Abstract
Oncogenic stimuli are thought to induce senescence in normal cells in order to protect against transformation and to induce proliferation in cells with altered p53 and/or retinoblastoma (Rb) pathways. In human fibroblasts, RAS initiates senescence through upregulation of the cyclin-dependent kinase inhibitor p16INK4A. We show here that in contrast to cultured fibroblast strains, freshly isolated normal fibroblasts are resistant to RAS-induced senescence and instead show some characteristics of transformation. RAS did not induce growth arrest or expression of senescence-associated β-galactosidase, and Rb remained hyperphosphorylated despite elevated levels of p16. Instead, RAS promoted anchorage-independent growth of normal fibroblasts, although expression of hTert with RAS increased colony formation and allowed normal fibroblasts to bypass contact inhibition. To test the hypothesis that p16 levels determine how cells respond to RAS, we expressed RAS in freshly isolated fibroblasts that expressed very low levels of p16, in hTert-immortalized fibroblasts that had accumulated intermediate levels of p16, and in IMR90 fibroblasts with high levels of p16. RAS induced growth arrest in cells with higher p16 levels, and this effect was reversed by p16 knockdown in the hTert-immortalized fibroblasts. These findings indicate that culture-imposed stress sensitizes cells to RAS-induced arrest, whereas early passage cells do not arrest in response to RAS.
Normal cells have several mechanisms in place to protect against uncontrolled proliferation and tumorigenesis. One line of defense is cellular senescence, a permanent growth arrest that occurs after extended periods of cell division, exposure to oxidative stress, or expression of activated oncogenes (26, 40). Senescent cells remain alive and metabolically active but are arrested in the G1 phase of the cell cycle, are resistant to growth factor stimulation, and show common biochemical markers such as expression of an acidic senescence-associated β-galactosidase (SA-β-Gal) activity (6). In addition, senescent cells show altered differentiation functions that are cell type specific. For example, human fibroblasts secrete growth factors and extracellular matrix-remodeling enzymes upon the induction of senescence (6). While senescence has been characterized primarily in cultured cells, there is also evidence that it occurs in vivo. Cells expressing markers of senescence such as SA-β-Gal have been identified in normal tissues (7), and senescence can be induced following some types of chemotherapeutic treatments (38).
The best-characterized example of oncogene-induced senescence is the response of normal fibroblasts to expression of an activated allele of H-ras (H-rasV12). Normal ras proteins are important for transducing mitogenic signals in the cell and are mutated to constitutively active forms in approximately 20% of human cancers (4). These activated alleles contribute to transformation by increasing proliferation, angiogenesis, and invasion of tumors, as well as desensitizing cells to apoptosis (8).
All normal cells with intact p53 and retinoblastoma (Rb) pathways are thought to senesce in response to RAS. Expression of viral oncoproteins that disrupt these pathways, such as simian virus 40 (SV40) T Ag (27), human papillomavirus E6 or E7 (25, 34), or adenovirus E1A (37), block senescence and cooperate with RAS to transform cells. In murine cells, an intact p53/Arf pathway is required for both RAS- and passage-induced senescence (18, 32, 41). In human cells, the p16/Rb pathway plays a more significant role. Levels of p16 increase in response to either culture (3, 35)- or oncogene (41)-induced stress, and high levels of p16 cause cell cycle arrest by inhibiting the G1 cyclin-dependent kinases Cdk4 and Cdk6. Inhibition of these kinases by p16 prevents phosphorylation and inactivation of Rb, resulting in repression of E2F-dependent promoters and a G1 cell cycle arrest (36). Recent studies have suggested that loss of p16 renders human fibroblasts resistant to RAS-induced senescence, since fibroblasts established from individuals carrying inactivating mutations in the p16 gene do not arrest in response to RAS (5, 14). However, it is controversial whether p16 loss is sufficient to prevent RAS-induced senescence. Data from several groups indicate that IMR90 (41), BJ (12), and LF1 (45, 46) fibroblasts require disruption of both the p16/Rb and p53/p21 pathways in order to prevent senescence induced by RAS. Moreover, another group has reported that p53 disruption is sufficient to prevent RAS-induced senescence (44). Further investigation to clarify these issues is needed.
It has been proposed previously that RAS-induced senescence in cultured cells models what happens when a normal cell acquires a RAS mutation in vivo and that senescence protects cells against RAS-mediated transformation. However, there is little evidence that expression of activated RAS in vivo leads to a senescence response. Several lines of transgenic mice expressing activated alleles of RAS have been generated, and the tissues in these mice proliferate normally (1, 11, 16). This discrepancy raises the question of whether cultured cells accurately represent cells in vivo or if the process of establishment in culture somehow sensitizes cells to RAS-induced arrest.
In this study, we demonstrate that normal fibroblasts isolated directly from primary tissue are resistant to RAS-induced senescence because, unlike other cultured fibroblasts, they lack p16 expression at early passages. Furthermore, exposing normal fibroblasts to the stress of extended passaging in culture leads to an accumulation of p16, and increased p16 levels sensitize cells to RAS-induced senescence. We also show that fibroblasts expressing RAS alone are capable of anchorage-independent growth and that coexpression of hTert increases the frequency of colony formation in soft agar and is required for cells to be able to bypass contact inhibition. These data suggest that expression of RAS in early passage normal fibroblasts is similar to what happens when a cell acquires an activating RAS mutation in vivo and that RAS only causes senescence in cells that have been exposed to additional stress.
MATERIALS AND METHODS
Cell culture.
Human foreskin fibroblasts (HFF1 and HFF2) were isolated from neonatal human foreskins and infected with retroviruses within the first 20 population doublings. IMR90, BJ, and WI38 fibroblasts were obtained from the American Type Culture Collection and used within 10 population doublings. Extended-passage HFFs expressing hTert have been described previously (19) and were infected with pBABE or 16-1 retroviruses at population doubling level 180 after selection. SV40-transformed Cl39T cells have also been described previously (29). All cells were cultured in Dulbecco's modified Eagle's medium that was supplemented with 10% fetal bovine serum and penicillin-streptomycin.
Retroviral infections.
Retroviruses were produced and concentrated as previously described (2). H-rasV12 was provided in pBABE-puro and pWZL-hygro retroviral vectors by Manuel Serrano (Spanish National Centre of Biotechnology) and subcloned into LXSN (LXSN/H-rasV12). pBABE-puro, LXSN, LXSH, and LXSN/p16 have been described elsewhere (3). The hTert cDNA was cloned by PCR from a HeLa cDNA library (Clontech) and was subsequently transferred into LXSN for retroviral expression. The p16 short hairpin RNA vector (16-1) was provided by Scott Lowe (Cold Spring Harbor Laboratory). Twenty-four hours after infection, cells were expanded 1:2 into complete media containing 1 mg of G418 per ml, 1.5 μg of puromycin per ml, or 200 μg of hygromycin per ml, as appropriate. Selection in G418 or puromycin was usually complete within 3 to 5 days, and selection in hygromycin was usually complete within 1 week. In all experiments, the day on which a parallel plate of uninfected target cells was completely killed in selective media is referred to as day 0. On day 0, cells were photographed, labeled with bromodeoxyuridine (BrdU) and fixed for immunofluorescence, and lysed for Western blot analysis. Also on day 0, cells were split and replated for analysis at later time points. In each case, experiments were repeated multiple times, and a representative experiment is shown.
BrdU staining.
Cells were labeled with 40 μM BrdU for 4 h, fixed in 4% paraformaldehyde, and permeabilized with methanol-acetone. Fixed cells were then stained for immunofluorescence by using either mouse anti-BrdU antibodies provided with DNase I from the manufacturer (Amersham-Pharmacia Biotech) or mouse anti-BrdU (BU-33; Sigma) in blocking buffer (5% goat serum, 5% horse serum, 0.2% Tween 20 in phosphate-buffered saline) and 20 U of DNase I (Sigma) per ml. Nuclei were also stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma) in order to determine the total number of cells in a given field. One hundred to 500 nuclei per condition were photographed, and the percentage of BrdU-positive cells was determined. Typically, 30 to 50% of nuclei in LXSN control populations were BrdU positive. For comparison, we show at each time point the percentage of BrdU-positive cells in p16- and RAS-expressing populations relative to that of LXSN control cells.
Western blotting.
Preparation of whole-cell lysates and Western blotting were performed as previously described (3). Forty to 80 μg of protein was analyzed with antibodies to H-ras (F235; Santa Cruz Biotechnology), p16 (BD PharMingen), actin (I-19; Santa Cruz Biotechnology), APA-1 (3), p53 (antibody 6; Oncogene Research Products), p21 (Waf1 antibody 1; Oncogene Research Products), Rb (BD PharMingen), p130 (C-20; Santa Cruz Biotechnology), and p27 (clone 57; BD Transduction Laboratories).
SA-β-Gal staining.
Acidic β-Gal staining was done as previously described (7).
p16 immunofluorescence.
Cells were grown on glass coverslips, fixed in 2% paraformaldehyde, and permeabilized with methanol-acetone. Fixed cells were then stained with mouse anti-p16 (E6H4; molecular tools in medicine) and rhodamine-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch). Nuclei were visualized by DAPI (Sigma) staining. The anti-p16 E6H4 antibody was developed specifically to detect p16 in tissue sections and is accepted as a tool for diagnosing cervical interepithelial neoplasia (20, 21). Staining patterns were confirmed by using mouse anti-p16 antibody 2 from Pharmingen (clone ZJ11).
Telomerase activity.
Telomerase activity was measured by using the TRAPeze telomerase detection kit (Serologicals Corporation).
Quiescence and cell cycle analysis.
To arrest cells in G0, confluent cultures were incubated in complete growth medium for 3 days and then refed with low-serum medium (Dulbecco's modified Eagle's medium containing 0.1% fetal bovine serum) and incubated for an additional 3 days. Cells were then replated at approximately 50% confluence in low-serum medium and incubated for an additional 2 days. At this stage (density-low serum [DS] arrest), three plates of each cell type were labeled with 10 μM BrdU for 4 h, followed by fixation in 70% ethanol for flow cytometry. In addition, cells were harvested for Western blot analysis as described above. Remaining plates were refed with complete medium containing 10% serum and harvested in the same way after 24 h. For cell cycle analysis, nuclei were isolated from fixed cells and stained with fluorescein isothiocyanate-conjugated anti-BrdU antibodies (Becton Dickinson) and propidium iodide, as previously described (3). Cell cycle fractions were quantitated with CellQuest software (Becton Dickinson).
In vitro transformation assays.
To assay anchorage-independent growth, HFFs transduced with the indicated retroviruses were seeded into soft agar. A total of 50,000 cells were resuspended in 0.3% Noble agar-growth medium and plated in triplicate in six-well dishes on top of solidified 0.6% Noble agar-growth medium. Fresh top agar was added every 4 days, and cells were photographed and counted after 3 weeks. Three fields of cells were counted in each of three wells per cell type. To assay contact inhibition, we plated cells in six-well dishes at a concentration of 100,000 cells/well. Cells were trypsinized and counted in triplicate 4, 7, and 10 days after plating.
RESULTS
HFFs do not senesce in response to RAS expression.
While characterizing the response of freshly isolated HFFs to senescence induced by different signals, we found that, unlike other human fibroblasts, HFFs did not arrest following expression of RAS. These experiments were repeated in HFFs obtained from different donors, with similar results (Fig. 1 and 5 and data not shown). We therefore decided to investigate the response of HFFs to RAS in greater detail.
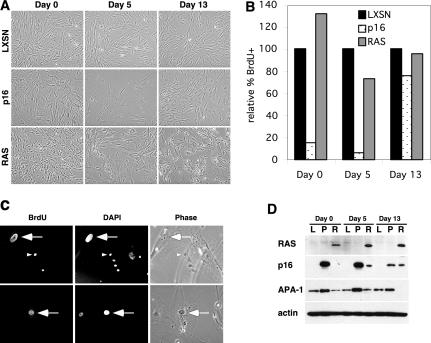
FIG. 1.
HFFs do not senesce in response to RAS expression. (A) Early passage HFF1 cells were infected with LXSN-, p16-, or RAS-expressing retroviruses. Cells were photographed immediately after selection (day 0) as well as 5 and 13 days later. (B) LXSN cells, p16 cells, and RAS cells from panel A were labeled with BrdU, fixed, stained with anti-BrdU antibody, and counted. The percentages of BrdU-positive cells, relative to those of LXSN controls, are shown. (C) Representative BrdU staining in RAS-expressing HFFs on day 5. As seen in panel A, RAS cultures were a heterogeneous population consisting of large, apparently senescent nuclei (large arrows) and smaller nuclei (small arrowheads). A fraction of both large and small nuclei were BrdU positive. (D) LXSN (L)-, p16 (P)-, and RAS (R)-expressing cells from panel A were harvested on days 0, 5, and 13. Levels of RAS, p16, and APA-1 proteins were examined by Western blot analysis. Actin is shown as a loading control.
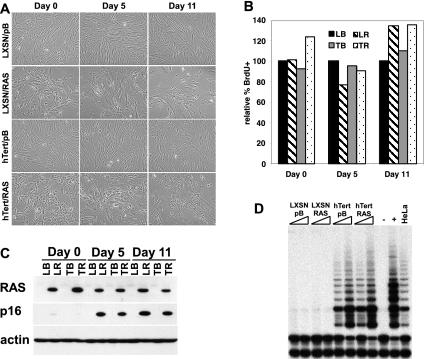
FIG. 5.
Expression of RAS in early passage HFF2 cells. (A) Early passage HFF2 fibroblasts expressing LXSN or hTert were infected with retroviruses expressing pB empty vector or pB/RAS. Cells were photographed immediately after selection (day 0) as well as 5 and 11 days later. (B) LXSN/pB (LB), LXSN/RAS (LR), hTert/pB (TB), and hTert/RAS (TR) cells from panel A were labeled with BrdU, fixed, stained with anti-BrdU antibody, and counted. The percentages of BrdU-positive cells, relative to those of LXSN controls, are shown. (C) RAS, p16, and actin protein levels were examined in cells described in panel A on days 0, 5, and 11, as indicated. (D) Telomerase activity in cells from panel A was analyzed 14 days after selection by using the TRAPeze kit. For each cell population, 0.2 and 2 μg of protein lysate was analyzed and compared to a buffer-alone control (−), the positive control provided with the kit (+), and 0.1 μg of HeLa extract.
Early passage HFFs were infected with retroviruses expressing empty vector (LXSN), p16, or RAS, and cells were analyzed immediately following selection (day 0) as well as 5 and 13 days after selection. LXSN-transduced cells resembled uninfected HFFs, and mitotic cells were evident in the population throughout the experiment (Fig. 1A). In contrast, no mitotic cells could be detected in p16-infected cultures on day 0, and by day 5 most cells in the culture had adopted an enlarged and flattened shape, indicative of senescence. However, by day 13, the majority of p16-expressing cells appeared to have bypassed the senescent arrest and were indistinguishable from LXSN control cells. As reported previously by others, RAS-expressing cells appeared smaller and more refractile than did controls immediately following selection—a finding similar to cells that were transformed by RAS (23). However, unlike other fibroblasts that arrest approximately 5 to 6 days after selection (23), RAS-expressing HFFs did not appear senescent on day 5. A large number of small dividing cells persisted in the culture throughout the experiment. Notably, the population was heterogeneous and large, flat cells were also present. This heterogeneity may be due in part to the heterogeneous nature of the population of fibroblasts that were established from the primary tissue.
We next labeled cells with BrdU and analyzed them by immunofluorescence. This type of analysis allowed us to determine if the flat, apparently senescent cells in the RAS-expressing cultures were truly arrested or if they were capable of entering S phase (BrdU positive). On day 0, most of the cells in the p16-infected culture were arrested, while RAS-infected cells continued to proliferate (Fig. 1B). As suggested by their appearance, the p16-expressing cells remained arrested on day 5, while a large percentage of the RAS cells remained BrdU positive. BrdU incorporation in RAS cells persisted through day 13 and, consistent with their visual appearance, a large fraction of the p16 cells were also BrdU positive and appeared to have escaped cell cycle arrest on day 13. We also found that many of the senescent-looking flat cells in the RAS-infected population stained positive for BrdU (Fig. 1C). Since senescence is defined by a G1 cell cycle arrest, we concluded that RAS-expressing HFFs were not senescent.
Western blotting confirmed that RAS protein levels were similar at each time point and showed the presence of p16 protein in RAS-expressing cells by days 5 and 13 (Fig. 1D). Importantly, the amount of p16 expressed in RAS cells was significantly lower than the amount expressed directly from the p16 retrovirus, a finding consistent with the fact that RAS-expressing cells were not arrested in the cell cycle. In addition, on day 13 when the p16 cells appeared to resume cycling, the levels of p16 protein were decreased to levels similar to that in RAS cells. One possibility for the observed decrease in p16 levels on day 13 is that p16 expression levels may have been heterogeneous following retroviral infection and that cells expressing insufficient levels of p16 to cause cell cycle arrest had overtaken the culture. No similar decrease in RAS protein levels was observed in RAS-infected cultures, a finding supporting the argument that there was no selection against cells expressing high levels of RAS and supporting the observation that RAS does not arrest HFFs. These data raised the possibility that RAS does not induce senescence in HFFs because p16 is not expressed at levels high enough to arrest the cell cycle.
The transcription factor APA-1 regulates transcription of extracellular matrix-remodeling genes during senescence and was examined as a marker of the functional changes that accompany senescence in human fibroblasts. As described previously (3), induction of premature senescence following p16 expression led to elevated levels of APA-1 protein (Fig. 1D). In contrast, RAS expression led to a reduction in APA-1 levels, a result similar to what is observed in fibroblasts transformed by SV40 (3). This pattern of APA-1 expression supported the model that RAS does not lead to senescence of HFFs but instead causes changes that are indicative of transformation.
High levels of p16 in IMR90 cells correlate with sensitivity to RAS-induced senescence.
In order to understand the reasons that HFFs were resistant to RAS-induced senescence, we repeated infection experiments in IMR90 fetal lung fibroblasts for comparison. We found that, consistent with previous studies (23, 41), IMR90 cells did undergo RAS-induced senescence (Fig. 2). Similar to HFFs, RAS-expressing IMR90 cells were still cycling and appeared refractile immediately following selection (Fig. 2A and B). However, on days 5 and 11, RAS cells appeared senescent and were arrested in the cell cycle, similar to the p16-expressing cells. IMR90 cells also differed from HFFs in their ability to bypass the growth arrest induced by p16 expression. On day 11 following selection, the majority of p16-expressing IMR90 cells still appeared senescent and were BrdU negative (Fig. 2A and B), whereas in HFF populations, cells expressing lower levels of p16 grew out at later time points (Fig. 1).
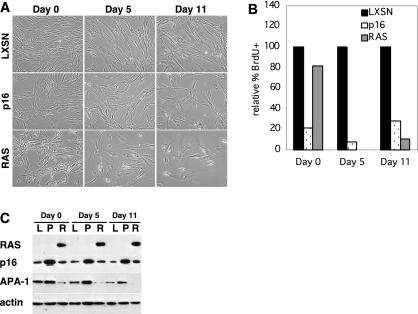
FIG. 2.
IMR90 cells arrest following RAS expression. (A) IMR90 cells were infected with LXSN-, p16-, or RAS-expressing retroviruses. Cells were photographed immediately after selection (day 0) as well as 5 and 11 days later. (B) LXSN cells, p16 cells, and RAS cells from panel A were labeled with BrdU, fixed, stained with anti-BrdU antibody, and counted. The percentages of BrdU-positive cells, relative to those of LXSN controls, are shown. (C) LXSN (L)-, p16 (P)-, and RAS (R)-expressing cells from panel A were harvested on days 0, 5, and 13. Levels of RAS, p16, and APA-1 proteins were examined by Western blot analysis. Actin is shown as a loading control.
Expression of RAS and p16 in IMR90 cells was confirmed by Western blotting (Fig. 2C). Unlike HFFs, which did not express any detectable p16 prior to infection, control IMR90 cells expressed p16 protein whose levels increased after infection with the p16 retrovirus. RAS expression also led to increased p16 levels on day 5 and day 11; however, the increases were not as dramatic as those occurring in HFFs, probably due to their high levels of p16 prior to RAS infection. As with HFFs, APA-1 was increased in IMR90 cells in response to p16 expression (Fig. 2C). Surprisingly, APA-1 levels were again reduced following RAS expression in IMR90 cells, a finding suggesting that downregulation of APA-1 by RAS overrides the upregulation of APA-1 in response to senescence.
Recent studies have shown that human fibroblasts with inactivating mutations in the p16 gene fail to undergo RAS-induced senescence (5, 14). This finding suggested that HFFs may have failed to arrest in response to RAS because p16 was not induced to sufficient levels. Direct comparison of HFF and IMR90 cells 5 days after selection supported this model. As shown in Fig. 3A, a comparison of cells transduced with empty vector revealed that IMR90 control cells expressed p16 protein, while none was detectable in HFFs. As noted above, p16 increased in both HFF and IMR90 cells following RAS expression. However, RAS-expressing HFFs had much lower levels of p16—even lower than those of control IMR90 cells. These data suggested that since IMR90 cells had high initial levels of p16, RAS expression increased p16 past a threshold that resulted in cell cycle arrest. The difference in p16 induction between cell types could not be accounted for by differences in RAS expression, as both expressed equivalent levels of RAS protein.
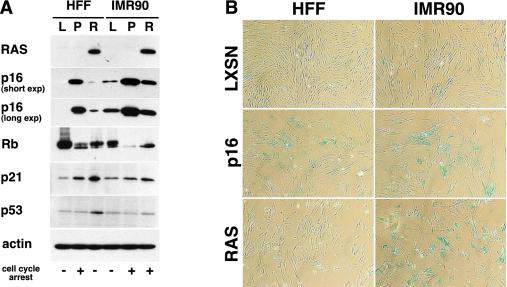
FIG. 3.
Comparison of HFF1 and IMR90 cells 5 days after selection. (A) Shown are Western blots of lysates from LXSN (L)-, p16 (P)-, and RAS (R)-expressing HFF1 and IMR90 cells harvested 5 days after selection. Blots were probed with antibodies to RAS, p16 (short and long exposures are shown), Rb, p21, p53, and actin. The corresponding cell cycle arrest is indicated beneath the blots. (B) HFF1 and IMR90 fibroblasts infected with LXSN, p16, or RAS were fixed 5 days after selection and stained for SA-β-Gal expression. Representative fields are shown.
Rb expression was also compared in infected HFF and IMR90 cells 5 days after selection. During senescence induced by p16 or RAS, Cdk4/6-associated kinase activity should be inhibited and Rb should be present in a hypophosphorylated, active form. Consistent with this model, Rb was exclusively hypophosphorylated in IMR90 cells expressing p16 or RAS (Fig. 3A). Expression of p16 in HFFs also caused a shift in Rb phosphorylation to primarily the hypophosphorylated form. In contrast, the majority of Rb in RAS-expressing HFFs was hyperphosphorylated, similar to vector-transduced controls, although overall levels of Rb were reduced.
The cyclin-dependent kinase inhibitor p21 increases in response to RAS in human fibroblasts (41, 45). We examined p21 and p53 levels in HFF and IMR90 cells and found little difference between the cell types (Fig. 3A). p21 increased slightly in both RAS-expressing HFFs and IMR90 cells; however, HFFs were not arrested. This finding supported the model that p21 was not the critical factor leading to RAS-induced growth arrest.
Finally, we compared the expression of SA-β-Gal in HFFs and IMR90 cells on day 5 following selection. IMR90 cells induced robust SA-β-Gal staining following expression of either p16 or RAS (Fig. 3B). In HFFs, p16 expression also induced SA-β-Gal, albeit to a lesser extent than that occurring in IMR90 cells (19 and 38%, respectively). We found that, consistent with other experiments, HFFs expressing RAS were primarily SA-β-Gal negative. Approximately 1% of HFFs with RAS stained positive, compared to 48% in IMR90 cells. These data supported the conclusion that RAS does not induce senescence in HFFs.
p16 levels sensitize HFFs to RAS-induced senescence.
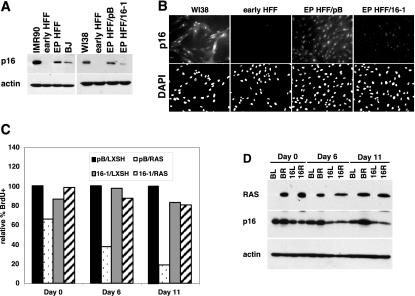
p16 levels increase when human fibroblasts are exposed to extended passaging in culture or to stress from suboptimal growth conditions (3, 35). Since high p16 levels correlated with sensitivity to RAS-induced senescence, we hypothesized that in vitro aging may sensitize HFFs to RAS-induced senescence and provide a mechanistic explanation for the resistance seen in early passage cells. To address this possibility, we decided to examine the consequences of RAS expression in hTert-immortalized HFFs that were approximately 150 population doublings past the time when control HFFs reached replicative senescence and had elevated levels of p16 (Fig. 4A, EP HFF). p16 immunofluorescence confirmed that p16 levels were increased in extended-passage HFFs (Fig. 4B, EP HFF/pB), whereas early passage HFFs appeared completely p16 negative. Overall, p16 levels appeared to increase in all cells in the population, although some variability in intensities was observed. Staining for p16 appeared to be specific, since levels were high in WI38 fibroblasts as previously reported (15) and were reduced in extended-passage cells expressing a short hairpin RNA that silenced p16 expression (28) (EP HFF/16-1, Fig. 4A and B). In addition, similar staining patterns were obtained by using two different p16 monoclonal antibodies (Fig. 4B; data not shown).
FIG. 4.
Expression of RAS in extended-passage, hTert-immortalized HFFs. (A) Western blot of p16 protein in IMR90 fibroblasts, early passage HFFs (early HFF), hTert-immortalized HFFs at 195 population doublings after selection (EP HFF), BJ fibroblasts, WI38 fibroblasts, and extended-passage HFFs transduced with empty vector (EP HFF/pB) or a short hairpin RNA-targeting p16 (EP HFF/16-1). Actin is shown as a loading control. (B) p16 immunofluorescence in WI38 fibroblasts, early passage HFFs (early HFF), and extended-passage HFFs expressing a control vector (EP HFF/pB) or p16 short hairpin RNA (EP HFF/16-1). Nuclei were visualized by corresponding DAPI staining. Overall p16 protein levels from cells at the identical passages are shown in panel A. (C) pB/LXSH, pB/RAS, 16-1/LXSH, and 16-1/RAS cells were labeled with BrdU, fixed, stained with anti-BrdU antibody, and counted. The percentages of BrdU-positive cells, relative to those of LXSN controls, are shown. (D) pB/LXSH (BL), pB/RAS (BR), 16-1/LXSH (16L), and 16-1/RAS (16R) cells from panel C were analyzed by Western blotting on days 0, 6, and 11. Levels of RAS, p16, and actin are shown.
The effects of RAS expression were next analyzed in extended-passage HFFs and extended-passage HFFs with reduced levels of p16 due to expression of the p16 short hairpin RNA. Control (pB) or p16 hairpin (16-1)-expressing cells were transduced with retroviruses expressing a second empty vector (LXSH) or RAS. Cells were selected in hygromycin and analyzed immediately following selection (day 0) as well as 6 and 11 days later. As shown earlier, all cells were cycling on day 0 (Fig. 4C). However, on days 6 and 11 there were obvious differences between pB/RAS and 16-1/RAS cells. While small dividing cells were continually seen in the 16-1/RAS population, pB/RAS cells took on a senescent shape and arrested in the cell cycle (Fig. 4C, data not shown). Western blot analysis confirmed that pB control cells had increased levels of p16 protein on days 6 and 11 following RAS expression. In contrast, 16-1 cells had a reduced level of p16 protein at all time points, and the protein level did not increase following expression of RAS (Fig. 4D). These data demonstrated that p16 levels were the critical factor in determining whether HFFs will senesce in response to expression of RAS.
Growth properties of RAS-expressing HFFs.
In most cell types, disruption of the p53 and Rb pathways allows cells to bypass RAS-induced senescence (41). When RAS is expressed in these cell types, they exhibit properties that are indicative of transformed cells, such as the ability to grow in an anchorage-independent manner and to bypass contact inhibition. In human cells, telomerase (hTert) is also often required for transformation, as the proliferation of many cell types is quickly limited by telomere length. Since early passage HFFs did not undergo senescence in response to RAS, we wished to test whether cells expressing RAS exhibited any properties of transformed cells. Although telomere length is not limiting in early passage HFFs, cells expressing hTert were also tested in order to determine if telomerase could contribute to transformation by RAS.
Directly following isolation from the primary tissue, HFFs were infected with retroviruses expressing an empty vector (LXSN) or hTert. Following selection in G418, cells were infected with retroviruses expressing a second empty vector (pB) or RAS, selected in puromycin, and analyzed as described above. As described earlier, small dividing cells were observed immediately following selection (day 0) as well as on days 5 and 11 (Fig. 5A). All cells incorporated BrdU into their DNA at similar levels (Fig. 5B), and RAS expression led to increased p16 levels by day 5 following infection (Fig. 5C). In addition, RAS expression had no effect on the amount of active telomerase in the cell (Fig. 5D). All four cell types were continually passaged in culture until LXSN/pB and LXSN/RAS cells reached senescence after approximately 46 population doublings following selection. Therefore, RAS expression did not affect the life span of HFFs. hTert/pB and hTert/RAS cells were passaged for approximately 20 additional population doublings and showed no signs of senescence when the experiment was ended.
We next determined whether RAS expression had an effect on the ability of cells to enter quiescence in response to contact inhibition and serum starvation. LXSN/pB, LXSN/RAS, hTert/pB, and hTert/RAS fibroblasts were first held at confluence for 3 days in 10% serum and were then refed with medium containing 0.1% serum for 3 days; finally, they were replated at subconfluent densities in 0.1% serum. Cells were harvested for Western blotting and labeled with BrdU and fixed for cell cycle analysis. At the same time, a second set of cells was stimulated to reenter the cell cycle with 10% serum and analyzed 24 h later. All four cell populations had approximately 2% of cells in S phase after this protocol, a result indicating that all cells could be arrested by a combination of density and serum starvation (Fig. 6A). By comparison, approximately 20% of cells in all populations were in S phase 24 h following serum addition.
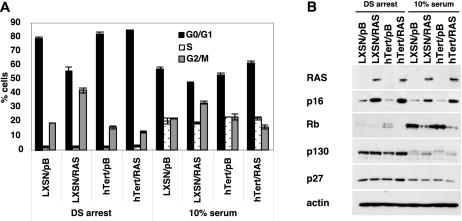
FIG. 6.
Quiescence in RAS-expressing HFFs. (A) Triplicate plates of the cells described in the legend of Fig. 5 were arrested by density and serum starvation as described in the text, labeled with BrdU, and fixed for cell cycle analysis (DS arrest). A second set of plates was stimulated to reenter the cell cycle by the addition of 10% serum and was then harvested 24 h later (10% serum). The percentages of cells in corresponding phases of the cell cycle are indicated; G0/G1 cells have a 2N DNA content, BrdU-positive cells are in S phase, and cells in the G2/M phase have a 4N DNA content. (B) An additional set of plates from panel A was harvested and analyzed by Western blotting for RAS, p16, Rb, p130, p27, and actin.
Although RAS-expressing cells arrested following density and serum starvation, RAS altered the cell cycle profiles of the arrested cells. While 79% of the LXSN/pB control cells arrested with a 2N DNA content, consistent with a G0-phase arrest, only 56% of the LXSN/RAS cells arrested with 2N DNA. Instead, 42% of LXSN/RAS cells arrested with a DNA content typical of the G2/M phase compared to 19% in controls, a finding suggesting that RAS expression may allow cells to bypass G0 arrest and to instead arrest in G2/M. Surprisingly, it appeared that expression of hTert reversed the effects of RAS. hTert/RAS cells contained 84% of cells arrested in G0 and only 13% in G2/M, a result that was more similar to LXSN/pB and hTert/pB cells than to cells expressing RAS alone. An increased fraction of cells in G2/M was also observed in normally cycling LXSN/RAS cells that had reentered the cell cycle following serum addition; however, the differences were more apparent following DS arrest. RAS and p16 expression was similar in all cells, regardless of whether they were cycling (Fig. 6B). In addition, all four cell types were found to have reduced Rb, increased p130, and increased p27 proteins following DS arrest. These changes are expected in quiescent cells (9, 13).
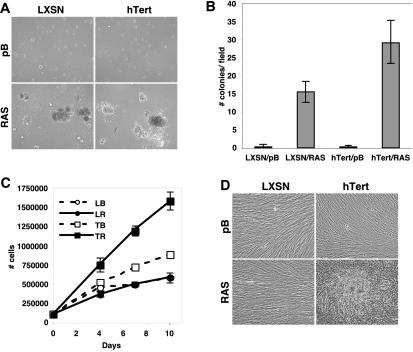
Early passage RAS-expressing cells were next seeded into soft agar and tested for their ability to grow in an anchorage-independent manner. After 21 days in culture, both LXSN/RAS and hTert/RAS cells formed colonies of similar size (Fig. 7A). However, hTert/RAS cells formed approximately twice as many colonies as did LXSN/RAS cells (Fig. 7B), suggesting that hTert contributed to anchorage-independent growth. Soft-agar assays were initiated with cells at an early time point after selection and with cells that had been passaged for approximately 20 population doublings, with similar results.
FIG. 7.
In vitro transformation of RAS-expressing HFFs. (A) Cells described in Fig. 5 were seeded into soft agar at a concentration of 50,000 cells/well and fed every 4 days. Cells were photographed after 21 days. (B) Quantitation of colonies from panel A. Colonies in three representative fields per well were counted for LXSN/pB (LB), LXSN/RAS (LR), hTert/pB (TB), and hTert/RAS (TR) cells, and the results were averaged. Average values from triplicate wells are represented in the graph. (C) Cells from Fig. 5 were plated in six-well dishes at a concentration of 100,000 cells per well and fed every 3 days. Triplicate wells were counted on days 4, 7, and 10 after plating. Average cell numbers are indicated for LB, LR, TB, and TR cells. (D) Photomicrographs of cells from panel C on day 13 after plating. LB, LR, and TB cells all appeared to be monolayers, while TR cells grew in multiple layers.
RAS-transformed cells can also bypass contact inhibition and form foci in culture dishes. To determine if RAS-expressing HFFs could bypass contact inhibition, cells were seeded into culture dishes and counted in triplicate on the indicated days. All cells appeared near confluent on day 4, although a few mitotic cells could be seen. HFFs expressing RAS alone were similar to vector-alone controls (Fig. 7C), demonstrating that expression of RAS alone did not allow cells to bypass contact inhibition. Surprisingly, hTert/RAS cells were considerably denser at every time point than were cells expressing either hTert or RAS alone. Cells expressing hTert alone reached higher densities than did controls at each time point; however, they were always less dense than were hTert/RAS cells and were more similar in appearance to controls than to hTert/RAS cells (Fig. 7D). By day 13 after plating, hTert/RAS cells did not form distinct foci but had grown in multiple layers on top of one another and came off the plate in a sheet following trypsin treatment. It was not possible to achieve an accurate cell count at this time point, since hTert/RAS cells could not easily be dissociated. This result indicated that the hTert/RAS cells had not only bypassed contact inhibition but also were capable of growing in the absence of adherence to a substratum. These growth characteristics were in striking contrast to those of control cells and cells expressing either RAS or hTert alone, which remained in monolayers. We observed that all HFF lines did not easily undergo contact inhibition, since even on day 13 mitotic cells were found in all cultures (Fig. 7D). Experiments were repeated in cells at an early passage following selection and in cells at approximately 24 population doublings after selection with similar results. These data demonstrated that although RAS-expressing HFFs have the ability to grow in an anchorage-independent manner, coexpression of hTert increases the frequency of colony formation in soft agar. In addition, expression of hTert was required for RAS-expressing HFFs to bypass contact inhibition.
DISCUSSION
In this study, we examined the response of freshly isolated HFFs to expression of RAS and demonstrated that, in contrast to other isolates of normal cells, HFFs are resistant to RAS-induced senescence. While RAS arrests IMR90 fibroblasts by 5 days following selection, HFFs continue to proliferate. Consistent with this observation, RAS-expressing HFFs are capable of entering S phase, have hyperphosphorylated Rb, and are predominantly negative for SA-β-Gal expression. In addition, these cells are capable of anchorage-independent growth—one hallmark of transformed cells.
Experiments performed with other strains of human fibroblasts have demonstrated that the cyclin-dependent kinase inhibitor p16 is important for RAS-induced senescence. RAS expression activates the mitogen-activated protein kinase pathway, which leads to induction of the Ets1 and Ets2 transcription factors and to activation of p16 transcription (23, 31, 48). However, in fibroblasts that carry inactivating mutations in the p16 gene, RAS does not induce senescence and cells are able to grow in soft agar (5, 14). Our finding that HFFs are resistant to RAS-induced senescence is consistent with this model. Although p16 levels did increase in HFFs following RAS expression, our results suggest that the level of p16 was not sufficient to cause growth arrest. It is known that human fibroblasts vary in their basal level of p16 expression (15), and it is therefore likely that fibroblasts will have variable responses following RAS expression. Similarly, the mechanism of passage-induced senescence in human fibroblasts varies depending on p16 levels. Some strains of human fibroblasts, such as WI-38 and IMR90, express high levels of p16, and p16 limits the life span of these cell types (15; Benanti and Galloway, unpublished results). In contrast, strains such as BJ as well as HFFs express lower levels of p16, and their life spans are limited primarily by telomere length (15).
One proposed explanation for these differences between fibroblast strains is that they are derived from different tissue types. Both IMR90 and WI-38 cells were derived from lung tissue, whereas BJ cells, like HFFs, were derived from neonatal foreskin. However, the tissue of origin cannot account for the differences in response to RAS expression. BJ cells are known to senesce following RAS expression (39), yet HFFs do not and are derived from the same tissue type. A more likely explanation is that BJ cells, like WI-38 and IMR90 cells, have been exposed to a greater amount of culture-induced stress when established. In support of this supposition, early passage BJ cells have higher levels of p16 than do HFFs (Fig. 4A) and are sensitive to RAS-induced senescence. Freshly established HFFs have not been exposed to prolonged stress, do not express any detectable p16, and are therefore resistant to RAS-induced senescence. This idea is supported by our finding that extended passaging of HFFs leads to increased levels of p16 and sensitizes cells to RAS-mediated arrest. Some evidence suggests that this effect may be mediated by increased mitochondrial activity and levels of oxidative stress during passage- and RAS-induced senescence (22, 47). It is possible that differences in mitochondrial function will explain the variable p16 levels in different fibroblast strains.
It is noteworthy that there is much controversy concerning the relative roles of the p16/Rb and p53/p21 pathways in RAS-induced senescence of human fibroblasts. Although we and others (5, 14) find that p16 is the key mediator of RAS-induced senescence, other groups have found that both the p16/Rb and p53/p21 pathways need to be disrupted in order to prevent RAS-induced arrest (12, 41, 45). Moreover, a recent report claims that disruption of p53 alone is sufficient for human fibroblasts to bypass RAS-induced arrest (44). There are two important factors that contribute to these differences. First, as described above, different strains of fibroblasts have variable levels of p16. It is likely that in cases when disruption of the p16/Rb pathway has been required to render cells resistant to RAS-induced arrest, cells had higher initial levels of p16. The other major factor contributing to the differing results appears to be whether the p53 pathway is activated following RAS expression. Other groups have found that expression of RAS in IMR90, LF1, and BJ fibroblasts (41, 44-46) activates the p53 pathway; however, we and others (5) see no evidence for p53 activation by RAS. The reason for this discrepancy remains unclear. However, because the p53 pathway is not strongly activated following RAS expression in our system, it therefore follows that disruption of the p53 pathway would not be required to prevent RAS-mediated arrest. We believe that HFFs have an intact p53 pathway, as p53-mediated arrest following DNA damage has been characterized in great detail previously (33).
In other types of human fibroblasts, disruption of the p16/Rb pathway and expression of RAS are sufficient to induce the ability to grow in soft agar; however, these conditions are not sufficient to permit cells to form tumors in nude mice (5, 39). Consistent with these reports, early passage HFFs expressing RAS were capable of growth in soft agar. Surprisingly, we found that hTert expression increased the frequency of soft-agar colony formation and appeared to be required for RAS to promote the bypass of contact inhibition. Although these cells show characteristics of transformation in culture, it is likely that they would not be tumorigenic in vivo. When compared to soft-agar colonies formed by the transformed 293T cell lines (data not shown), RAS-expressing HFFs formed relatively small colonies. In addition, expression of RAS and hTert in Leiden fibroblasts lacking functional p16 protein allows growth in soft agar and the bypass of contact inhibition; however, these conditions are not sufficient for tumorigenicity in vivo (5). Moreover, LF1 and BJ fibroblasts expressing RAS along with disruptions in the p16 and p53 pathways are capable of producing small colonies in soft agar but cannot produce tumors in mice (39, 46). In BJ and LF1 cells, expression of another cooperating gene such as SV40 ST is required for cells to be able to form tumors in vivo (12, 46), and it is likely that this would be the case for HFFs as well.
There is a growing body of evidence supporting a role for hTert in transformation that is independent of its ability to lengthen telomeres and immortalize cells. Evidence from mice that overexpress mTert in skin or cardiac myocytes argues that forced telomerase expression can promote cell growth (10, 30), and telomerase has been shown previously to affect transcription of growth-promoting genes in human cells (24, 42). In addition, human fibroblasts that are immortalized by a telomerase-independent mechanism are not transformed by RAS unless hTert is also expressed (43). Early passage HFFs should provide a good system with which to study the contribution of hTert to transformation, since these cells proliferate for 70 to 90 population doublings in culture before telomere length becomes limiting. A second advantage to studying transformation in HFFs is that the p16/Rb pathway does not need to be inactivated to study the effects of RAS. We have found that RAS expression affects the cell cycle distribution of HFFs but that it does not impair the ability of cells to enter quiescence. Moreover, coexpression of hTert modifies this cell cycle effect. It will be of considerable interest to determine the mechanism of these cell cycle alterations and how hTert interacts with RAS in this setting.
In conclusion, we have shown that normal human fibroblasts do not undergo senescence in response to RAS expression. Our findings are also consistent with what is known about RAS expression in vivo. Several cancer models have been developed by generating mice that express activated alleles of RAS. The tissues in these transgenic animals proliferate normally, suggesting that RAS may not in fact induce senescence in vivo (1, 11, 16). In addition, there is no evidence that RAS can cause senescence in human tissues. Activating mutations in RAS are believed to be early events during the development of human cancer (4). RAS mutations are thought to cause a burst of hyperproliferation, allowing for clonal expansion and an increased chance of acquiring additional mutations in tumor suppressor gene pathways. If a cell were to undergo senescence within a few population doublings after RAS activation, it would be improbable that additional growth-promoting mutations would arise and that RAS would have a tumor-promoting effect (17). In this way, early passage human fibroblasts may be representative of cells in vivo and provide a good system with which to study the effects of RAS expression in human cells.
Acknowledgments
We thank Thomas Fazzio, Carla Grandori, Beatrice Knudsen, and Joseph Carter for critical reading of the manuscript; Jim Roberts for advice and support; Kristin Robinson for establishing HFF cultures; Greg Wipf for cloning hTert; and members of the Galloway laboratory for their continued support and suggestions. In addition, we thank Magnus von Knebel Doeberitz for the E6H4 antibody, Scott Lowe for his gift of the p16 shRNA vector prior to publication, and Manuel Serrano for RAS expression vectors.
This work was supported by grant number CA64795 from the NCI to D.A.G. J.A.B. was supported in part by grant PHS NRSA T32 GM07270 from NIGMS.
REFERENCES
- 1.Adams, J. M., and S. Cory. 1991. Transgenic models of tumor development. Science 254:1161-1167. [DOI] [PubMed] [Google Scholar]
- 2.Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337-342. [DOI] [PubMed] [Google Scholar]
- 3.Benanti, J. A., D. K. Williams, K. L. Robinson, H. L. Ozer, and D. A. Galloway. 2002. Induction of extracellular matrix-remodeling genes by the senescence-associated protein APA-1. Mol. Cell. Biol. 22:7385-7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos, J. L. 1989. ras oncogenes in human cancer: a review. Cancer Res. 49:4682-4689. [PubMed] [Google Scholar]
- 5.Brookes, S., J. Rowe, M. Ruas, S. Llanos, P. A. Clark, M. Lomax, M. C. James, R. Vatcheva, S. Bates, K. H. Vousden, D. Parry, N. Gruis, N. Smit, W. Bergman, and G. Peters. 2002. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 21:2936-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campisi, J., G. Dimri, and E. Hara. 1996. Control of replicative senescence, p. 121-149. In T. E. Johnson, N. J. Holbrook, and J. H. Morrison (ed.), Handbook of the biology of aging. Academic Press, San Diego, Calif.
- 7.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, M. Peacocke, and J. Campisi. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downward, J. 2003. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3:11-22. [DOI] [PubMed] [Google Scholar]
- 9.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Suarez, E., E. Samper, A. Ramirez, J. M. Flores, J. Martin-Caballero, J. L. Jorcano, and M. A. Blasco. 2001. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 20:2619-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra, C., N. Mijimolle, A. Dhawahir, P. Dubus, M. Barradas, M. Serrano, V. Campuzano, and M. Barbacid. 2003. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 4:111-120. [DOI] [PubMed] [Google Scholar]
- 12.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hengst, L., and S. I. Reed. 1998. Inhibitors of the Cip/Kip family. Curr. Top. Microbiol. Immunol. 227:25-41. [DOI] [PubMed] [Google Scholar]
- 14.Huot, T. J., J. Rowe, M. Harland, S. Drayton, S. Brookes, C. Gooptu, P. Purkis, M. Fried, V. Bataille, E. Hara, J. Newton-Bishop, and G. Peters. 2002. Biallelic mutations in p16INK4a confer resistance to Ras- and Ets-induced senescence in human diploid fibroblasts. Mol. Cell. Biol. 22:8135-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itahana, K., Y. Zou, Y. Itahana, J. L. Martinez, C. Beausejour, J. J. Jacobs, M. van Lohuizen, V. Band, J. Campisi, and G. P. Dimri. 2003. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol. Cell. Biol. 23:389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson, L., K. Mercer, D. Greenbaum, R. T. Bronson, D. Crowley, D. A. Tuveson, and T. Jacks. 2001. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410:1111-1116. [DOI] [PubMed] [Google Scholar]
- 17.Jones, C. J., D. Kipling, M. Morris, P. Hepburn, J. Skinner, A. Bounacer, F. S. Wyllie, M. Ivan, J. Bartek, D. Wynford-Thomas, and J. A. Bond. 2000. Evidence for a telomere-independent “clock” limiting RAS oncogene-driven proliferation of human thyroid epithelial cells. Mol. Cell. Biol. 20:5690-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 19.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 20.Klaes, R., A. Benner, T. Friedrich, R. Ridder, S. Herrington, D. Jenkins, R. J. Kurman, D. Schmidt, M. Stoler, and D. M. von Knebel. 2002. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am. J. Surg. Pathol. 26:1389-1399. [DOI] [PubMed] [Google Scholar]
- 21.Klaes, R., T. Friedrich, D. Spitkovsky, R. Ridder, W. Rudy, U. Petry, G. Dallenbach-Hellweg, D. Schmidt, and D. M. von Knebel. 2001. Overexpression of p16INK4A as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int. J. Cancer 92:276-284. [DOI] [PubMed] [Google Scholar]
- 22.Lee, A. C., B. E. Fenster, H. Ito, K. Takeda, N. S. Bae, T. Hirai, Z. X. Yu, V. J. Ferrans, B. H. Howard, and T. Finkel. 1999. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 274:7936-7940. [DOI] [PubMed] [Google Scholar]
- 23.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12:3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindvall, C., M. Hou, T. Komurasaki, C. Zheng, M. Henriksson, J. M. Sedivy, M. Bjorkholm, B. T. Teh, M. Nordenskjold, and D. Xu. 2003. Molecular characterization of human telomerase reverse transcriptase-immortalized human fibroblasts by gene expression profiling: activation of the epiregulin gene. Cancer Res. 63:1743-1747. [PubMed] [Google Scholar]
- 25.Liu, Z., J. Ghai, R. S. Ostrow, R. C. McGlennen, and A. J. Faras. 1994. The E6 gene of human papillomavirus type 16 is sufficient for transformation of baby rat kidney cells in cotransfection with activated Ha-ras. Virology 201:388-396. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg, A. S., W. C. Hahn, P. Gupta, and R. A. Weinberg. 2000. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 12:705-709. [DOI] [PubMed] [Google Scholar]
- 27.Michalovitz, D., L. Fischer-Fantuzzi, C. Vesco, J. M. Pipas, and M. Oren. 1987. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J. Virol. 61:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narita, M., S. Nunez, E. Heard, M. Narita, A. W. Lin, S. A. Hearn, D. L. Spector, G. J. Hannon, and S. A. Lowe. 2003. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113:703-716. [DOI] [PubMed] [Google Scholar]
- 29.Neufeld, D. S., S. Ripley, A. Henderson, and H. L. Ozer. 1987. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol. Cell. Biol. 7:2794-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh, H., G. E. Taffet, K. A. Youker, M. L. Entman, P. A. Overbeek, L. H. Michael, and M. D. Schneider. 2001. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl. Acad. Sci. USA 98:10308-10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtani, N., Z. Zebedee, T. J. Huot, J. A. Stinson, M. Sugimoto, Y. Ohashi, A. D. Sharrocks, G. Peters, and E. Hara. 2001. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409:1067-1070. [DOI] [PubMed] [Google Scholar]
- 32.Palmero, I., C. Pantoja, and M. Serrano. 1998. p19ARF links the tumour suppressor p53 to Ras. Nature 395:125-126. [DOI] [PubMed] [Google Scholar]
- 33.Passalaris, T. M., J. A. Benanti, L. Gewin, T. Kiyono, and D. A. Galloway. 1999. The G2 checkpoint is maintained by redundant pathways. Mol. Cell. Biol. 19:5872-5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelps, W. C., C. L. Yee, K. Munger, and P. M. Howley. 1988. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell 53:539-547. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez, R. D., C. P. Morales, B. S. Herbert, J. M. Rohde, C. Passons, J. W. Shay, and W. E. Wright. 2001. Putative telomere-independent mechanisms of replicative aging reflect inadequate growth conditions. Genes Dev. 15:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruas, M., and G. Peters. 1998. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378:F115-F177. [DOI] [PubMed] [Google Scholar]
- 37.Ruley, H. E. 1983. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature 304:602-606. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt, C. A., J. S. Fridman, M. Yang, S. Lee, E. Baranov, R. M. Hoffman, and S. W. Lowe. 2002. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109:335-346. [DOI] [PubMed] [Google Scholar]
- 39.Seger, Y. R., M. Garcia-Cao, S. Piccinin, C. L. Cunsolo, C. Doglioni, M. A. Blasco, G. J. Hannon, and R. Maestro. 2002. Transformation of normal human cells in the absence of telomerase activation. Cancer Cell 2:401-413. [DOI] [PubMed] [Google Scholar]
- 40.Serrano, M., and M. A. Blasco. 2001. Putting the stress on senescence. Curr. Opin. Cell Biol. 13:748-753. [DOI] [PubMed] [Google Scholar]
- 41.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 42.Smith, L. L., H. A. Coller, and J. M. Roberts. 2003. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell Biol. 5:474-479. [DOI] [PubMed] [Google Scholar]
- 43.Stewart, S. A., W. C. Hahn, B. F. O'Connor, E. N. Banner, A. S. Lundberg, P. Modha, H. Mizuno, M. W. Brooks, M. Fleming, D. B. Zimonjic, N. C. Popescu, and R. A. Weinberg. 2002. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA 99:12606-12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voorhoeve, P. M., and R. Agami. 2003. The tumor-suppressive functions of the human INK4A locus. Cancer Cell 4:311-319. [DOI] [PubMed] [Google Scholar]
- 45.Wei, W., R. M. Hemmer, and J. M. Sedivy. 2001. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21:6748-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei, W., W. A. Jobling, W. Chen, W. C. Hahn, and J. M. Sedivy. 2003. Abolition of cyclin-dependent kinase inhibitor p16Ink4a and p21Cip1/Waf1 functions permits Ras-induced anchorage-independent growth in telomerase-immortalized human fibroblasts. Mol. Cell. Biol. 23:2859-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu, D., and T. Finkel. 2002. A role for mitochondria as potential regulators of cellular life span. Biochem. Biophys. Res. Commun. 294:245-248. [DOI] [PubMed] [Google Scholar]
- 48.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12:2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]