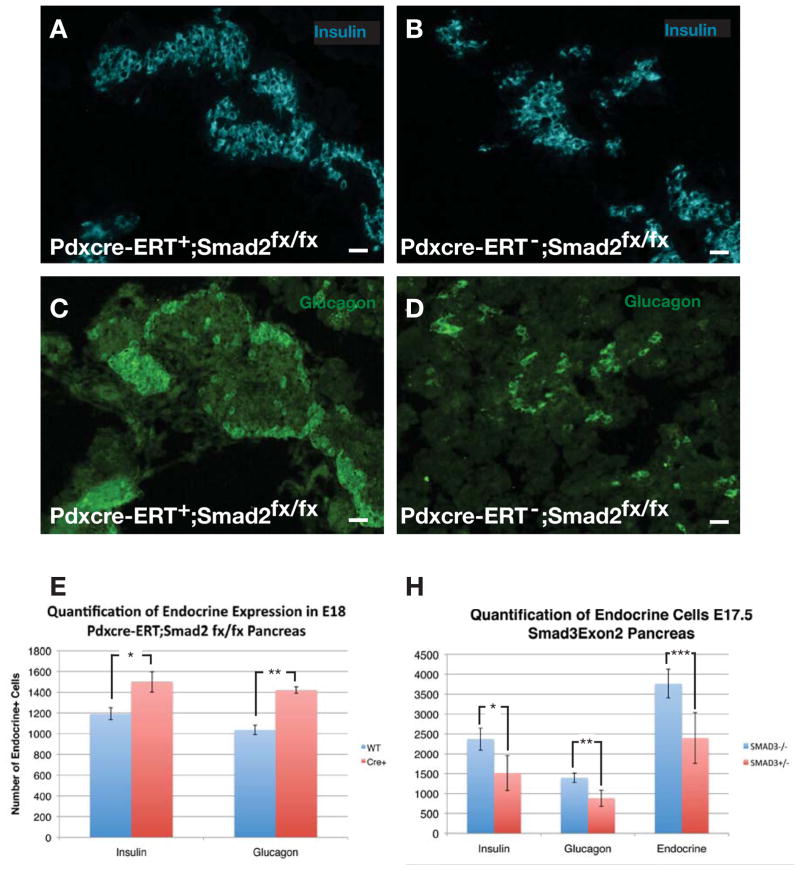
To expand on these in vitro observations of the role of smad2 and 3 in embryonic pancreatic differentiation, we then obtained smad2 conditional (Smad2fx/fx)(Ju et al., 2006) and smad3 global mutant (exon2 deletion) mice (Datto et al., 1999). Smad3−/− (exon2) global null mutant mice are viable and fertile (Datto et al., 1999), and to our knowledge no smad3 conditional mutant mice exist. Smad2fx/fx mice were crossed with a pdx1-cre-ERT mouse to create tamoxifen-inducible smad2 conditional mutants for the pancreas. For smad2 mutants, tamoxifen was given at E10.5 of gestation. In pdx1-cre-ERT;R26R-lsl-lacZ embryos, consistent with previous reports (Gu et al., 2002), we confirmed that by E13.5 essentially all (>95%) of pancreatic epithelial and endocrine cells were lineage-tagged after the single E10.5 tamoxifen injection (data not shown). Quantification of the number of endocrine cells in the smad mutant animals revealed that at E15 there were not significant differences in the number of pancreatic endocrine cells between mutant smad2 or smad3 mice and littermate controls, and no difference in the percentage of BrdU positive cells (data not shown). By E17–18, however, significant increases in the number of endocrine cells were seen with ablation of smad2 (Fig. 3A–E) or smad3 (Fig. 3H). In pdx1-cre-ERT;smad2fx/fx conditional mutants, there was a 30% increase in the number of endocrine cells (insulin+ or glucagon+ only, other endocrine cells made up only a small fraction of the cells present), which consisted of a 20% increase in the number of insulin-positive cells, and a 40% increase in the number of glucagon-positive cells (Fig. 3E). In smad3−/− mice there was a greater (50%) increase in the number of endocrine cells, with a relatively equal percentage increase in insulin+ and glucagon+ cells (Fig. 3H). This endocrine augmentation, especially with the greater pro-endocrine effect in smad3 mutants than in smad2 mutants, is similar to our findings with the in vitro antisense inhibition (data not shown). We did not see an additive effect between these two mutations, as mice bred to be pancreatic double smad mutants (pdx1-cre-ERT;smad2fx/fx; smad3−/−), showed a phenotype similar to the smad3−/− mutants (data not shown). Thus, given that we found that both smads are expressed in the endocrine progenitor pool, it seems likely that both smads are necessary for endocrine maturation, and absence of these smads leads to an inappropriate expansion of immature pancreatic endocrine cells, and thus the two smads may work in a synergistic or overlapping way.
Figure 3.
(A–D) Insulin and glucagon expression pattern in E18 pdx1-cre- ERT;smad2fx/fx pancreas compared with littermates that are Cre negative. (E) Quantification of the number of endocrine cells in E18 pdx1-cre-ERT;smad2fx/fx pancreas (n=5, *p<0.0002, **p<0.0001, ±SEM). (H) Quantification of endocrine cells in smad3-exon2−/− and smad3-exon2+/− E18 pancreas (n=3, *p<0.05, **p=0.02, ***p<0.04, ±SEM. Scale bar is 20μm.
Expression of smad7 during pancreatic development
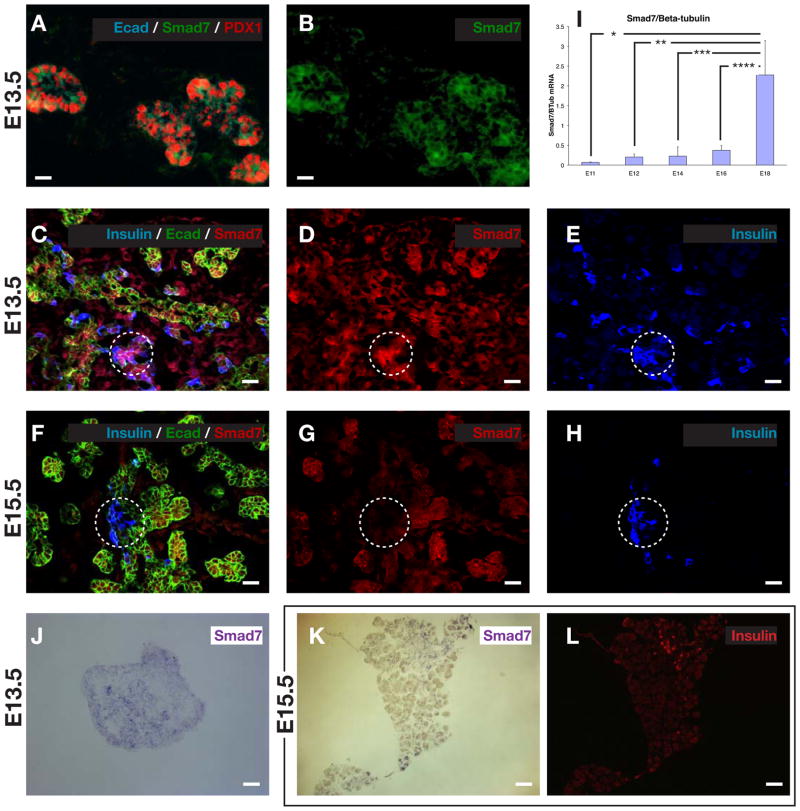
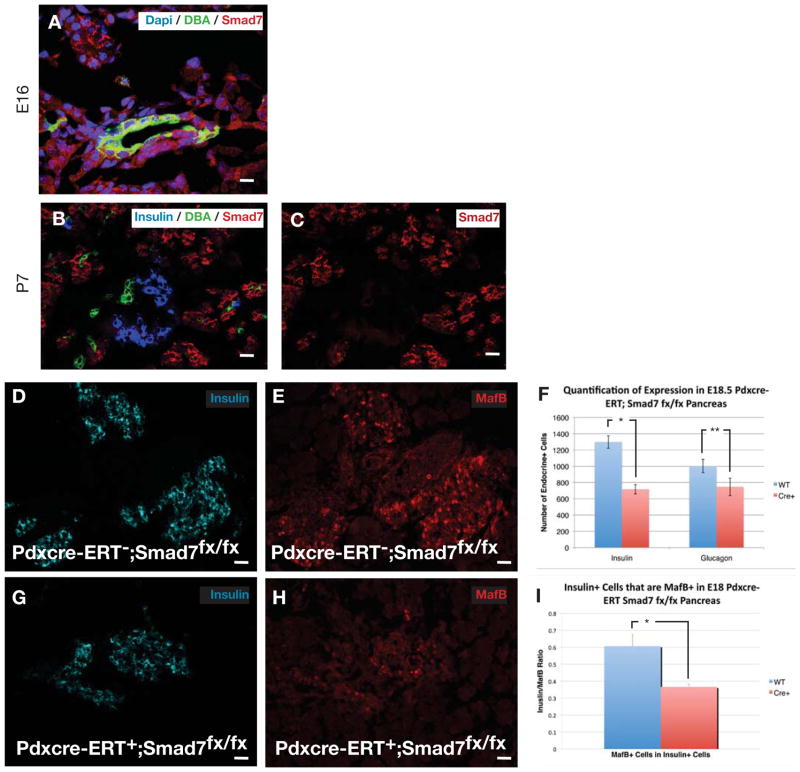
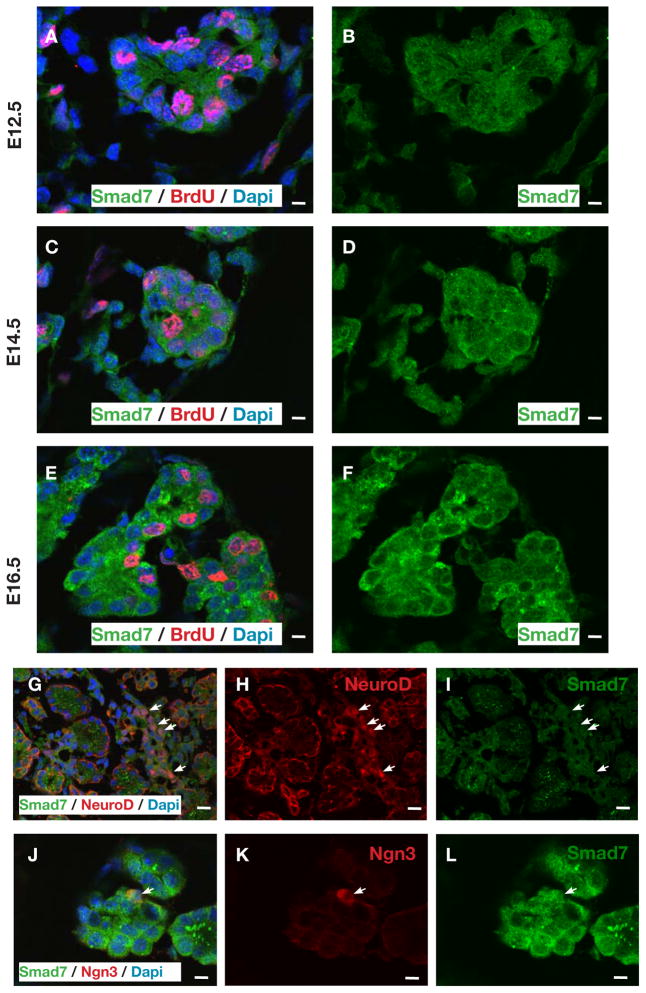
Because of the known role of smad7 as an inhibitor of smad signaling (Park, 2005; Yan et al., 2009), and since smad7 overexpression, but not smad6 overexpression in the developing pancreas led to pancreatic endocrine hypoplasia (Goulley et al., 2007; Smart et al., 2006), we wished to examine the presence and role of endogenous smad7 in the pancreas. To better characterize these smad7+ cells during embryonic development, we performed confocal immunofluorescence for smad7. Smad7 was present throughout the early (E13.5) pancreatic epithelium, both by immunohistochemistry and by in situ hybridization (Fig. 4A–E,J). By QPCR, however, this expression seemed relatively low, and then was upregulated late in gestation (Fig. 4I), perhaps due to an increased contribution from acinar cells (see below and Fig. 6C). Most of the smad7-expressing cells in the younger embryo (E13.5) co-localized with E-cadherin+ epithelial cells, but a few were hormone+ endocrine cells (Fig. 4A–E), perhaps representing immature newly-formed endocrine cells. Interestingly, as development progressed (E15.5), the smad7 co-localization with the hormone+ endocrine cells was diminished and weaker, but persisted in the E-cadherin+/hormone− cells (Fig. 4F–H,K,L and Fig. 6C). This transition of smad7 expression from being positive in a few early hormone-positive, presumably more immature endocrine cells at E13.5 to being diminished in late-gestational, presumably more mature hormone-positive cells, is consistent with a role for smad7 in suppressing smad2/3 in immature endocrine cells. Then, as smad7 shuts off, the endocrine cells are able to mature in association with smad2/3 activity. Consistent with this observation, we saw that many smad7+ cells were BrdU+ throughout gestation (Fig. 5A–F), and many smad7+ cells co-stained for ngn3+ (Fig. 5J–L), marking a proliferative endocrine progenitor pool. Interestingly, smad7+ cells typically did not co-stain for neuroD (Fig. 5G–I), a marker of post-mitotic endocrine lineage cells, consistent with smad7 serving to maintain the proliferative state of the immature endocrine cells. Later in gestation, at E16.5 and at P7, the acinar tissue was positive for smad7 (Fig. 5E,F and Fig. 6C), which may explain the high levels of smad7 by QPCR (Fig. 4I), and may reflect the fact that the acinar population is rapidly proliferative in late gestation. At E16 the ducts and some endocrine cells were weakly positive for smad7 (Fig. 6A). By post-natal day 7 (P7), smad7 was almost exclusively localized to the acinar tissue (Fig. 6B,C).
Figure 4.
Ontogeny of smad7 expression in the embryonic pancreas. (A–E) Smad7 is initially expressed throughout the pancreatic epithelium during early pancreatic development (E13.5), including co-expression with insulin positive cell. (F–H) During later embryonic pancreatic development (E15.5), insulin-positive cells no longer co-express smad7. (I) QPCR ontogeny for smad7 at different stages of embryonic development (n=5, *p<0.001, **p<0.0001, ***p<0.001, ***p<0.002, ±S.D). Smad7 in-situ hybridization in E13.5 pancreas (J) and in E15.5 pancreas (K), with E15.5 insulin counterstaining in (L). Scale bar, (A,B) is 10μm, (C–H) is 20μm, (J–L) is 50μm.
Figure 6.
Smad7 initially co-localizes with DBA positive ducts at E16 (A), and then is absent from the ducts by P7 (B,C). (I) Quantification of the number of endocrine cells in E18.5 pdx1-cre-ERT;smad7fx/fx pancreas following tamoxifen treatment at E10.5 (n=5, *p<0.0001,3 *p=0.0001, ±S.D). (G,H,J,K) Insulin and mafB co-expressing cells are diminished in E18 pdx1-cre-ERT;smad7fx/fx pancreas, quantified in (L) (n=5, *p<0.0001, ±SEM). Scale bar, (A–F) is 10μm, (I,J, L,M) is 20μm.
Figure 5.
Proliferation analysis of smad7-expressing cells in the embryonic pancreas. (A–F) Proliferating smad7 cells during embryonic development. (A,B) At E12.5, 49.4% ± 4.3 SD, n=7, smad7 positive cells are BrdU positive. (C,D) At E14.5, 55.4% ± 4.3 SD, n=7, smad7 positive cells are BrdU positive. (E–F) At E16.5, 26.5% ± 3.6 SD, n=7, smad7 positive cells are BrdU positive. (G–I) NeuroD positive cells specifically do not coexpress smad7 at E16.5 (arrows), with none of the 6,727 NeuroD-positive cells from 7 embryos co-localizing with smad7. (J–L) Ngn3 positive cells co-express smad7 in pancreatic epithelium at E16.5 (arrow), with 17.1% ± 3.4 SD, n=5, of Ngn3 positive cells co-localizing with smad7 positive cells. Scale bar, (A–F) and (J–L) is 10μm, (G–I) is 20 μm.
Smad7 inhibition in vitro or in vivo leads to suppression of endocrine development
When cultured E11.5 embryonic pancreas explants were treated with morpholino antisense against smad7, we saw a diminution in the number of endocrine cells and in the endocrine-positive area in the explants after 7-days in culture (Figure 2F,G,I n=4, p<0.001). These results suggest that smad2/3 inhibition may normally prevent premature endocrine maturation, and thus allow for endocrine progenitor expansion. As with the smad2/3 antisense inhibition earlier, Western blot confirmed smad7 knock-down by the antisense (Figure 2B). BrdU analysis revealed that there was a marked decrease in the percentage of endocrine cells that were BrdU+ (insulin and glucagon only were quantified)(Figure 2J, Figure S2H).
To determine a possible role for smad7 in pancreatic endocrine cell development, we generated a smad7fx/fx knock-in mouse. These mice were created with loxP sites flanking exon5 (the TGF-β receptor interacting domain)(Park, 2005; Yan et al., 2009, El-Gohary, et al. Unpublished data) of the smad7 locus. We then conditionally deleted smad7 in the pancreas by crossing the smad7fx/fx mice with pdx1-cre-ERT mice to allow tamoxifen-inducible ablation of smad7 in embryonic pancreatic epithelial cells and in developing islets, acini, and ducts. We gave tamoxifen at E10.5. We saw no phenotypic effect early on, at E13–15, but by E17.5, similar to smad7 antisense inhibition in vitro, we saw diminished numbers of hormone+ cells (insulin and glucagon) (Fig. 6I). Here again, consistent with a role for smad7 in suppressing premature maturation of endocrine cells, we saw a significant decrease in the number of (presumably immature) mafB+/insulin+ double-positive cells (Fig. 6G,H,J–L). MafB+/insulin+ cells have been proposed as an immature β-cell, and as the cells mature mafB is turned off and mafA turned on (Artner et al., 2010).
Discussion
Here we have detailed a mechanism that regulates embryonic pancreatic endocrine expansion and maturation, involving a smad signaling network. This regulation is achieved through the intracellular smads 2, 3, and 7. TGF-β superfamily signaling has been shown to play an important role in regulating many developmental and physiologic processes in the pancreas and in the β-cell (Goto et al., 2007; Harmon et al., 2004; Kim et al., 2000; Miralles et al., 1998a; Miralles et al., 1998b; Rane et al., 2006; Ritvos et al., 1995; Sanvito et al., 1994; Shi and Massague, 2003; Tulachan et al., 2007; Zhang et al., 2004; Zhang et al., 2008). We have previously reported that signals mediated through TGF-β-receptor-II to the embryonic ductal structures suppress the recruitment of endocrine progenitors and suppress their proliferation (Tulachan et al., 2007). We show here that these signals appear to be mediated by the intracellular signaling molecules, smad2 and smad3, which are downstream effectors of TGF-β-receptor-II (Shi and Massague, 2003).
These same smads are potential regulators of the transdifferentiation of AR42J cells (a duct cell line) into β-cells in vitro (Yew et al., 2004; Zhang et al., 1999). In keeping with the results that we now show here, heterozygous smad2 global null mutant mice (smad2+/−) have been shown to have increased numbers of ngn3+ progenitor cells (Harmon et al., 2004), and increased numbers of nkx2.2+ and nkx6.1+ progenitor cells (Harmon et al., 2004) (homozygous global smad2−/− mutant mice are early embryonic lethal (Nomura and Li, 1998)). The significant increase in endocrine cell number that we found at E18 with smad2 and/or smad3 deletion, coupled with the normal strong nuclear localization of p-smad2/3 in hormone positive cells at E12.5 (Fig 1C,D), E18.5 (Fig. 1E,F) and 10wks (Fig. 1G) supports the notion that inhibiting smad2/3, and thus TGF-β signaling, may be an endogenous mechanism for allowing proliferation of β-cells. Furthermore, semi-quantitative RT-PCR analysis of smad expression revealed that smads 2, 3, and 4 all showed a rise toward the end of gestation, consistent with a possible role in mediating the maturation of endocrine cells (Fig. 1H,I and S1E).
Our results show an important regulatory role for smad7 in both developmental endocrine differentiation, and maturation. Although there are two known inhibitory smads, smad6 and smad7, we believe the effects here are specific to smad7. Smad6 is thought to be a specific inhibitor of the BMP smads 1,5, and 8 (Massague and Gomis, 2006; Park, 2005; Yan et al., 2009). Transgenic overexpression of smad6 under the pdx1 promoter showed no developmental phenotype, and in the adult there was mainly only an effect on insulin secretion (Goulley et al., 2007) rather than an effect on islet growth. Smad7, on the other hand, can inhibit both the BMP smads (1,5, 8) and the TGF-β and activin smads (2/3)(Massague and Gomis, 2006; Park, 2005; Yan et al., 2009). Thus, phenotypes attributable to alterations of smad7, but not smad6, would likely be due to effects on smad2/3 signaling. When Smart, et al. transgenically overexpressed smad7 under the pdx1 promoter in a tetracycline-regulatable system to thus create an inducible inhibition of smad signaling in pdx1-expressing cells (Smart et al., 2006), there was an enhanced number of glucagon cells, but a drastically reduced number of β-cells. This latter study could contradict ours. However, since smad7 was expressed under the pdx1 promoter, coupled with the fact that pdx1 expression is typically down-regulated as embryonic pancreatic epithelial cells commit to becoming endocrine progenitor cells, it would suggest that the transgenic smad7 would not be strongly expressed in endocrine progenitors. Therefore, phenotypic effects of forced smad7 overexpression in early pancreatic epithelium may have effects on endocrine cell formation only as an epiphenomenon.
Thus, we have identified an important and necessary regulatory point for proper β-cell expansion in the form of a smad2/3/7 network. This pathway could represent a legitimate target for regulating β-cell proliferation, and for generating more β-cells for the future treatment of diabetes.
Statistical analysis
Statistical significance was determined by two-tailed Student’s t-test.
Supplementary Material
Supplemental Figure 1. (A–D) Individual p-smad2 or p-smad3 staining in CD-1 wild-type pancreas at E15 and E18. (E) QPCR ontogeny for smad4 at different embryonic stages of development (+/− S.D.). (F–H) QPCR ontogeny for TGFβ ligand 1,2 and 3.
Supplemental Figure 2. (A–D) Efficacy of smad2 and smad3 morpholino treatment in E11.5 pancreas culture demonstrating decreased immunostaining signal for these two molecules compared to controls. (E–H) BrdU incorporation in (E) control E11.5 cultured pancreas, (F) smad2 morpholino treated E11.5 pancreas, (G) smad3 morpholino treated E11.5 pancreas, and (H) smad7 morpholino treated. (I–L) QPCR analysis for insulin, glucagon, neuroD and ngn3 mRNA after treatment with smad2 or smad3 morpholino for 5 days. (I) n=5, ±S.D. (J) n=5, *p<0.02, **p=0.0002, ±S.D. (K) n=5, *p=0.006, **p<0.002, ±S.D. (L) n=5, *p=0.0003, ±S.D. (M–O) Pax6 and glucagon expression in cultured E11.5 pancreas treated with (E) scrambled morpholino (control), (F) smad2 morpholino, or (G) smad3 morpholino harvested and analyzed after only 1 day of culture. Scale bar, (A–H) is 20μm, (M–O) is 10μm.
Acknowledgments
Special thanks to Lauren Brink, Jessica Thomas, and Sean-Paul Williams for technical assistance in flow cytometry and mouse genotyping. Thanks to Sanjay Mishra, Csaba Galambos, Farzad Esni, Angela Criscimanna, Sohail Shah, Marcus Malek, Nikesh Lath for helpful discussions. Thanks to Christine Kalinyak, Anne L Meiner and Tamara Daviston for administrative assistance. Financial support was from NIH (G.K.G., RO1 DK064952, R01 DK083541-01) and the Children’s Hospital of Pittsburgh. M.D. was supported by Project Z01 ES101603 from the Division of Intramural Research of the NIH (NIEHS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- Bottinger EP, Jakubczak JL, Roberts IS, Mumy M, Hemmati P, Bagnall K, Merlino G, Wakefield LM. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–2633. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson M, Hougaard DM, Nielsen JH, Tornehave D, Larsson LI. Expression of SMAD signal transduction molecules in the pancreas. Histochemistry and cell biology. 2001;116:263–267. doi: 10.1007/s004180100316. [DOI] [PubMed] [Google Scholar]
- Crisera CA, Rose MI, Connelly PR, Li M, Colen KL, Longaker MT, Gittes GK. The ontogeny of TGF-beta1, -beta2, -beta3, and TGF-beta receptor-II expression in the pancreas: implications for regulation of growth and differentiation. J Pediatr Surg. 1999;34:689–693. doi: 10.1016/s0022-3468(99)90357-3. discussion 693–684. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Molecular and cellular biology. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund H. Developmental biology of the pancreas. Diabetes. 2001;50(Suppl 1):S5–9. doi: 10.2337/diabetes.50.2007.s5. [DOI] [PubMed] [Google Scholar]
- El-Gohary Y, Tulachan S, Branca M, Sims-Lucas S, Guo P, Prasadan K, Shiota C, Gittes GK. Whole-mount imaging demonstrates hypervascularity of the pancreatic ducts and other pancreatic structures. Anat Rec (Hoboken) 2012;295:465–473. doi: 10.1002/ar.22420. [DOI] [PubMed] [Google Scholar]
- El-Gohary Y, Tulachan S, Guo P, Welsh C, Wiersch J, Prasadan K, Paredes J, Shiota C, Xiangwei X, Wada Y, Diaz M, Gittes G. A smad signaling network regulates islet cell proliferation. (unpublished) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, Masui T, Kawaguchi M, Takaori K, Doi R, Nishi E, Kakinoki R, Deng JM, Behringer RR, Nakamura T, Uemoto S. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Goto Y, Nomura M, Tanaka K, Kondo A, Morinaga H, Okabe T, Yanase T, Nawata H, Takayanagi R, Li E. Genetic interactions between activin type IIB receptor and Smad2 genes in asymmetrical patterning of the thoracic organs and the development of pancreas islets. Dev Dyn. 2007;236:2865–2874. doi: 10.1002/dvdy.21303. [DOI] [PubMed] [Google Scholar]
- Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Harmon EB, Apelqvist AA, Smart NG, Gu X, Osborne DH, Kim SK. GDF11 modulates NGN3+ islet progenitor cell number and promotes beta-cell differentiation in pancreas development. Development (Cambridge, England) 2004;131:6163–6174. doi: 10.1242/dev.01535. [DOI] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Ju W, Ogawa A, Heyer J, Nierhof D, Yu L, Kucherlapati R, Shafritz DA, Bottinger EP. Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Molecular and cellular biology. 2006;26:654–667. doi: 10.1128/MCB.26.2.654-667.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nature genetics. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. Activin receptor patterning of foregut organogenesis. Genes & development. 2000;14:1866–1871. [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS letters. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Miralles F, Battelino T, Czernichow P, Scharfmann R. TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. The Journal of cell biology. 1998a;143:827–836. doi: 10.1083/jcb.143.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development (Cambridge, England) 1998b;125:1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. Journal of biochemistry and molecular biology. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- Prasadan K, Daume E, Preuett B, Spilde T, Bhatia A, Kobayashi H, Hembree M, Manna P, Gittes GK. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes. 2002;51:3229–3236. doi: 10.2337/diabetes.51.11.3229. [DOI] [PubMed] [Google Scholar]
- Prasadan K, Koizumi M, Tulachan S, Shiota C, Lath N, Paredes J, Guo P, El-Gohary Y, Malek M, Shah S, Gittes GK. The expression and function of glucose-dependent insulinotropic polypeptide in the embryonic mouse pancreas. Diabetes. 2011;60:548–554. doi: 10.2337/db09-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane SG, Lee JH, Lin HM. Transforming growth factor-beta pathway: role in pancreas development and pancreatic disease. Cytokine & growth factor reviews. 2006;17:107–119. doi: 10.1016/j.cytogfr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ritvos O, Tuuri T, Eramaa M, Sainio K, Hilden K, Saxen L, Gilbert SF. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mechanisms of development. 1995;50:229–245. doi: 10.1016/0925-4773(94)00342-k. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development (Cambridge, England) 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvito F, Herrera PL, Huarte J, Nichols A, Montesano R, Orci L, Vassalli JD. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development (Cambridge, England) 1994;120:3451–3462. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart NG, Apelqvist AA, Gu X, Harmon EB, Topper JN, MacDonald RJ, Kim SK. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006;4:e39. doi: 10.1371/journal.pbio.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Tulachan SS, Tei E, Hembree M, Crisera C, Prasadan K, Koizumi M, Shah S, Guo P, Bottinger E, Gittes GK. TGF-beta isoform signaling regulates secondary transition and mesenchymal-induced endocrine development in the embryonic mouse pancreas. Dev Biol. 2007;305:508–521. doi: 10.1016/j.ydbio.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liu Z, Chen Y. Regulation of TGF-beta signaling by Smad7. Acta biochimica et biophysica Sinica. 2009;41:263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew KH, Prasadan KL, Preuett BL, Hembree MJ, McFall CR, Benjes CL, Crowley AR, Sharp SL, Li Z, Tulachan SS, Mehta SS, Gittes GK. Interplay of glucagon-like peptide-1 and transforming growth factor-beta signaling in insulin-positive differentiation of AR42J cells. Diabetes. 2004;53:2824–2835. doi: 10.2337/diabetes.53.11.2824. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Cleary MM, Si Y, Liu G, Eto Y, Kritzik M, Dabernat S, Kayali AG, Sarvetnick N. Inhibition of activin signaling induces pancreatic epithelial cell expansion and diminishes terminal differentiation of pancreatic beta-cells. Diabetes. 2004;53:2024–2033. doi: 10.2337/diabetes.53.8.2024. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Kanzaki M, Furukawa M, Shibata H, Ozeki M, Kojima I. Involvement of Smad proteins in the differentiation of pancreatic AR42J cells induced by activin A. Diabetologia. 1999;42:719–727. doi: 10.1007/s001250051220. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Sterling L, Stotland A, Hua H, Kritzik M, Sarvetnick N. Nodal and lefty signaling regulates the growth of pancreatic cells. Dev Dyn. 2008;237:1255–1267. doi: 10.1002/dvdy.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A–D) Individual p-smad2 or p-smad3 staining in CD-1 wild-type pancreas at E15 and E18. (E) QPCR ontogeny for smad4 at different embryonic stages of development (+/− S.D.). (F–H) QPCR ontogeny for TGFβ ligand 1,2 and 3.
Supplemental Figure 2. (A–D) Efficacy of smad2 and smad3 morpholino treatment in E11.5 pancreas culture demonstrating decreased immunostaining signal for these two molecules compared to controls. (E–H) BrdU incorporation in (E) control E11.5 cultured pancreas, (F) smad2 morpholino treated E11.5 pancreas, (G) smad3 morpholino treated E11.5 pancreas, and (H) smad7 morpholino treated. (I–L) QPCR analysis for insulin, glucagon, neuroD and ngn3 mRNA after treatment with smad2 or smad3 morpholino for 5 days. (I) n=5, ±S.D. (J) n=5, *p<0.02, **p=0.0002, ±S.D. (K) n=5, *p=0.006, **p<0.002, ±S.D. (L) n=5, *p=0.0003, ±S.D. (M–O) Pax6 and glucagon expression in cultured E11.5 pancreas treated with (E) scrambled morpholino (control), (F) smad2 morpholino, or (G) smad3 morpholino harvested and analyzed after only 1 day of culture. Scale bar, (A–H) is 20μm, (M–O) is 10μm.