Abstract
BACKGROUND
The mechanisms that underlie allergic transfusion reactions (ATRs) are not well characterized, but likely involve recipient, donor, and product factors. To assess product factors associated with ATRs, we investigated candidate mediators in apheresis platelet products associated with ATRs and controls.
STUDY DESIGN AND METHODS
Using bead-based and standard ELISA immunoassays, we tested supernatants from 20 consecutive apheresis platelet transfusions associated with ATRs and 30 control products for concentrations of mediators in 3 categories: acute inflammatory mediators, direct agonists of basophils and mast cells, and growth/priming factors of basophils and mast cells.
RESULTS
Median concentrations of the direct allergic agonists C5a, brain derived neurotrophic factor (BDNF), and CCL5 (RANTES) were 16.6%, 41.8%, and 13.9% higher, respectively, in the supernatant of apheresis platelet products that were most strongly associated with ATRs (P < 0.05 for each mediator). Other direct agonists (MIP-1α, MCP-1, eotaxin-1, IL-8) were similar between groups. Concentrations of acute inflammatory mediators and basophil growth/priming factors were also similar between groups (P > 0.2 for all associations).
CONCLUSION
The allergic agonists C5a, BDNF, and CCL5 may be mediators of ATRs in apheresis platelet products. Acute inflammatory proteins and basophil/mast cell growth and priming factors do not appear to be associated with apheresis platelet products that cause ATRs.
Keywords: allergy, transfusion reaction, IgE, platelet
INTRODUCTION
Allergic transfusion reactions (ATRs) are the most common adverse reaction to transfused platelets and plasma and have an incidence of approximately 1–3%1–3. Although they are often self-limited reactions that involve erythematous rash, urticaria, and/or pruritus, more severe reactions with angioedema, bronchospasm, and anaphylaxis can occur. Furthermore, ATRs are problematic because prevention methods are limited4,5. The mechanisms that underlie ATRs are not well characterized, with the exception of selective protein deficiencies and rare cases of passive transfer of IgE or allergen6–10. These mechanisms have been associated with severe ATRs and likely do not account for the high frequency of common, mild ATRs.
Recipient, donor, and product characteristics are associated with ATRs. Previous studies have shown that recipient atopic predisposition is an important factor associated with ATRs2,11,12. At the same time, a plasma component is necessary to cause an ATR5,13. Thus, ATRs are likely the result of a necessary plasma mediator that is introduced into a susceptible recipient, but the plasma factors are unclear. Specific agonists of basophils and mast cells, e.g. CCL5 (Regulated upon Activation, Normal T-cell Expressed, and Secreted; RANTES) have been evaluated with inconclusive results14,15.
IgE and complement have classically been associated with allergic mediator release from basophils and mast cells16, but other chemokines and cytokines have known effects on these cells. These factors can generally be categorized as growth/priming factors or agonists that can directly induce release of mediators from basophils and mast cells, e.g. histamine and leukotrienes. Factors that promote effector cell growth and survival include IL-4, IL-5, IL-13, and GM-CSF16,17. Factors that have been reported to directly induce effector cell mediator release include C5a, CCL2 (monocyte chemotactic protein-1; MCP-1), CCL3 (macrophage inflammatory protein-1α; MIP-1α), CCL5 (RANTES), CCL11 (eotaxin-1), and IL-818,19. Brain derived neurotrophic factor (BDNF) is not yet known to be a direct agonist for basophils or mast cell histamine release, but it is thought to stimulate production of neuropeptides that cause pruritus, and is therefore categorized as an agonist20,21.
The goal of this study is to evaluate the association of ATRs with concentrations of three classes of plasma proteins in apheresis platelet products. Specifically, we investigated proteins that mediate acute inflammation, proteins that are agonists for acute mediator release from basophils and mast cells, and proteins that are primarily priming and growth factors for basophils and mast cells. Many of these factors are stored and released by platelets, making them plausible candidates for ATR mediators22.
METHODS
Study Samples
We evaluated products from 20 platelet transfusions that were associated with ATRs and returned to the blood bank between February 2010 and April 2010. In seven cases during the study period, the reporting of ATRs was not accompanied by a product returned to the blood bank; therefore the ATRs studied were not sequential. According to hospital policy, ATRs were identified by reports of ATRs to the Division of Transfusion Medicine at Johns Hopkins Hospital by the primary clinical team and/or a team of oncology platelet coordinators who provide active surveillance of platelet transfusions. Transfusion reactions were adjudicated as allergic by the attending physician, according to CDC Hemovigilance criteria (http://www.cdc.gov/nhsn/wc_bio_hemo_overview.html)23, which include at least two signs and/or symptoms of urticaria, pruritus, morbilliform rash, angioedema, flushing, bronchospasm, chemosis, and hypotension. All allergic reactions were included, regardless of severity. All platelet products at this institution are leukoreduced, apheresis platelets. The study was approved by the Johns Hopkins University School of Medicine IRB.
Control platelet products were obtained from sequential apheresis products issued from the blood bank that were confirmed transfused and did not cause any transfusion reaction. To improve the specificity of our analysis, we matched controls to cases at a 1.5:1 ratio. Samples were centrifuged twice for 5 minutes @ 15,000g at 4°C and supernatant was stored at −80°C until assayed. All laboratory testing was performed at the same time to reduce technical variation.
Quantitative immunoassays
Chemokines and cytokines were measured using the Bioplex 200 platform (Bio-Rad, Hercules, CA). This multiplexed bead-based immunoassay utilized a 25-plex human cytokine kit (Invitrogen, Carlsbad, CA) and brain derived neurotrophic factor (BDNF) kit (Millipore, Billerica, MA), which were performed according to the manufacturer’s protocols and supplied standards. The concentration was determined using a 5 parameter log curve fit using the supplied software.
C5a concentrations were determined by measurement of C5a-desArg using an OptEIA immunoassay according to the manufacturer’s instructions (BD Biosciences, Franklin Lakes, NJ). C5a-desArg is a stable degradation product of C5a, which is rapidly cleaved by carboxypeptidases in plasma24. C5a-desArg testing was performed on a subset of 13 control and 18 ATR samples that had sufficient volume for this assay.
Laboratory testing for total and allergen-specific IgE in pre-transfusion patient samples and supernatants from apheresis platelet products was performed using the ImmunoCAP system (Phadia, Kalamazoo, MI, USA), as described previously11.
Statistics
Differences in concentrations between groups were compared using two-tailed Mann-Whitney rank sum tests. Correlations were assessed using Spearman rho coefficients unless otherwise indicated. Area under receiver-operator characteristic curve (AUC) was calculated to summarize the discriminatory ability of candidate mediators to classify apheresis products as ATR or control in exploratory analyses. For analyses investigating allergen-specific IgE, a sum of quantitative results of food and aeroallergen specific-IgE screens (reported previously11) was used. In order to assess the combined associations of C5a, BDNF, and CCL5 with ATRs, concentrations of these mediators were converted to an ordinal scale according to order of increasing concentrations. The sum of the three ranked concentrations represents the combined levels of these mediators compared to the other products in the study for the subset of products that had C5a concentrations measured. P <0.05 was considered statistically significant, and no adjustments were made for multiple comparisons. For the five instances in which two apheresis products were associated with an ATR and returned to the blood bank, values for candidate mediators were averaged between the two products. Statistics were calculated using Stata v11.1 (StataCorp, College Station, TX).
RESULTS
Allergic reactions to apheresis platelet products
Twenty apheresis platelet transfusions associated with ATRs (n=25 apheresis platelet products) and 30 control apheresis products were evaluated. Five transfusions consisted of two apheresis units transfused consecutively before the ATR: three transfusions consisted of two apheresis units that were split products from the same apheresis collection, and two transfusions consisted of two apheresis units that were from different donors. None of the apheresis products were plasma concentrated or washed. Urticaria or pruritus was reported in all ATRs. Wheezing (1/20, 5%) and angioedema (1/20, 5%) were reported in addition to pruritus in two ATRs. No patient had anaphylaxis.
Relationship of candidate mediators to ATRs
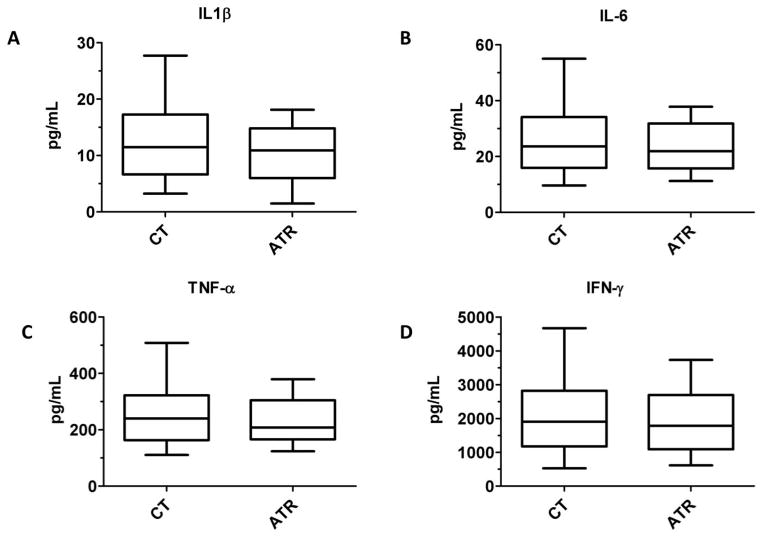
Figure 1 shows the concentrations of the acute inflammatory proteins IL-1β, IL-6, TNF-α, and IFN-γ in control apheresis products and apheresis products that caused ATRs. Levels of these proteins that induce acute inflammation were not statistically significantly different between groups (P > 0.4 for all observations).
Figure 1. Acute inflammatory mediators in apheresis platelet supernatants are not associated with ATRs.
Concentrations of (A) IL-1β, (B) IL-6, (C) TNF-α, and (D) IFN-γ are compared between control apheresis platelet products and products that were associated with ATRs. P > 0.4 for all comparisons.
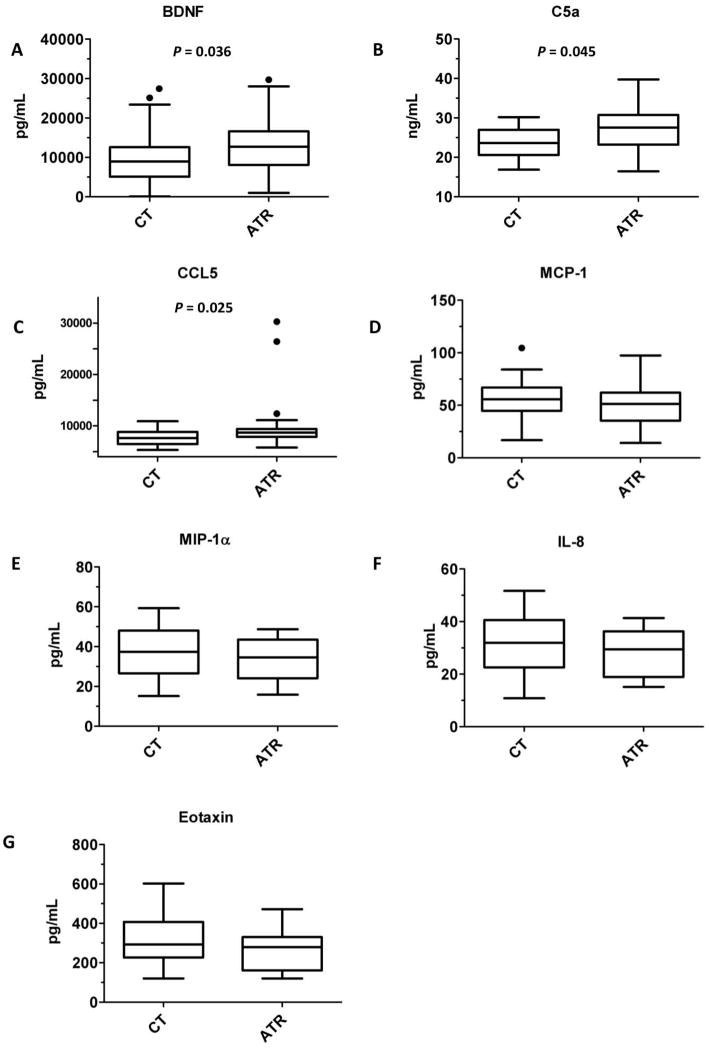
Figure 2 shows concentrations of the effector cell agonists brain-derived neurotrophic factor (BDNF), C5a, CCL5 (RANTES), MCP-1, MIP-1α, IL-8, and CCL11 (eotaxin-1) in control and ATR-associated platelet product supernatants. Figure 3 shows concentrations of the priming/growth factors IL-4, IL-5, IL-13, and GM-CSF. The statistically significant differences between control and ATR groups were increases in BDNF, CCL5, and C5a among apheresis products that caused ATRs. As BDNF is a candidate mediator of pruritus, the association of BDNF was stronger when an ATR is defined as the presence of pruritus (P = 0.01), as compared to the presence of either urticaria or pruritus (P = 0.04).
Figure 2. Three agonists of allergic effector cells are associated with ATRs in apheresis platelet supernatants.
Concentrations of (A) brain-derived neurotrophic factor (BDNF), (B) C5a, (C) and CCL5 (RANTES) were elevated in ATR products as compared to controls. Concentrations of other agonists were not associated with ATRs (P >0.2 for all comparisons): (D) macrophage chemoattractant protein-1 (MCP-1, CCL2), (E) macrophage inflammatory protein 1α (MIP-1α, CCL3), (F) IL-8 (CXCL8), and (G) eotaxin-1 (CCL11).
Figure 3. Mast cell and basophil growth and priming factors are not associated with ATRs.
Concentrations of (A) IL-4, (B) IL-5, (C) IL-13, and (D) GM-CSF are compared between control apheresis platelet products and products that were associated with ATRs. P > 0.2 for all comparisons.
Previous studies have shown that BDNF25 and CCL525–27 concentrations increase during platelet storage. Increasing concentrations of these factors during storage were one reason to select them as candidate mediators. On the other hand, varying storage times could confound the relationship between ATRs and concentrations of BDNF and CCL5. To address this concern, we correlated BDNF and CCL5 concentrations with time to supernatant freezing among ATR products and found no statistically significant correlations by either Pearson or Spearman methods.
Although exploratory, candidate ATR mediators CCL5, BDNF, and C5a were modestly able to discriminate ATR versus control products, with C5a having a slightly higher discriminatory ability (AUC 0.71, 95% CI 0.53–0.90) than CCL5 (AUC 0.69, 95% CI 0.53–0.84) and BDNF (AUC 0.68, 95% CI 0.52–0.84).
Relationships among mediators
To evaluate the possibility of synergistic effects among allergic agonists in apheresis platelet products that cause ATRs, we analyzed correlations among the candidate mediators BDNF, C5a, and CCL5. Levels among these mediators were not statistically significantly associated with levels of the other mediators (rho −0.04 to 0.22; P > 0.3 for all comparisons). However, when concentrations of these three mediators were combined into a single measurement, the sum of these mediators differentiated controls from ATR platelet products, which had higher total levels of these factors (P = 0.003, Figure 4).
Figure 4. The sum of C5a, BDNF, and CCL5 concentrations more strongly distinguish ATR and control platelet products than concentrations of individual mediators.

In order to combine concentrations of C5a, BDNF, and CCL5 into a single metric, concentrations were converted to an ordinal scale according order of increasing concentrations. The sum of the three ranked concentrations is presented on the y-axis and compared between ATR and control platelet products. P = 0.003.
To investigate whether concentrations of these mediators were associated with allergic sensitization in donors, we further correlated BDNF, C5a, and CCL5 with total and allergen-specific IgE concentrations in the plasma of apheresis products. No statistically significant correlation was found (rho −0.21 to 0.19; P > 0.1 for all comparisons).
In five ATR events, two apheresis products were transfused sequentially prior to an ATR. Our primary analysis used an average of the mediators in the two products, but it remained a possibility that an ATR could be due to a high mediator level in only one of two products transfused. Among the two product transfusions, concentrations of all mediators in each unit were in the ranges of concentrations presented in Figures 1–3. To summarize the variability of mediator concentrations in two apheresis unit transfusions, we calculated the coefficient of variations (CVs) between the two apheresis products for each of the 15 mediators. For transfusions of split products derived from the same apheresis collection, the average of the 15 CVs for all mediators was 18.0%. For transfusions of two products collected from two donors, the average of the 15 CVs was 45.6%.
DISCUSSION
We evaluated the associations of acute inflammatory proteins, allergic effector cell agonists, and allergic cell priming/growth factors in the supernatants of apheresis platelet products that did or did not cause an ATR. Increased concentrations of C5a, BDNF, and CCL5 in apheresis platelet products are associated with ATRs. There appears to be a stronger association of these mediators with ATRs when the sum of their concentrations is analyzed in aggregate.
If multiple plasma factors can contribute to ATRs to varying degrees, then the inconsistent association of CCL5 with ATRs 14,15 may be explained by varying contributions of CCL5 to the ATRs studied. It will be helpful in future studies of ATRs to measure multiple mediators simultaneously so that the relative contribution of each can be determined.
Given that ATRs occur during or within two hours of transfusion, we hypothesized that allergic effector cell agonists would be lead candidates for mediators of ATRs. In contrast, we demonstrate that acute inflammatory proteins and effector cell priming and growth factors that do not typically mediate clinical signs and symptoms within minutes would not be elevated in apheresis platelet products associated with ATRs. Nevertheless, cumulative exposure to acute inflammatory and growth/priming factors from prior transfusions may have a role in increasing recipient susceptibility to ATRs on subsequent transfusions. Platelet recipients had a median of 22 apheresis platelet exposures prior to the ATR captured in this study, and studies that evaluate cumulative exposure of allergic mediators are needed to address the role of allergic effector modulation in transfusion recipients.
Acute inflammatory mediators have previously been reported to be associated with febrile non-hemolytic transfusion reactions (FNHTRs), as leukoreduction decreases the concentrations of these mediators and the incidence of FNHTRs28. The increasing prevalence of leukoreduction has led to a decrease in FNHTR, but not ATRs29,30. Thus, we hypothesized that these cytokines/chemokines would not be associated with ATRs, and our data confirm prior observations.
The observation that BDNF, CCL5, and C5a are associated with ATRs does not necessarily indicate that they are part of the causal mechanism for ATRs. First, there is large overlap in concentrations of these mediators between ATR and control products. Second, an alternative explanation could be that these markers are surrogates for allergic status of the donor and that other, unidentified factors are causing ATRs. However, previous studies11,12,31 did not find an association of donor food or environmental sensitization with ATRs, and we did not find an association of donor IgE sensitization and BDNF, CCL5, or C5a concentrations in this study.
It is still possible that qualitative differences among donors or a storage effect may contribute to the plasma mediator milieu that causes ATRs. For example, platelet-derived mediators (e.g. CCL5) may be released during storage or in vivo to varying degrees because of qualitative platelet differences32 and not storage time33,34. Correlating the allergic histories of donors with mediator levels in future studies may help more precisely define any qualitative associations. In vitro generation of pro-allergic mediators may depend on qualitative differences in how platelets and plasma proteins react to the forces, temperatures, and material exposures in apheresis platelet processing.
In summary, apheresis platelet levels of acute inflammatory mediators and growth/chemotactic factors of basophils and mast cells do not appear to be associated with ATRs. BDNF, C5a, and CCL5 are candidate mediators of ATRs that may serve as necessary plasma factors in a two-prong model of ATRs that requires both recipient susceptibility and plasma factors in the donor product.
Acknowledgments
Sources of support: This study was supported in part from internal funds provided by the Johns Hopkins DACI Reference Laboratory. WS acknowledges funding support from 5K12HL087169. JHS acknowledges support from T32AI007056-31.
Footnotes
Disclaimers: none
Conflicts of interest: none
References
- 1.Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2004;31:111–22. doi: 10.1016/j.transci.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic transfusion reactions to platelets are associated more with recipient and donor factors than with product attributes. Transfusion. 2011 doi: 10.1111/j.1537-2995.2010.03009.x. Epub Jan 7, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roback J. Technical Manual. 16. AABB Press; 2008. [Google Scholar]
- 4.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–96. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 5.Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011 doi: 10.1111/j.1537-2995.2010.03008.x. Epub Jan 7, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Wibaut B, Mannessier L, Horbez C, Coupez B, Courbon B, Mizon P, Goudemand J. Anaphylactic reactions associated with anti-Chido Antibody following platelet transfusions. Vox Sang. 1995;69:150–1. doi: 10.1111/j.1423-0410.1995.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimada E, Tadokoro K, Watanabe Y, Ikeda K, Niihara H, Maeda I, Isa K, Moriya S, Ashida T, Mitsunaga S, Nakajima K, Juji T. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion. 2002;42:766–73. doi: 10.1046/j.1537-2995.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 8.Vassallo RR. Review: IgA anaphylactic transfusion reactions. Part I Laboratory diagnosis, incidence, and supply of IgA-deficient products. Immunohematology. 2004;20:226–33. [PubMed] [Google Scholar]
- 9.Arnold DM, Blajchman MA, Ditomasso J, Kulczycki M, Keith PK. Passive transfer of peanut hypersensitivity by fresh frozen plasma. Arch Intern Med. 2007;167:853–4. doi: 10.1001/archinte.167.8.853. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JF, Baumert JL, Brons PP, Joosten I, Koppelman SJ, van Pampus EC. Anaphylaxis from passive transfer of peanut allergen in a blood product. The New England journal of medicine. 2011;364:1981–2. doi: 10.1056/NEJMc1101692. [DOI] [PubMed] [Google Scholar]
- 11.Savage WJ, Tobian AA, Savage JH, Hamilton RG, Ness PM. Atopic predisposition of recipients in allergic transfusion reactions to apheresis platelets. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03160.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm D, Kluter H, Klouche M, Kirchner H. Impact of allergy screening for blood donors: relationship to nonhemolytic transfusion reactions. Vox Sang. 1995;69:217–21. doi: 10.1111/j.1423-0410.1995.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 13.Buck SA, Kickler TS, McGuire M, Braine HG, Ness PM. The utility of platelet washing using an automated procedure for severe platelet allergic reactions. Transfusion. 1987;27:391–3. doi: 10.1046/j.1537-2995.1987.27587320530.x. [DOI] [PubMed] [Google Scholar]
- 14.Wakamoto S, Fujihara M, Kuzuma K, Sato S, Kato T, Naohara T, Kasai M, Sawada K, Kobayashi R, Kudoh T, Ikebuchi K, Azuma H, Ikeda H. Biologic activity of RANTES in apheresis PLT concentrates and its involvement in nonhemolytic transfusion reactions. Transfusion. 2003;43:1038–46. doi: 10.1046/j.1537-2995.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 15.Kluter H, Bubel S, Kirchner H, Wilhelm D. Febrile and allergic transfusion reactions after the transfusion of white cell-poor platelet preparations. Transfusion. 1999;39:1179–84. doi: 10.1046/j.1537-2995.1999.39111179.x. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–61. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- 17.Sin AZ, Roche EM, Togias A, Lichtenstein LM, Schroeder JT. Nerve growth factor or IL-3 induces more IL-13 production from basophils of allergic subjects than from basophils of nonallergic subjects. J Allergy Clin Immunol. 2001;108:387–93. doi: 10.1067/mai.2001.117459. [DOI] [PubMed] [Google Scholar]
- 18.Petersen LJ, Brasso K, Pryds M, Skov PS. Histamine release in intact human skin by monocyte chemoattractant factor-1, RANTES, macrophage inflammatory protein-1 alpha, stem cell factor, anti-IgE, and codeine as determined by an ex vivo skin microdialysis technique. The Journal of allergy and clinical immunology. 1996;98:790–6. doi: 10.1016/s0091-6749(96)70128-8. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder JT, MacGlashan DW, Jr, Lichtenstein LM. Human basophils: mediator release and cytokine production. Advances in immunology. 2001;77:93–122. doi: 10.1016/s0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- 20.Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. The Journal of allergy and clinical immunology. 2006;117:583–9. doi: 10.1016/j.jaci.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 21.Stander S, Raap U, Weisshaar E, Schmelz M, Mettang T, Handwerker H, Luger TA. Pathogenesis of pruritus. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology: JDDG. 2011 doi: 10.1111/j.1610-0387.2011.07585.x. [DOI] [PubMed] [Google Scholar]
- 22.Boehlen F, Clemetson KJ. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus Med. 2001;11:403–17. doi: 10.1046/j.1365-3148.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuehn BM. CDC launches surveillance system to improve blood transfusion safety. JAMA. 2010;303:1467. doi: 10.1001/jama.303.15.1467. [DOI] [PubMed] [Google Scholar]
- 24.Bokisch VA, Muller-Eberhard HJ. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. The Journal of clinical investigation. 1970;49:2427–36. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glenister KM, Payne KA, Sparrow RL. Proteomic analysis of supernatant from pooled buffy-coat platelet concentrates throughout 7-day storage. Transfusion. 2008;48:99–107. doi: 10.1111/j.1537-2995.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 26.Cardigan R, Sutherland J, Wadhwa M, Dilger P, Thorpe R. The influence of platelet additive solutions on cytokine levels and complement activation in platelet concentrates during storage. Vox Sang. 2003;84:28–35. doi: 10.1046/j.1423-0410.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 27.Fujihara M, Ikebuchi K, Wakamoto S, Sekiguchi S. Effects of filtration and gamma radiation on the accumulation of RANTES and transforming growth factor-beta1 in apheresis platelet concentrates during storage. Transfusion. 1999;39:498–505. doi: 10.1046/j.1537-2995.1999.39050498.x. [DOI] [PubMed] [Google Scholar]
- 28.Heddle NM, Klama L, Meyer R, Walker I, Boshkov L, Roberts R, Chambers S, Podlosky L, O’Hoski P, Levine M. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion. 1999;39:231–8. doi: 10.1046/j.1537-2995.1999.39399219278.x. [DOI] [PubMed] [Google Scholar]
- 29.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 30.King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44:25–9. doi: 10.1046/j.0041-1132.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 31.Stern A, van Hage-Hamsten M, Sondell K, Johansson SG. Is allergy screening of blood donors necessary? A comparison between questionnaire answers and the presence of circulating IgE antibodies. Vox Sang. 1995;69:114–9. doi: 10.1111/j.1423-0410.1995.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa S, Tashiro N, Matsubara T, Furukawa S, Ra C. A comparison of FcepsilonRI-mediated RANTES release from human platelets between allergic patients and healthy individuals. Int Arch Allergy Immunol. 2001;125 (Suppl 1):42–7. doi: 10.1159/000053852. [DOI] [PubMed] [Google Scholar]
- 33.Sarkodee-Adoo CB, Kendall JM, Sridhara R, Lee EJ, Schiffer CA. The relationship between the duration of platelet storage and the development of transfusion reactions. Transfusion. 1998;38:229–35. doi: 10.1046/j.1537-2995.1998.38398222865.x. [DOI] [PubMed] [Google Scholar]
- 34.Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, Garraud O. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–9. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]