Abstract
With their ability to lyse Gram-positive bacteria, phage lytic enzymes (or lysins) have received a great deal of attention as novel anti-infective agents. The number of known genes encoding these peptidoglycan hydrolases has increased markedly in recent years, due in large part to advances in DNA sequencing technology. As the genomes of more and more bacterial species/strains are sequenced, lysin-encoding open reading frames (ORFs) can be readily identified in lysogenized prophage regions. In the current study, we sought to assess lysin diversity for the medically relevant pathogen Clostridium perfringens. The sequenced genomes of nine C. perfringens strains were computationally mined for prophage lysins and lysin-like ORFs, revealing several dozen proteins of various enzymatic classes. Of these lysins, a muramidase from strain ATCC 13124 (termed PlyCM) was chosen for recombinant analysis based on its dissimilarity to previously characterized C. perfringens lysins. Following expression and purification, various biochemical properties of PlyCM were determined in vitro, including pH/salt-dependence and temperature stability. The enzyme exhibited activity at low µg/ml concentrations, a typical value for phage lysins. It was active against 23 of 24 strains of C. perfringens tested, with virtually no activity against other clostridial or nonclostridial species. Overall, PlyCM shows potential for development as an enzybiotic agent, demonstrating how expanding genomic databases can serve as rich pools for biotechnologically relevant proteins.
Keywords: Lysin, Prophage, Enzybiotic, Muramidase, Clostridium perfringens
Introduction
Phage lytic enzymes (a.k.a. lysins or endolysins) have been the focus of a great deal of recent applied microbiological research (Fischetti 2010; Fischetti 2008; Borysowksi et al. 2005; Loessner 2005). Encoded by virtually all dsDNA phages, lysins are expressed late in the infective cycle. They hydrolyze the bacterial peptidoglycan and, along with membrane-permeabilizing holin proteins, lysins allow progeny viruses to escape the host cytoplasm. Biotechnological interest in these proteins stems from their ability to lyse Gram-positive species when applied exogenously, as the peptidoglycan of these organisms is accessible from the extracellular space. Phage lysins have been proposed as novel enzybiotic (enzyme antibiotic) agents against human and veterinary pathogens, in addition to other potential uses in food (Deutsch et al. 2004), agricultural (Kim et al. 2004), and industrial (Ye and Zhang 2008) science. Numerous lysins have been expressed and characterized to date, including successful in vivo trials involving animal models of colonization/infection (Daniel et al. 2010; Grandgirard et al. 2008; Nelson et al. 2001). Their appeal lies in both their potency and their specificity toward individual bacterial species, typically the host organism of the encoding phage. Studies to date have observed a lack of acquired bacterial resistance following lysin treatment, an additional beneficial feature (Loeffler et al. 2001; Schuch et al. 2002; Fischetti 2010).
Lysins are highly diverse proteins both enzymatically and architecturally. Gram-positive lysins are classically modular, 250–400 amino acids in length, with an N-terminal enzymatic domain and a C-terminal binding domain (Fischetti 2008). The enzymatic domain can possess various bond specificities, including muramidase (Porter et al. 2007), glucosaminidase (Pritchard et al. 2007), alanine-amidase (Schuch et al. 2002), alanoyl-glutamate endopeptidase (Korndörfer et al. 2008), glutaminyl-lysine endopeptidase (Pritchard et al. 2007), and cross-bridge endopeptidase (Baker et al. 2006). The C-terminal domain binds one of various epitopes within the target cell envelope (e.g., surface carbohydrate, choline, or peptidoglycan itself), and it is largely responsible for the specificity of a lysin toward particular bacteria (Diaz et al. 1991). Gram-negative lysins, by contrast, are typically smaller and are comprised of an enzymatic domain alone. Occasionally, lysins are identified that do not conform to standard architectures, including Gram-positive lysins with multiple enzymatic domains (Baker et al. 2006), Gram-negative lysins with an N-terminal binding domain (Briers et al. 2007), and a multimeric lysin (Nelson et al. 2006).
Most lysins characterized to date were cloned through traditional phage genomic techniques (Schmitz et al. 2010). Here, a phage is isolated through environmental sampling or prophage induction, and its genomic DNA is extracted following laboratory culture. Either the phage genome is sequenced and the lysin-encoding open reading frame (ORF) identified through homology analysis, or the DNA is subjected to a recombinant functional screen. In the latter case, a lysin-encoding fragment is identified by its ability to confer a bacteriolytic phenotype on a host clone. While generally successful, these methods are still rather time-consuming and dependent on the isolation/propagation of the initial phage. Moreover, they cannot predict how novel the identified lysin will be relative to previously characterized enzymes, nor can they assess total lysin diversity for a particular bacterial host.
In this regard, the rapidly expanding number of bacterial genome sequences could prove quite valuable. If a particular strain is lysogenized, its genome becomes an easy source of lysins, which can be rapidly polymerase chain reaction (PCR) cloned. For poly-lysogenized strains or species with multiple sequenced strains, one can systematically compare the different lysins and their predicted properties (e.g., enzymatic activity) before choosing which one(s) to express. In the current study, we wished to do precisely this—analyze all known prophage lysins for a particular Gram-positive pathogen, using this as a guide for subsequent recombinant analysis.
We chose to focus on the spore-forming, anaerobic rod Clostridium perfringens, one of the most frequently encountered clostridial species in clinical and environmental laboratories. C. perfringens is a component of healthy human feces, and it is found in the digestive tracts of other vertebrate and non-vertebrate animals, as well as soil samples (Matches et al. 1974). Despite its ubiquity, C. perfringens can nevertheless induce various pathologies. The species is taxonomically subdivided into five toxinotypes (A–E) based on the combinatorial presence of 15+ exotoxins genes that determine a strain’s pathogenic potential (Smedly et al. 2004). The most commonly encountered toxinotype of the healthy human bowel (enterotoxin-negative type A, Carman et al. 2008) can induce myonecrosis in the context of wounds (Bryant and Stevens 1997). Enterotoxin-positive type A strains are prevalent causes of food-borne illness, while type C strains are the agents of the potentially fatal (albeit rare) condition enteritis necroticans (Lawrence 1997). All five toxinotypes have likewise been implicated in various maladies of poultry and livestock (Van Immerseel et al. 2009; Uzal and Songer 2008).
To date, a single enzymatic class of phage lysin for C. perfringens has been subject to recombinant expression and analysis. Zimmer et al. (2002a) induced and sequenced a prophage from toxinotype B strain ATCC 3626. The gene encoding the lytic enzyme (Ply3626, a type 3 alanine-amidase) was subsequently identified by sequence homology and PCR-cloned into an Escherichia coli expression plasmid (Zimmer et al. 2002b). The authors demonstrated that extracts of the induced clone were capable of lysing viable suspensions of all C. perfringens strains tested, while non-perfringens clostridia and other Gram-positive genera demonstrated virtually no susceptibility. It has been suggested that Ply3626 could potentially serve as a topical agent or food additive (Jay et al. 2005; Zimmer et al. 2008). Very recently, two additional C. perfringens lysins (PlyCP39O and PlyCP26F) were cloned from the sequenced genomes of environmentally isolated lytic phages (Simmons et al. 2010; Seal et al. 2010). These enzymes were also predicted to be type 3 alanine-amidases, although their C-terminal binding regions were unrelated to that of Ply3626.
In recent years, the genomes of nine different strains of C. perfringens have been sequenced, all of which contain identifiable prophage regions (Shimizu et al. 2002; Myers et al. 2006; gsc.jcvi.org/projects/msc/clostridium/). In the present study, we systematically examined the prophage lysins in these sequences, comparing them based on sequence homology and domain composition. In the process, we identified 14 lysin genes and 31 lysin-like genes. These proteins could be categorized into three families of enzymatic domains: type 2 alanine-amidase, type 3 alanine-amidase, and muramidase (GH-25). Of the identified genes, a muramidase lysin from strain ATCC 13124 was selected for cloning and expression based on its catalytic mechanism. Following purification, the enzyme (termed PlyCM, for C. perfringens muramidase) was subject to various in vitro tests to determine its potency, specificity, and biochemical properties. Overall, this work adds another phage lysin to the growing list of candidate enzybiotics, demonstrating an important role for comparative genomics in their development.
Materials and methods
Computational identification/analysis of lytic enzymes
The genomes of the following sequenced strains of C. perfringens were analyzed: ATCC 13124 (toxinotype A, NCBI genome project #304); SM101 (A, #2521); 13 (A, #79); F4969 (A, #20031); NCTC 8239 (A, #20033); ATCC 3626 (B, #20027); JGS1495 (C, #20025); JGS1721 (D, #28587); JGS1987 (E, #20029). The first step in compiling a comprehensive list of lysins was to identify obvious proviral regions, accomplished through a combination of manual inspection and the prophage prediction algorithm Prophinder (Lima-Mendez et al. 2008).
The lysin-encoding genes within each prophage regions were next selected. In many cases, these ORFs were already designated explicitly (e.g., “endolysin”) by the database annotation. For other prophages, the existing annotations were insufficient—in these cases, pfam domain analysis was performed on the proteins in the region (Finn et al. 2010). A set of several criteria (described in detail in the “Results” section) were applied to the pfam predictions to designate a lysin for each case, and a preliminary list of genes was compiled. To ensure no enzymes were overlooked (e.g., ones encoded within short prophage remnants) the list was subjected to iterative blast analysis (Altschul et al. 1990). The protein sequences on the initial list were blastp-queried against C. perfringens sequences. Any newly identified homologs were added to the original list, and the process was repeated until no new genes were revealed. The aforementioned criteria were then applied to the expanded list to eliminate any genes included erroneously.
The sequences comprising this final list were phylogenetically compared through the Phylip v3.67 package (Felsenstein 1989). The translated protein sequences were subject to multiple sequence alignment through the ClustalX algorithm, followed by 1,000 rounds of boot strapping with Seqboot. These alignments were analyzed in turn by the Protdist and Fitch algorithms with default settings, and an unrooted consensus tree was generated with Consense. Signal peptide predictions were made via SignalP v3.0 (Bendtsen et al. 2005; hidden Markov and neural network methods; Gram-positive organism group).
Expression and purification of PlyCM
Genomic DNA from C. perfringens ATCC 13124 was PCR amplifed with the following PlyCM-targeted primers: ACCATGGAAAGTA GAAACAATAATAATTTAAAAGG (fwd) and GTCAGA TATTACTCTAACTAACCTTAAAA (rev). The underlined G in the forward primer was intentionally altered from the wild-type C (a Q2E mutation) in order to introduce an NcoI restriction site that overlaps with the start codon. The PCR amplicon was topoisomerase cloned into pBad-TOPO (Invitrogen), an arabinose-inducible E. coli expression plasmid. This construct was subsequently purified, NcoI-digested, and re-circularized to eliminate a plasmid-encoded N-terminal leader sequence and ensure translation from the native start codon. This final construct was maintained and expressed in TOP10 E. coli.
To express PlyCM, the clone was grown in LB broth to OD600≈0.5 and induced with 0.2% L-(+)-arabinose. The induced culture was shaken overnight at 30°C; this temperature was important, as inclusion bodies formed at 37°C. The cells were pelleted, resuspended in 15 mM phosphate buffer pH 7.4, and lysed by three passages through an EmulsiFlex C-5 homogenizer. Cellular debris was pelleted (1 h, 35,000×g), and the supernatant was (NH4)2SO4-precipitated to 40% saturation (226 g/L). The precipitated protein (including the predominant amount of PlyCM) was pelleted, resolubilized in 15 mM phosphate buffer pH 7.4 (buffer A), and dialyzed against this buffer.
The protein solution was passed through a DEAE anion-exchange column equilibrated against buffer A (fast flow resin, GE). Based on PlyCM’s theoretical isoelectric point of 5.1, one would predict it to bind to DEAE at pH=7.4. Nevertheless, the protein demonstrated an unusual chromatographic response: it neither bound the column in earnest nor flowed directly through it. Rather, there was a transient interaction in which PlyCM would initially bind the resin, but then slowly elute over 5+ column volumes as the column was washed with buffer A. Although atypical, this property provided a fortuitous purification step, as PlyCM could be separated from the proteins in both the flow-through and tightly bound fractions. The PlyCM-containing component was immediately passed through a ceramic hyroxyapatite column (Macro-Prep type II, 40 µm, Bio-Rad) equilibrated against the same buffer A. The lysin demonstrated non-transient binding to this resin, and it was subsequently concentrated/purified through a 20 column– volume elution with buffer B (500 mM phosphate buffer pH 7.4), eluting at ~80 mM.
Following purification, we observed that PlyCM would undergo irreversible precipitation in phosphate buffer. Addition of L-arginine mitigated the precipitation, as has been reported for other recombinant proteins (Hamada et al. 2009). As a result, 100 mM L-arginine (pH=7.4) was included in the PlyCM stock preparation prior to freezing, lyophilization, and storage at −20°C. Overall, this protocol generated ~10 mg of purified PlyCM per liter E. coli culture.
In vitro analysis of PlyCM
PlyCM activity was examined predominately through optical density analysis. Bacterial strains were grown on agar plates at 37°C. For clostridial strains, Schaedler agar with vitamin K1 and 5% sheep’s blood was employed with anaerobic conditions. For nonclostridial strains, brain–heart infusion agar was employed with aerobic culture. Following overnight growth, bacteria were scraped from the plates and suspended directly in buffer (which varied depending on the particular experiment) to the desired optical density. PlyCM or lysin vehicle was added immediately prior to the start of each experiment, and OD600 measurments were conducted in 96-well plate format at 22°C. For colony-forming unit (CFU) counts, C. perfringens samples were diluted over five orders of magnitude, with triplicate plating at each dilution onto Schaedler agar.
Results
We sought to compile a comprehensive list of prophage lysins within all sequenced genomes of C. perfringens. In doing so, the following criteria were used for designating a given ORF as a probable lysin: (1) the presence of an N-terminal enzymatic domain, (2) the presence of a C-terminal binding region1, (3) the absence of an N-terminal signal peptide, and (4) the absence of additional domains with extraneous function. The third criterion is important because bacteria encode chromosomal peptidoglycan hydrolases (autolysins) that are involved in processes such as cell wall turnover and sporulation (Vollmer et al. 2008). Some autolysins possess the same domain architecture as phage lysins, except that they also often include a signal peptide for secretion. The fourth criterion is significant because phages can encode other proteins (i.e., not lysins proper) that include peptidoglycan hydrolase domains, particularly structural proteins involved with DNA-injection (Rashel et al. 2008). Usually, however, these structural lysins are readily discernable by their greater size and the presence of additional domains.
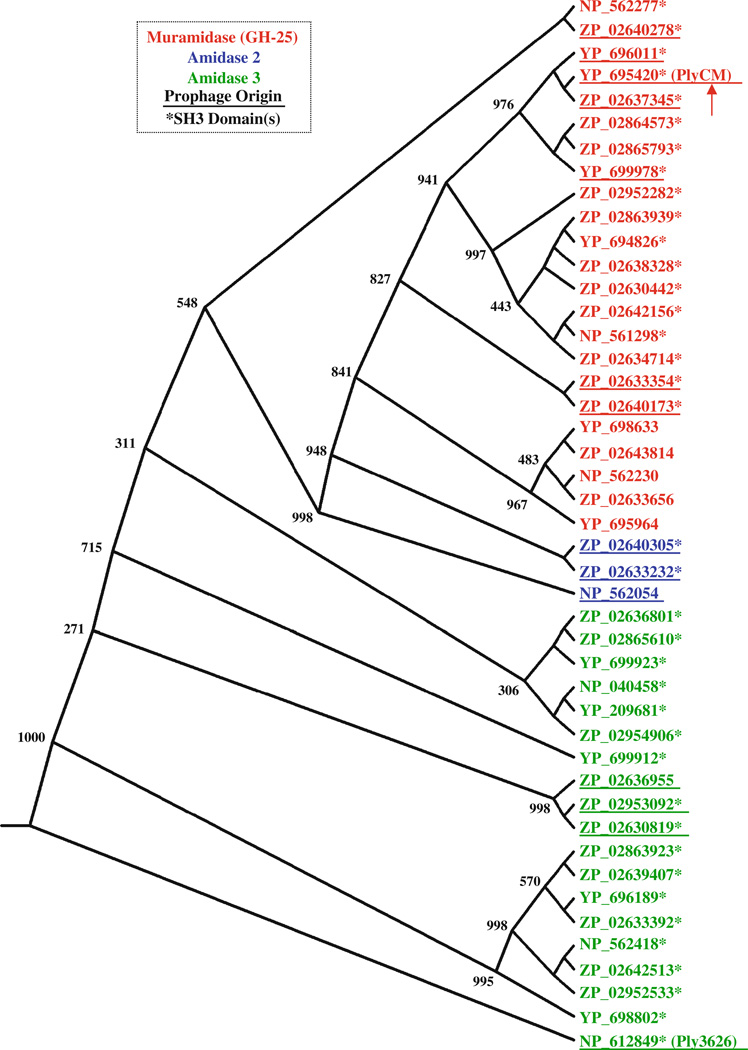
In total, 45 ORFs were identified with the above criteria. Their GenBank accession numbers are provided in Fig. 1, which depicts the predicted evolutionary relationships among the enzymes as a consensus phylogram. Of the 45 proteins, 23 were predicted to possess N-acetylmuramidase activity with an N-terminal GH-25 domain (glycosyl hydrolase type 25, pfam accession number PF01183). The 22 others were predicted to possess N-acetylmuramoyl-L-alanine-amidase activity: three encoded a type 2 alanine-amidase domain (PF01510), while 19 encoded a type 3 alanine-amidase domain (PF01520). Although these two alanine– amidase families diverge sequentially, they target the same bond at the beginning of peptidoglycan’s pentapeptide stem.
Fig. 1.
The phylogenetic relationship among C. perfringens enzymes with phage-lysin-like architectures is depicted here as a distance-based phylogenetic tree; the numbers at select nodes represent the consensus values following 1,000 rounds of bootstrap analysis. The catalytic domain of each protein is indicated with the corresponding color scheme (inset). The proteins for which pfam analysis predicted a C-terminal SH3 type 3 domain (either a single or a dual) are marked with an asterisk. Of these 45 proteins, 14 appear to be phage lysins proper, in that they are encoded within proviral regions within the C. perfringens genomes. These are denoted with an underline. The other lysin-like proteins are highly homologous to the phage enzymes, but reside elsewhere in the chromosome or in an associated plasmid
At their C-termini, 38 of 45 enzymes (also denoted in Fig. 1) contained either a single or a double SH3-3 binding domain (PF08239). For some of the proteins, the degree of alignment with the SH3-3 consensus logo was low (E value range: 10−7–0.03). Bacterial SH3 domains are commonly found in autolysins and phage lysins, and they presumed to function in binding the bacterial cell wall (Xu et al. 2009). The other seven enzymes possessed extended C-terminal regions (see footnote 1), although pfam failed to recognize any conserved domains. One of these proteins, ZP_02636955, demonstrated BLAST homology with the C-terminal regions of several of the other 45 enzymes. Most likely, ZP_02636955 possesses the same binding functionality, although its C-terminal domain differs too greatly from the SH3-3 consensus for pfam recognition. Another enzyme, NP_562054, demonstrated closest C-terminal alignment (E value ≈10−35) to a segment found within the C. perfringens bacteriocin BCN5 (Garnier and Cole 1986). Finally, a group of five muramidases (all clustered together in Fig. 1), contained C-terminal regions that— while nearly identical to one another—showed no homology to any other C. perfringens proteins. Notably, none of the 45 enzymes possessed the SPOR domain (PF05036) observed at the C-terminus of the recently described PlyCP39O and PlyCP26F lysins (Simmons et al. 2010).
Based on the selection criteria, we initially assumed all 45 of these proteins to represent legitimate phage lysins of proviral origin. After inspecting their positions within the respective genomes, however, it became apparent that this was not the case. Many of the proteins (31 of 45) were encoded in regions that did not correspond to prophages or prophage remnants. In fact, several patterns existed as to where these genes were found, including in the vicinity of UV-inducible bacteriocins. The commonality of these lysinlike proteins raises several interesting questions, which will be reflected upon further in the “Discussion” section. Fourteen of the enzymes did reside in clear prophage regions. Underlined in Fig.1, these include a combination of muramidases, type 2 alanine-amidases, and type 3 alanine-amidases. The first two categories are of particular interest, as lysins of these classes have not been characterized for C. perfringens.
We decided to focus subsequent efforts on a muramidase encoded by strain ATCC 13124 (the C. perfringens type strain). We hereafter refer to this protein (YP_695420) as PlyCM (for C. perfringens muramidase). Several other nonperfringens phage lysins with GH-25 lytic domains have been characterized to date. These enzymes are summarized in Fig. 2a, along with the binding domains with which they are paired. In Fig. 2b, the GH-25 domain of PlyCM is aligned with that of two non-clostridial lysins for which crystal structures have been solved (Hermoso et al. 2003; Porter et al. 2007). As shown, the catalytic residues are conserved in all three cases.
Fig. 2.
a Several phage lytic enzymes containing a GH-25 muramidase domain (all from non-clostridial phages) have been recombinantly expressed and studied to date. These are depicted here along with the variety of C-terminal binding domains with which they are paired. Lysins for which a crystal structure has been solved are indicated with an asterisk. Included are: CPL-1 from Streptococcus pneumoniae, choline bindings repeats (pfam PF01473; GenBank ABC88204; Hermoso et al. 2003); CPL-7 from S. pneumoniae, eponymous binding repeats (PF08230; AAA72844; García et al. 1990); PlyB from Bacillus anthracis, SH3–5 binding domain (PF08460; 2NW0_A; Porter et al. 2007); PlyPH from B. anthracis, conserved hypothetical binding domain (PF12123; NP_845154; Yoong et al. 2006); Lyb5 from Lactobacillus fermentum, LysM binding domains (PF01476; ABP88927; Wang et al. 2008); and PlyGBS from Streptococcus agalactiae, single SH3-3 binding domain (PF08239; AAR99416; Cheng et al. 2005). Also listed (in the inset) are several basic characteristics of the PlyCM lysin studied here. b The GH-25 catalytic domain of PlyCM is aligned with those of CPL-1 and PlyB. Three-way and pair-wise identities are indicated with a blue/pink/yellow/gray color scheme. Putative catalytic residues (see Porter et al. 2007) are denoted with arrows
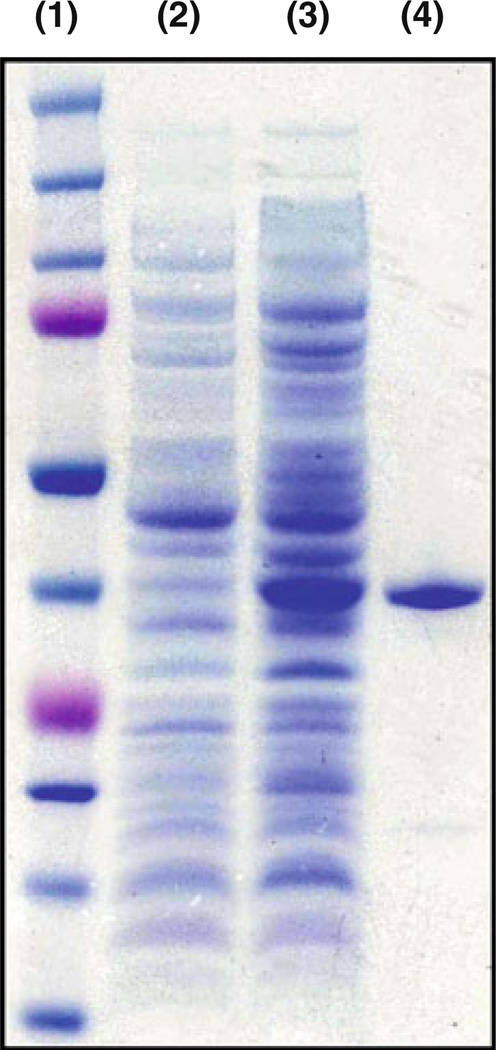
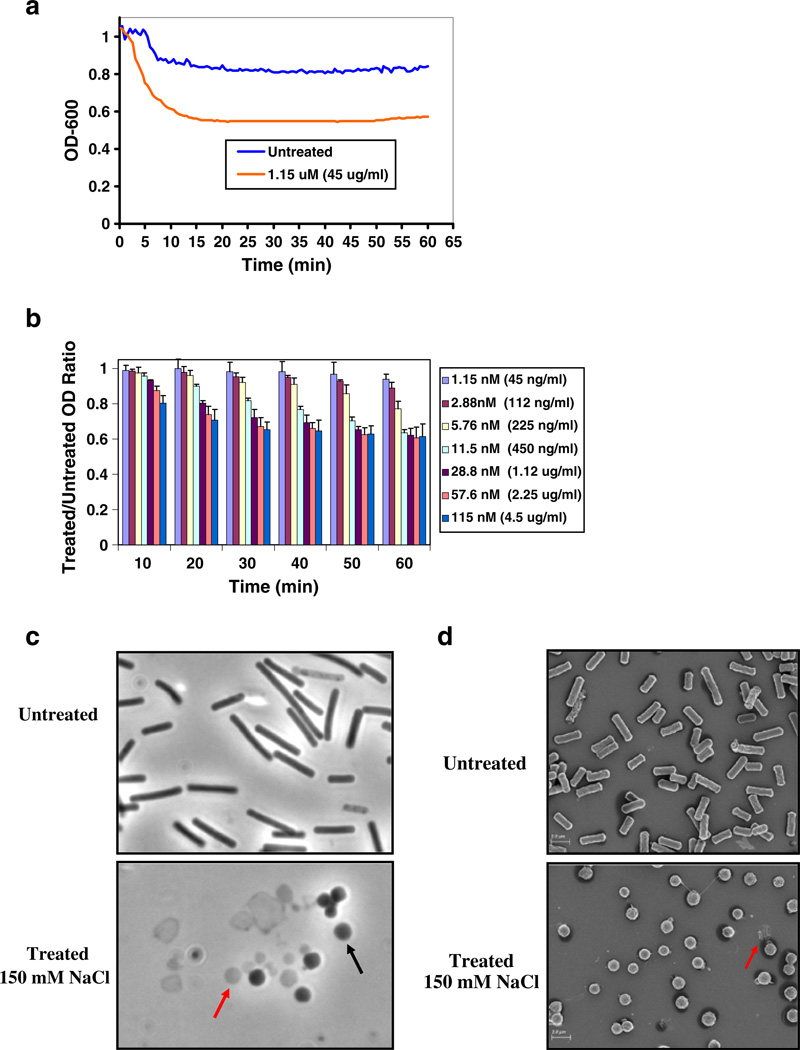
PlyCM was expressed in E. coli and purified chromato-graphically (Fig. 3). The lysin showed clear bacteriolytic activity in vitro against the encoding strain ATCC 13124. When added to a buffered suspension (20/10 mM phos-phate/citrate pH 6.4) of live cells for 1 h, PlyCM generated a ~70% reduction in bacterial turbidity at low nM (low-to-sub µg/ml) concentrations (Fig. 4). The time-versus-OD response for PlyCM is similar to that as observed for Ply3626 against its host strain (Zimmer et al. 2002b). The required concentration of PlyCM is also commensurate with values observed for other Gram-positive bacteria and their respective lysins (Fischetti 2008). The residual turbidity after 1 h of treatment can be considered a baseline value for these buffering conditions—neither increasing the lysin concentration nor the incubation time lead to further OD declines. The reader should note that this baseline OD does not correspond to quantitative viability levels (see CFU analysis below).
Fig. 3.
PlyCM was purified following recombinant expression in E. coli. 1 Molecular weight ladder; 2 crude extract of encoding strain prior to induction; 3 crude extract of encoding strain ~16 h after induction; 4 final product following isolation protocol. By visual approximation, PlyCM is >90% pure; its band appears at the level of the 37 kDa marker (predicted MW=38.7 kDa)
Fig. 4.
a Time course. Depicted here is a representative example of an OD drop experiment in which lysin was added to a buffered suspension (20/10 mM phosphate/citrate pH 6.4) of live host strain ATCC 13124. A 1.15-µM (45 µg/ml) enzyme-treated sample and an untreated control are shown. b Variable lysin concentration. The PlyCM concentration was decreased 10–1,000-fold from the level shown in a, and cell lysis was monitored over an hour. The figure reports treated/untreated OD ratios (to account for slight possible fluctuation in the untreated) at 10-min intervals; the average of three independent experiments is depicted. As the graph indicates, concentrations as low as 11.5 nM (450 ng/ml) were able to bring the OD to a baseline level within 1 h. In comparison, hen egg white lysozyme (HEWL, a non-specific eukaryotic muramidase, NP_990612, PF00062) failed to affect the cells’ turbidity or microscopic morphology after 1 h at 500 µg/ml
Acidity represents one of the most important variables affecting lysin activity, so we sought to determine the effect of pH on PlyCM. Two sets of OD drop experiments were conducted in which pH was varied at a constant lysin concentration. First, a broad-range buffer (25 mM boric/ phosphoric acid) was utilized with a variable pH of 3.0– 10.5 and a PlyCM concentration of 115 nM (4.5 µg/ml). Enzymatic activity was observed between pH=4.0–9.5, with a maximum from 6.5 to 8.0 (Fig. 5a). To confirm these observations (and fine tune an optimal pH), a narrow-range buffer (20/10 mM phosphate/citrate) was utilized with smaller pH degradations and a lower PlyCM concentration (11.5 nM, 450 ng/ml). A similar profile was observed here (Fig. 5b), with pH=6.4 as the center of activity.
Fig. 5.
PlyCM pH dependence; a 115 nM PlyCM was incubated for 2 h with suspensions of ATCC 13124 in a variable-pH boric/phosphoric acid buffer (pH 3.0–10.5, 0.5 intervals, n= 3). By the end of the experiment, some degree of activity was observed between pH=4–9.5, although lysis was maximal from 6.5 to 8. b The experiments in a were repeated under more sensitive digestion conditions. This include a 10-fold reduction in PlyCM concentration and the use of a phosphate/citrate buffer (pH= 5.0–9.0, 0.2 intervals, n=3). After 1 h, maximal activity was centered around pH 6.4. c Aliquots of PlyCM were diluted in 20/10 mM phosphate/citrate buffers of various pH values, yielding solutions whose final pHs are indicated in the graph. Each aliquot was titrated back to pH 6.5 with dibasic phosphate; a positive control was maintained at pH 6.5 for the entire experiment. Following volume normalization (with phosphate/ citrate pH 6.5), the enzyme samples were added to cells (final PlyCM concentration= 58 nM, final pH 6.5) and lysis was observed for 2 h. The sample brought to a pH 4.3 showed identical activity to the positive control, while the others failed to induce lysis
Considering the acidic environment of the stomach (as well as many food items), we wished to determine whether the loss-of-activity at low pH represented a mechanistic inhibition or an irreversible denaturation. The latter scenario turned out to be the case. PlyCM was buffered at a range of acidic pHs, followed by titration back to pH=6.5. Activity was subsequently lost for samples at pH=3.3 and below. The pH=4.3 sample, however, exhibited an identical lytic profile to the positive control maintained at pH=6.5 (Fig. 5c).
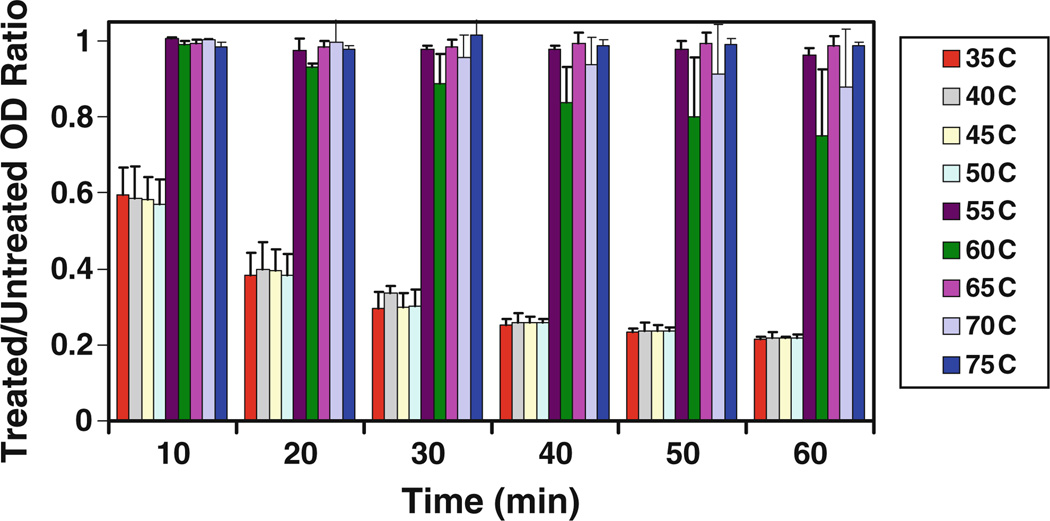
Several other biochemical properties of PlyCM were likewise evaluated, in particular the temperature stability of the enzyme. Following incubation at various temperatures for 30 min, PlyCM demonstrated a sharp loss of activity (with concomitant flocculation) between 50°C and 55°C (Fig. 6). In separate experiments, molar excesses of either EDTA or DTT failed to inhibit lysin activity (data not shown), indicating that PlyCM is not dependent upon chelatable cations or intramolecular disulfide binds for activity.
Fig. 6.
Stock concentrations of PlyCM were incubated for 30 min at the indicated temperatures, after which they were added to buffered suspensions (pH 6.4) of C. perfringens ATCC 13124 at 11.5 nM (450 ng/ml) for 1 h. The figure reports the treated/untreated OD-ratio for each temperature at 10-min intervals (n=4)
The above experiments were all conducted in low-osmolarity suspensions in which the only salt was the buffering agent. Seeing as any real-world application would likely occur in a less hypotonic environment, we evaluated the effect of salt on PlyCM-induced lysis. The experiments in Fig. 4 were repeated with 150 mM NaCl in the lysis buffer. Although the concentration dependence of PlyCM was virtually identical, the baseline OD of the treated cells jumped to ~60% of the untreated value (Fig. 7a and b). Microscopic inspection of the cells indicated the reason: in the presence of 150 mM NaCl, many of the cells collapsed to spheroplast forms without extrusion of their cytoplasmic contents (see Fig. 7c and d, respectively, for phase contrast and scanning electron micrographs). In rod-shaped bacteria, the peptidoglycan layer both prevents osmotic lysis and maintains the bacilloid morphology, the natural lowest energy form being coccoid (Pichoff and Lutkenhaus 2007). Presumably, the addition of NaCl reduced the pressure on the cells to lyse, without affecting the pressure-independent collapse to spheroplasts. Indeed, when these lysin-treated cells were pelleted and resuspended in salt-free solution, immediate lysis was observed.
Fig. 7.
a Time course. A single lytic time course is shown with 1.15 mM PlyCM (45 µg/ml) and buffering conditions that include 150 mM NaCl (pH=6.4). b Variable lysin concentration. The experiment in Fig. 4b was repeated, again with the addition of 150 mM NaCl in the lysis buffer (for B, n=3). The clostridia responded to PlyCM at nearly identical concentrations as in 4b, except that the OD would fall to a maximum of 60% the untreated value. c The image compares (×1,000 magnification, phase contrast) the gross morphology of untreated ATCC 13124 with that of cells treated with lysin for 1 h in the presence of 150 mM NaCl. Within the treated samples, there exists a combination of collapsed spheroplasts (black arrow) that have maintained their cytoplasmic contents and contribute to optical density, as well as clostridial ghosts and ghost-fragments (red arrow) that have undergone complete lysis. d Scanning electron microscopy was also conducted on treated and untreated ATCC 13124 cells. The treated image is dominated by clostridial spheroplasts; a presumptive ghost is denoted with the red arrow
The preceding observations raise the question of whether the spheroplast-like clostridia are viable. Ultimately, bacterial death following lysin treatment is a more significant metric than simple optical density. Accordingly, CFU analysis was conducted on cells treated in the presence of 150 mM NaCl. Despite the residual turbidity, the cells were overwhelmingly non-viable. The following percentages killed were observed after 1 h (relative to untreated, n=5 independent experiments): 11.5 nM (86–99.2%), 115 nM (>99.7%), and 1.15 µM (>99.99%). These data indicate that, even in environments that are incompatible with osmotic lysis, PlyCM exerts a potent lethal effect on ATCC 13124.
We next attempted to gauge the effect of PlyCM on actively-dividing cells. Unfortunately, minimum inhibitory concentration (MIC) analysis in liquid culture proved unsuccessful. At the concentrations employed in Figs. 4 and 7, PlyCM failed to prevent proliferation of ATCC 13124. Although we would have liked to repeat these experiments at higher lysin concentrations, a technical barrier prevented it. As mentioned in “Materials and methods” section, 100 mM L-arginine was added to the PlyCM stock solution to prevent enzyme precipitation. Higher lysin concentrations would have likewise necessitated elevated amounts of L-arginine in the culture broth. In preliminary experiments, however, we observed that mM concentrations of this amino acid could itself significantly impact actively-dividing C. perfringens (the cells would proliferate with defective division, yielding markedly elongated rod forms2). With this confounding variable, we did not feel MIC analysis would be rigorously meaningful.
Finally, the activity of PlyCM was tested in vitro against a panel of other bacterial species and strains. These include: 24 isolates of C. perfringens (with representative examples of each toxinotype), 10 non-perfringens clostridia, and 16 non-clostridial species of Gram-positive bacteria. We found it necessary to conduct these experiments in a buffer (phosphate/citrate pH 6.4) that included 150 mM NaCl; without this, many strains of C. perfringens would self-adhere into macroscopic aggregates. Given the above observations involving spheroplast formation at 150 mM NaCl, we did not want to rely exclusively on OD measurements in evaluating the panel (as is typically done for assessing a lysin’s target range). Consequently, a semi-quantitative scoring system was devised that relied upon microscopic inspection of the samples. Strains were ranked on scale from 1 (equally sensitive or more sensitive than host train ATCC 13124) to 4 (insensitive). The results of this panel are summarized in Table 1 (the reader is referred to the table caption for the specific details of the scoring system). Overall, 23 of 24 strains of C. perfringens demonstrated susceptibility to the lysin, although the level varied quantitatively from strain to strain. Outside of C. perfringens, only 3 of 10 other clostridia demonstrated susceptibility (at the lowest level), while all non-clostridia were completely insensitive to treatment.
Table 1.
The species/strains of bacteria that were evaluated for their susceptibility to PlyCM
| Species/Strain | Sensitivity |
|---|---|
| C. perfringens ATCC 13124 (type A, CPE−) | I (reference) |
| C. perfringens ATCC 3624 (type A, CPE−) | II |
| C. perfringens ATCC 12915 (type A, CPE+) | II |
| C. perfringens ATCC 12916 (type A, CPE+) | III |
| C. perfringens ATCC 12917 (type A, CPE+) | III |
| C. perfringens ATCC 12919 (type A, CPE+) | II |
| C. perfringens ATCC 3626 (type B) | I |
| C. perfringens ATCC 3628 (type C) | I |
| C. perfringens NCTC 8346 (type D) | II |
| C. perfringens ATCC 27324 (type E) | III |
| C. perfringens: 3 untyped human fecal isolates | Two—I |
| One—II | |
| C. perfringens: 12 untyped canine fecal isolates | Three—I |
| Seven—II | |
| One—III | |
| One—IV | |
| C. tetani ATCC 19406, C. septicum ATCC 12464, C. beijerinckii ATCC 8260 | III |
|
C. difficile ATCC 43593, C. difficile ATCC 700057, C. difficile ATCC 9689, C. histolyticum ATCC 19401, C. sordelli ATCC 9714, C. sporogenes ATCC 3584, C. bifermentans ATCC 638 |
IV |
|
S. pyogenes D471, S. agalactiae 090R, S. pneumoniae R36, S. mutans in-house strain, E. faecalis V583, E. faecium EFSK-2, S. aureus RN4220, S. epidermidis ATCC 12228, M. lysodeikticus ATCC 4698, L. monocytogenes HER1083, B. anthracis 222, B. cereus ATCC 14579, B. subtilis SL4, B. thuringiensis HD-73, B. megaterium in-house strain, G. vaginalis ATCC 14018, P. aeruginosa PAO1, E. coli TOP10 |
IV |
If known, the toxinotype and CPE-status (for toxinotype A) are listed for C. perfringens strains. Each bacteria was suspended in phosphate/citrate buffer (pH=6.4)+150 mM NaCl and exposed to three PlyCM-concentrations (11.5 nM, 115 nM, and 1.15 µM); 11.5 nM represents the lowest concentration that brought the host strain ATCC 13124 to baseline OD (see Figs. 4 and 7). After 1 h, the samples were visualized at ×1,000 magnification and the OD was measured
Each bacteria was assigned a rank based on the following semi-quantitative criteria: I 11.5 nM PlyCM induced complete lysis, or >9/10 cells had converted from rod-forms to spheroplasts, II 115 nM PlyCM lead to either preceding observation, III 1.15 µM PlyCM induced lysis/spheroplast conversion (complete or partial); IV no lysis or spheroplast conversion was observed after 1 h, even at 1.15 µM
Discussion
The work presented here illustrates the utility of genomic sequencing in the identification of candidate enzybiotics. Moreover, the specific protein expressed and characterized (PlyCM) exhibits potent bacteriolytic ability in vitro, demonstrating potential as a novel antibacterial agent. In theory, phage lysins seem well suited as food additives to combat C. perfringens. This is especially true given that C. perfringens enteritis is caused by ingestion of vegetative bacteria (estimated dose necessary for clinical symptoms >108 cells) and not preformed toxin or dormant spores (USFDA 2009). As a result, an agent that could selectively reduce the overall bacterial load could prove quite useful.
Despite different enzymatic mechanisms, PlyCM and the previously characterized C. perfringens phage lysins (Ply3626, PlyCP39O, and PlyCP26F) demonstrated several noteworthy similarities to one another (Zimmer et al. 2002b; Simmons et al. 2010). Most importantly, the enzymes all possessed broad activity against C. perfringens strains, with little effect against other species and genera. Quantitatively speaking, however, the level of sensitivity among C. perfringens varied somewhat from strain to strain for each lysin. With both Ply3626 and PlyCM, the treatment–response of the lysogenized strain was more pronounced than many others. This observation underscores the importance of developing complimentary lysins with varied sequences and component domains. In fact, the ability to accomplish this rationally and rapidly is an inherent strength of multigenomic sequence analysis.
In this regard, it would be informative for future work to consider the combined effect of a muramidase-like PlyCM and the various alanine-amidases. For instance, one could observe whether they act synergistically with one another or normalize the strain-to-strain response. In the process, it could be possible to ascertain whether definitive trends exist as to the sensitivity of a given bacterial strain toward particular lysins. For instance, are certain toxinotypes inherently more sensitive to certain enzymes? Or does lysogenization by a prophage encoding a given lysin affect the strain’s sensitivity to that class of lysins in general?
Going further, one could even include non-viral enzymes in combined treatment pools. Just recently, Camiade et al. (2010) characterized an endogenous C. perfringens peptidoglycan hydrolase (an autolysin) involved in cell division and stress responses. This protein (Acp) encodes a glucosaminidase domain (PF01832), distinct from all sequences considered here. While autolysins have not received as much attention, nothing in theory would prevent them from functioning as enzybiotics alongside phage enzymes.
In terms of future work on PlyCM, the immediate next steps must involve the fine tuning of certain biochemical properties. In particular, the issue of solubility must be addressed; this would allow for increased concentrations of lysin to be tested against actively proliferating cells. Possible strategies include the use of additives other than arginine or the re-engineering of its sequence to increase solubility, such as the design of a chimeric lysin that combines PlyCM’s enzymatic domain with a different binding domain. This strategy has been employed successfully to optimize the behavior of other past lysins (Daniel et al. 2010).
Aside from the goal of enzybiotic development, one other relevant issue warrants further discussion here. Our genomic analysis revealed numerous enzymes that possessed the architectural properties of a lysin (catalytic and binding domains, no signal peptide), but that did not reside in a recognizable prophage region. Conceivably, they could represent small prophage remnants from their hosts’ evolutionary history. Nevertheless, many demonstrated conserved genomic arrangements from strain-to-strain, suggesting a dedicated in vivo role. These lysin-like proteins are probably better classified as host autolysins, and their existence raises several noteworthy questions regarding their biological purpose and their means of accessing the peptidoglycan without a secretion signal.
Several (ZP_02863923, ZP_02639407, YP_696189, ZP_02633392, NP_562418, ZP_02642513, ZP_02952533, YP_698802) are encoded in the vicinity of a putative histidine kinase and metallo-beta-lactamase. They are classified as type 3 alanine–amidases, and are clustered together near the bottom of the phylogram in Fig. 1. Also clustered together are several additional type 3 alanine–amidases (YP_699923, NP_040458, YP_209681, ZP_02954906, YP_699912); for these enzymes, the genomic positioning suggests an intriguing biological function. They are all encoded near UV-inducible C. perfringens bacteriocins known as BCN5 proteins.3 Four of these lysins are located on plasmids, while one is chromosomal (ZP_02954906, strain JGS1721). Ongoing research will explore the in vivo role of these genes, as well as analyze whether similar elements exist in other clostridia and Gram-positive bacteria.
In conclusion, the increasing ease of genomic sequencing is clearly transforming both basic and applied microbiological research. These genomes shed light on both biological mechanisms and useful molecules, and their inspection might well lead to new avenues for improving human health.
Acknowledgments
This research was funded by NIH/NIAID grants AI057472 and AI11822 to VAF. JES acknowledges the kind support of the NIH MSTP program (Weill Cornell/Rockefeller/Sloan-Kettering grant GM 07739). The authors would like to thank Dr. Davise Larone of New York Hospital for providing clostridial strains. We also acknowledge Prof. Ezio Bottarelli of the University of Parma for his valued insights, as well as Ms. Cinzia Reverberi and Mr. Roberto Lurisi for technical assistance. Electron micrographs were acquired by Ms. Eleana Sphicas of the Rockefeller University Electron Microscopy Core Facility.
Footnotes
ORFs that encoded an extended C-terminal region without a pfamrecognized binding domain were not excluded on this fact alone. There exist a number of Gram-positive phage lysins for which this is indeed the case. Most likely, these C-termini do possess a cell-wall binding functions, albeit ones that have not yet been characterized and organized into conserved protein families.
It should be emphasized that L-arginine, in itself, had no affect on bacterial turbidity or viability when cells were exposed to the amino acid for a short time in buffered solution (as in previous experiments).
This is the same BCN5 mentioned previously in the text. It demonstrated sequence homology to the C-terminus of another C. perfringens lysin considered here (NP_562054, a type 2 alanine– amidase of evident proviral origin).
Contributor Information
Jonathan E. Schmitz, Email: jschmitz@rockefeller.edu, Laboratory of Bacterial Pathogenesis and Immunology, The Rockefeller University, 1230 York Avenue, Box 172, New York, NY 10065, USA.
Maria Cristina Ossiprandi, Department of Animal Health, Microbiology and Immunology, Section, University of Parma, Faculty of Veterinary Medicine, Via del Taglio, 10, 43100, Parma, Italy.
Kareem R. Rumah, Laboratory of Bacterial Pathogenesis and Immunology, The Rockefeller University, 1230 York Avenue, Box 172, New York, NY 10065, USA
Vincent A. Fischetti, Laboratory of Bacterial Pathogenesis and Immunology, The Rockefeller University, 1230 York Avenue, Box 172, New York, NY 10065, USA
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baker JR, Liu C, Dong S, Pritchard DG. Endopeptidase and glycosidase activities of the bacteriophage B30 lysin. Appl Environ Microbiol. 2006;72:6825–6828. doi: 10.1128/AEM.00829-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: signalP 3.0. J Mol Biol. 2005;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Borysowksi J, Weber-Dabrowska B, Gorski A. Bacteriophage endolysins as a novel class of antibacterial reagents. Exp Biol Med. 2005;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- Briers Y, Volckaert G, Cornelissen LS, Michiels CW, Hertveldt K, Lavigne R. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages φKZ and EL. Mol Microbiol. 2007;65:1334–1344. doi: 10.1111/j.1365-2958.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- Bryant AE, Stevens DL. The pathogenesis of gas gangrene. In: Rood JI, McClane BA, Songer JG, Titball RW, editors. The clostridia: molecular biology and pathogenesis. 1st edn. London: Academic; 1997. pp. 185–196. [Google Scholar]
- Camiade E, Peltier J, Bourgeois I, Couture-Tosi E, Courtin P, Antunes A, Chapot-Chartier MP, Dupuy B, Pons JL. Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis. J Bacteriol. 2010;192:2373–2384. doi: 10.1128/JB.01546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman RJ, Sayeed S, Li J, Genheimer CW, Hiltonsmith MF, Wilkins TD, McClane BA. Clostridium perfringens toxin genotypes in the feces of healthy North Americans. Anaerobe. 2008;14:102–108. doi: 10.1016/j.anaerobe.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Nelson D, Zhu S, Fischetti VA. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother. 2005;49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2010;54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch S, Guezenec S, Piot M, Foster S, Lortal S. Mur-LH, the broad-spectrum endolysin of Lactobacillus helveticus temperate bacteriophage phi-0303. Appl Environ Microbiol. 2004;70:96–103. doi: 10.1128/AEM.70.1.96-103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E, López R, Garcia JL. Chimeric pneumococcal cell wall lytic enzymes reveal important physiological and evolutionary traits. J Biol Chem. 1991;266:5464–5471. [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP—Phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A. The Pfam protein families database. Nucleic Acid Res. 2010;38:D211–D222. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol. 2010;300:357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008;11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P, García JL, García E, Sánchez-Puelles JM, López R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–88. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- Garnier Cole ST. Characterization of a bacteriocinogenic plasmid from Clostridium perfringens and molecular genetic analysis of the bacteriocin-encoding gene. J Bacteriol. 1986;168:1189–1196. doi: 10.1128/jb.168.3.1189-1196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis. 2008;197:1519–1522. doi: 10.1086/587942. [DOI] [PubMed] [Google Scholar]
- Hamada H, Arakawa T, Shiraki K. Effect of additives on protein aggregation. Curr Pharm Biotechnol. 2009;10:400–407. doi: 10.2174/138920109788488941. [DOI] [PubMed] [Google Scholar]
- Hermoso JA, Monterroso B, Albert A, Galán B, Ahrazem O, García P, Martínez-Ripoll M, García JL, Menéndez M. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure. 2003;11:1239–1249. doi: 10.1016/j.str.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Jay JM, Loessner MJ, Golden DA. Modern Food Microbiology. 7th edn. New York: Springer; 2005. Food protection with chemicals, and by biocontrol; pp. 301–350. [Google Scholar]
- Kim W, Salm H, Geider K. Expression of bacteriophage phiEa1h lysozyme in Escherichia coli and its activity in growth inhibition of Erwinia amylovora . Microbiology. 2004;150:2702–2714. doi: 10.1099/mic.0.27224-0. [DOI] [PubMed] [Google Scholar]
- Korndörfer IP, Kanitz A, Danzer J, Zimmer M, Loessner MJ, Skerra A. Structural analysis of the L-alanoyl-D-glutamate endopeptidase domain of Listeria bacteriophage endolysin Ply500 reveals a new member of the LAS peptidase family. Acta Cryst. 2008;64:644–650. doi: 10.1107/S0907444908007890. [DOI] [PubMed] [Google Scholar]
- Lawrence GW. The pathogenesis of enteritis necroticans. In: Rood JI, McClane BA, Songer JG, Titball RW, editors. The clostridia: molecular biology and pathogenesis. 1st edn. London: Academic; 1997. pp. 197–210. [Google Scholar]
- Lima-Mendez G, Van Helden J, Toussaint A, Leplae R. Prophinder: a computational tool for prophage prediction in prokaryotic genomes. Bioinformatics. 2008;24:863–865. doi: 10.1093/bioinformatics/btn043. [DOI] [PubMed] [Google Scholar]
- Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- Loessner MJ. Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol. 2005;8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Matches JR, Liston J, Curran D. Clostridium perfringens in the environment. Appl Microbiol. 1974;28:655–660. doi: 10.1128/am.28.4.655-660.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GS, Rasko DA, Cheung JK, Ravel J, Seshadri R, DeBoy RT, Ren Q, Varga J, Awad MM, Brinkac LM, Daugherty SC, Haft DH, Dodson RJ, Madupu R, Nelson WC, Rosovitz MJ, Sullivan SA, Khouri H, Dimitrov GI, Watkins KL, Mulligan S, Benton J, Radune D, Fisher DJ, Atkins HS, Hiscox T, Jost BH, Billington SJ, Songer JG, McClane BA, Titball RW, Rood JI, Melville SB, Paulsen IT. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens . Genome Res. 2006;16:1031–1040. doi: 10.1101/gr.5238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D, Loomis L, Fischetti VA. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci USA. 2001;98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D, Schuch R, Chahales P, Zhu S, Fischetti VA. PlyC: a multimeric bacteriophage lysin. Proc Natl Acad Sci USA. 2006;103:10765–10770. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CJ, Schuch R, Pelzek AJ, Buckle AM, McGowan S, Wilce MC, Rossjohn J, Russell R, Nelson D, Fischetti VA, Whisstock JC. The 1.6-A crystal structure of the catalytic domain of PlyB, a bacteriophage lysin active against Bacillus anthracis . J Mol Biol. 2007;366:540–550. doi: 10.1016/j.jmb.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. LambdaSa1 and lambdaSa2 prophage lysins of Streptococcus agalactiae . Appl Environ Microbiol. 2007;73:7150–7154. doi: 10.1128/AEM.01783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichoff S, Lutkenhaus J. Overview of cell shape: cytoskeletons shape bacterial cells. Curr Opin Microbiol. 2007;10:601–605. doi: 10.1016/j.mib.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashel M, Uchiyama J, Takemura I, Hoshiba H, Ujihara T, Takatsuji H, Honke K, Matsuzaki S. Tail-associated structural protein gp61 of Staphylococcus aureus phage phi MR11 has bifunctional lytic activity. FEMS Microbiol Lett. 2008;284:9–16. doi: 10.1111/j.1574-6968.2008.01152.x. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Schuch R, Fischetti VA. Identifying phage lytic enzymes: past, present, and future. In: Villa TG, Veiga-Crespo P, editors. Enzybiotics: antibiotic enzymes as drugs and therapeutics. 1st edn. Hoboken: Wiley; 2010. pp. 219–251. [Google Scholar]
- Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis . Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- Seal BS, Fouts DE, Simmons M, Garrish JK, Kuntz RL, Woolsey R, Schegg KM, Kropinski AM, Ackermann HW, Siragusa GR. Clostridium perfringens bacteriophages ΦCP39O and ΦCP26F: genomic organization and proteomic analysis of the virions. Arch Virol. 2010 doi: 10.1007/s00705-010-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. Complete genome sequence of Clostridium perfringens, an anaerobic flesheater. Proc Natl Acad Sci. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons M, Donovan DM, Siragusa GR, Seal BS. Recombinant expression of two bacteriophage proteins that lyse Clostridium perfringens and share identical sequences in the C-terminal cell wall binding domain of the molecules but are dissimilar in their N-terminal active domains. J Agric Food Chem. 2010;58:10330–10337. doi: 10.1021/jf101387v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedly JG, III, Fisher DJ, Sayeed S, Chakrabarti G, McClane BA. The enteric toxins of Clostridium perfringens . Rev Physiol Biochem Pharmacol. 2004;152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration. [Accessed 22 October 2010];The bad bug book: foodborne pathogenic microorganisms and natural toxins handbook. 2009 http://www.fda.gov/Food/FoodSafety/FoodborneIllness/default.htm.
- Uzal FA, Songer JG. Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. J Vet Diagn Invest. 2008;20:253–265. doi: 10.1177/104063870802000301. [DOI] [PubMed] [Google Scholar]
- Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17:32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Wang S, Kong J, Zhang X. Identification and characterization of the two-component cell lysis cassette encoded by temperate bacteriophage phiPYB5 of Lactobacillus fermentum . J Appl Microbiol. 2008;105:1939–1944. doi: 10.1111/j.1365-2672.2008.03953.x. [DOI] [PubMed] [Google Scholar]
- Xu Q, Sudek S, McMullen D, Miller MD, Geierstanger B, Jones DH, Krishna SS, Spraggon G, Bursalay B, Abdubek P, Acosta C, Ambing E, Astakhova T, Axelrod HL, Carlton D, Caruthers J, Chiu HJ, Clayton T, Deller MC, Duan L, Elias Y, Elsliger MA, Feuerhelm J, Grzechnik SK, Hale J, Han GW, Haugen J, Jaroszewski L, Jin KK, Klock HE, Knuth MW, Kozbial P, Kumar A, Marciano D, Morse AT, Nigoghossian E, Okach L, Oommachen S, Paulsen J, Reyes R, Rife CL, Trout CV, van den Bedem H, Weekes D, White A, Wolf G, Zubieta C, Hodgson KO, Wooley J, Deacon AM, Godzik A, Lesley SA, Wilson IA. Structural basis of murein peptide specificity of a γ-D-glutamyl-L-diamino acid endopeptidase. Structure. 2009;17:303–313. doi: 10.1016/j.str.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Zhang X. Characterization of a lysin from deep-sea thermophilic bacteriophage GVE2. Appl Microbiol Biotechnol. 2008;78:635–641. doi: 10.1007/s00253-008-1353-1. [DOI] [PubMed] [Google Scholar]
- Yoong P, Schuch R, Nelson D, Fischetti VA. PlyPH, a bacteriolytic enzyme with a broad pH range of activity and lytic action against Bacillus anthracis . J Bacteriol. 2006;188:2711–2714. doi: 10.1128/JB.188.7.2711-2714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Loessner M, Morgan AJ. US Patent. 2008;7:371. 375. [Google Scholar]
- Zimmer M, Scherer S, Loessner MJ. Genomic analysis of Clostridium perfringens bacteriophage phi3626, which integrates into guaA and possibly affects sporulation. J Bacteriol. 2002a;184:4359–4368. doi: 10.1128/JB.184.16.4359-4368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Vukov N, Scherer S, Loessner MJ. The murein hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl Environ Microbiol. 2002b;68:5311–5317. doi: 10.1128/AEM.68.11.5311-5317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]