Abstract
Allergic transfusion reactions (ATRs) are a spectrum of hypersensitivity reactions that are the most common adverse reaction to platelets and plasma, occurring in up to 2% of transfusions. Despite the ubiquity of these reactions, little is known about their mechanism. In a small subset of severe reactions, specific antibody has been implicated as causal, although this mechanism does not explain all ATRs. Evidence suggests that donor, product, and recipient factors are involved, and it is possible that many ATRs are multi-factorial. Further understanding of the mechanisms of ATRs is necessary so that rationally designed and cost-effective prevention measures can be developed.
Keywords: allergy, transfusion reaction, platelets, plasma, red cell, hypersensitivity, urticaria, pruritus
Allergic transfusion reactions (ATRs) are the most common adverse events associated with platelet and plasma transfusion, and ATRs are second in incidence to febrile reactions among red cell transfusions1. Reported incidence rates depend on the degree of active surveillance vs. passive reporting to the blood bank. Best estimates using active surveillance of transfusions show that ATRs are associated with about 2% of platelet transfusion2,3. There is less active surveillance data for red cell transfusion, but the incidence rate is about 0.1–0.5%1.
ATRs most commonly manifest with urticaria, pruritus, erythematous rash, angioedema, bronchospasm, and/or hypotension. These manifestations occur on a spectrum of severity and most commonly are mild, involving localized pruritus and/or urticaria only. More severe reactions involving angioedema, bronchospasm, or hypotension occur in less than 10% of ATRs4,5.
Regardless of severity, all ATRs cause patient morbidity and incur costs of transfusion reaction evaluation and possible product wastage6. The high incidence of these reactions makes them a cumulative burden on transfusion medicine specialists and patients, particularly chronically transfusion-dependent patients with recurrent reactions. A more comprehensive understanding of the mechanisms of ATRs will lead to strategies that reduce the incidence of these reactions. The goal of this review is to summarize our limited understanding of ATR mechanisms and their prevention.
The scope of our understanding of ATRs
Because ATRs are characterized by the development of allergic symptoms, it has been assumed that the mechanism of IgE mediated, Type I immediate hypersensitivity reactions to a specific allergen (discussed below) explains ATRs. Indeed, it has been established that ATRs can occur due to 1) the passive transfer of specific antibody to a transfusion recipient and subsequent allergen exposure or 2) the development in a transfusion recipient of antibody specific to donor protein. These mechanisms are conceptually elegant, and there is a long list of reports consistent with these mechanisms7–27. However, most of these reports describe severe reactions, and there are no data that support the generalization of these mechanisms to the most common pruritic and urticarial reactions that occur almost daily in large centers. Furthermore, ATRs typically occur in a small proportion of transfusions for a given patient, if they are recurrent at all28, leaving uncertainty as to why some products seem to cause ATRs and others do not.
The clinical manifestations of ATRs are similar to the signs and symptoms of other allergic reactions. Although the intravenous route of administration differs from most allergic exposures, e.g. topical, inhalational, gastrointestinal, the manifestations are similar. Data available from allergic reactions to radiocontrast media, which is another exclusively intravascular exposure, demonstrate the same spectrum of signs and symptoms as seen in ATRs and non-vascular allergic exposures: pruritus, urticaria, flushing, bronchospasm, emesis, abdominal pain, and hypotension29,30. The diagnosis of ATRs is complicated by the overlapping manifestations of hypotensive, fluid overload, TRALI, and septic reactions. The presence of cutaneous signs, the absence of fever, and the clinical and radiologic differences between pulmonary edema and bronchospasm usually make the diagnosis apparent among the differential diagnosis of transfusion reactions. Among anaphylaxis cases in an emergency room setting, cutaneous findings of pruritus, urticaria, edema, and flushing were present in the majority of patients with allergic reactions31. Isolated hypotension or bronchospasm is unusual among allergic reactions in general. The distribution of ATR signs and symptoms according to standardized criteria or a comparison of anaphylaxis from transfusion to anaphylaxis from other exposures has not been specifically studied.
History of ATRs
Modern observations of hypersensitivity responses to blood components began at the end of the 19th century when immediate hypersensitivity reactions and serum sickness were noted after immunizations in humans and animals. The first theories about the mechanisms of sensitization with foreign antigen were proposed separately in 1903 by Nicolas Arthus32 and Bela Schick and Clemens von Pirquet33. Indeed, the introduction of the term “allergy” (Greek allos “other” + ergon “reaction”) was borne out of the work of von Pirquet and Schick34. While the work is not specifically identified as transfusion-specific, many of the experiments involved transfusion of serum intravenously. Von Pirquet noted that upon re-challenge of horse serum into children, there was sometimes an “immediate reaction” that consisted of urticaria, redness, and edema, and the reaction was “sometimes accompanied by collapse.”35 Their work helped lay the framework for the landmark 1963 Gell and Coombs classification of the four types of hypersensitivity reactions36, with type I reactions being immediate hypersensitivity reactions, which include anaphylaxis.
The first reported ATRs were identified as passive transfer of horse allergy into previously non-allergic recipients7,8. Human experiments investigating passive sensitization to food and aero-allergens began in 193910. Remarkably, the initial experiments accurately depict the same time course of passive sensitization that was observed in experiments done 66 years later with specific IgE kinetic studies10,22, suggesting passive transfer of IgE as a mechanism of allergy. As early as 1944, it was reported that some transfusion recipients who had experienced ATRs to serum had less severe reactions upon repeat exposure to the same product. The author concluded that it was possible to desensitize subjects to blood products37, as is often done for treatment of other hypersensitivity reactions, although other mechanisms may have been involved.
A major breakthrough in the modern understanding of ATRs came in the late 1960s with reports by Vyas and Schmidt of IgA deficiency and anti-IgA antibodies as a specific mechanism for ATRs11,12. These were the first molecular descriptions of a specific hypersensitivity that caused allergic reactions through transfusion. The paradigm of protein-antibody reactions is the most extensively described model for ATRs. Associations of other protein deficiencies/polymorphisms, e.g. C415 and haptoglobin21, with ATRs followed. Unusual antibody-mediated mechanisms of anti-CD36 antibody23 and multimeric IgE in donor plasma25 have been described recently. While many of these reports support a causal role for protein-specific antibodies in ATRs, they do not explain all ATRs.
Of note, the literature of ATRs to date reflects the terminology used in the fields of allergy and immunology. Historically, the terms allergic, anaphylactoid, and anaphylactic have been applied to describe hypersensitivity reactions to transfusion. “Anaphylactoid” has been used variably to describe reactions of moderate severity that do not qualify as anaphylaxis or reactions that are not mediated by IgE. Some allergy experts suggest that the term “anaphylactoid” is confusing and discourage its use38,39. Newer terms of “immunologic” and “non-immunologic” hypersensitivity have been proposed38. Immunologic mechanisms refer to specific antigen recognition by antibody or T cells. Non-immunologic mechanisms are thought to directly increase the susceptibility of mast cells to release histamine and other mediators of anaphylaxis. Non-immunologic mechanisms are thought to be involved with radiocontrast media40 and opioid41 reactions, for example. Anaphylaxis is currently defined as an acute, life-threatening reaction involving skin, mucosal tissue (e.g. lips), or both, and at least one symptom of respiratory compromise or hypotension (≥30% decrease from baseline or symptom consistent with hypotension)42.
General mechanisms of allergic reactions
Because urticaria, pruritus, angioedema, bronchospasm, and shock are familiar allergic manifestations, it is assumed that the same allergic pathophysiologic mechanisms that underlie other allergic diseases are responsible for ATRs. Therefore, a basic understanding of the pathophysiology of immediate (Type 1) hypersensitivity reactions provides a context in which to discuss the pathophysiology of ATRs. The pathophysiology of type I hypersensitivity reactions is most relevant because most allergic reactions to transfused blood products occur immediately or within a few hours of exposure and present clinically with symptoms similar to type I hypersensitivity reactions. Type I hypersensitivity reactions are classically mediated by allergen specific IgE, which can be quantified.
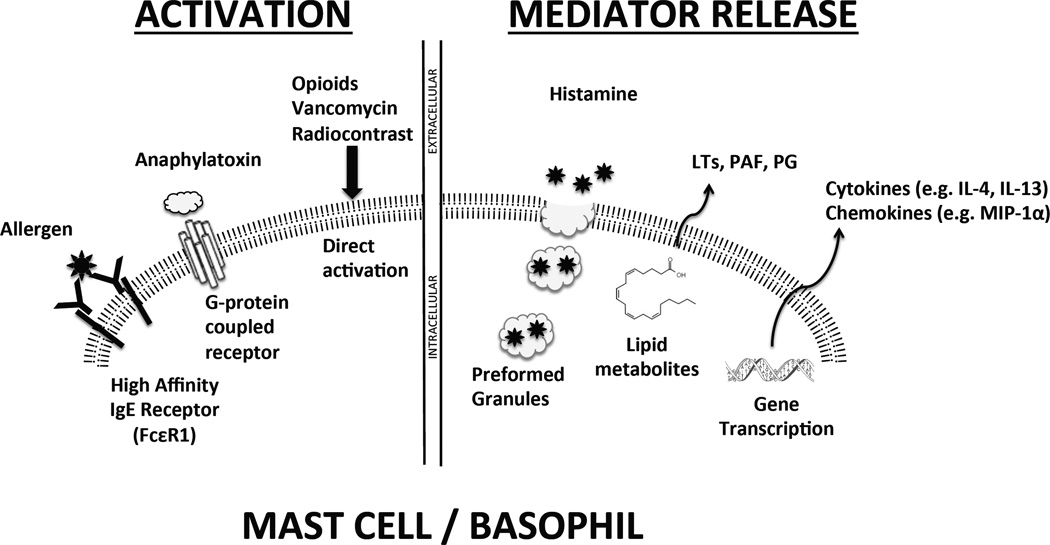
Activation of mast cells and basophils, the primary allergic effectors of immediate hypersensitivity reactions, typically occurs after cell surface high-affinity IgE receptors (FcεRI) aggregate in response to cell surface IgE binding specific antigen (Figure 1). Details of the cell signaling in this context have been recently reviewed43–45. IgE/antigen interactions lead to a signal transduction cascade that results in the immediate release of preformed mediators such as histamine. There is also de novo synthesis of lipid mediators such as leukotrienes and platelet activating factor. Changes in gene expression and cytokine and chemokine generation are consistent with the onset time of the so-called “late-phase” of an allergic reaction, which peaks 6–8 hours after exposure46.
Figure 1. Summary of mast cell/basophil activation and mediator release.
The best understood mechanism for activation is the aggregation of high affinity IgE receptor on the cell surface after exposure to allergen, but IgE-independent mechanisms of activation have also been described. After activation, mediators may be released immediately via preformed granule release or immediate synthesis of lipid mediators; products of gene transcription may be synthesized within hours. LT- leukotriene; PAF- platelet activating factor, PG- prostaglandin; MIP-1α – macrophage inflammatory protein-1α
IgE-independent mechanisms that can lead to clinical manifestations of immediate hypersensitivity reactions have also been described. IgG can directly induce anaphylaxis by binding the low affinity IgG receptor FcγRIII in mouse models47. The significance of IgG in anaphylactic reactions in humans is not well established but limited case series provide evidence for this mechanism, which may involve direct complement activation48–50. Iodinated, hyperosmolar radiocontrast compounds can cause IgE-independent reactions. Investigations into the mechanism of these reactions show that they may be mediated by anaphylatoxin (complement component C3a, C4a, or C5a) activation, hyperosmolar effect on mast cells, or direct, osmolarity-independent induction of histamine release, as with opioids40,41. Evidence for non-immunologic mechanisms also comes from studies of direct injection of allergic agonists, e.g. bradykinins, leukotrienes, and platelet activating factor, which stimulate histamine release from human basophils and mast cells51–54.
Clinical observations that give insight into the mechanisms of ATRs
Clinical studies have helped define in broad strokes the recipient and product factors that are involved in the mechanisms of allergic transfusion reactions. Notable observations are listed below.
Plasma as the culprit
It has been known for decades that plasma reduction of products is associated with a lower incidence of ATRs55,56. There appears to be a dose response relationship between the amount of plasma and the incidence of ATRs. Tobian et al showed that in a selected cohort of platelet recipients with recurrent ATRs, the ATR incidence rate of unmanipulated, concentration, washed platelet components was 5.5%, 1.7%, and 0.5%, respectively57. Furthermore, platelet components prepared with platelet additive solution (PAS) have a reduced plasma component and are associated with a lower incidence of ATRs58–60. It is not known whether the plasma agents responsible for ATRs are present in the donor at the time of collection or arise during platelet processing and storage, but there is not an obvious association between storage time and ATRs3. A recent study suggests that ABO incompatible platelet transfusion results in higher rates ATRs61, but the contributions of incompatible plasma vs. incompatible platelets were not described.
Plasma proteins are obvious suspects for the etiology of antibody-mediated ATRs, as a limited number of examples demonstrate that antibody-mediated hypersensitivity to proteins underlies some ATRs. This mechanism requires an initial exposure to develop antibody and subsequent sensitization. Ahmed et al evaluated the incidence of ATRs to first red cell transfusion in multiparous women, who are known to be to be exposed and sensitized to a variety of antigens during pregnancy. This suggestive study found that the incidence of mild ATRs to first transfusion increased from 0% with 0 or 1 prior pregnancy to 3.8%, 8.3%, 21.7% and 37.5% with two, three, four, and five prior pregnancies, respectively.62 Thus, the frequency of fetal exposure directly correlates with the risk of ATR on initial transfusion. Sensitization to specific allergen can occur with prior transfusion, but auto-antibodies can be formed in the absence of identifiable exposure, as has been demonstrated with anti-IgA63. Nevertheless, prior sensitization does not necessarily lead to reactions64. Sensitization can rarely occur through passive transfer of plasma containing specific antibody, as demonstrated by cases of passively acquired food and drug allergy14,24,65. It has been considered that food allergen could be consumed by a donor and transmitted via plasma to a sensitized recipient26, but this hypothesis has not been proven yet66.
No apparent role of leukocytes
Leukocytes in blood components do not appear to be directly involved with the pathogenesis of ATRs, even though they are capable of producing several mediators that could produce symptoms of ATRs. First, there is the observation that acellular plasma components are a common cause of ATRs67. Second, as universal leukoreduction became universally adopted in certain centers, reductions in febrile, but not allergic, reactions were noted68–70.
Atopic predisposition of recipients, not donors
Allergic individuals tend to have multiple manifestations of allergic disease, and it is reasonable to suspect that an atopic predisposition is a risk factor for ATRs. Maunsell experimentally found that an atopic history to environmental allergens is associated with an increased risk of an ATR37. Wilhelm et al found that 91% of platelet recipients tested positive for IgE specific to environmental allergens71, and Savage et al. reported that median total IgE, a crude measure of atopic predisposition, was 6.7-fold higher in subjects who experienced an ATR as compared to controls who never had an ATR, and IgE specific to common environmental allergens was 58% higher than controls4. The lack of concordance of ATRs in two different recipients of the same apheresis platelet collections also corroborates the concept that a recipient, not an intrinsic product characteristics increases susceptibility to ATRs28. These clinical observations are corroborated by in vitro data that show the threshold and magnitude (i.e. priming) of histamine release from mast cells and basophils varies among individuals72 and that transfusion recipients who experience ATRs have plasma factors that increase susceptibility to mast cell calcium influx and histamine degranulation73. It does not seem plausible that the IgE specific to environmental allergens is causing the ATR per se, but the general atopic predisposition of the patient that increases susceptibility to an ATR. Of note, an atopic predisposition may be acquired22, as in a case of passively transferred peanut-specific IgE24. Genetic predispositions to ATRs have not been studied, as they have in other allergic diseases74.
There is limited evidence that certain donors are associated more frequently with ATRs than the general donor population25,28. However, there are no reported estimates of the frequency of suspect donors in a large donor population. Atopic disease in donors does not appear to confer a risk of ATRs4,75, even though atopy is prevalent in donor populations76. Nevertheless, if it is confirmed that some donors are particularly associated with ATRs independent of the recipient, it remains unknown to what extent the increased risk is intrinsic to the donor, as reported in rare cases23,25, or a susceptibility that is unmasked during component processing and storage.
Relationship of specific mediators and predispositions to ATRs
Histamine
The fact that nearly all ATRs occur during or very soon after transfusion suggests that pre-formed or quickly synthesized mediators contribute to the clinical manifestations of ATRs. A primary mediator of these type I hypersensitivity manifestations is histamine. It is also important to understand the role of histamine in allergic reactions because anti-histamines are the most widely used drugs to treat ATRs.
Histamine is a histidine-derived small molecule for which there are four histamine receptor subtypes. Clinical manifestations attributable to histamine include bronchospasm, headache, flushing, palpitations, angioedema, hypotension, and rhinitis77. Histamine is stored preformed in mast cells and basophils and can be released after IgE aggregation or other antibody independent stimuli, e.g. opioids41 or activated complement78. Histamine increases vascular permeability and activates sensory neurons in conjunction with other mediators79. Histamine has a half-life of minutes in blood and is released into blood almost immediately after allergen challenge or mast cell or basophil activation80,81.
Histamine is present in blood components. Levels of histamine in blood components depend on the number of leukocytes and duration of storage; however, leukodepletion markedly reduces plasma concentrations in blood components82–86. The lack of an effect of leukoreduction on incidence rates of ATRs argues against a role for passive transfer of histamine as a cause of ATRs. One study found higher in vivo plasma histamine in recipients who experience ATRs, but it is not clear if this elevation precedes or is a consequence of ATRs84.
Complement
The complement system is a coordinated set of plasma proteins that has a primary role in attacking extracellular pathogens. Dysregulated and maladaptive complement activation is an inflammatory pathway commonly involved in anaphylaxis and many diseases. Anaphylatoxins are activated cleavage products of the complement pathway87. Among the anaphylatoxins C3a, C4a, and C5a, C5a is the most potent; they cause increases in vascular permeability, bronchospasm, and histamine release. Mast cells and basophils express complement receptors for C3a (which also binds C4a) and C5a88,89.
The extensive literature on the platelet storage lesion90 leads to the hypothesis that complement component accumulation during storage could lead to ATR development. C3a and C4a, but not C5a have been demonstrated to increase during storage91–94. C3a increases markedly during blood collection but is apparently rapidly degraded and/or adsorbed93. Accumulation of anaphylatoxins during storage is a possible mechanism of ATRs in platelet concentrates, but there does not appear to be an association between storage time of platelets and the development of ATRs3. Although levels of C5a remain low, there is an association of products that contain higher C5a levels with ATRs95.
Specific protein deficiencies and polymorphisms
Exposure of a transfusion recipient to a foreign protein is a known mechanism of sensitization. IgA12 and haptoglobin21 deficiencies are classic examples; however, protein deficiency is not a universal set-up for ATRs. There is extensive experience with coagulation factor replacement that shows a low rate of allergic reactions, ∼1 in 10,000 doses96 or 3% of recipients97. Other experience of plasma and plasma protein replacement in subjects with severe alpha-1-antitrypsin deficiency shows a similarly low incidence98,99. Reports of C4 polymorphisms and ATRs raise the possibility that differences in protein polymorphisms between donors and recipients could be a common source of intermittent ATRs in recipients when given donor products mismatched for a polymorphic protein. Indeed, in the report from Shimada et al on haptoglobin deficient patients, one was noted to have “relatively mild reactions.”21 Antibodies appear to be made frequently to transfused proteins, e.g. albumin, fibrinogen, C2, and C4, but the formation of these antibodies has not been studied in the context of ATRs100.
Other product-derived factors
Platelets are a rich source of several molecules that have been demonstrated to either prime allergic effector cells or directly activate these cells to release allergic mediators101. The observation of ATRs after autologous transfusion suggests a product-specific mechanism in some cases5`. CCL5 (RANTES) is one such candidate because it is abundant in platelets, is released during storage102,103, and can activate allergic effector cells104. Three studies have evaluated CCL5 concentrations in platelets: two found minimally higher levels in products associated with ATRs95,105 and the third study, which was augmented by in vitro functional assays, found no difference106. The literature that describes molecules released by platelets during storage may serve as a list for candidate mediators of ATRs107. While little is known about the role of molecules like CCL5 in ATRs, even less is known about potential direct allergic agonists in red cell and plasma transfusion. Nevertheless, the observation that ATRs can occur with autologous transfusion supports the concept that factors elaborated during storage underlie some ATRs5.
A non-blood derived product source of allergen is ethylene oxide, which has been described as a cause for allergic reactions in apheresis donors16,17, but the applicability of ethylene oxide antibodies to transfusion recipients has not been published. Some transfusion recipients may develop IgE antibodies against plasticizer compounds108. The relationship of these antibodies to transfusion reactions has yet to be demonstrated.
Synthesizing the data: a two-prong model of ATRs
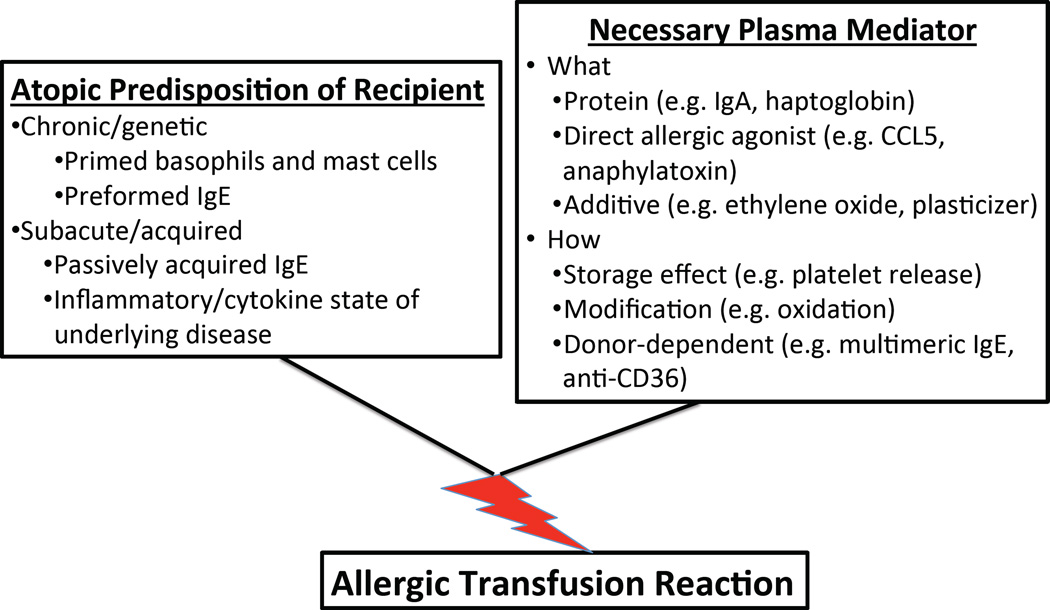
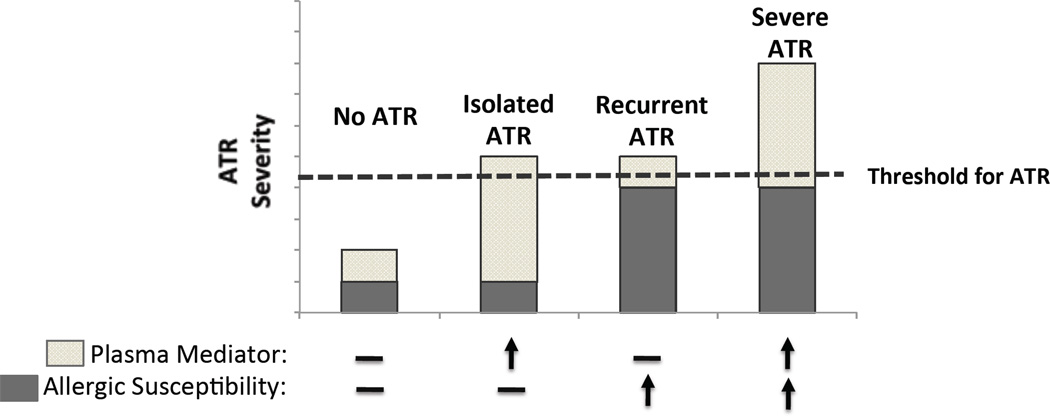
The current evidence summarized in this review supports the concept that both atopic susceptibility in the recipient as well as particular donor/product characteristics are unique risk factors for the development of ATRs. Thus, we propose a model in which the frequency and possibly even the severity of ATRs would depend on the combination of how strong the recipient predisposition and specific donor or product factors (Figure 2).
Figure 2. Conceptual model of allergic transfusion reactions.
(A) ATRs may result from a combination of recipient atopic predisposition and a necessary plasma mediator in the blood component. Known and speculative factors are shown. (B) The degree of recipient susceptibility at the time of transfusion and magnitude of the plasma mediator(s) may determine the severity of an ATR.
The concept of recipient, product, and donor contributions to the development of an ATR has implications for research. Studies that evaluate only one part of the recipient, product, or donor aspects of transfusion may miss key aspects of the process compared to those that control for the other parts of the donor-product-recipient chain.
Prevention of allergic reactions
A primary motivation for understanding of the mechanisms of ATRs is to develop effective, rational prevention strategies. Vamvakis summarized ATR prevention strategies as component-centered vs. patient-centered approaches109. As stated previously, plasma reduction is an intervention shown to reduce the incidence of ATRs. Based on available data, universal adoption of PAS platelets might be expected to reduce the overall burden of ATRs, as ATRs are most commonly reported to platelets. In the absence of PAS platelets, plasma reduction is not always feasible, given the time and labor requirements of plasma reducing individual products. However, plasma reduction methods often come at a cost of reduced platelet yields and corrected count increments59,110,111.
Antihistamines are commonly used as transfusion premedications in an attempt to reduce the incidence of ATRs1,112,113. The sedating H1 receptor antagonist diphenhydramine is the most frequently used antihistamine. A recent systematic review by Marti-Carvajal et al evaluated three RCTs114–116 and concluded no benefit of antihistamines117. Other studies also do not report a difference in ATR rates with premedication118,119. In spite of the lack of evidence for the practice, many hospitals have placed premedication orders in their transfusion order sets, encouraging the continuing use of a wasteful practice.
Despite the lack of evidence for efficacy as a prophylactic agent, there is extensive, unpublished clinical experience of symptomatic benefit of antihistamines once an ATR manifests, which is consistent with studies demonstrating efficacy in other allergic diseases120. The combination of H1 antagonists with H2 receptor or other non-sedating antihistamines has not been studied in the setting of transfusion. In vivo data suggest a synergistic effect of H1 and H2 antagonists in the alleviation of symptoms during histamine infusion77, but the relevance of this to transfusion is not clear.
Using glucocorticoids for the prevention and treatment of severe ATRs has not been studied but is a common practice that is borrowed from experience from severe allergic reactions in other settings121. It is hoped that other antihistamines or other classes of medications will be able to prevent ATRs in the future as we learn more about the mechanisms of ATR and perform rationally designed clinical research studies based on knowledge of these mechanisms.
Challenges and future directions in ATR research
Anaphylactic reactions understandably receive special attention in research, and certainly our understanding is relatively restricted to these reactions. It is not known to what extent the common, mild ATRs are less intense manifestations of severe forms or whether they represent a different pathophysiology altogether. Common ATRs tend to be studied in aggregate, and it is not known if there are patterns or clusters of certain manifestations (e.g. respiratory) with certain types of transfusion recipients (e.g. asthma). More detailed study of recipient susceptibility factors and the clinical manifestations of ATRs may help parse categories of ATRs beyond “mild” or “severe.”
Studies of ATRs would appear to be at a disadvantage because any transfused allergic mediator is disseminated systemically and no one site is a target, as in allergic rhinitis or asthma. Furthermore, blood components represent complex, heterogeneous exposures to potential allergen. Nevertheless, the most common manifestations of ATRs are cutaneous4,5, and this distribution suggests involvement of cutaneous mast cells or basophils, which are amenable to direct study51,52,54,104,122.
After an initial transfusion, subjects can be actively and passively sensitized. That is, recipients may not only develop antibodies to transfused proteins, but they receive antibodies from donor plasma. Disentangling the contribution of passive sensitization from transfusion of different donors’ plasma will not be possible until a more comprehensive understanding of ATR mechanisms is known. In vitro and animal models have been applied sparingly to questions about ATRs. Some of the limitations of clinical ATR research can be controlled for in laboratory settings. A combination of clinical and translational lab research is needed to enhance our understanding of ATRs.
In summary, ATRs are common, problematic transfusion reactions that cause morbidity and consume time and money. Only with a deeper understanding of the pathophysiology of ATRs will rationally designed prevention strategies be possible.
Acknowledgments
Supported in part by ASH Scholar Award, R21HL107828
Footnotes
Presented in part at the Annual Meeting, AABB, 2011, Emily Cooley Memorial Lecture.
The authors declare no conflicts of interest.
References
- 1.Geiger TL, Howard SC. Acetaminophen and diphenhydramine premedication for allergic and febrile nonhemolytic transfusion reactions: good prophylaxis or bad practice? Transfusion medicine reviews. 2007;21:1–12. doi: 10.1016/j.tmrv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heddle NM, Klama L, Meyer R, Walker I, Boshkov L, Roberts R, Chambers S, Podlosky L, O'Hoski P, Levine M. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion. 1999;39:231–238. doi: 10.1046/j.1537-2995.1999.39399219278.x. [DOI] [PubMed] [Google Scholar]
- 3.Sarkodee-Adoo CB, Kendall JM, Sridhara R, Lee EJ, Schiffer CA. The relationship between the duration of platelet storage and the development of transfusion reactions. Transfusion. 1998;38:229–235. doi: 10.1046/j.1537-2995.1998.38398222865.x. [DOI] [PubMed] [Google Scholar]
- 4.Savage WJ, Tobian AA, Savage JH, Hamilton RG, Ness PM. Atopic predisposition of recipients in allergic transfusion reactions to apheresis platelets. Transfusion. 2011;51:2337–2342. doi: 10.1111/j.1537-2995.2011.03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domen RE, Hoeltge GA. Allergic transfusion reactions: an evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–320. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 6.Riley W, Smalley B, Pulkrabek S, Clay ME, McCullough J. Using lean techniques to define the platelet (PLT) transfusion process and cost-effectiveness to evaluate PLT dose transfusion strategies. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03539.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez M. Horse asthma following blood transfusion. JAMA. 1919;73:984–985. [Google Scholar]
- 8.Mills C, Schiff L. Passive anaphylaxis in a hemophiliac. Am J Med Sci. 1926;171:854–855. [Google Scholar]
- 9.Tedstrom M. Active transmission of urticaria by blood transfusion. J Allergy. 1934;5:303–305. [Google Scholar]
- 10.Loveless M. Passive sensitization of man through transfusion. Journal of Immunology. 1941;41:15–34. [Google Scholar]
- 11.Schmidt AP, Taswell HF, Gleich GJ. Anaphylactic transfusion reactions associated with anti-IgA antibody. The New England journal of medicine. 1969;280:188–193. doi: 10.1056/NEJM196901232800404. [DOI] [PubMed] [Google Scholar]
- 12.Vyas GN, Perkins HA, Fudenberg HH. Anaphylactoid transfusion reactions associated with anti-IgA. Lancet. 1968;2:312–315. doi: 10.1016/s0140-6736(68)90527-8. [DOI] [PubMed] [Google Scholar]
- 13.Sandler SG, Eckrich R, Malamut D, Mallory D. Hemagglutination assays for the diagnosis and prevention of IgA anaphylactic transfusion reactions. Blood. 1994;84:2031–2035. [PubMed] [Google Scholar]
- 14.Routledge RC, De Kretser DM, Wadsworth LD. Severe anaphylaxis due to passive sensitisation by donor blood. Br Med J. 1976;1:434. doi: 10.1136/bmj.1.6007.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambin P, Le Pennec PY, Hauptmann G, Desaint O, Habibi B, Salmon C. Adverse transfusion reactions associated with a precipitating anti-C4 antibody of anti-Rodgers specificity. Vox sanguinis. 1984;47:242–249. doi: 10.1111/j.1423-0410.1984.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 16.Leitman SF, Boltansky H, Alter HJ, Pearson FC, Kaliner MA. Allergic reactions in healthy plateletpheresis donors caused by sensitization to ethylene oxide gas. The New England journal of medicine. 1986;315:1192–1196. doi: 10.1056/NEJM198611063151904. [DOI] [PubMed] [Google Scholar]
- 17.Dolovich J, Sagona M, Pearson F, Buccholz D, Hiner E, Marshall C. Sensitization of repeat plasmapheresis donors to ethylene oxide gas. Transfusion. 1987;27:90–93. doi: 10.1046/j.1537-2995.1987.27187121484.x. [DOI] [PubMed] [Google Scholar]
- 18.Westhoff CM, Sipherd BD, Wylie DE, Toalson LD. Severe anaphylactic reactions following transfusions of platelets to a patient with anti-Ch. Transfusion. 1992;32:576–579. doi: 10.1046/j.1537-2995.1992.32692367205.x. [DOI] [PubMed] [Google Scholar]
- 19.Take H, Tamura J, Sawamura M, Murakami H, Naruse T, Tsuchiya J, Miyawaki S, Hirabayashi H. Severe anaphylactic transfusion reaction associated with HLA-incompatible platelets. British journal of haematology. 1993;83:673–674. doi: 10.1111/j.1365-2141.1993.tb04712.x. [DOI] [PubMed] [Google Scholar]
- 20.Wibaut B, Mannessier L, Horbez C, Coupez B, Courbon B, Mizon P, Goudemand J. Anaphylactic reactions associated with anti-Chido Antibody following platelet transfusions. Vox Sang. 1995;69:150–151. doi: 10.1111/j.1423-0410.1995.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 21.Shimada E, Tadokoro K, Watanabe Y, Ikeda K, Niihara H, Maeda I, Isa K, Moriya S, Ashida T, Mitsunaga S, Nakajima K, Juji T. Anaphylactic transfusion reactions in haptoglobin-deficient patients with IgE and IgG haptoglobin antibodies. Transfusion. 2002;42:766–773. doi: 10.1046/j.1537-2995.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 22.Johansson SG, Nopp A, van Hage M, Olofsson N, Lundahl J, Wehlin L, Soderstrom L, Stiller V, Oman H. Passive IgE-sensitization by blood transfusion. Allergy. 2005;60:1192–1199. doi: 10.1111/j.1398-9995.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- 23.Morishita K, Wakamoto S, Miyazaki T, Sato S, Fujihara M, Kaneko S, Yasuda H, Yamamoto S, Azuma H, Kato T, Ikeda H. Life-threatening adverse reaction followed by thrombocytopenia after passive transfusion of fresh frozen plasma containing anti-CD36 (Nak) isoantibody. Transfusion. 2005;45:803–806. doi: 10.1111/j.1537-2995.2005.04320.x. [DOI] [PubMed] [Google Scholar]
- 24.Arnold DM, Blajchman MA, Ditomasso J, Kulczycki M, Keith PK. Passive transfer of peanut hypersensitivity by fresh frozen plasma. Arch Intern Med. 2007;167:853–854. doi: 10.1001/archinte.167.8.853. [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Matsumoto C, Shimada E, Mazda T, Takanashi M, Kawaguchi K, Hamasaki T, Mita H, Akiyama K, Okazaki H, Satake M, Tadokoro K. Immunoglobulin E oligomers identified in blood components activate mast cells: relevance to anaphylactic transfusion reaction. Transfusion. 2011;51:2327–2336. doi: 10.1111/j.1537-2995.2011.03126.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs JF, Baumert JL, Brons PP, Joosten I, Koppelman SJ, van Pampus EC. Anaphylaxis from passive transfer of peanut allergen in a blood product. The New England journal of medicine. 2011;364:1981–1982. doi: 10.1056/NEJMc1101692. [DOI] [PubMed] [Google Scholar]
- 27.Michel J, Sharon R. Non-haemolytic adverse reaction after transfusion of a blood unit containing penicillin. British medical journal. 1980;280:152–153. doi: 10.1136/bmj.280.6208.152-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage WJ, Tobian AA, Fuller AK, Wood RA, King KE, Ness PM. Allergic transfusion reactions to platelets are associated more with recipient and donor factors than with product attributes. Transfusion. 2011;2011 doi: 10.1111/j.1537-2995.2010.03009.x. Epub Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology. 2009;253:372–379. doi: 10.1148/radiol.2532090465. [DOI] [PubMed] [Google Scholar]
- 30.Simon MR. Allergic-type adverse reactions to low osmolality contrast media in patients with a history of allergy or asthma. Invest Radiol. 1995;30:285–290. doi: 10.1097/00004424-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lin RY, Schwartz LB, Curry A, Pesola GR, Knight RJ, Lee HS, Bakalchuk L, Tenenbaum C, Westfal RE. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. The Journal of allergy and clinical immunology. 2000;106:65–71. doi: 10.1067/mai.2000.107600. [DOI] [PubMed] [Google Scholar]
- 32.Arthus. Injections répétées de sérum de cheval chez le lapin. Compt. rendu Soc de Biol. 1903;50:20. [Google Scholar]
- 33.von Pirquet C, Schick B. Zur theorie der inkubationszeit. Wuen. klin. Wchnschr. 1903;16:1244. [Google Scholar]
- 34.Wagner R, von Pirquet Clemens. His Life and Work. Baltimore: The Johns Hopkins Press; 1968. [Google Scholar]
- 35.von Pirquet C. Allergy. Arch Intern Med. 1911;7:259–288. [Google Scholar]
- 36.Coombs R, Gell P. The classification of allergic reactions underlying disease. Oxford, England: Blackwel; 1963. [Google Scholar]
- 37.Maunsell K. Desensitization in Allergic Recipients after Serum Transfusions. British medical journal. 1944;2:236–239. doi: 10.1136/bmj.2.4363.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemp SF, Lockey RF, Simons FE. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63:1061–1070. doi: 10.1111/j.1398-9995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 39.Simons FE. Anaphylaxis: Recent advances in assessment and treatment. The Journal of allergy and clinical immunology. 2009;124:625–636. doi: 10.1016/j.jaci.2009.08.025. quiz 37–8. [DOI] [PubMed] [Google Scholar]
- 40.Lieberman PL, Seigle RL. Reactions to radiocontrast material. Anaphylactoid events in radiology. Clinical reviews in allergy & immunology. 1999;17:469–496. doi: 10.1007/BF02737651. [DOI] [PubMed] [Google Scholar]
- 41.Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesthesia and analgesia. 2004;98:364–370. doi: 10.1213/01.ANE.0000097168.32472.0D. [DOI] [PubMed] [Google Scholar]
- 42.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr., Bock SA, Branum A, Brown SG, Camargo CA, Jr., Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD, Jr., Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O'Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. The Journal of allergy and clinical immunology. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 43.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. The Journal of allergy and clinical immunology. 2009;124:639–646. doi: 10.1016/j.jaci.2009.08.035. quiz 47–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilfillan AM, Peavy RD, Metcalfe DD. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunologic research. 2009;43:15–24. doi: 10.1007/s12026-008-8046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 46.Lemanske RF, Jr, Kaliner MA. Late phase allergic reactions. International journal of dermatology. 1983;22:401–409. doi: 10.1111/j.1365-4362.1983.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 47.Finkelman FD. Anaphylaxis: lessons from mouse models. The Journal of allergy and clinical immunology. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 16–7. [DOI] [PubMed] [Google Scholar]
- 48.Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, Plevy S. The incidence and management of infusion reactions to infliximab: a large center experience. The American journal of gastroenterology. 2003;98:1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 49.Hakim RM, Breillatt J, Lazarus JM, Port FK. Complement activation and hypersensitivity reactions to dialysis membranes. The New England journal of medicine. 1984;311:878–882. doi: 10.1056/NEJM198410043111403. [DOI] [PubMed] [Google Scholar]
- 50.Bergamaschini L, Mannucci PM, Federici AB, Coppola R, Guzzoni S, Agostoni A. Posttransfusion anaphylactic reactions in a patient with severe von Willebrand disease: role of complement and alloantibodies to von Willebrand factor. The Journal of laboratory and clinical medicine. 1995;125:348–355. [PubMed] [Google Scholar]
- 51.Petersen LJ, Church MK, Skov PS. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. The Journal of allergy and clinical immunology. 1997;99:640–647. doi: 10.1016/s0091-6749(97)70026-5. [DOI] [PubMed] [Google Scholar]
- 52.Lawrence ID, Warner JA, Cohan VL, Lichtenstein LM, Kagey-Sobotka A, Vavrek RJ, Stewart JM, Proud D. Induction of histamine release from human skin mast cells by bradykinin analogs. Biochemical pharmacology. 1989;38:227–233. doi: 10.1016/0006-2952(89)90031-2. [DOI] [PubMed] [Google Scholar]
- 53.Maxwell DL, Atkinson BA, Spur BW, Lessof MH, Lee TH. Skin responses to intradermal histamine and leukotrienes C4, D4, and E4 in patients with chronic idiopathic urticaria and in normal subjects. The Journal of allergy and clinical immunology. 1990;86:759–765. doi: 10.1016/s0091-6749(05)80180-0. [DOI] [PubMed] [Google Scholar]
- 54.Nitschke M, Sohn K, Dieckmann D, Gibbs BF, Wolff HH, Amon U. Effects of basophil-priming and stimulating cytokines on histamine release from isolated human skin mast cells. Archives of dermatological research. 1996;288:463–468. doi: 10.1007/BF02505236. [DOI] [PubMed] [Google Scholar]
- 55.Silvergleid AJ, Hafleigh EB, Harabin MA, Wolf RM, Grumet FC. Clinical value of washed-platelet concentrates in patients with non-hemolytic transfusion reactions. Transfusion. 1977;17:33–37. doi: 10.1046/j.1537-2995.1977.17177128881.x. [DOI] [PubMed] [Google Scholar]
- 56.Buck SA, Kickler TS, McGuire M, Braine HG, Ness PM. The utility of platelet washing using an automated procedure for severe platelet allergic reactions. Transfusion. 1987;27:391–393. doi: 10.1046/j.1537-2995.1987.27587320530.x. [DOI] [PubMed] [Google Scholar]
- 57.Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;2011 doi: 10.1111/j.1537-2995.2010.03008.x. Epub Jan 7. [DOI] [PubMed] [Google Scholar]
- 58.Azuma H, Hirayama J, Akino M, Miura R, Kiyama Y, Imai K, Kasai M, Koizumi K, Kakinoki Y, Makiguchi Y, Kubo K, Atsuta Y, Fujihara M, Homma C, Yamamoto S, Kato T, Ikeda H. Reduction in adverse reactions to platelets by the removal of plasma supernatant and resuspension in a new additive solution (M-sol) Transfusion. 2009;49:214–218. doi: 10.1111/j.1537-2995.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 59.Kerkhoffs JL, Eikenboom JC, Schipperus MS, van Wordragen-Vlaswinkel RJ, Brand R, Harvey MS, de Vries RR, Barge R, van Rhenen DJ, Brand A. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108:3210–3215. doi: 10.1182/blood-2006-04-020131. [DOI] [PubMed] [Google Scholar]
- 60.de Wildt-Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: a prospective, randomized study. Transfusion. 2000;40:398–403. doi: 10.1046/j.1537-2995.2000.40040398.x. [DOI] [PubMed] [Google Scholar]
- 61.Henrichs KF, Howk N, Masel DS, Thayer M, Refaai MA, Kirkley SA, Heal JM, Blumberg N. Providing ABO-identical platelets and cryoprecipitate to (almost) all patients: approach, logistics, and associated decreases in transfusion reaction and red blood cell alloimmunization incidence. Transfusion. 2011 doi: 10.1111/j.1537-2995.2011.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahmed SG, Kyari O, Ibrahim UA. Urticarial reactions in obstetric transfusion in Maiduguri, north east Nigeria. The Nigerian postgraduate medical journal. 2002;9:137–139. [PubMed] [Google Scholar]
- 63.Vyas GN, Perkins HA, Yang YM, Basantani GK. Healthy blood donors with selective absence of immunoglobulin A: prevention of anaphylactic transfusion reactions caused by antibodies to IgA. J Lab Clin Med. 1975;85:838–842. [PubMed] [Google Scholar]
- 64.Sandler SG, Mallory D, Malamut D, Eckrich R. IgA anaphylactic transfusion reactions. Transfusion medicine reviews. 1995;9:1–8. doi: 10.1016/s0887-7963(05)80026-4. [DOI] [PubMed] [Google Scholar]
- 65.Branch DR, Gifford H. Allergic reaction to transfused cephalothin antibody. JAMA : the journal of the American Medical Association. 1979;241:495–496. [PubMed] [Google Scholar]
- 66.Vickery BP, Burks AW, Sampson HA. Anaphylaxis from peanuts ingested by blood donors? The New England journal of medicine. 2011;365:867–868. doi: 10.1056/NEJMc1106934. author reply 8. [DOI] [PubMed] [Google Scholar]
- 67.Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2004;31:111–122. doi: 10.1016/j.transci.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Paglino JC, Pomper GJ, Fisch GS, Champion MH, Snyder EL. Reduction of febrile but not allergic reactions to RBCs and platelets after conversion to universal prestorage leukoreduction. Transfusion. 2004;44:16–24. doi: 10.1046/j.0041-1132.2004.00608.x. [DOI] [PubMed] [Google Scholar]
- 69.King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44:25–29. doi: 10.1046/j.0041-1132.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 70.Yazer MH, Podlosky L, Clarke G, Nahirniak SM. The effect of prestorage WBC reduction on the rates of febrile nonhemolytic transfusion reactions to platelet concentrates and RBC. Transfusion. 2004;44:10–15. doi: 10.1046/j.0041-1132.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 71.Wilhelm D, Kluter H, Klouche M, Kirchner H. Impact of allergy screening for blood donors: relationship to nonhemolytic transfusion reactions. Vox Sang. 1995;69:217–221. doi: 10.1111/j.1423-0410.1995.tb02598.x. [DOI] [PubMed] [Google Scholar]
- 72.Schroeder JT, MacGlashan DW, Jr, Lichtenstein LM. Human basophils: mediator release and cytokine production. Advances in immunology. 2001;77:93–122. doi: 10.1016/s0065-2776(01)77015-0. [DOI] [PubMed] [Google Scholar]
- 73.Azuma H, Yamaguchi M, Takahashi D, Fujihara M, Sato S, Kato T, Ikeda H. Elevated Ca(2+) influx-inducing activity toward mast cells in pretransfusion sera from patients who developed transfusion-related adverse reactions. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02172.x. [DOI] [PubMed] [Google Scholar]
- 74.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nature reviews. Immunology. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 75.Stern A, van Hage-Hamsten M, Sondell K, Johansson SG. Is allergy screening of blood donors necessary? A comparison between questionnaire answers and the presence of circulating IgE antibodies. Vox Sang. 1995;69:114–119. doi: 10.1111/j.1423-0410.1995.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 76.Johansson SG, Nopp A, Florvaag E, Lundahl J, Soderstrom T, Guttormsen AB, Hervig T, Lundberg M, Oman H, van Hage M. High prevalence of IgE antibodies among blood donors in Sweden and Norway. Allergy. 2005;60:1312–1315. doi: 10.1111/j.1398-9995.2005.00896.x. [DOI] [PubMed] [Google Scholar]
- 77.Kaliner M, Shelhamer JH, Ottesen EA. Effects of infused histamine: correlation of plasma histamine levels and symptoms. The Journal of allergy and clinical immunology. 1982;69:283–289. doi: 10.1016/s0091-6749(82)80005-5. [DOI] [PubMed] [Google Scholar]
- 78.MacGlashan D, Jr, Warner J. Stimulus-dependent leukotriene release from human basophils: a comparative study of C5a and Fmet-leu-phe. Journal of leukocyte biology. 1991;49:29–40. doi: 10.1002/jlb.49.1.29. [DOI] [PubMed] [Google Scholar]
- 79.Simons FE, Simons KJ. Histamine and H1-antihistamines: celebrating a century of progress. The Journal of allergy and clinical immunology. 2011;128:1139–1150. e4. doi: 10.1016/j.jaci.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 80.McBride P, Bradley D, Kaliner M. Evaluation of a radioimmunoassay for histamine measurement in biologic fluids. The Journal of allergy and clinical immunology. 1988;82:638–646. doi: 10.1016/0091-6749(88)90977-3. [DOI] [PubMed] [Google Scholar]
- 81.Pollock I, Murdoch RD, Lessof MH. Plasma histamine and clinical tolerance to infused histamine in normal, atopic and urticarial subjects. Agents and actions. 1991;32:359–365. doi: 10.1007/BF01980899. [DOI] [PubMed] [Google Scholar]
- 82.Nielsen HJ, Edvardsen L, Vangsgaard K, Dybkjaer E, Skov PS. Time-dependent histamine release from stored human blood products. The British journal of surgery. 1996;83:259–262. [PubMed] [Google Scholar]
- 83.Muylle L, Beert JF, Mertens G, Bult H. Histamine synthesis by white cells during storage of platelet concentrates. Vox Sang. 1998;74:193–197. [PubMed] [Google Scholar]
- 84.Frewin DB, Jonsson JR, Frewin CR, Russell WJ, Russell MW, Davis KG, Beal RW. Influence of blood storage time and plasma histamine levels on the pattern of transfusion reactions. Vox Sang. 1989;56:243–246. doi: 10.1111/j.1423-0410.1989.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 85.Muylle L, Laekeman G, Herman AG, Peetermans ME. Histamine levels in stored platelet concentrates. Relationship to white cell content. Transfusion. 1988;28:226–228. doi: 10.1046/j.1537-2995.1988.28388219148.x. [DOI] [PubMed] [Google Scholar]
- 86.Frewin DB, Jonsson JR, Head RJ, Russell WJ, Beal RW. Histamine levels in stored human blood. Transfusion. 1984;24:502–504. doi: 10.1046/j.1537-2995.1984.24685066810.x. [DOI] [PubMed] [Google Scholar]
- 87.Walport MJ. Complement. First of two parts. The New England journal of medicine. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 88.Martin U, Bock D, Arseniev L, Tornetta MA, Ames RS, Bautsch W, Kohl J, Ganser A, Klos A. The human C3a receptor is expressed on neutrophils and monocytes, but not on B or T lymphocytes. The Journal of experimental medicine. 1997;186:199–207. doi: 10.1084/jem.186.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fureder W, Agis H, Willheim M, Bankl HC, Maier U, Kishi K, Muller MR, Czerwenka K, Radaszkiewicz T, Butterfield JH, Klappacher GW, Sperr WR, Oppermann M, Lechner K, Valent P. Differential expression of complement receptors on human basophils and mast cells. Evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. Journal of immunology. 1995;155:3152–3160. [PubMed] [Google Scholar]
- 90.Shrivastava M. The platelet storage lesion. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2009;41:105–113. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Miletic VD, Popovic O. Complement activation in stored platelet concentrates. Transfusion. 1993;33:150–154. doi: 10.1046/j.1537-2995.1993.33293158048.x. [DOI] [PubMed] [Google Scholar]
- 92.Cardigan R, Sutherland J, Wadhwa M, Dilger P, Thorpe R. The influence of platelet additive solutions on cytokine levels and complement activation in platelet concentrates during storage. Vox Sang. 2003;84:28–35. doi: 10.1046/j.1423-0410.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 93.Gyongyossy-Issa MI, McLeod E, Devine DV. Complement activation in platelet concentrates is surface-dependent and modulated by the platelets. The Journal of laboratory and clinical medicine. 1994;123:859–868. [PubMed] [Google Scholar]
- 94.Schleuning M, Bock M, Mempel W. Complement activation during storage of single-donor platelet concentrates. Vox Sang. 1994;67:144–148. doi: 10.1111/j.1423-0410.1994.tb01649.x. [DOI] [PubMed] [Google Scholar]
- 95.Savage WJ, Savage JH, Tobian AA, Thoburn C, Hamilton RG, Schroeder JT, Ness PM. Allergic agonists in apheresis platelet products are associated with allergic transfusion reactions. Transfusion. 2012;52:575–581. doi: 10.1111/j.1537-2995.2011.03310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franchini M, Makris M, Santagostino E, Coppola A, Mannucci PM. Non-thrombotic-, non-inhibitor-associated adverse reactions to coagulation factor concentrates for treatment of patients with hemophilia and von Willebrand’s disease: a systematic review of prospective studies. Haemophilia : the official journal of the World Federation of Hemophilia. 2012 doi: 10.1111/j.1365-2516.2011.02745.x. [DOI] [PubMed] [Google Scholar]
- 97.Recht M, Pollmann H, Tagliaferri A, Musso R, Janco R, Neuman WR. A retrospective study to describe the incidence of moderate to severe allergic reactions to factor IX in subjects with haemophilia B. Haemophilia : the official journal of the World Federation of Hemophilia. 2011;17:494–499. doi: 10.1111/j.1365-2516.2011.02436.x. [DOI] [PubMed] [Google Scholar]
- 98.Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, Crystal RG. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. The New England journal of medicine. 1987;316:1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- 99.Stoller JK, Fallat R, Schluchter MD, O’Brien RG, Connor JT, Gross N, O'Neil K, Sandhaus R, Crystal RG. Augmentation therapy with alpha1-antitrypsin: patterns of use and adverse events. Chest. 2003;123:1425–1434. doi: 10.1378/chest.123.5.1425. [DOI] [PubMed] [Google Scholar]
- 100.Heal JM, Cowles J, Masel D, Rowe JM, Blumberg N. Antibodies to plasma proteins: an association with platelet transfusion refractoriness. British journal of haematology. 1992;80:83–90. doi: 10.1111/j.1365-2141.1992.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 101.Orchard MA, Kagey-Sobotka A, Proud D, Lichtenstein LM. Basophil histamine release induced by a substance from stimulated human platelets. Journal of immunology. 1986;136:2240–2244. [PubMed] [Google Scholar]
- 102.Wadhwa M, Seghatchian MJ, Dilger P, Sands D, Krailadisiri P, Contreras M, Thorpe R. Cytokines in WBC-reduced apheresis PCs during storage: a comparison of two WBC-reduction methods. Transfusion. 2000;40:1118–1126. doi: 10.1046/j.1537-2995.2000.40091118.x. [DOI] [PubMed] [Google Scholar]
- 103.Glenister KM, Payne KA, Sparrow RL. Proteomic analysis of supernatant from pooled buffy-coat platelet concentrates throughout 7-day storage. Transfusion. 2008;48:99–107. doi: 10.1111/j.1537-2995.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 104.Petersen LJ, Brasso K, Pryds M, Skov PS. Histamine release in intact human skin by monocyte chemoattractant factor-1, RANTES, macrophage inflammatory protein-1 alpha, stem cell factor, anti-IgE, and codeine as determined by an ex vivo skin microdialysis technique. The Journal of allergy and clinical immunology. 1996;98:790–796. doi: 10.1016/s0091-6749(96)70128-8. [DOI] [PubMed] [Google Scholar]
- 105.Kluter H, Bubel S, Kirchner H, Wilhelm D. Febrile and allergic transfusion reactions after the transfusion of white cell-poor platelet preparations. Transfusion. 1999;39:1179–1184. doi: 10.1046/j.1537-2995.1999.39111179.x. [DOI] [PubMed] [Google Scholar]
- 106.Wakamoto S, Fujihara M, Kuzuma K, Sato S, Kato T, Naohara T, Kasai M, Sawada K, Kobayashi R, Kudoh T, Ikebuchi K, Azuma H, Ikeda H. Biologic activity of RANTES in apheresis PLT concentrates and its involvement in nonhemolytic transfusion reactions. Transfusion. 2003;43:1038–1046. doi: 10.1046/j.1537-2995.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 107.Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, Garraud O. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–1189. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 108.Salkie ML, Hannon JL. Anti-plasticizer specific IgE is present in the serum of transfused patients. Clin Invest Med. 1995;18:419–423. [PubMed] [Google Scholar]
- 109.Vamvakas EC. A patient-centric approach to preventing allergic reactions to platelet transfusions. Transfusion. 2011;51:1651–1653. doi: 10.1111/j.1537-2995.2011.03246.x. [DOI] [PubMed] [Google Scholar]
- 110.Karafin M, Fuller AK, Savage WJ, King KE, Ness PM, Tobian AA. The impact of apheresis platelet manipulation on corrected count increment. Transfusion. 2012 doi: 10.1111/j.1537-2995.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vesilind GW, Simpson MB, Shifman MA, Colman RE, Kao KJ. Evaluation of a centrifugal blood cell processor for washing platelet concentrates. Transfusion. 1988;28:46–51. doi: 10.1046/j.1537-2995.1988.28188127952.x. [DOI] [PubMed] [Google Scholar]
- 112.Tobian AA, King KE, Ness PM. Prevention of febrile nonhemolytic and allergic transfusion reactions with pretransfusion medication: is this evidence-based medicine? Transfusion. 2008;48:2274–2276. doi: 10.1111/j.1537-2995.2008.01924.x. [DOI] [PubMed] [Google Scholar]
- 113.Tobian AA, King KE, Ness PM. Transfusion premedications: a growing practice not based on evidence. Transfusion. 2007;47:1089–1096. doi: 10.1111/j.1537-2995.2007.01242.x. [DOI] [PubMed] [Google Scholar]
- 114.Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48:2285–2291. doi: 10.1111/j.1537-2995.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 115.Wang SE, Lara PN, Jr, Lee-Ow A, Reed J, Wang LR, Palmer P, Tuscano JM, Richman CM, Beckett L, Wun T. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70:191–194. doi: 10.1002/ajh.10119. [DOI] [PubMed] [Google Scholar]
- 116.Wang JS, Sackett DJ, Yuan YM. [Randomized clinical controlled cross-over trial (RCT) in the prevention of blood transfusion febrile reactions with small dose hydrocortisone versus anti-histamines] Zhonghua nei ke za zhi [Chinese journal of internal medicine] 1992;31:536–538. 85–86. [PubMed] [Google Scholar]
- 117.Marti-Carvajal AJ, Sola I, Gonzalez LE, Leon de Gonzalez G, Rodriguez-Malagon N. Pharmacological interventions for the prevention of allergic and febrile non-haemolytic transfusion reactions. Cochrane database of systematic reviews. 2010:CD007539. doi: 10.1002/14651858.CD007539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanders RP, Maddirala SD, Geiger TL, Pounds S, Sandlund JT, Ribeiro RC, Pui CH, Howard SC. Premedication with acetaminophen or diphenhydramine for transfusion with leucoreduced blood products in children. Br J Haematol. 2005;130:781–787. doi: 10.1111/j.1365-2141.2005.05670.x. [DOI] [PubMed] [Google Scholar]
- 119.Patterson BJ, Freedman J, Blanchette V, Sher G, Pinkerton P, Hannach B, Meharchand J, Lau W, Boyce N, Pinchefsky E, Tasev T, Pinchefsky J, Poon S, Shulman L, Mac KP, Thomas K, Blanchette N, Greenspan D, Panzarella T. Effect of premedication guidelines and leukoreduction on the rate of febrile nonhaemolytic platelet transfusion reactions. Transfusion medicine. 2000;10:199–206. doi: 10.1046/j.1365-3148.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- 120.Park JH, Godbold JH, Chung D, Sampson HA, Wang J. Comparison of cetirizine and diphenhydramine in the treatment of acute food-induced allergic reactions. The Journal of allergy and clinical immunology. 2011;128:1127–1128. doi: 10.1016/j.jaci.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choo KJ, Simons FE, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane database of systematic reviews. 2012;4:CD007596. doi: 10.1002/14651858.CD007596.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hennino A, Berard F, Guillot I, Saad N, Rozieres A, Nicolas JF. Pathophysiology of urticaria. Clinical reviews in allergy & immunology. 2006;30:3–11. doi: 10.1385/CRIAI:30:1:003. [DOI] [PubMed] [Google Scholar]