Abstract
BACKGROUND
The biologic mechanisms of allergic transfusion reactions (ATRs) are largely unknown. We sought to compare the atopic predisposition of platelet recipients who experienced an ATR to non-reactive control recipients.
STUDY DESIGN AND METHODS
We identified 37 consecutive apheresis platelet recipients who experienced an ATR and 26 matched controls. Total IgE and aero- and food-allergen-specific IgE were quantified in plasma by ImmunoCAP (Phadia, Phadiatop and Fx5). IgE testing of apheresis platelet supernatants was also performed.
RESULTS
Pruritus and urticaria were manifest in 91.9% and 83.8% of all ATRs, with more severe respiratory symptoms and angioedema occurring in <15% of cases. No subject had anaphylaxis. Sex, age, and primary diagnosis were balanced between the two groups. Total and aero-allergen specific IgE was higher among subjects experiencing an ATR in comparison to control subjects (median total IgE 55.5 kU/L vs. 8.3 kU/L, P=0.002; and median aero-allergen specific IgE 0.57 kUa/L vs. 0.36 kUa/L, P=0.046). IgE antibody levels in apheresis products associated with ATRs were similar to control products (P>0.1 for all IgE tests).
CONCLUSION
Recipient atopic predisposition, as defined by IgE sensitization, is a risk factor associated with ATRs.
Keywords: allergy, transfusion reaction, IgE, platelet
Introduction
Allergic transfusion reactions (ATRs) are common adverse reactions associated with the administration of blood components1–3. Most reactions are mild and typically manifest as pruritus and urticaria, and occasionally as angioedema and airway hypersensitivity. Uncommon protein deficiencies underlie the mechanism of severe allergic reactions in some transfusion recipients, but these deficiencies are too rare to account for the 1–3% incidence of mild ATRs4–6. Similarly, passive transfusion of allergens or allergen-specific IgE rarely results in an ATR7,8.
The mechanisms of ATR induction are largely unknown, but they are important to elucidate so that more effective ATR prevention strategies can be developed. Despite the ubiquitous use of antihistamines for the prevention of ATRs, two randomized controlled trials and a large observational study have demonstrated that antihistamines do not prevent ATRs9–12, At best, anti-histamines may mitigate symptoms to some extent. Rationally designed ATR prevention measures require that the mechanisms of the ATRs be understood.
Both recipient and donor factors appear to play roles in the development of common ATRs. Prior studies on ATRs and clinical experience show that ATRs tend to recur repeatedly in some patients, suggesting that a recipient’s hypersensitivity or susceptibility underlies the mechanism of common ATRs2. On the other hand, the plasma component of products appears to also be necessary for the development of an ATR, but plasma alone is not thought to be sufficient to induce an ATR13.
One model to explain the combination of donor and recipient factors needed to produce an ATR proposes that recipients who experience an ATR harbor allergic or atopic tendencies and are thus more likely to experience an ATR when necessary plasma factors are infused. Laboratory testing for IgE antibodies specific for common food and environmental allergens is an accepted method for assessing detecting patient’s state of sensitization to allergens. These measurements have been used to characterize the atopic phenotype of platelet recipients who have experienced an ATR. A previous study qualitatively assessed allergen-specific IgE levels and found that recipients who experienced an ATR were more likely to have an atopic predisposition than controls14. However the magnitude and specificity of the IgE antibody levels have not been evaluated. The goal of this study was to quantitatively evaluate atopic status as indicated by the IgE antibody levels in platelet recipients who experience ATRs and in the apheresis products they received.
Methods
Study Subjects
Sequential patients who experienced ATRs and control recipients were identified from all apheresis platelet recipients from January 2010 through April 2010. ATRs were identified by reports of ATRs to the Division of Transfusion Medicine at Johns Hopkins Hospital. Transfusion reactions were adjudicated as allergic by the attending physician, according to CDC Hemovigilance criteria (http://www.cdc.gov/nhsn/wc_bio_hemo_overview.html)15, which include at least two signs and symptoms of urticaria, pruritus, morbilliform rash, angioedema, flushing, bronchospasm, chemosis, and hypotension. Symptoms must occur during or within two hours after transfusion.
Subjects were stratified into diagnostic categories that generally encompass similar treatments and pathophysiologies. Control subjects without a history of an ATR were matched with ATR recipients for age, sex, and primary diagnostic category. Control subjects were selected to have a more extensive transfusion history without an ATR to ensure that they had ample opportunity to have experienced and ATR. Clinical histories and laboratory data were obtained through retrospective evaluation of the clinical and transfusion electronic records. Discarded plasma from blood bank samples and clinical data were obtained through an IRB approved protocol.
Sample Collection
Plasma from pre-transfusion blood bank samples was obtained for IgE studies and stored in the blood bank at 4 °C until it was retrieved for analysis. Collected plasma was re-spun at 1500g for 8 minutes and the supernatant was stored at −80 °C. Samples were ≤7 days old at the time of freezing, although IgE is stable for at least 6 months at 4 °C16. Supernatant from apheresis platelet products was also isolated for IgE studies. Residual apheresis platelet products returned to the blood bank after an ATR were collected and spun at 1500g for 5 minutes at 4 °C. The supernatant was re-spun for 5 minutes at 4 °C to remove any residual platelets and then stored at −80 °C. The control apheresis platelet products tested represent sequential platelet products issued from the blood bank that did not cause an ATR. These products were not the products transfused to the platelet recipients that served as controls.
IgE Quantitation Methods
Laboratory testing for total and allergen-specific IgE in pre-transfusion patient samples and supernatants from apheresis platelet products was performed using the ImmunoCAP system (Phadia, Kalamazoo, MI, USA). In brief, allergen-specific antibody was initially bound to one of two solid phase allergen cellulose matrices (called CAPs). The Phadiatop analysis employed the use of a CAP on which aero-allergens from a mixture of weed, grass and tree pollen, pet epidermal, dust mite and mold allergen groups were covalently bound. The Fx5 used a similar multi-allergen CAP except that allergens from 6 foods (cow’s milk, chicken egg, peanut, soybean, wheat and cod fish) were covalently bound. Following this initial binding reaction and a buffer wash to remove unbound plasma proteins, bound IgE was quantitatively detected with a fluorescently labeled anti-IgE. The level of fluorescence detected was proportional to the quantity of IgE antibody in the original test sample. Fluorescence levels were interpolated from an IgE calibration curve referenced to the World Health Organization IgE standard in kUa/L units, where 1 unit equals 2.4 nanograms of IgE. The total IgE assay differed from the allergen-specific IgE assay only in the use of an anti-IgE CAP which bound all IgE, irrespective of its specificity, in the first incubation step. The combined use of the Phadiatop and the Fx5 specific IgE screening assays are known to display the highest negative predictive value of any set of serological tests for IgE sensitization and thus the are considered useful indicators of atopic status.
On eight occasions, the dose of the platelet transfusion was two products and both products implicated in the ATR were returned to the blood bank. In these cases, supernatants from both apheresis products were tested, and IgE values were averaged between the two products and analyzed as a single, averaged value.
Statistics
Continuous variables were compared by the Wilcoxon rank sum test between ATR and control groups. Categorical variable comparisons between groups were performed using the Fisher exact test. P <0.05 was considered statistically significant. Analyses were conducted using Stata v11.1 (StataCorp, College Station, TX).
Results
Subject Characteristics
Baseline characteristics of the control and ATR subjects are shown in Table 1. Plasma and clinical data were collected on 37 patients experiencing an ATR. Seven patients had a second ATR during the study period, but only data from the initial ATR has been included in the analysis. Demographic and diagnostic distributions between the two study groups were similar. However, control subjects without a history of any ATR had an average of 53.9 transfusions of all product component types and subjects experiencing an ATR had an average of 35.0 transfusions.
Table 1.
Characteristics of platelet recipients
| Control Group (n=26) | ATR Group (n=37) | P | |
|---|---|---|---|
| Age, years, median (IQR) | 53 (43–61) | 49 (31–58) | 0.13 |
| Male, n (%) | 18 (69) | 25 (68) | 1 |
| Primary Diagnosis, n (%) | |||
| Acute Luekemia | 15 (57.7) | 18 (48.7) | 0.6 |
| HSCT | 3 (11.5) | 8 (21.6) | 0.5 |
| Other hematologic | 2 (7.7) | 5 (13.5) | 0.7 |
| Solid tumor | 1 (3.9) | 4 (10.8) | 0.4 |
| Lymphoma | 3 (11.5) | 2 (5.4) | 0.6 |
| Non-oncology medical | 1 (3.9) | 0 (0) | 0.4 |
| Surgical | 1 (3.9) | 0 (0) | 0.4 |
| Peripheral Blood Cell Counts, median (IQR) | |||
| Absolute neutrophil count | 842 (0–4100) | 244 (0–629) | 0.08 |
| Absolute eosinophil count | 0 (0–5) | 0 (0–15) | 0.9 |
| Absolute basophil count | 0 (0–0) | 0 (0–0) | 0.9 |
| Prior Transfusions, median units (IQR) | |||
| Platelet (apheresis units) | 18 (6–31) | 7 (2–26) | 0.06 |
| RBC | 22 (10–44) | 14 (6–23) | 0.06 |
| Number of Prior ATRs, median (IQR) | 0 (0–0) | 0 (0–1) | 0.002 |
IQR= Interquartile range
HSCT= Hematopoietic Stem Cell Transplant
Atopic disease can be associated with peripheral blood eosinophilia, but as most patients had oncologic primary diagnoses, bone marrow aplasia was common. Thus, peripheral blood leukocyte counts were generally low (Table 1) and no statistically significant differences in leukocyte counts were observed, although neutrophil counts tended to be lower in the ATR group.
ATR Clinical Features
The clinical features of the ATRs studied are presented in Table 2. Consistent with clinical experience and prior reports9,17,18 most ATRs involved pruritus and urticaria. Reports of pruritus and urticaria together without other symptoms accounted for 22/37 (59.5%) reactions. No patient developed anaphylaxis or required epinephrine.
Table 2.
Symptoms reported in transfusions with an ATR (n=37 total)
| n | % | |
|---|---|---|
|
|
||
| Pruritus, (non-airway) | 34 | 91.9 |
| Urticaria | 31 | 83.8 |
| Rash/Flusing | 6 | 16.2 |
| Wheezing/Dyspnea | 4 | 10.8 |
| Upper airway pruritus or angioedema | 3 | 8.1 |
Atopic Status based on Serological IgE Antibody Screening
Laboratory evidence of allergic sensitization was determined by a combined evaluation of the total and allergen-specific IgE levels. Figure 1 shows higher pre-transfusion total IgE and aero-allergen specific IgE levels in subjects experiencing ATRs as compared to controls. Median total IgE was 55.5 kU/L vs. 8.3 kU/L (P=0.002). Median Phadiatop IgE levels were 0.57 kUa/L vs. 0.36 kUa/L (P=0.046) in ATR vs. control subjects, respectively. Median food-specific IgE was similar between control and ATR groups (median of 0 in both groups), even though the proportion with a positive Fx5 tended to be higher in the ATR group (13.5% vs. 3.9%); however, this difference was not statistically significant (P = 0.4). Since IgE antibody specific for food allergens tends to be most common in children, the low frequency of food-specific IgE antibody observed in this adult study population was anticipated.
Figure 1. Total and allergen-specific IgE in platelet recipients.
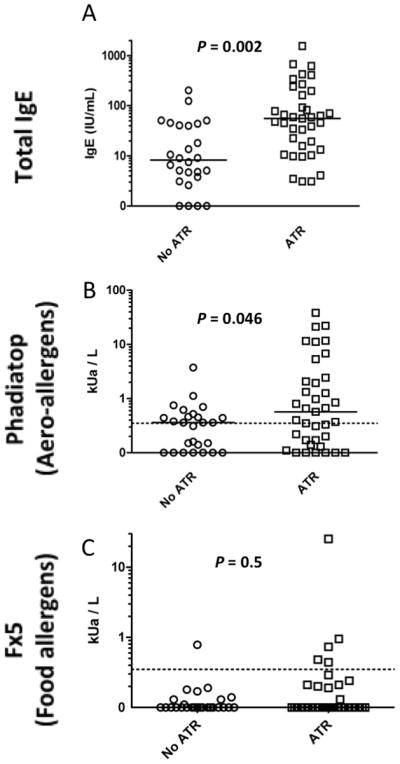
Total serum IgE and allergen-specific IgE, as measured by Phadiatop (aero-allergens) and Fx5 (food allergens) are compared between control platelet recipients (n=26) and recipients who experienced an ATR (n=37). Dashed lines represent the threshold for positivity in Phadiatop and Fx5 analyses. Bars represent median values.
Our previous work has suggested that certain platelet donors may be more strongly associated with ATRs13. To explore this possibility further, we tested platelet product supernatants for the presence of IgE antibody as an indicator of donor sensitization. Total and aero- and food-allergen specific IgE antibodies were measured in 31 platelet transfusions that resulted in ATRs and 30 control platelet products that did not result in an ATR (Figure 2). There were no statistically significant differences in any IgE levels among the study groups (P>0.1 for all comparisons).
Figure 2. Total and allergen-specific IgE in apheresis platelet products.
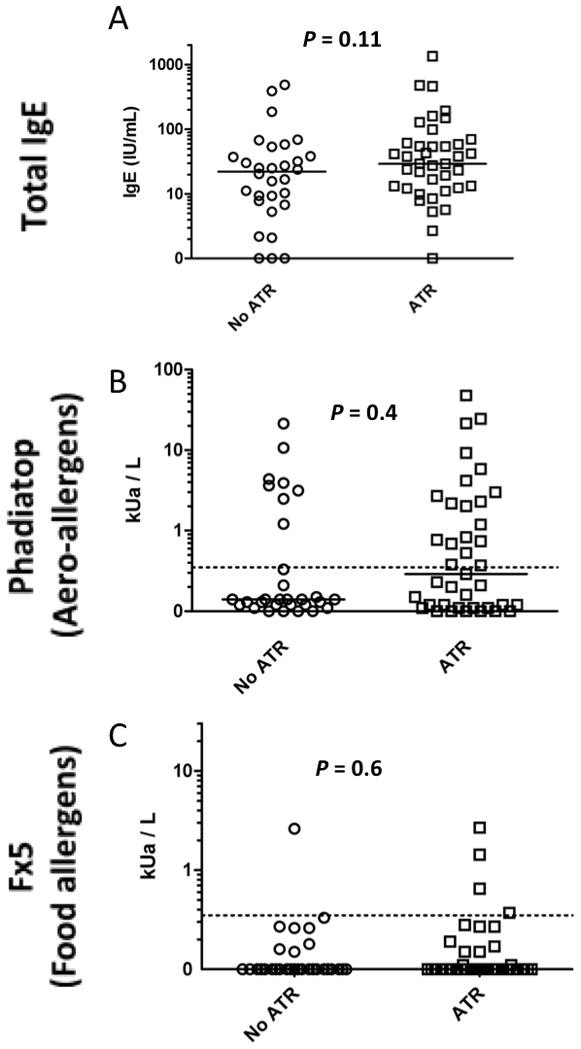
Total serum IgE and allergen-specific IgE, as measured by Phadiatop (aero-allergens) and Fx5 (food allergens) are compared between apheresis platelet products that were (n=31) or were not (n=30) implicated in an ATR. Dashed lines represent the threshold for positivity in Phadiatop and Fx5 analyses. Bars represent median values.
Discussion
In the present study, we provide evidence for atopy as one important risk factor that is associated with ATRs in apheresis platelet recipients. We observed that the median total IgE levels were 6.7-fold higher in recipients experiencing an ATR as compared to controls. Moreover, the median aeroallergen-specific IgE levels were 58% higher among recipients experiencing an ATR. The association of IgE sensitization appears to be more strongly associated with platelet recipients rather than platelet donors.
We chose to use the Phadiatop and Fx5 multi-allergen screens as indicators for atopy status based on data from several studies. In 2001, an American population of children and adolescents (ages 6–18 yrs) with history of rhinitis and an asthma prevalence of 59%, the Phadiatop displayed a diagnostic sensitivity of 98% against puncture skin test reference and 83.2% against a clinical history reference using a 0.35 kUa/L positive cutpoint19. The combined use of the Phadiatop and Fx5 has been shown to effectively predict the atopic state by age 4. Their combined degree of positivity correlated with the severity of recurrent wheeze and limited peak flows in a pediatric asthma population20,21.
It does not seem plausible that aeroallergens per se are being transfused and actually inducing the ATRs. We thus hypothesize that it is the predisposition of a platelet recipient’s basophils and mast cells that may be in a “primed” state to release allergic mediators in response to plasma proteins or small molecule mediators present in the plasma of donor blood components.
Food allergens can be passively transfused and have been rarely reported to be responsible for ATRs22. However, food allergy is uncommon in adults, in whom most ATRs occur. An estimate of the incidence of transfusion related food allergen exposure would require recipient allergy testing and a donor diet history. As laboratory testing to quantify the level of common food allergens in plasma is not possible, especially with food-specific antibodies being present in the plasma of most individuals, this hypothesis cannot be adequately tested. However, in the current study, most platelet recipients did not have a sensitivity to common food allergens. Only 13.5% of recipients who experienced an ATR showed allergic sensitization to any of the six most common food allergens that drive most food allergic responses. Thus, food allergen exposure appears to be a minor concern related to ATRs. Nevertheless, estimates of the incidence of food allergen-mediated ATRs could be clinically important because a simple dietary avoidance by donors could reduce the incidence of ATRs if there was an identifiable association.
One possible confounder in characterizing recipient atopic status is that most patients who experience ATRs are multiply transfused oncology patients. Passive transfer of IgE could possibly influence recipient IgE testing by sensitizing mast cells and basophils and in rare cases lead to an allergen-specific, IgE-induced ATR. However, the control subjects in this study had received almost twice as many prior transfusions, and total IgE was lower in controls than in ATR subjects. Thus, passive transfer of IgE does not appear to be related to ATRs on a quantitative level. The qualitative nature of the donor IgE transfused may be important, although one study found no association between specific IgE levels in donors and the development of ATRs8. Further research involving a longitudinal study of individual IgE specificities in the patient and products transfused is needed.
There are limitations to this study. Laboratory screening for atopy is not diagnostic of allergic disease; rather, IgE antibody is necessary but not sufficient for allergic disease manifestation, and thus it is viewed as a risk factor or an indicator of a predisposition for a hypersensitivity reaction. In general, it is important to link a patient’s clinical history with laboratory evidence for sensitization to confirm allergic disease. Clinical history and, in most cases, IgE antibody detection by skin prick tests or specific IgE tests, are required to confirm the diagnosis of allergic disease. Estimates of drug, food, and environmental allergies need to be evaluated in individuals experiencing an ATR using a prospective questionnaire which is combined with laboratory testing. However, performing skin prick testing in a safe manner is not feasible in a transfusion population that at the time of an ATR is frequently neutropenic and thrombocytopenic.
We evaluated apheresis platelet product supernatants rather than donor blood samples for this study. While testing the apheresis collection may skew IgE testing results away from values obtained from peripheral blood, ultimately it is the apheresis product exposure that is associated with the ATR in the recipient. Thus, testing levels IgE antibody in an apheresis product may be a more relevant measure of potential risk for becoming sensitized and later experience an ATR.
We conclude that the atopic status of the recipient is a predisposing risk factor for the development of an ATR. Thus, additional studies that examine both recipient and donor factors that could contribute to an ATR are needed.
Acknowledgments
Sources of support: This study was supported in part from internal funds provided by the Johns Hopkins DACI Reference Laboratory. WS acknowledges funding support from 5K12HL087169. JS acknowledges support from T32AI007056-31.
We thank the staff and technologists of the Johns Hopkins Hospital blood bank and Dermatology Allergy and Clinical Immunology (DACI) Reference Laboratory who were instrumental in sample acquisition and specimen analysis for this study. This study was supported in part from internal funds provided by the Johns Hopkins DACI Reference Laboratory. WS acknowledges funding support from 5K12HL087169. JS acknowledges support from T32AI007056-31.
Footnotes
Disclaimers: none
Conflicts of interest: none
References
- 1.Enright H, Davis K, Gernsheimer T, McCullough JJ, Woodson R, Slichter SJ. Factors influencing moderate to severe reactions to PLT transfusions: experience of the TRAP multicenter clinical trial. Transfusion. 2003;43:1545–52. doi: 10.1046/j.1537-2995.2003.00529.x. [DOI] [PubMed] [Google Scholar]
- 2.Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011 doi: 10.1111/j.1537-2995.2010.03008.x. [DOI] [PubMed] [Google Scholar]
- 3.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimada E, Tadokoro K, Watanabe Y, Ikeda K, Niihara H, Maeda I, Isa K, Moriya S, Ashida T, Mitsunaga S, Nakajima K, Juji T. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion. 2002;42:766–73. doi: 10.1046/j.1537-2995.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 5.Wibaut B, Mannessier L, Horbez C, Coupez B, Courbon B, Mizon P, Goudemand J. Anaphylactic reactions associated with anti-Chido Antibody following platelet transfusions. Vox Sang. 1995;69:150–1. doi: 10.1111/j.1423-0410.1995.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 6.Vamvakas E. Transfusion Reactions. 3. Bethesda: AABB Press; 2007. [Google Scholar]
- 7.Routledge RC, De Kretser DM, Wadsworth LD. Severe anaphylaxis due to passive sensitisation by donor blood. Br Med J. 1976;1:434. doi: 10.1136/bmj.1.6007.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern A, van Hage-Hamsten M, Sondell K, Johansson SG. Is allergy screening of blood donors necessary? A comparison between questionnaire answers and the presence of circulating IgE antibodies. Vox Sang. 1995;69:114–9. doi: 10.1111/j.1423-0410.1995.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–91. doi: 10.1111/j.1537-2995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanders RP, Maddirala SD, Geiger TL, Pounds S, Sandlund JT, Ribeiro RC, Pui CH, Howard SC. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130:781–7. doi: 10.1111/j.1365-2141.2005.05670.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang SE, Lara PN, Jr, Lee-Ow A, Reed J, Wang LR, Palmer P, Tuscano JM, Richman CM, Beckett L, Wun T. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70:191–4. doi: 10.1002/ajh.10119. [DOI] [PubMed] [Google Scholar]
- 12.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–96. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 13.Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic transfusion reactions to platelets are associated more with recipient and donor factors than with product attributes. Transfusion. 2011 doi: 10.1111/j.1537-2995.2010.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilhelm D, Kluter H, Klouche M, Kirchner H. Impact of allergy screening for blood donors: relationship to nonhemolytic transfusion reactions. Vox Sang. 1995;69:217–21. doi: 10.1111/j.1423-0410.1995.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuehn BM. CDC launches surveillance system to improve blood transfusion safety. JAMA. 2010;303:1467. doi: 10.1001/jama.303.15.1467. [DOI] [PubMed] [Google Scholar]
- 16.Bazaral M, Hamburger RN. Standardization and stability of immunoglobulin E (IgE) The Journal of allergy and clinical immunology. 1972;49:189–91. doi: 10.1016/0091-6749(72)90113-3. [DOI] [PubMed] [Google Scholar]
- 17.Heddle NM, Klama L, Meyer R, Walker I, Boshkov L, Roberts R, Chambers S, Podlosky L, O’Hoski P, Levine M. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion. 1999;39:231–8. doi: 10.1046/j.1537-2995.1999.39399219278.x. [DOI] [PubMed] [Google Scholar]
- 18.Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–20. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 19.Williams PB, Siegel C, Portnoy J. Efficacy of a single diagnostic test for sensitization to common inhalant allergens. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2001;86:196–202. doi: 10.1016/S1081-1206(10)62691-9. [DOI] [PubMed] [Google Scholar]
- 20.Wickman M, Ahlstedt S, Lilja G, van Hage Hamsten M. Quantification of IgE antibodies simplifies the classification of allergic diseases in 4-year-old children. A report from the prospective birth cohort study. BAMSE Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2003;14:441–7. doi: 10.1046/j.0905-6157.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 21.Wickman M, Lilja G, Soderstrom L, van Hage-Hamsten M, Ahlstedt S. Quantitative analysis of IgE antibodies to food and inhalant allergens in 4-year-old children reflects their likelihood of allergic disease. Allergy. 2005;60:650–7. doi: 10.1111/j.1398-9995.2004.00764.x. [DOI] [PubMed] [Google Scholar]
- 22.Arnold DM, Blajchman MA, Ditomasso J, Kulczycki M, Keith PK. Passive transfer of peanut hypersensitivity by fresh frozen plasma. Arch Intern Med. 2007;167:853–4. doi: 10.1001/archinte.167.8.853. [DOI] [PubMed] [Google Scholar]


