Abstract
Posttraumatic stress disorder (PTSD) has been linked to numerous negative outcomes in persons living with HIV (PLH) and there is evidence that PTSD symptoms may play a role in maintaining alcohol use problems. The opioid receptor mu-1 (OPRM1) gene may play a role in both PTSD and alcohol use. We examined the association between PTSD and drinking motives as well as variation in the OPRM1 as a predictor of both PTSD and drinking motives in a sample of 201 PLH reporting recent binge drinking. Self-reported PTSD symptom severity was significantly associated with drinking motives for coping, enhancement, and socialization. OPRM1 variation was associated with decreased PTSD symptom severity as well as enhancement motives for drinking.
Posttraumatic stress disorder (PTSD) is a prevalent concern among persons living with HIV (PLH) and is linked to comorbid substance concerns as well as a range of negative health behaviors and outcomes. Estimates of PTSD diagnosis in HIV-infected populations range from 15% to 64% (1-7). Among PLH, PTSD symptoms and diagnosis have been linked to poor medication adherence (5, 8-10), poor health functioning and quality of life (2, 11), and comorbid substance use (12, 13). To date, no studies have examined the relationship between PTSD symptoms and self-reported drinking motivations in PLH.
Research with uninfected populations has found that drinking both to cope with negative emotions as well as drinking to enhance positive emotions partially mediates the link between trauma and alcohol problems (14-16). Furthermore, drinking to cope has been associated with PTSD symptoms (15) and women in substance use treatment with PTSD report greater expectancies of positive enhancement from drinking relative to women without PTSD (17). Although PTSD and substance use problems are common among PLH and are linked to multiple negative functional outcomes (7, 18-20), we are unaware of any prior studies that have examined how PTSD may influence drinking motivations among PLH. Of note, associations between PTSD and alcohol use may occur through multiple, non-exclusive pathways. It is possible that pre-trauma substance use may place individuals at increased risk for PTSD, that PTSD symptoms may exacerbate or promote development of substance use problems to self-medicate distress, and/or that constitutional factors such as genetic variation place individuals at risk for both substance use and PTSD symptoms. Interestingly, prior research does point to the potential for some shared influence of specific genetic variation on both PTSD and substance abuse, including the OPRM1 gene.
The opioid neurotransmitter system is believed to play a role in development of PTSD (21). For example, morphine administration in the acute aftermath of trauma reduces the risk of developing PTSD (22-25), likely via activation of mu-opioid receptors and downstream influences on the acute stress response (26, 27). Specifically, mu-opioid receptors exert inhibitory effects on the hypothalamic-pituitary-adrenal (HPA) axis, the major stress response system implicated in the development of PTSD (28). OPRM1 A118G, the most widely investigated polymorphism within the OPRM1 gene, codes for the Asn40Asp substitution, with the Asp40 (i.e., G) allele binding three times more strongly to the endogenous ligand beta-endorphin than the Asn40 allele (29). It has been suggested that individuals with the G allele evidence greater receptor response but reduced stable receptor expression (28). Carriers of the G allele appear to show enhanced HPA axis dynamics in response to opiate blockade (30). Further, G allele carriers evidence higher baseline and post-naloxone cortisol levels, greater peak cortisol response, and greater cortisol area under the curve than participants homozygous for the A allele (31). Acute peritraumatic elevations in cortisol may contain the HPA response and thereby may actually be protective against the development of PTSD (32-34). Thus, as we have proposed elsewhere (35), it is possible that the G allele may protect against the development of PTSD through its potential influence on containment of peritraumatic stress response.
Importantly, the neurobiologic influences on the development of a given disorder may differ greatly from the influences on maintenance of the disorder. The role of peritraumatic neurobiologic response in later development of PTSD is well-known and widely-accepted, with acute neurobiologic predictors often found to differ significantly from neurobiologic correlates of chronic PTSD (34). Although no studies have examined naturally-occuring peritraumatic opioid levels, chronic PTSD has been linked to abnormalities in endogenous opioids (36-38). Combat veterans with PTSD showed reduced mu-opioid receptor binding in the anterior cingulate relative to non-PTSD control groups with and without combat exposure (39). Interestingly, both combat-exposed groups showed lower mu-opioid receptor binding in amygdala, nucleus accumbens, and dorsal frontal and insular cortex in conjunction with higher binding in the orbitofrontal cortex. The authors suggest that trauma exposure may lead to down-regulation of limbic forebrain, thalamus, and associated cortical regions in conjunction with up-regulation of the orbital prefrontal cortex; thus, altered binding in PTSD patients may reflect deficient initial activation and/or impaired subsequent up-or down-regulation of these areas. Although we were unable to test this hypothesis in the present design, we expected that genetic differences on OPRM1 would influence PTSD symptom severity primarily through its impact on participant neurobiology during acute trauma and initial consolidation and reconsolidation processes and that the G allele would be protective.
In contrast to the expected influence of OPRM1, the G allele has been linked to increased risk for substance use disorders. Substance use researchers have postulated that G allele carriers might evidence increased drug-induced euphoria, analgesia, and withdrawal based on differences in binding. OPRM1 has been associated with a differential response to alcohol and response to alcohol-related cues (40, 41). Carriers of the G allele report higher subjective feelings of intoxication, stimulation, sedation, and mood (42). Further, OPRM1 carriers of the OPRM1 G allele evidenced increased naltrexone-induced blunting of alcohol-induced “high” (43). Ten days following randomization to naltrexone, G carriers endorsed higher alcohol craving in response to both alcohol and neutral beverage cue trials relative to a homozygotes (41). G allele carriers in the medical management COMBINE study evidenced increased abstinence and decreased number of heavy drinking days in naltrexone treatment relative to placebo treatment, whereas individuals homozygous for the A allele evidenced no medication differences (44). Carriers of the G allele showed greater brain response on mesocorticolimbic areas both before and after administration of a priming dose of alcohol (45). Further, striatum brain response in G carriers was highly associated with elevations in alcohol use frequency and quantity. OPRM1 appears to modulate striatal dopamine response to alcohol in humans and an animal model reports a fourfold greater peak dopamine response in the AA genotype relative to the GG genotype (46). A recent investigation using an approach avoidance task reported strong automatic approach tendencies (approach bias) for alcohol in heavy drinking carriers of the G-allele (47). Of particular note, heavy drinking carriers of the G-allele showed an approach bias across additional appetitive stimuli. Interestingly, a recent manipulation of stress- and cue-induced craving in heavy drinkers suggested that cue-induced craving was associated with OPRM1 in the neutral but not in the stressful condition (48). Taken together, these findings suggest that the G allele’s influence on alcohol use functions through an appetitive reward system, rather than as a mechanism for coping.
OPRM1 may play a particularly important role in psychological functioning in medical populations. Prior research with medical populations has found that genetic variation in OPRM1 is associated with symptoms of depression (49) and health-related quality of life (50). There is also some evidence that OPRM1 Asp40 carriers may be associated with suppression of peripheral proinflammatory cytokine secretion, secondary to enhanced sensitivity of endogenous opioid system (50). Accordingly, OPRM1 may be especially important among PLH.
The present investigation provides an examination of PTSD related to HIV diagnosis and motives for drinking in a sample of PLH who report recent binge drinking; binge drinking was defined as greater than 4 drinks for women and 5 drinks for men consumed on a single occasion in the past six months. We expected that PTSD symptom severity would predict drinking to cope. Although PLH may have symptoms of PTSD secondary to a range of experiences, we selected PTSD secondary to HIV diagnosis to permit a common anchor across all participants. We also planfully examined PTSD symptom severity rather than diagnosis, as a wealth of prior research supports a robust linear association between number of symptoms and degree of functional impairment (51-56). In addition, the present investigation is the first study to examine how the OPRM1 A118G variant is related to PTSD symptom severity and self-reported motivations to drink. Given the role of opioid response to the HPA axis and peritrauma stress response, we expected that the G allele would be associated with less severe PTSD symptom reporting in an additive fashion. However, based on evidence that the G allele is associated with increased approach bias towards alcohol, we expected that the G allele would be associated with self-reported motivation to drink for enhancement.
METHODS
Participants
201 HIV-infected adults (22-64) were recruited from a medical clinic. Participants were predominantly male (72.6%). Consistent with clinic demographics, the majority of participants were male (72.6%) and Caucasian (non-Hispanic White: n = 83, 41.3%; Hispanic White: n=6, 3.0%). Another 17.9% of participants were African American, 2.5% (n=5) endorsed American Indian, 1% (n = 2) African (from the African subcontinent), 1% (n = 2) Native Hawaiian, and 2% (n = 4) Other. Nearly 20% of the sample reported mixed heritage (n=32).
Procedures
Participants were recruited by a clinic nurse during regularly scheduled appointments; patients were provided with a detailed description of the investigation. To minimize participant over-reported adherence and under-reports of risk-taking behaviors, data were collected using audio-assisted computer (ACASI) methodology. ACASI has been found to increase participant willingness to disclose personally sensitive information such as sex risk and drug use (57) and to decrease the impact of social desirability on assessments (58). Prior research with PLH has cross-validated ACASI sexual activity questions with face-to-face interview questions, with participants evidencing similar responses in both formats (59).
Genotyping
Genomic DNA was isolated from buccal cells using a modification of published methods (Freeman et al., 1997; Lench et al., 1988; Meulenbelt et al., 1995; Spitz et al., 1996). After being rubbed against the participants’ inner cheek cells, swabs were placed in a 50-mL capped polypropylene tube containing lysis buffer (500 mL of 1 M Tris-HCl; 200 mM disodium ethylene diaminetetracetic acid (EDTA), pH 8.0; 500 mL of 10% sodium docecyl sulfate; and 100 mL of 5 M sodium chloride). Participants then rinsed out their mouth vigorously with 10 mL of distilled water and this was added to the 50-mL tube. Tubes were stored at 4°C until the DNA was extracted, usually within 48 hours. Proteinase K solution (100 mL of 20 mg/mL) and sodium chloride (100 mL of 5 M) were added to each of the tubes. The tubes were incubated at 65°C for 60 minutes. Residual lysis buffer was removed from the saturated swabs by centrifugation for 5 minutes at 1000 rpm, and the collected buffer was added back to the original 50-mL collection tube. An equal volume of 100% isopropyl alcohol was then added to each tube to precipitate the DNA, which was collected by centrifugation at 3500 g for 10 min at room temperature. The liquid was decanted, and the DNA pellet was washed with 1 mL of fresh 50% isopropyl alcohol. After drying at 65°C, the pellet was resuspended in 1 mL of 20 mM Tris-EDTA, pH 8.0. The yield of DNA was quantified by absorbance at 260 nm (1 optical density unit (O.D.) = 50 mg/mL), and an aliquot was diluted to a concentration of ≤ 20 ng/mL for a working sample. Samples were genotyped for the rs1799971 (A118G) polymorphism using Taqman assay C___8950074_1_ on an ABI 7900HT Real Time PCR system.
Drinking
Participants were referred for the investigation if they reported at least one occasion of binge drinking over the past 6 months during their regularly-scheduled HIV clinic visits. Binge drinking was defined consistent with the extant literature as consisting of 4 or more drinks for women and 5 or more drinks for men on one occasion. Participants also completed the AUDIT (60, 61), a widely used measure of alcohol use devised by World Health Organization (WHO) investigators as a brief screening method appropriate for general medical settings in developing and developed countries. The measure is comprised of 10 items and respondents use likert-type scaling to indicate engagement in a range of behaviors indicative of drinking problems. A likely alcohol use disorder score was defined as a cut-off score of 8 or more points, a cutoff recommended by the developers that has been applied across a wide range of settings and populations (60, 61).
Posttraumatic stress disorder symptoms
Participants completed the Impact Event Scale-Revised (IES-R; (62), a widely used measure of PTSD symptoms that may be scored for diagnosis as well as for symptom severity. To permit examination of a trauma experienced by all participants, symptoms were anchored to the experience of being diagnosed with HIV. Respondents rated the frequency of each symptom in the past seven days using four frequency anchors (i.e., not at all, rarely, sometimes, and often). Possible scores range from 0 to 88, with higher scores indicating more frequent symptoms.
Drinking Motives
Participants completed the Drinking Motive Questionnaire (63), a 15-item self report measure in which respondents indicate frequency of drinking for a range of possible reasons. Each item is rated on a scale of 1 (almost never/never) to 4 (almost always). The measure includes three factor-derived subscales including drinking to cope, drinking for enhancement, and drinking for social motives.
Covariates
Given evidence that PTSD is highly comorbid with depression, which has also been previously associated with OPRM1, we also assessed for self-reported history of depression using a single item asking whether any mental health professional has ever “diagnosed you with, or told you that you had, depression?” Participants were also asked to indicate any medications (prescribed or otherwise) that they were taking and responses were coded to indicate current opioid use. Finally, as a very rough index of disease progression, respondents were asked “have you ever had an AIDS-defining illness?”
Data analysis
As this, to our knowledge, is the first behavioral candidate gene investigation in HIV-infected adults, we first provide descriptive information related to the sample characteristics across demographic, psychobehavioral, and genetic variables. Preliminary descriptive analyses were conducted using SPSS Version 17.0. Tests of hypotheses were conducted using MPlus Version 6.11 maximum likelihood estimation procedures with robust standard errors (MLR). Outcomes evidencing a poisson distribution were estimated with a poisson model. Analyses were conducted assuming an additive model (AA = 0, AG = 1, GG = 2). Data were complete across nearly all variables; covariance coverage was high, ranging from .53 to 1.0 and falling well above the minimum recommended coverage of .10 (64). Multiple imputation was implemented using Bayesian estimation procedures. Consistent with prior psychosocial research with PLH (65, 66), all tests of hypotheses controlled for age, gender, race, and socioeconomic status (SES education levels were used as these are less biased in the sample of interest); we also conservatively covaried for participant-reported history of an AIDS-defining illness. Models involving OPRM1 conservatively excluded participants reporting current opioid use (N=4).
RESULTS
On average, participants reported relatively little education, with the largest proportion of participants indicating that they did not graduate high school or complete a GED (n = 62, 30.8%). Twenty-six percent of participants reported obtaining a high school diploma and another 20% reported completing a GED. Just over half (53.7%) of participants reported their annual income to be $10,000 or below. Consistent with recruitment of participants on the basis of drinking behavior, most (n=129; 64.2%) of the sample met the AUDIT threshold for a likely alcohol use disorder (M = 12.66, SD = 8.46), with participants particularly likely to endorse hazardous drinking. Consistent with prior research with PLH (1, 4), roughly one quarter of participants reported symptoms of PTSD in the clinically significant range (25.8% exceeded a cut-off of 30; 21.7% exceeded the more stringent cutoff of 33 (67, 68). OPRM1 allele frequencies (AA = 167, AG = 29, GG = 3) did not differ from Hardy-Weinberg Equilibrium (χ2 = 1.67, p > .05) and were consistent with observed allelic frequencies in the literature (69).
PTSD symptoms and drinking motives
MLR estimation procedures were conducted, controlling for age, gender, education, ethnicity, and history of an AIDS-defining illness to examine whether endorsement of PTSD symptoms was associated with concurrent reports of drinking motives. Shown in Table 2, PTSD symptom severity was found to be positively associated with all assessed drinking motives. The strongest effect of PTSD symptom severity was observed in increased endorsement of coping motives for drinking, estimate = .02, S.E. = .00, z-score = 7.29, p < .001. Age, gender, education, ethnicity, and history of an AIDS-defining illness were not related to coping motives. PTSD symptom severity was also found to significantly predict increased enhancement drinking motives, estimate = .01, S.E. = .00, z-score = 5.64, p< .001. Again, no significant effect was observed for the covariates in the model predicting enhancement motives. However, females, as compared with males, were significantly less likely to report drinking for social motives, estimate = −.22, S.E. = .10, z-score = −2.23, p < .05. Symptoms of PTSD were also found to significantly predict social motives for drinking estimate = .01, S.E. = .00, z-score = 4.99, p < .001.
Table 2.
PTSD Symptom Severity as a Predictor of Drinking Motives
Note: All analyses control for age, gender, education, self-reported race, and history of an AIDS-defining illness.
p < .001
OPRM1 and PTSD
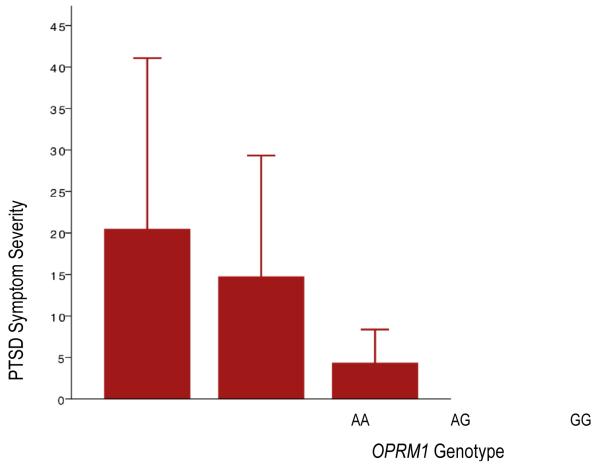
Given the distribution of PTSD symptom severity scores observed across participants (see Table 1 for descriptives), Poisson regression with robust standard error modeling, controlling for age, gender, education, ethnicity, and history of an AIDS-defining illness was conducted to examine whether OPRM1 G alleles were protective against PTSD. Given high comorbidity of PTSD and depression, we also covaried for self-reported lifetime history of depression in models with PTSD as the outcome. As this research question was pertinent in the selected sample of binge drinkers but was not intended to examine alcohol use specifically, we also covaried for hazardous drinking total score. Findings are illustrated in Figure 1. Age, gender, education, race, and history of an AIDS-defining illness were unrelated to PTSD symptom severity. Consistent with known comorbidity, self-reported depression history was highly associated with current PTSD symptom severity in the composite model, estimate = .91, S.E. = .16, z-score = 5.61, p < .001. Similarly consistent with known associations between PTSD and substance use, hazardous drinking was positively associated with PTSD, estimate = .03, S.E. = .01, z-score = 2.91, p < .01. However, even after accounting for the substantial effects of both depression and drinking in the model, consistent with our hypothesis, G alleles were associated with less severe PTSD symptoms, estimate = −.48, S.E. = .18, z-score = −2.66, p < .01.
Table 1.
Participant Descriptives
| M | (SD) | Range | |
|---|---|---|---|
| Posttraumatic Stress Symptoms | 19.39 | (19.78) | 0-87 |
| Drinking Motives: | |||
| Social | 2.17 | (.69) | 1-4 |
| Coping | 2.01 | (.83) | 1-4 |
| Enhancement | 2.11 | (.81) | 1-4 |
Figure 1.
PTSD Symptom Severity Score Means and Standard Deviations across Genotype
OPRM1 and drinking motives
MLR estimation procedures were conducted to examine drinking motives; all models adjusted for effects of age, gender, education, ethnicity, and history of an AIDS-defining illness. A nonsignificant trend toward increased coping motives in G allele carriers was found, estimate = .01, S.E. = .00, z-score = 1.70, p < .10 (Table 3). There were no significant effects of covariates on coping motives. Consistent with the literature supporting the reward-driven effect of OPRM1 on alcohol use, increased endorsement of enhancement motives in G allele carriers was observed, estimate = .01, S.E. = .00, z-score = 2.00, p < .05 (Table 3). No significant effects of covariates on enhancement motives were observed. Shown in Table 3, analyses did not reveal a significant effect of genotype on social drinking motives. However, a trend toward a gender effect was observed such that females were less likely to endorse social motives for drinking than males, estimate = −.20, S.E. = .10, z-score = −1.91, p < .10.
Table 3.
OPRM1 as a Predictor of Drinking Motives
| Estimate | SE | Est/SE | |
|---|---|---|---|
| Coping | .01 | .00 | 1.70 |
| Enhancement | .01 | .00 | 2.00* |
| Social | .00 | .00 | 1.14 |
Note: All analyses involving OPRM1 excluded participants endorsing current opioid use (n = 4). Analyses covary for age, gender, education, self-reported race, and history of an AIDS-defining illness.
p < .05,
DISCUSSION
The present investigation integrates, informs, and extends the substance use, PTSD, HIV, and behavioral genetic literatures in three ways. First, the present investigation sheds light on the relationship between PTSD symptoms and drinking motives in a sample of binge drinkers who are living with HIV. Second, this is the first investigation to examine the relationship between OPRM1 and PTSD symptoms in any sample. Last, this is the first study to explore OPRM1 as related to self-reported drinking motives in a sample of HIV-infected individuals.
Findings from the present investigation confirm the expected association between self-reported PTSD symptoms and self-reported drinking to cope. This is consistent with prior evidence in healthy samples, and is consistent with a self-medication hypothesis (14-16). Our finding that PTSD symptoms also predicted drinking for enhancement motives is also consistent with prior evidence from women in substance use treatment (17). Although we did not explicitly expect increased endorsement of drinking for social reasons among individuals with more severe symptoms of PTSD, this association has been observed in prior studies with samples of adolescents (70). Although it is important to note that respondents’ PTSD symptoms and motives for drinking were measured at the same time, preventing directional conclusions, these findings suggest that symptoms of PTSD may influence drinking motivations through multiple pathways; a clinically-significant implication of this finding is the importance of assessing and treating PTSD symptoms in substance abusing PLH. Prior treatment research in uninfected samples suggests that reduction of PTSD symptoms may precipitate improvements in substance use behaviors (12, 13). Thus, even when the goal is change in substance abuse behavior, interventions targeting PTSD symptoms may provide significant gains across multiple outcomes. Trauma-focused cognitive-behavioral and exposure interventions for PLH have shown promise in reduction of symptoms and promotion of health behaviors. However, evidence for psychosocial treatment is preliminary and not all patients are willing to engage in psychosocial treatment approaches. As such, characterization of neurobiologic pathways influencing both PTSD symptoms and drinking motives may facilitate the development of pharmacologic interventions.
We expected that the G allele would be associated with lower PTSD symptom severity, possibly due to early peritraumatic containment of the stress response. Consistent with our hypothesis, findings from the present investigation suggest that the OPRM1 G allele may be protective in the development of PTSD. In our relatively small sample, this was observed to be a dose-effect such that the highest symptoms were reported by participants with the AA genotype, intermediary levels of PTSD were reported by participants with the AG genotype, and the lowest symptoms were reported by participants with the GG genotype. Although these findings appear to support our expectations, it is also possible that OPRM1 may play a role in maintenance of PTSD symptoms through opioid influence on fear extinction processes (71). As the first investigation of OPRM1 and PTSD, this finding provides intriguing evidence that genetically-influenced endogenous opioids may be relevant to either the development or maintenance of PTSD. However, the clinical implications of this finding (such as use of OPRM1 as a biomarker of risk for PTSD or genetically-informed pharmacologic prevention) are not known and would need to be evaluated in a prospective study..
In contrast to the dearth of research on OPRM1 in PTSD, numerous studies are available for the characterization of OPRM1 in alcohol use, with prior evidence generally supporting a link between the G allele and an approach bias. In the present investigation, we expected that this would translate to increased endorsement of enhancement motives for drinking in G allele carriers. Consistent with our hypotheses, OPRM1 G alleles were associated with enhancement motives in the present sample. As the majority of studies related to drinking and OPRM1 have incorporated biological measures and/or laboratory paradigms, it is remarkable that this effect was observed in the context of a simple self-report measure. Demonstrating the discriminant validity of this effect, no evidence was found for an association of OPRM1 and drinking to cope or for social reasons. This is consistent with prior evidence that the association between cue-induced craving and OPRM1 is found under neutral but not stressful conditions (48). If these findings are replicated in other samples of PLH, it is possible that psychosocial or pharmacological interventions for G carriers who are struggling with substance abuse might benefit from genetically-tailored psychosocial or pharmacological interventions.
The present findings demonstrate the complexity of genetic, and biologic, research as the same polymorphism that was found to be protective for PTSD was also found to increase self-reported use of alcohol for enhancement. Although this may seem counter-intuitive, it is not surprising given that consistently and strongly deleterious variants would not be preserved as common variants; also, there are many such examples in nature whereby the same variation that is protective under certain circumstances can be problematic under others. It is also important to keep in mind that the presence of HIV, the biology of HIV disease progression, and even the pharmacology of antiretroviral treatment may influence the biology of PTSD as well as the biological underpinnings of motivations to drink. Research to date has largely focused on ways that psychiatric factors influence health perception, immune function, and/or disease progression in medical populations but it will be important for future research efforts to also examine how medical illness may influence the biology of development and maintenance of psychiatric concerns.
OPRM1 may be especially relevant to psychological and physical health in PLH. Prior research in medical populations has implicated a role for OPRM1 variation in depression (49) and psychoneuroimmunology research with healthy populations has linked OPRM1 to health quality of life (50) and immune function (50). More specifically, G carriers evidenced significantly lower proinflammatory cytokines in addition to significantly higher self-reported general health (50). As noted in the context of PTSD development, the sensitivity of the opioid system associated with the G allele may suppress HPA axis activation and corresponding peripheral immune activation. In turn, decreases in peripheral immune activation may result in the perception of good health. These potential psychoneuroimmunological relationships are greatly complicated by the presence of HIV. For example, there is evidence that opioids may exert a multitude of effects on HIV and may increase HIV-coreceptor expression and replication, enhance infection of macrophages, promote trafficking of HIV infected cells and enhance disease progression (72-74).
Also of note, the size of the observed effects for OPRM1 on both PTSD and enhancement motives for drinking were relatively small. This is consistent with other psychiatric research studies of single variants as related to complex disorders. However it is also possible that OPRM1 might be associated with either other motives or with unmeasured motives for use of other substances; it is possible that current use of other substances or history of opioid use (which we did not assess in the current investigation) may represent important influences on the role of OPRM1 variation in substance use. Moreover, OPRM1 is only one variant in multiple genes influencing the stress system pathway. Future studies may model combined influences of multiple genes in this pathway. Another reason for the relatively small effect size observed for OPRM1 in this investigation is the fact that symptoms of PTSD are a distal outcome relative to genes; behavioral genetic research paradigms have increasingly endorsed the use of intermediary phenotypes, or phenotypes believed to underlie the relationship between genes and observed outcomes. Accordingly, future research in this area could explore potential mediators of these associations such as cortisol or psychophysiological reactivity. However, if OPRM1’s effects on development of PTSD are indeed strongest in the acute phase of PTSD development, efforts to disentangle the effects of OPRM1 would need to prospectively characterize biological and/or psychological functioning over the course of exposure to a traumatic experience. In prior studies, we have used urine samples collected in hospital for the twelve hours following admission for injury to inform our understanding of peritraumatic hormones (75, 76). More recently, we demonstrated that CRHR1 predicted trajectories PTSD symptoms and symptom course in pediatric injury patients followed longitudinally in-hospital and over the course of a year post-injury (Amstadter et al., in press).
Limitations to the present investigation include the use of a relatively small sample, the use of a self-report measure of PTSD rather than a clinical interview, the use of a single item to assess history of depression, and the absence of a thorough assessment of other types of traumas experienced by participants. Accordingly, although symptoms were anchored to a common event (diagnosis of PTSD), some symptoms such as enhanced startle or emotional numbing are not clearly tied to a specific trauma and thus symptom endorsement may have been influenced by other unrelated traumatic experiences. As such, it possible that observed relationships between PTSD and both drinking motives and OPRM1 are not specific to PTSD secondary to HIV-diagnosis but rather are secondary to any current symptoms of PTSD. It is also possible that OPRM1 may play a role in the general negative affect that serves as a risk factor for PTSD, depression, and a range of other psychiatric concerns. Additionally, although the ethnic diversity of the present sample is a strength in terms of generalizability of findings, OPRM1 is less frequent in certain ethnic groups, introducing concerns about population stratification. [For an account of recently-identified population-specific effects see (77) and for discussion of factors that complicate genetic findings with OPRM1, see (78).] Another limitation of the present investigation is the absence of a comparison of matched participants without HIV. As noted, OPRM1 has been shown to influence immune functioning and it is possible that effects observed in the present investigation may not generalize to healthy samples. Future studies should include a healthy comparison group and may also benefit from a more thorough characterization of the relationship between OPRM1 and immune functioning in healthy and medically-involved participants. Although we argue that binge drinking is common and represents an important personal and public health concern for PLH, selection for binge drinkers further limits generalizability of findings. Finally, clinic-based recruitment may have biased against recruitment of participants with the highest levels of avoidance symptoms, as attendance at clinic inherently serves as a reminder of HIV diagnosis.
Nonetheless, the present investigation is the first to test the association between OPRM1 and PTSD and is the first to examine OPRM1 variation as related to psychological outcomes in a sample of HIV-infected participants. Moreover, the present investigation extends extant literature on the relationship between PTSD and drinking motives. Taken together, these findings provide some evidence for a role of OPRM1 variation in PTSD and in drinking for enhancement motives and provide continued evidence for the importance of PTSD symptoms in drinking motivation. The present findings suggest that trauma-focused psychosocial interventions may be beneficial for substance use outcomes in PLH and future studies may explore how genetic variation may influence response to these psychosocial interventions and/or guide development of novel pharmacotherapies.
Acknowledgments
Author Note: This research was funded by a 2008 developmental grant from the Lifespan/Tufts/Brown Center for AIDS Research. The project described was supported by Grant Number P30AI042853 from the National Institute Of Allergy And Infectious Diseases. Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institute of Health. Dr. Nugent is supported by US-NIMH K01 MH087240. Work was supported by 1S10RR023457 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs, awarded to J. E. McGeary. This material is the result of work supported with resources and the use of facilities at the Providence VA Medical Center.
References
- 1.Kelly B, Raphael B, Judd F, Perdices M, Kernutt G, Burnett P, et al. Posttraumatic stress disorder in response to HIV infection. General Hospital Psychiatry. 1998;20(6):345–52. doi: 10.1016/s0163-8343(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 2.Leserman J, Whetten K, Lowe K, Strangl D, Schwartz M, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the South. Psychosomatic Medicine. 2005;67:500–7. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 3.Safren SA, Gershuny BS, Hendriksen E. Symptoms of Posttraumatic Stress and Death Anxiety in Persons with HIV and Medication Adherence Difficulties. AIDS Patient Care and STDs. 2003;17(12):657–64. doi: 10.1089/108729103771928717. [DOI] [PubMed] [Google Scholar]
- 4.Katz S, Nevid JS. Risk Factors Associated with Posttraumatic Stress Disorder Symptomatology in HIV-Infected Women. AIDS Patient Care and STDs. 2005;19(2):110–20. doi: 10.1089/apc.2005.19.110. [DOI] [PubMed] [Google Scholar]
- 5.Vranceanu AM, Safren SA, Lu M, Coady WM, Skolnik PR, Rogers WH, et al. The Relationship of Post-traumatic Stress Disorder and Depression to Antiretroviral Medication Adherence in Persons with HIV. AIDS Patient Care and STDs. 2008;22(4):313–21. doi: 10.1089/apc.2007.0069. [DOI] [PubMed] [Google Scholar]
- 6.Martin L, Kagee A. Lifetime and HIV-Related PTSD Among Persons Recently Diagnosed with HIV. AIDS and Behavior. 2011;15(1):125–31. doi: 10.1007/s10461-008-9498-6. [DOI] [PubMed] [Google Scholar]
- 7.Martinez A, Israelski D, Walker C, Koopman C. Posttraumatic Stress Disorder in Women Attending Human Immunodeficiency Virus Outpatient Clinics. AIDS Patient Care and STDs. 2002;16(6):283–91. doi: 10.1089/10872910260066714. [DOI] [PubMed] [Google Scholar]
- 8.Boarts J, Sledjeski E, Bogart L, Delahanty D. The Differential Impact of PTSD and Depression on HIV Disease Markers and Adherence to HAART in People Living with HIV. AIDS and Behavior. 2006;10(3):253–61. doi: 10.1007/s10461-006-9069-7. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MA, Alfonso CA, Hoffman RG, Milau V, Carrera G. The impact of PTSD on treatment adherence in persons with HIV infection+ General Hospital Psychiatry. 2001;23(5):294–6. doi: 10.1016/s0163-8343(01)00152-9. 2001/10// [DOI] [PubMed] [Google Scholar]
- 10.Delahanty DL, Bogart LM, Figler JL. Posttraumatic stress disorder symptoms, salivary cortisol, medication adherence, and CD4 levels in HIV-positive individuals. AIDS Care. 2004;16:247–60. doi: 10.1080/09540120410001641084. [DOI] [PubMed] [Google Scholar]
- 11.Olley BO, Bolajoko AJ. Psychosocial determinants of HIV-related quality of life among HIV-positive military in Nigeria. Int J STD AIDS. 2008 Feb 1;19(2):94–8. doi: 10.1258/ijsa.2007.007134. 2008. [DOI] [PubMed] [Google Scholar]
- 12.Back SE, Brady KT, Sonne SC, Verduin ML. Symptom Improvement in Co-Occurring PTSD and Alcohol Dependence. The Journal of Nervous and Mental Disease. 2006;194(9):690–6. doi: 10.1097/01.nmd.0000235794.12794.8a. 10.1097/01.nmd.0000235794.12794.8a. [DOI] [PubMed] [Google Scholar]
- 13.Hien DA, Jiang H, Campbell ANC, Hu M-C, Miele GM, Cohen LR, et al. Do Treatment Improvements in PTSD Severity Affect Substance Use Outcomes? A Secondary Analysis From a Randomized Clinical Trial in NIDA’s Clinical Trials Network. Am J Psychiatry. 2010 Jan 1;167(1):95–101. doi: 10.1176/appi.ajp.2009.09091261. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grayson CE, Nolen-Hoeksema S. Motives to drink as mediators between childhood sexual assault and alcohol problems in adult women. Journal of Traumatic Stress. 2005;18(2):137–45. doi: 10.1002/jts.20021. [DOI] [PubMed] [Google Scholar]
- 15.Ullman SE, Filipas HH, Townsend SM, Starzynski LL. Trauma exposure, posttraumatic stress disorder and problem drinking in sexual assault survivors. Journal of Studies on Alcohol. 2005;66:610–9. doi: 10.15288/jsa.2005.66.610. [DOI] [PubMed] [Google Scholar]
- 16.Miranda R, Meyerson LA, Long PJ, Marx BP, Simpson SM. Sexual Assault and Alcohol Use: Exploring the Self-Medication Hypothesis. Violence and Victims. 2002;17:205–17. doi: 10.1891/vivi.17.2.205.33650. [DOI] [PubMed] [Google Scholar]
- 17.Simpson TL. Childhood sexual abuse, PTS, and the functional roles of alcohol use among women drinkers. Substance Use & Misuse. 2003;38(2):249–70. doi: 10.1081/ja-120017248. [DOI] [PubMed] [Google Scholar]
- 18.Chander G, Himelhoch S, Moore R. Substance Abuse and Psychiatric Disorders in HIV-Positive Patients: Epidemiology and Impact on Antiretroviral Therapy. Drugs. 2006;66:769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 19.Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-Defined Mood, Anxiety, and Substance Use Disorders in an HIV Clinic in the Southeastern United States. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;42(3):298–306. doi: 10.1097/01.qai.0000219773.82055.aa. 10.1097/01.qai.0000219773.82055.aa. [DOI] [PubMed] [Google Scholar]
- 20.Haller DL, Miles DR. Suicidal Ideation Among Psychiatric Patients with HIV: Psychiatric Morbidity and Quality of Life. AIDS and Behavior. 2003;7(2):101–8. doi: 10.1023/a:1023985906166. [DOI] [PubMed] [Google Scholar]
- 21.Merenlender-Wagner A, Dikshtein Y, Yadid G. The -Endorphin Role in Stress-Related Psychiatric Disorders. Current Drug Targets. 2009;10:1096–108. doi: 10.2174/138945009789735147. [DOI] [PubMed] [Google Scholar]
- 22.Saxe G, Geary M, Bedard K, Bosquet M, Miller A, Koenen K, et al. Separation anxiety as a mediator between acute morphine administration and PTSD symptoms in injured children. Ann N Y Acad Sci. 2006 Jul;1071:41–5. doi: 10.1196/annals.1364.004. [DOI] [PubMed] [Google Scholar]
- 23.Saxe G, Stoddard F, Courtney D, Cunningham K, Chawla N, Sheridan R, et al. Relationship between acute morphine and the course of PTSD in children with burns. J Am Acad Child Adolesc Psychiatry. 2001 Aug;40(8):915–21. doi: 10.1097/00004583-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Holbrook TL, Galarneau MR, Dye JL, Quinn K, Dougherty AL. Morphine Use after Combat Injury in Iraq and Post-Traumatic Stress Disorder. N Engl J Med. 2010;362(2):110–7. doi: 10.1056/NEJMoa0903326. [DOI] [PubMed] [Google Scholar]
- 25.Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A Study of the Protective Function of Acute Morphine Administration on Subsequent Posttraumatic Stress Disorder. Biological Psychiatry. 2009;65(5):438–40. doi: 10.1016/j.biopsych.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Morgan CA, Krystal JH, Southwick SM. Toward early pharmacological posttraumatic stress intervention. Biological Psychiatry. 2003;53(9):834–43. doi: 10.1016/s0006-3223(03)00116-1. [DOI] [PubMed] [Google Scholar]
- 27.McGaugh JL, Introini-Collison IB, Nagahara AH. Memory-enhancing effects of posttraining naloxone: involvement of fl-noradrenergic influences in the amygdaloid complex. Brain Research. 1988;446(1):37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- 28.Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, et al. Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology. 2009;56:32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-Nucleotide Polymorphism in the Human Mu opioid Receptor Gene Alters Î2 -Endorphin Binding and Activity: Possible Implications for Opiate Addiction. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wand G, McCaul M, Gotjen D, Reynolds J, Lee S. Confirmation That Offspring From Families With Alcohol-Dependent Individuals Have Greater Hypothalamic-Pituitary-Adrenal Axis Activation Induced by Naloxone Compared With Offspring Without a Family History of Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2001;25(8):1134–9. [PubMed] [Google Scholar]
- 31.Hernandez-Avila C, Wand G, Luo X, Gelernter J, Kranzler H. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2003;118B(1):60–5. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R, Golier J. Is there a rationale for cortisol-based treatments for PTSD? Expert Review of Neurotherapeutics. 2009;9(8):1113–5. doi: 10.1586/ern.09.79. [DOI] [PubMed] [Google Scholar]
- 33.Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectrums. 2005 Feb;10(2):99–106. doi: 10.1017/s109285290001943x. [DOI] [PubMed] [Google Scholar]
- 34.Delahanty DL, Nugent NR. Predicting PTSD prospectively based on prior trauma history, trauma severity, and immediate biologic responses. Annals of the New York Academy of Sciences. 2006;1071:27–40. doi: 10.1196/annals.1364.003. [DOI] [PubMed] [Google Scholar]
- 35.Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals. 2009;39(6):358–67. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitman RK, van der Kolk BA, Orr SP, Greenberg MS. Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Arch Gen Psychiatry. 1990 Jun;47(6):541–4. doi: 10.1001/archpsyc.1990.01810180041007. [DOI] [PubMed] [Google Scholar]
- 37.van der Kolk BA, Greenberg MS, Orr SP, Pitman RK. Endogenous opioids, stress induced analgesia, and posttraumatic stress disorder. Psychopharmacol Bull. 1989;25(3):417–21. [PubMed] [Google Scholar]
- 38.Glover H. A preliminary trial of nalmefene for the treatment of emotional numbing in combat veterans with post-traumatic stress disorder. Isr J Psychiatry Relat Sci. 1993;30(4):255–63. [PubMed] [Google Scholar]
- 39.Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, et al. Altered Central mu-Opioid Receptor Binding After Psychological Trauma. Biol Psychiatry. 2006 Aug 28; doi: 10.1016/j.biopsych.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 40.van den Wildenberg E, Wiers RW, Dessers J, Janssen RGJH, Lambrichs EH, Smeets HJM, et al. A Functional Polymorphism of the mu-Opioid Receptor Gene (OPRM1) Influences Cue-Induced Craving for Alcohol in Male Heavy Drinkers. Alcoholism: Clinical and Experimental Research. 2007;31(1):1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 41.McGeary J, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R. Genetic Moderators of Naltrexone’s Effects on Alcohol Cue Reactivity. Alcoholism: Clinical and Experimental Research. 2006;30(8):1288–96. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 42.Ray LA, Hutchison KE. A Polymorphism of the Mu-Opioid Receptor Gene (OPRM1) and Sensitivity to the Effects of Alcohol in Humans. Alcoholism: Clinical and Experimental Research. 2004;28(12):1789–95. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- 43.Ray LA, Hutchison KE. Effects of Naltrexone on Alcohol Sensitivity and Genetic Moderators of Medication Response: A Double-blind Placebo-Controlled Study. Arch Gen Psychiatry. 2007 Sep 1;64(9):1069–77. doi: 10.1001/archpsyc.64.9.1069. 2007. [DOI] [PubMed] [Google Scholar]
- 44.Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, et al. An Evaluation of {micro}-Opioid Receptor (OPRM1) as a Predictor of Naltrexone Response in the Treatment of Alcohol Dependence: Results From the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. Arch Gen Psychiatry. 2008 Feb 1;65(2):135–44. doi: 10.1001/archpsyc.65.2.135. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filbey F, Ray L, Smolen A, Claus E, Audette A, Hutchinson KE. Differential neural response to alcohol proming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcohol Clin Exp Res. 2008;32(7):1113–23. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiers R, Rinck M. Relatively Strong Automatic Appetitive Action-Tendencies in Male Carriers of the OPRM1 G-Allele. Genes, Brain and Behavior. doi: 10.1111/j.1601-183X.2008.00454.x. In Press. [DOI] [PubMed] [Google Scholar]
- 48.Ray LA. Stress-Induced and Cue-Induced Craving for Alcohol in Heavy Drinkers: Preliminary Evidence of Genetic Moderation by the OPRM1 and CRH-BP Genes. Alcoholism: Clinical and Experimental Research. 2010;35(1):166–74. doi: 10.1111/j.1530-0277.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 49.Max M, Wu T, Atlas S, Edwards R, Haythornthwaite J, Bollettino A, et al. A clinical genetic method to identify mechanisms by which pain causes depression and anxiety. Molecular Pain. 2006;2(1):14. doi: 10.1186/1744-8069-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsunaga M, Isowa T, Murakami H, Kasugai K, Yoneda M, Kaneko H, et al. Association of polymorphism in the human [mu]-opioid receptor OPRM1 gene with proinflammatory cytokine levels and health perception. Brain, Behavior, and Immunity. 2009;23(7):931–5. doi: 10.1016/j.bbi.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Norman SB, Stein MB, Davidson JRT. Profiling Posttraumatic Functional Impairment. The Journal of Nervous and Mental Disease. 2007;195(1):48–53. doi: 10.1097/01.nmd.0000252135.25114.02. 10.1097/01.nmd.0000252135.25114.02. [DOI] [PubMed] [Google Scholar]
- 52.Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL. Comorbidity, impairment, and suicidality in subthreshold PTSD. American Journal of Psychiatry. 2001;158:1467–73. doi: 10.1176/appi.ajp.158.9.1467. [DOI] [PubMed] [Google Scholar]
- 53.Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in Primary Care: Prevalence, Psychiatric Disorders, Healthcare Use, and Functional Status. The Journal of Nervous and Mental Disease. 2005;193(10):658–64. doi: 10.1097/01.nmd.0000180740.02644.ab. [DOI] [PubMed] [Google Scholar]
- 54.Breslau N, Lucia VC, Davis GC. Partial PTSD versus full PTSD: an empirical examination of associated impairment. Psychological Medicine. 2004;34(07):1205–14. doi: 10.1017/s0033291704002594. [DOI] [PubMed] [Google Scholar]
- 55.Ccedil, orap, lolu A, uuml, Tural, mit, et al. Subthreshold post traumatic stress disorder in the survivors of Marmara earthquake. Primary Care Psychiatry. 2004;9(4):137–44. [Google Scholar]
- 56.Cukor J, Wyka K, Jayasinghe N, Difede J. The nature and course of subthreshold PTSD. Journal of Anxiety Disorders. 2010;24(8):918–23. doi: 10.1016/j.janxdis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 57.Galai N, Strathdee SA, Vlahov D, Bareta J, Macalino G, D CD. ACASI versus interview administered questionnaires for sensitive risk behaviors: Results of a cross-over randomized trial among injection drug users. International Conference on AIDS. 2004;15:11–6. [Google Scholar]
- 58.Waruru AK, Nduati R, Tylleskar T. Audio computer-assisted self-interviewing (ACASI) may avert socially desirable responses about infant feeding in the context of HIV. BMC Biomedical Informatics and Decision Making. 2005;5(24):1–7. doi: 10.1186/1472-6947-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson CM, Houser J, Partlow C, Rudy BJ, Futterman DC, Friedman LB. The REACH (Reaching for Excellence in Adolescent Care and Health) project: study design, methods, and population profile. Journal of Adolescent Health. 2001;29(3, Supplement 1):8–18. doi: 10.1016/s1054-139x(01)00291-9. [DOI] [PubMed] [Google Scholar]
- 60.Saunders JB, Aasland O, Babor T, dlF JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 61.Babor TF, de la Fuente JR, Saunders J, Grant M, editors. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. World Health Organization; Geneva, Switzerland: 1992. Revision, WHO Document No. WHO/PSA/92.4. [Google Scholar]
- 62.Weiss D, Marmar CR, editors. The Impact of Event Scale—Revised. Guilford; New York: 1997. [Google Scholar]
- 63.Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychological Assessment. 1992;4(123-132) [Google Scholar]
- 64.Muthen LK, Muthen BO. Mplus. Version 3.11 Muthen & Muthen; Los Angeles, CA: 2004. [Google Scholar]
- 65.O’Cleirigh C, Ironson G, Smits JAJ. Does Distress Tolerance Moderate the Impact of Major Life Events on Psychosocial Variables and Behaviors Important in the Management of HIV? Behavior Therapy. 2007;38(3):314–23. doi: 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ironson G, O’Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, et al. Psychosocial factors predict CD4 and viral load change in men and women with HIV in the era of HAART. Psychosomatic Medicine. 2005;67(1013-1021) doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Creamer M, Bell R, Failla S. Psychometric properties of the impact of event scale-revised. Behaviour Research and Therapy. 2003;41:1489–96. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Asukai N, Kato H, Kawamura N, Kim YH, Yamamoto K, Kishimoto J, et al. Reliabiligy and Validity of the Japanese-Language Version of the Impact of Event Scale-Revised (Ies-R-J): Four Studies of Different Traumatic Events. The Journal of Nervous and Mental Disease. 2002;190(3):175–82. doi: 10.1097/00005053-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 69.Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the [mu]-opioid receptor gene with substance dependence: A meta-analysis. Drug and Alcohol Dependence. 2006;83(3):262–8. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 70.Dixon LJ, Leen-Feldner EW, Ham LS, Feldner MT, Lewis SF. Alcohol use motives among traumatic event-exposed, treatment-seeking adolescents: Associations with posttraumatic stress. Addictive Behaviors. 2009;34(12):1065–8. doi: 10.1016/j.addbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu C, Li P, Hu B, Ouyang X, Fu J, Gao J, et al. Chronic Morphine Selectively Impairs Cued Fear Extinction in Rats: Implications for Anxiety Disorders Associated with Opiate Use. Neuropsychopharmacology. 2007;33(3):666–73. doi: 10.1038/sj.npp.1301441. [DOI] [PubMed] [Google Scholar]
- 72.Ho W-Z, Guo C-J, Yuan C-S, Douglas SD, Moss J. Methylnaltrexone Antagonizes Opioid-Mediated Enhancement of HIV Infection of Human Blood Mononuclear Phagocytes. Journal of Pharmacology and Experimental Therapeutics. 2003 Dec 1;307(3):1158–62. doi: 10.1124/jpet.103.056697. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309(1):99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 74.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid Therapy and Immunosuppression: A Review. American Journal of Therapeutics. 2004;11(5):354–65. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 75.Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Journal of Psychoneuroendocrinology. 2005;30(2):121–8. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accidents. Biological Psychiatry. 2000;48(9):940–7. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- 77.Hernandez-Avila C, Covault J, Wand G, Zhang H, Gelernter J, Kranzler H. Population-specific effects of the Asn40Asp polymorphism at the mu-opioid gene (OPRM1) on HPA-axis activation. Pharmacogenetics and Genomics. 2007;17(12):1031–8. doi: 10.1097/FPC.0b013e3282f0b99c. [DOI] [PubMed] [Google Scholar]
- 78.van der Zwaluw CS, van den Wildenberg E, Wiers RW, Franke B, Buitelaar J, Scholte RHJ, et al. Polymorphisms in the mu-opioid receptor gene (OPRM1) and the implications for alcohol dependence in humans. Pharmacogenomics. 2007;8(10):1427–36. doi: 10.2217/14622416.8.10.1427. [DOI] [PubMed] [Google Scholar]