Abstract
Background
Although adolescent major depressive disorder (MDD) is acknowledged to be a heterogeneous disorder, no studies have reported on biological correlates of its clinical subgroups. This study addresses this issue by examining whether adolescent MDD with and without melancholic features (M-MDD and NonM-MDD) have distinct biological features in the kynurenine pathway (KP). The KP is initiated by pro-inflammatory cytokines via induction of the enzyme indoleamine 2,3-dioxygenase (IDO), which degrades tryptophan (TRP) into kynurenine (KYN). KYN is further metabolized into neurotoxins linked to neuronal dysfunction in MDD. Hypotheses were that, compared to healthy controls and to NonM-MDD adolescents, adolescents with M-MDD would exhibit: (i) increased activation of the KP [i.e., increased KYN and KYN/TRP (reflecting IDO activity)]; (ii) greater neurotoxic loads [i.e., increased 3-hydroxyanthranilic acid (3-HAA, neurotoxin) and 3-HAA/KYN (reflecting production of neurotoxins)]; and (iii) decreased TRP. We also examined relationships between severity of MDD and KP metabolites.
Methods
Subjects were 20 adolescents with M-MDD, 30 adolescents with NonM-MDD, and 22 healthy adolescents. MDD episode duration had to be ≥ 6 weeks and Children’s Depression Rating Scale-Revised (CDRS-R) scores were ≥ 36. Blood samples were collected at AM after an overnight fast and analyzed using high-performance liquid chromatography. Group contrasts relied on analysis of covariance based on ranks, adjusted for age, gender, and CDRS-R scores. Analyses were repeated excluding medicated patients. Fisher’s protected least significant difference was used for multiple comparisons.
Results
As hypothesized, KYN/TRP ratios were elevated and TRP concentrations were reduced in adolescents with M-MDD compared to NonM-MDD adolescents (p = .001 and .006, respectively) and to healthy controls (p = .008 and .022, respectively). These findings remained significant when medicated patients were excluded from the analyses. Significant correlations were obtained exclusively in the M-MDD group between KYN and 3-HAA/KYN and CDRS-R.
Conclusions
Findings support the notion that adolescent M-MDD may represent a biologically distinct clinical syndrome.
Keywords: Adolescent depression, indoleamine 2, 3-dioxygenase (IDO), kynurenine (KYN), tryptophan (TRP), melancholic, MDD subtypes
It is generally recognized that major depressive disorder (MDD) is a heterogeneous syndrome of different clinical forms identified by distinct features, as reflected in the DSM-IV. Of these, MDD with melancholic features (M-MDD) has the longest history and evidence supporting its validity (Rush & Weissenburger, 1994). During the 1970s and 1980s, impaired hedonic capacity was proposed as the key distinguishing symptom between ‘endogenous/melancholia’ and ‘neurotic/reactive’ depression (Klein, 1974). Consistent with this formulation, the DSM-IV requires the presence of either anhedonia or nonreactive mood as essential features of melancholia. In addition, at least three of the following six features must be present: distinct quality of mood, diurnal variation (worse in AM), early morning awakening, psychomotor retardation or agitation, anorexia, and inappropriate guilt. Clinical reports support the presence of M-MDD in children and adolescents (Ryan et al., 1987; Kolvin et al., 1991; Wood, Moore, Harrington, & Jayson, 1996; Ambrosini, Bennett, Cleland, & Haslam, 2002), but no attempts have been made yet to investigate whether this clinical subtype is associated with specific neurobiological abnormalities.
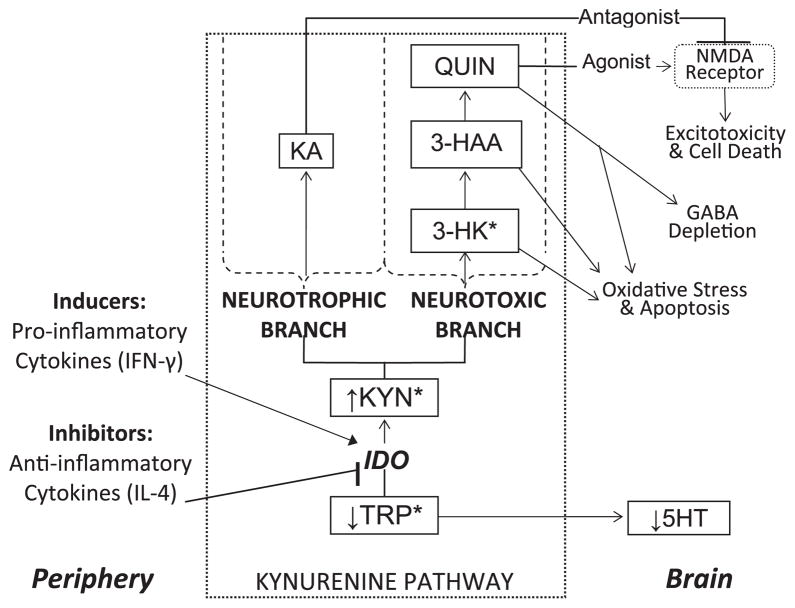
A large body of evidence links immune system dysregulation to adult MDD (Irwin & Miller, 2007). Consistent with findings in adults, we recently reported increased pro-inflammatory cytokines (immune system modulators), interferon (IFN)-γ and IFN-γ/interleukin (IL)-4 in adolescents with MDD relative to healthy controls, suggesting immune system dysregulation with a pro-inflammatory/anti-inflammatory imbalance in this age group (Gabbay et al., 2009). One possible mechanism linking cytokines and MDD involves the kynurenine pathway (KP), which is initiated by the enzyme indoleamine 2,3-dioxygenase (IDO). IDO is induced by proinflammatory cytokines and metabolizes tryptophan (TRP) into kynurenine (KYN), thereby decreasing brain TRP availability. KYN is further metabolized into 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA), and quinolinic acid (QUIN), which may induce neuronal damage through oxidative stress and over stimulation of the N-methyl- D-aspartate (NMDA) receptor (Goldstein et al., 2000; Schwarcz, Whetsell, & Mangano, 1983). KYN is also metabolized into kynurenic acid (KA), a competitive NMDA receptor antagonist that blocks the neurotoxic effects of QUIN (Sapko et al., 2006) (Figure 1 illustrates the KP).
Figure 1.
Potential neurotoxic consequences of kynurenine pathway activation resulting from peripheral induction of indoleamine 2,3-dioxygenase (IDO). Asterisks represent pathway metabolites that cross the blood-brain barrier. TRP, tryptophan; KYN, kynurenine; IFN, interferon; IL, interleukin; 3-HK, 3-hydroxykynurenine; 3-HAA, 3-hydroxyanthranilic acid; QUIN, quinolinic acid; KA, kynurenic acid; NMDA, N-methyl- D-aspartate; GABA, γ-aminobutyric acid
The KP has been hypothesized to play a role in MDD, accounting, in part, for glial and neuronal loss and atrophy in MDD based on clinical studies of adults with MDD and adults who developed depressive symptoms while undergoing immunotherapy (Myint et al., 2007; Wichers et al., 2005). Findings in these clinical groups include increased KYN/TRP (reflecting IDO activity), increased KYN, decreased TRP, and increased KYN/KA (reflecting increased neurotoxic load), consonant with the model implicating the KP and production of neurotoxins in MDD (Maes et al., 2001; Capuron et al., 2001; Capuron et al., 2003; Wichers et al., 2005; Myint et al., 2007). We recently reported positive relationships between plasma KP metabolites and striatal total choline (tCho, the cell membrane turnover biomarker) in adolescents with MDD, using proton magnetic resonance spectroscopy. It is only in the adolescent M-MDD group that positive correlations were found between KYN and 3-HAA and tCho, suggesting distinct neurobiological differences between adolescents with M-MDD and NonM-MDD (Gabbay et al., 2010).
This study was designed to extend the aforementioned findings by examining KP plasma metabolites to test for differences in KP activation between adolescents with M-MDD and NonM-MDD and controls. Hypotheses were that, compared to healthy controls and to NonM-MDD adolescents, adolescents with M-MDD would exhibit: (i) increased activation of the KP [i.e., increased KYN and KYN/TRP (reflecting IDO activity)]; (ii) greater neurotoxic loads [i.e., increased 3-HAA and 3-HAA/KYN (reflecting production of neurotoxins)]; and (iii) decreased TRP. We also examined relationships between severity of MDD and KP metabolites.
Methods and materials
I. Subjects
The study was approved by the New York University (NYU) School of Medicine Institutional Review Board and the New York City Health and Hospital Corporation. Participants ages 18 and over (N = 14) provided signed informed consent to participate in the study; subjects under age 18 provided assent, and a parent provided signed consent.
Exclusion criteria for all subjects included: immune-affecting medications taken in the past six months, any immunological or hematological disorder, chronic fatigue syndrome, any infection during the month prior to the blood draw (including the common cold), significant medical or neurological disorders, a positive urine toxicology test and, in females, a positive urine pregnancy test.
i) MDD adolescents
Fifty adolescents with MDD (27 females, 54%), ages 12–19 (15.9 ± 2.0), were enrolled. All MDD subjects were required to be in a current episode of at least 6 weeks’ duration and have a minimum severity score of 36 on the Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski et al., 1984). The following lifetime psychiatric disorders were exclusionary for subjects with MDD: (i) bipolar disorder, (ii) schizophrenia, (iii) pervasive developmental disorder, (iv) post-traumatic stress disorder, (v) obsessive-compulsive disorder, (vi) Tourette’s disorder, (vii) eating disorder, and (viii) a substance-related disorder in the past 12 months.
Adolescents with MDD were enrolled from the NYU Child Study Center, the NYU Tisch inpatient psychiatric unit, and the Bellevue Department of Psychiatry. Patient recruitment rates were proportionally equal in terms of site and season of recruitment for both the MDD subgroups.
Medication status
Of the 50 adolescents with MDD, 33 (66%) were not on medication; of these, 28 were medication-naïve and 5 had been medication-free for at least one year; 17 (34%) had been receiving psychotropic medications for periods ranging from one month to two-and-a-half years. All patients on medication had failed to respond to treatment at the time of blood draw. Medications included fluoxetine, sertraline, citalopram, mirtazapine, bupropion, lamotrigine, lithium, risperidone, quetiapine, methylphenidate, and mixed amphetamine salts. Medication use in each MDD group is described in Table 1.
Table 1.
Clinical and demographic characteristics of melancholic adolescents with major depressive disorder (MDD), non-melancholic adolescents with MDD, and healthy controls
| Characteristic | Melancholic MDD n = 20 | Non-melancholic MDD n = 30 | Healthy controls n = 22 |
|---|---|---|---|
| Age (years) | 16.3 ± 2.1 | 15.6 ± 2.0 | 16.0 ± 2.7 |
| Gender (female/male) | 11/9 1(55/45%) | 16/14 1(53/47%) | 13/9 1(59/41%) |
| Ethnicity (Caucasian/African American/Hispanic/Asian/Other) | 9/6/1/3/1 1(45/30/5/15/5%) | 17/0/11/2/0 1(57/0/37/7/0%) | 13/2/1/4/2 1(59/9/5/18/9%) |
| Illness history | |||
| Duration of Illness (months) | 25.3 ± 22.1 2(3–84) | 14.9 ± 10.8 2(1.5–36) | 0 |
| History of suicide attempts | .9 ± 1.0 2(0–3) | .4 ± .7 2(0–3) | 0 |
| Medication-naïve/Medication-free/Medicated | 11/3/6 1(55/15/30%) | 17/2/11 1(57/7/37%) | 0 |
| 3 CDRS-R | 66.5 ± 14.7 2(42–97) | 50.4 ± 9.0 2(36–75) | 18.3 ± 2.5 2(16–27) |
| 4 BDI-II | 24.3 ± 11.9 2(10–53) | 21.0 ± 9.3 2(4–42) | 1.9 ± 2.7 2(0–11) |
| Current comorbidity | |||
| 5 ADHD | 0 | 3 1(10%) | 0 |
| Any anxiety disorder | 4 1(20%) | 11 1(37%) | 0 |
| Any comorbidity | 4 1(20%) | 12 1(40%) | 0 |
Respective percentages (may not add up to 100% due to rounding);
Range;
Children’s Depression Rating Scale-Revised;
Beck Depression Inventory, 2nd edn;
Attention deficit hyperactivity disorder.
Melancholic MDD
The DSM-IV requires either severe anhedonia or lack of mood reactivity. We required the presence of both anhedonia and lack of mood reactivity. This was done because many adolescents with MDD may endorse severe anhedonia while experiencing marked mood amelioration in response to positive social situations. Therefore, to ensure that adolescents with M-MDD had impaired hedonic capacity, the core symptom of melancholia, we applied a relatively conservative diagnostic approach that required both these essential symptoms of melancholia. Following the DSM-IV, at least three of the six associated features noted above were required as well. By these criteria, 20 of the 50 MDD subjects (11 females, 55%), ages 12–19 (16.3 ± 2.1), had melancholic features.
Non-melancholic MDD
Thirty adolescents with MDD (16 females, 53%), ages 12–19 (15.6 ± 2.0), had non-melancholic, reactive MDD.
ii) Healthy controls
Twenty-two healthy control subjects (13 females, 59%), ages 11–20 (16.0 ± 2.7), were recruited from the New York metropolitan area by means of fliers, information on the NYU Child Study Center website, and through families of NYU staff. Control subjects could not meet criteria for any major current or past DSM-IV diagnosis and could not be taking psychotropic medications.
II. Assessments
Adolescents (patients and controls) and parents were interviewed by a child and adolescent psychiatrist using the Schedule for Affective Disorders and Schizophrenia- Present and Lifetime Version for Children (K-SADS-PL; Kaufman et al., 1997), a semi-structured psychiatric interview. Based on the clinical evaluation, the psychiatrist rated severity of depression on the CDRS-R and overall adjustment on the Children’s Global Assessment Scale (C-GAS; Shaffer et al., 1983). Baseline medical assessments included medical history and laboratory tests, including complete blood count, metabolic panel, liver and thyroid function tests, urine toxicology test (done at baseline and at the time of the blood draw assessing: amphetamines, barbiturates, benzodiazepines, cocaine, marijuana, methadone, opiates, phencyclidine, propoxyphene), and a urine pregnancy test for females.
III. Determination of kynurenine pathway metabolite concentrations
All blood samples (10 ml) were drawn between 08:00 and 09:00AM after an overnight fast (≥12h), processed within 20 minutes of collection, and stored at −80°C. All blood samples were analyzed concurrently by SM and LL, who were blind to the clinical status of subjects. Multiday validation procedures with calibration and precision standards to define the reproducible limits of detection and ruggedness of the assays in terms of the intra- and inter-assay coefficient of variations were done. TRP, KYN, 3-HAA, and 3-nitro-L-tyrosine were obtained from Sigma-Aldrich, Inc (St. Louis, MO).
Preparation of direct calibration and plasma standards
Calibration curves were prepared from stock solutions (100 μg/ml) to yield the respective levels of TRP (1, 2, 4, 8, 12, and 14 μg/ml); KYN (.05, .1, .2, .4, 1, 2, 4, and 5 μg/ml); and 3-HAA (.1, .2, .4, .8, 2, and 3 μg/ml) in double distilled (DD) grade water and human plasma. The internal standard (3-nitro-L-tyrosine, 1 mg/ml) was also added to all calibration standards for recovery purposes.
Sample preparation and extraction procedure
Plasma (.20 ml) was combined with 5 μl of 1 μg/μl 3-nitro-L-tyrosine followed by the addition of .20 ml of .05M KH2PO4 (pH 6.2). Subsequently, 50 μl of cold 2 M trichloroacetic acid were added, followed by vigorous vortexing. The mixture was centrifuged at 9,500 rpm for 10 minutes, and the supernatant was transferred into autosampler vials for analysis by high-performance liquid chromatography (HPLC). Aliquots of 200 μl of the supernatant were injected into the chromatographic system using a WISP model 717 autosampler (Waters Associates, Milford, MA).
Chromatographic procedure
The eluted components of interest were detected as follows: (i) KYN and 3-nitro-L-tyrosine by tandem monitoring with UV absorption at 360 nm; (ii) TRP and 3-HAA by fluorescence monitoring at 340 nm (with UV excitation at 285 nm). A gradient elution was used for development of the chromatography in the following manner: The gradient consisted of a combination of two buffers: A (40 mM citrate buffer/.1 mM succinic acid, disodium salt/.05% sodium azide), and B (60% methanol/40 mM citrate buffer/.1 mM succinic acid, disodium salt/.05% sodium azide). The following sequence of steps was employed: 0–3 min, 0% B; 3–12 min, 0% B to 65%B (linear gradient); 12–18 min, 65% B; 18– 20 min, 65% B to 100% B; 20–24 min 100% B; 24– 26 min, 100% B to 0% B (linear gradient); 26–30 min 0% B. A flow rate of .8 ml/min was used during the 30 minute elution sequence of the analytical HPLC C18 column (4.6 × 150-mm, 5 μm, Luna C18, Phenomenex, Torrance, CA).
IV. Data analyses
As data were not normally distributed, analysis of covariance (ANCOVA) based on ranks compared KP measures between adolescents with MDD and controls, adjusting for key covariates which included age, gender, and CDRS-R. The type 3 p values were used to assess group differences since it tests the effect of one factor adjusted for the effects of all other factors in the model. Analyses were repeated excluding medicated patients. Fisher’s protected least significant difference multiple comparison procedure tested for group differences. Specifically, the difference between each pair of subject groups was tested only when the composite F test for differences among the three groups was significant. We repeated the analysis to compare medicated and non-medicated MDD adolescents. An Exact Mann– Whitney test compared CDRS-R scores of the two MDD groups. Spearman rank correlation coefficients were used to characterize the association of KP metabolite plasma levels with severity of MDD as measured by CDRS-R. A permutation test was used to examine whether documented correlations are significantly different between the MDD groups.
Statistical significance was defined as two-sided p ≤ .05. SAS version 9.0 (SAS Institute, Cary, NC) was used for all statistical computations.
Results
I. Subjects
Demographics, diagnoses, and treatment profiles are compiled in Table 1.
We obtained body mass index (BMI) measures from roughly half of the depressed subjects. From these data, however, there were no differences in BMI between melancholic (N = 12; 23.09 ± 3.86) and non-melancholic (N = 14; 23.9 ± 3.66) depressed adolescents.
Adolescents with M-MDD had significantly more severe MDD than adolescents with NonM-MDD (CDRS-R ratings, t = 3.79; p = .0001).
II. KP plasma metabolite findings
Means and standard deviations of KYN, TRP, 3-HAA, KYN/TRP (estimating IDO activity), and 3-HAA/KYN (reflecting neurotoxic load) for MDD and control subjects are summarized in Table 2.
Table 2.
Mean (SD) plasma concentrations (in ng/ml) of kynurenine (KYN), tryptophan (TRP), 3-hydroxyanthranilic acid (3-HAA), KYN/TRP [estimate of indoleamine 2,3-dioxygenase (IDO) activity], and 3-HAA/KYN [estimate of neurotoxic load] in adolescents with melancholic major depressive disorder (MDD), non-melancholic MDD, and healthy controls.
| Measure | Melancholic MDD n = 20 | Non-melancholic MDD n = 30 | Healthy controls n = 22 |
|---|---|---|---|
| KYN | 375.60 (146.70) | 373.26 (133) | 408.65 (148.08) |
| TRP | 718.59a,c (727.12) | 1022.77 (643.03) | 982.87 (406.31) |
| 3-HAA | 9009.68 (2060.33) | 9235.25 (2695.44) | 9630.58 (2251.77) |
| KYN/TRP | .76b,d (.41) | .45 (.21) | .49 (.27) |
| 3-HAA/KYN | 25.76 (6.69) | 25.96 (5.99) | 24.77 (4.43) |
Compared to healthy controls (p = .022).
Compared to healthy controls (p = .008).
Compared to non-melancholic MDD (p = .006).
Compared to non-melancholic MDD (p = .001).
i) Group differences in KP measures
The composite F test yielded significant differences across the groups in TRP (F = 4.91, p = .01), and KYN/TRP (F = 3.92, p = .02). However, the groups did not differ significantly with respect to KYN, 3-HAA, and 3-HAA/KYN.
Whole MDD group versus controls
There were no significant differences in KP plasma metabolite measures between the whole adolescent MDD group (N = 50) and healthy control group (N = 22).
Adolescents with melancholic MDD versus healthy controls
As hypothesized, compared to healthy controls, adolescents with M-MDD had significantly decreased plasma TRP levels (718.59 ± 727.12 ng/ml versus 982.87 ± 406.31 ng/ml, t = 2.34, df = 65; p = .02), and significantly increased KYN/TRP quotient (.76 ± .41 versus .49 ± .27, t = −2.72, df = 65; p = .008).
Adolescents with melancholic MDD versus non-melancholic MDD
As predicted, relative to adolescents with NonM-MDD, adolescents with M-MDD had significantly decreased plasma TRP levels (718.59 ± 727.12 ng/ml versus 1022.77 ± 643.03 ng/ml, t = −2.81, df = 63; p = .006) and increased KYN/TRP (.76 ± .41 versus .45 ± .21, t = 3.33, df = 63; p = .001).
Adolescents with non-melancholic MDD versus controls
There were no significant differences between NonM-MDD adolescents and controls.
Medication status
KP metabolites did not differ significantly between medicated and non-medicated adolescents with MDD.
Excluding medicated patients
Findings described above remained significant when currently medicated subjects were excluded from the analyses. IDO was significantly higher and TRP significantly decreased in adolescents with M-MDD compared to controls (IDO: t = −2.2, p = .03; TRP: t = 2.9, p < .005) and NonM-MDD adolescents (IDO: t = 2.67, p = .01; TRP: t = −2.6, p < .01).
ii) Correlations between MDD severity (CDRS-R ratings) and KP measures
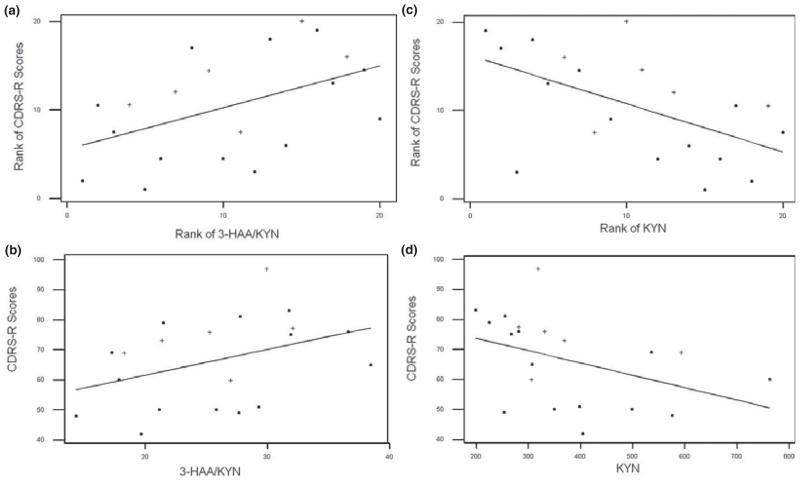
Across all MDD patients, no associations were observed between MDD severity and KP measures. Similarly, no associations were found within the NonM-MDD group. In contrast, significant relationships between MDD severity and KP measures were observed in the M-MDD group: KYN was negatively correlated with CDRS-R scores (r = −.55, p = .01; Figure 2c,d) and 3-HAA/KYN was positively correlated with CDRS-R scores (r = .47, p = .03; Figure 2a,b). Additionally, the M-MDD group differed significantly from the NonM-MDD group in terms of the correlation of 3-HAA/KYN with CDRS-R (p < .05), and with a trend toward significant difference for the correlation of KYN with CDRS-R (p < .06).
Figure 2.
Scatter plots with regression lines characterizing associations of kynurenine pathway measures and CDRS-R severity scores for the twenty adolescents with melancholic major depressive disorder. Medication-free/naïve individuals represented by (■); medicated individuals represented by (+). (a) Ranks of plasma 3-HAA/KYN ratios and CDRS-R scores (Spearman correlation: r = 0.47, p = 0.03); (b) Raw plasma 3-HAA/KYN ratios and CDRS-R scores; (c) Ranks of plasma KYN concentrations and CDRS-R scores (Spearman correlation: r = −0.55, p = 0.01); (d) Raw plasma KYN concentrations (ng/ml) and CDRS-R scores. CDRS-R, Children’s Depression Rating Scale– Revised; 3-HAA, 3-Hydroxyanthranilic acid; KYN, Kynurenine
Discussion
The study addressed whether melancholic features in adolescent MDD comprise a distinct clinical syndrome. To our knowledge, this is the first attempt to examine possible specific abnormalities in the neurobiology of adolescent M-MDD.
As expected, adolescents with M-MDD exhibited more severe depressive ratings compared to their NonM-MDD counterparts and displayed different neurobiological profiles. Major findings are of significantly decreased plasma TRP levels and increased KYN/TRP (estimating IDO activity) in adolescents with M-MDD compared to both NonMMDD and control adolescents. Importantly, findings remained significant when controlled for CDRS-R scores, suggesting that they are not related to increased symptom severity observed in the M-MDD group. The NonM-MDD group did not differ from healthy controls with respect to any KP measure. We also found associations between KP measures (e.g., KYN and 3-HAA/KYN) and severity of MDD episode (measured by CDRS-R) in the adolescent M-MDD, exclusively. However, we did not detect differences in other KP measures (i.e., KYN, 3-HAA, and 3-HAA/KYN). On balance, findings support the notion that the neurobiology of adolescent M-MDD may be distinct from other clinical subtypes of adolescent MDD.
To date, biological research and/or treatment studies in adolescent M-MDD have been scarce. An earlier study by Robbins et al. found that melancholic features are associated with failure to respond to psychosocial treatment (Robbins, Alessi, & Colfer, 1989). The recent Treatment for Adolescents with Depression Study also found that melancholic features were predictive of relatively less favorable response to selective serotonin reuptake inhibitors (Curry et al., 2006). These treatment findings support the concept of a distinct neurobiology in adolescent M-MDD.
Our findings of decreased TRP and increased KYN/TRP in the M-MDD group compared to both the NonM-MDD and control groups are consistent with reports in adults citing lower plasma TRP and TRP/competing amino acids levels in adults with M-MDD compared to controls as well as NonM-MDD subjects (Maes et al., 1994; Anderson, Parry-Billings, News-holme, Poortmans, & Cowen, 1990; Maes et al., 1996; Cowen, Parry-Billings, & Newsholme, 1989; Maes, De Ruyter, Hobin, & Suy, 1987). In an early study Curzon and Bridges found that women with ‘endogenous’ depression (i.e., M-MDD) exhibited increased urinary excretion of KYN and 3-HK compared to controls after an oral load of TRP (Curzon & Bridges, 1970). More recently, in adult cancer patients undergoing treatment with IFN-α or IL-2, a positive correlation was reported between the development of neurovegetative symptoms – which are prominent in M-MDD – and reduction of plasma TRP (Capuron et al., 2002). Also supporting a possible role of IDO in M-MDD are findings of highest plasma levels of neopterin, a pteridine released from macrophages/monocytes in parallel to IDO activity, in adults with M-MDD (Maes et al., 1994).
As dietary intake may affect plasma TRP concentrations, we required all blood samples to be collected in the morning following an overnight fast in order to minimize confounding effects of diet on TRP levels (Badawy, 2009).
While the increased KYN/TRP ratios found in the M-MDD group suggest increased IDO activation, this finding may be attributed to the decreased TRP as KYN levels did not differ across the groups. However, the significant negative relationship observed between MDD severity and KYN in the M-MDD group indicates increased KYN metabolism in this clinical group. This hypothesis is further supported by the significant positive correlation also found between 3-HAA/KYN and MDD severity only in the M-MDD group. Taken together, these findings suggest that KYN is preferentially metabolized toward the neurotoxic branch, and that production of neurotoxins may be a factor in M-MDD (see Figure 1).
Several studies reinforce our hypothesis that KYN metabolism is shifted toward the neurotoxic branch, including our recent findings of positive correlations between plasma KYN and 3-HAA and striatal tCho (cell membrane turnover biomarker) exclusively in M-MDD adolescents (Gabbay et al., 2010). Relatedly, in an adult immunotherapy study Wichers et al. reported positive correlations between KYN/KA (reflecting neurotoxic metabolite load) and symptoms that are prominent in M-MDD (e.g., observed sadness, tension/irritability, decreased appetite, sleep problems, and social withdrawal) (Wichers et al., 2005). This investigation also failed to find changes in KYN plasma levels (Wichers et al., 2005).
The concept that neuroplasticity impairment may play a specific role in M-MDD is also supported by studies reporting more pronounced reductions of γ-aminobutyric acid (GABA) concentrations in the plasma (Petty, Kramer, Gullion, & Rush, 1992), cerebrospinal fluid (Roy, Dejong, & Ferraro, 1991) and the occipital lobe (Sanacora et al., 2004), as well as increased brain glutamate concentrations (Sanacora et al., 2004), in adults with M-MDD compared to controls.
The study has a number of limitations. We have not assessed other relevant metabolites such as KA (NMDA receptor antagonist) as well as 3-HK and QUIN (neurotoxins) (illustrated in Figure 1). Additionally, the availability of TRP uptake to the brain is proportionally affected by plasma concentrations of several other amino acids (tyrosine, valine, phenylalanine, leucine, isoleucine), collectively termed competing amino acids (CAA). As we did not measure CAA concentrations we cannot conclude that TRP availability to the brain was decreased in the adolescents with M-MDD. Further, a substantial proportion of MDD adolescents (34%) were on psychotropic medications, which have anti-inflammatory effects and, as such, may have limited the identification of other differences in KP measures (e.g., KYN levels). Additionally, the groups were not matched with respect to their ethnic distribution having higher proportions of African Americans (AA) in the M-MDD group and higher proportions of Hispanics in the NonM-MDD group. A recent study reported no ethnic differences in TRP, 3-HK, and KA, while 3-HAA was decreased in AA compared to Caucasians and Hispanics and KYN decreased in AA and Hispanics compared to Caucasians (Badawy et al., 2007). These data suggest decreased activation of the KP and of the neurotoxic pathway in AA. Since our findings suggest opposite directions (i.e., activation of the KP and increased neurotoxicity) in M-MDD, our findings are likely not the result of having increased proportions of AA in the melancholic group but instead suggest that we may have detected a greater effect if the groups were better matched. Last, we did not assess menstrual cycle, which may affect KP activity (Hrboticky, Leiter, & Anderson, 1989).
In summary, our findings suggest a possible specific role of the KP in adolescent M-MDD. The identification of homogenous and valid clinical subtypes is important to inform the nosology and guide clinical care through better understanding of the disorder’s biological underpinnings.
Findings should be replicated, optimally with medication-free adolescents with MDD while examining other KP metabolites, such as 3-HK and KA, to elucidate the role of the KP in M-MDD. Additionally, future studies should examine whether specific symptoms associated with melancholia (e.g., sleep, eating disturbances) account for KP abnormalities.
Failure of much of the research to identify biological correlates of adolescent MDD may be related to the heterogeneity of the syndrome. Study findings point to the potential importance of distinguishing between adolescents with and without M-MDD, whether addressing family history, biology, treatment, or course.
Key points.
Adolescent major depressive disorder (MDD) is well recognized as a clinically heterogeneous syndrome; however, research on neurobiological correlates of its clinical subtypes has been limited to date.
Immune system activation has been repeatedly documented in MDD studies, which could be explained by induction of the neurotoxin-forming kynurenine pathway (KP).
The present study addresses these issues by examining KP metabolite differences between depressed adolescents with and without melancholic features (M-MDD; NonM-MDD) and healthy controls.
We found that the KP activation was prominent in adolescents with M-MDD, suggesting a possible role of the KP in this clinical subgroup.
Our findings support the notion that adolescent M-MDD may represent a biologically distinct clinical syndrome.
Acknowledgments
This study was supported by grants from the NIH (AT002395, MH077072, AT004576), the American Foundation for Suicide Prevention, the NYU School of Medicine General Clinical Research Center grant (M01-RR00096), the Leon Levy Foundation, and generous gifts from the Anita Saltz Foundation and from Bruce and Claude Wasserstein. The authors thank Dr. F. Xavier Castellanos for his helpful comments on this manuscript.
Abbreviations
- MDD
major depressive disorder
- M-MDD
melancholic MDD
- NonM-MDD
non-melancholic MDD
- KP
kynurenine pathway
- IDO
indoleamine 2,3-dioxygenase
- TRP
tryptophan
- KYN
kynurenine
- 3-HAA
3-hydroxyanthranilic acid
- CDRS-R
Children’s Depression Rating Scale-Revised
- IFN
interferon
- IL
interleukin
- 3-HK
3-hydroxykynurenine
- NMDA
N-methyl-D-aspartate
- QUIN
quinolinic acid
- KA
kynurenic acid
- tCho
total choline
- ANCOVA
analysis of covariance
- GABA
gamma (γ)-aminobutyric acid
- CAA
competing amino acids
Footnotes
Conflict of interest statement: Dr. Vilma Gabbay serves as a Sub-Investigator on a study sponsored by Boehringer Ingelheim and Otsuka Pharmaceuticals, but has received no compensation for this work. At the time of data collection, Dr. Babb had consulting contracts with E-Z-EM, Inc. and Applied NeuroSolutions, Inc, but received no compensation over the last three years for these services and has since allowed all such contracts to expire. Drs. Klein, Alonso, Hirsch and Liebes, and Sandra Mendoza, Yisrael Katz and Leah E. Guttman report no conflicts of interest.
References
- Ambrosini PJ, Bennett DS, Cleland CM, Haslam N. Taxonicity of adolescent melancholia: A categorical or dimensional construct? Journal of Psychiatric Research. 2002;36:247–256. doi: 10.1016/s0022-3956(02)00011-0. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Parry-Billings M, Newsholme EA, Poortmans JR, Cowen PJ. Decreased plasma tryptophan concentration in major depression: Relationship to melancholia and weight loss. Journal of Affective Disorders. 1990;20:185–191. doi: 10.1016/0165-0327(90)90143-v. [DOI] [PubMed] [Google Scholar]
- Badawy AB. Plasma free tryptophan revisited: What you need to know and do before measuring it. Journal of Psychopharmacology. 2009 doi: 10.1177/0269881108098965. [DOI] [PubMed] [Google Scholar]
- Badawy AAB, Morgan CJ, Turner JA, Dougherty DM, Marsh DM, Mathias CW, Addicott MA, Jagar AA. Assessment of the kynurenine pathway in humans: I. Normal plasma values, ethnic differences and their clinical implications. International Congress Series. 2007;1304:335–343. [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism: Relationship to depression and paroxetine treatment. Biological Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26:797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Cowen PJ, Parry-Billings M, Newsholme EA. Decreased plasma tryptophan levels in major depression. Journal of Affective Disorders. 1989;16:27–31. doi: 10.1016/0165-0327(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Curry J, Rohde P, Simons A, Silva S, Vitiello B, Kratochvil C, Reinecke M, Feeny N, Wells K, Pathak S, Weller E, Rosenberg D, Kennard B, Robins M, Ginsburg G, March J. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS) Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1427–1439. doi: 10.1097/01.chi.0000240838.78984.e2. [DOI] [PubMed] [Google Scholar]
- Curzon G, Bridges PK. Tryptophan metabolism in depression. Journal of Neurology, Neurosurgery and Psychiatry. 1970;33:698–704. doi: 10.1136/jnnp.33.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ. Immune system dysregulation in adolescent major depressive disorder. Journal of Affective Disorders. 2009;115:177–182. doi: 10.1016/j.jad.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Liebes L, Katz Y, Liu S, Mendoza S, Babb JS, Klein RG, Gonen O. The kynurenine pathway in adolescent depression: Preliminary findings from a proton MR spectroscopy study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:37– 44. doi: 10.1016/j.pnpbp.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LE, Leopold MC, Huang X, Atwood CS, Saunders AJ, Hartshorn M, Lim JT, Faget KY, Muffat JA, Scarpa RC, Chylack LT, Jr, Bowden EF, Tanzi RE, Bush AI. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39:7266– 7275. doi: 10.1021/bi992997s. [DOI] [PubMed] [Google Scholar]
- Hrboticky N, Leiter LA, Anderson GH. Menstrual cycle effects on the metabolism of tryptophan loads. American Journal of Clinical Nutrition. 1989;50:46–52. doi: 10.1093/ajcn/50.1.46. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klein DF. Endogenomorphic depression: A conceptual and terminological revision. Archives of General Psychiatry. 1974;31:447–454. doi: 10.1001/archpsyc.1974.01760160005001. [DOI] [PubMed] [Google Scholar]
- Kolvin I, Barrett ML, Bhate SR, Berney TP, Famuyiwa OO, Fundudis T, Tyrer S. The Newcastle Child Depression Project. Diagnosis and classification of depression. British Journal of Psychiatry Supplement. 1991:9–21. [PubMed] [Google Scholar]
- Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Molecular Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- Maes M, De Ruyter M, Hobin P, Suy E. Relationship between the dexamethasone suppression test and the L-tryptophan/competing amino acids ratio in depression. Psychiatry Research. 1987;21:323– 335. doi: 10.1016/0165-1781(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpe S, Meltzer HY, Okayli G, Bosmans E, D’Hondt P, Vanden Bossche BV, Cosyns P. Increased neopterin and interferon-gamma secretion and lower availability of L-tryptophan in major depression: Further evidence for an immune response. Psychiatry Research. 1994;54:143–160. doi: 10.1016/0165-1781(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Maes M, Wauters A, Verkerk R, Demedts P, Neels H, Van Gastel A, Cosyns P, Scharpe S, Desnyder R. Lower serum L-tryptophan availability in depression as a marker of a more generalized disorder in protein metabolism. Neuropsychopharmacology. 1996;15:243–251. doi: 10.1016/0893-133X(95)00181-C. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. Journal of Affective Disorders. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Petty F, Kramer GL, Gullion CM, Rush AJ. Low plasma gamma-aminobutyric acid levels in male patients with depression. Biological Psychiatry. 1992;32:354– 363. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children’s Depression Rating Scale. Journal of the American Academy of Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Robbins DR, Alessi NE, Colfer MV. Treatment of adolescents with major depression: Implications of the DST and the melancholic clinical subtype. Journal of Affective Disorders. 1989;17:99–104. doi: 10.1016/0165-0327(89)90031-1. [DOI] [PubMed] [Google Scholar]
- Roy A, Dejong J, Ferraro T. CSF GABA in depressed patients and normal controls. Psychological Medicine. 1991;21:613–618. doi: 10.1017/s0033291700022248. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Weissenburger JE. Melancholic symptom features and DSM-IV. American Journal of Psychiatry. 1994;151:489–498. doi: 10.1176/ajp.151.4.489. [DOI] [PubMed] [Google Scholar]
- Ryan ND, Puig-Antich J, Ambrosini P, Rabinovich H, Robinson D, Nelson B, Iyengar S, Twomey J. The clinical picture of major depression in children and adolescents. Archives of General Psychiatry. 1987;44:854–861. doi: 10.1001/archpsyc.1987.01800220016003. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Archives of General Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sapko MT, Guidetti P, Yu P, Tagle DA, Pellicciari R, Schwarcz R. Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: Implications for Huntington’s disease. Experimental Neurology. 2006;197:31–40. doi: 10.1016/j.expneurol.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: An endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A Children’s Global Assessment Scale (CGAS) Archives of General Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: A shift in hypothesis from tryptophan depletion to neurotoxicity. Molecular Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Wood A, Moore A, Harrington R, Jayson D. Clinical validity of major depression-endogenous sub-type in adolescent patients. European Child and Adolescent Psychiatry. 1996;5:155–161. doi: 10.1007/BF00571675. [DOI] [PubMed] [Google Scholar]