Abstract
This study presents the results of a secondary analysis of data collected during a trial of reflexology that aimed to improve health-related quality of life (HRQOL) among women with advanced breast cancer in treatment. A comparison of HRQOL (functioning, symptoms, spirituality) of those with (n = 298) and without (n = 87) distant metastasis is presented. Following the intake interview, 385 women were randomised to reflexology, lay foot manipulation or conventional care control, and were interviewed again at weeks 5 and 11. Those with distant metastasis were older, had fewer comorbid conditions, and a smaller proportion were employed. Longitudinal analysis of HRQOL at intake, 5 and 11 weeks revealed that those with distant metastasis had lower functioning and more pain; however, no differences were found on fatigue, nausea, shortness of breath, sleep quality, anxiety, depressive symptoms or spirituality. Despite advanced disease, 56% of all women in this study were below the clinical screening cut-off for depressive symptoms. These findings may indicate that patients with advanced breast cancer have adapted emotionally and spiritually; however, the management of physical symptoms remains a priority.
Keywords: metastatic breast cancer, quality of life, symptoms, physical functioning, spirituality
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer among women worldwide, ranging from 20% to 27% in various countries, and the leading cause of cancer deaths (World Cancer Research Fund International 2008). In the USA and among those with advanced breast cancer (38%), approximately 5% have distant metastases (National Cancer Institute 2011). While advanced breast cancer is documented as a devastating diagnosis (Cohen 2002), few reports have considered the difference between those with distant metastases compared with those without distant metastases (Siddiqi et al. 2009). This paucity in reports on the assessed needs of this subset of breast cancer patients was highlighted in a current review of literature (Vilhauer 2008). Thornton et al. (2008) reported retrospectively on differences in symptoms 17 months before recurrence. Those who ultimately experienced local recurrence reported fatigue, pain and emotional distress as well as elevated biomarkers, whereas those who later developed distant recurrence had only elevated biomarkers. Other investigators have compared breast cancer patients with recurrent disease versus those with a first diagnosis, and results revealed poorer physical, functional and emotional health-related quality of life (HRQOL) for recurrent patients (Northouse et al. 2002). Another study reported that women with distant versus local/regional metastatic recurrence of breast cancer were more similar than different on their quality of life ratings, although women with distant metastatic recurrence reported significantly lower perceived health status (Thornton et al. 2005). Thus, the type of disease and its progression can influence the course of treatment as well as symptoms and side effects associated with treatment. However, as the literature demonstrates, it cannot always be assumed that the poorest diagnosis translates to the most severe symptom experience and limitation in physical function.
This secondary analysis begins to address a gap in the literature for breast cancer patients with distant metastatic and local/regional metastatic disease by comparing the two groups on patient-reported outcomes such as symptoms and physical functioning. Both symptoms and functioning contribute to perceived HRQOL, which is a high priority for women with advanced breast cancer (Aranda et al. 2005). For this work, HRQOL is conceptualised by the theoretical framework proposed by Wilson and Cleary (1995) and further developed by Ferrans et al. (2005). Overall HRQOL is defined as subjective well-being related to how happy or satisfied someone is with life. Conceptually, HRQOL has four central components: biological, symptoms, functioning and general health perception, as well as factors that influence the central components, that is, characteristics of the environment and individual. Theoretically, by demonstrating improvement in symptoms and/or functional status, overall HRQOL is enhanced.
The following research questions were addressed in this analysis: among women with advanced breast cancer who are undergoing chemotherapy and/or hormonal therapy:
Are there differences in characteristics (socio-demographics and comorbid conditions) between women with distant metastatic disease and those with local/regional metastatic disease?
Are there difference in physical functioning and/or symptoms reported at three time points between women with distant metastatic disease and those with local/regional metastatic disease?
Among those with distant metastatic disease, is there an association of symptoms and physical functioning with patient characteristics (age and comorbidity), time since metastasis diagnosis and type of treatment (chemotherapy with and without concurrent hormonal therapy versus hormonal therapy alone)?
METHODS
Sample
The sample for this secondary analysis consisted of 385 women with advanced breast cancer who were on chemotherapy and/or hormonal therapy at the time of enrolment. Inclusion criteria were: 21 years of age or older; diagnosis of stage III or IV breast cancer, metastasis or recurrence; able to perform basic activities of daily living; cognitively intact and free of a charted diagnosis of mental illness; able to speak and understand English; access to a telephone; able to hear normal conversation; receiving chemotherapy at intake into the study; and a score of 11 or lower on the Palliative Prognostic Score (Pirovano et al. 1999). Exclusion criteria were: receiving hospice care at intake; residing in a nursing home or similar care facility; being bedridden; regularly using therapies similar to those used in the protocol (e.g. reflexology, foot massage or pedicure with massage); participating in a new experimental chemotherapy; or undergoing bone marrow transplantation.
Setting
The sample was recruited from 13 community-based medical oncology sites in the Midwest of the USA. The study had human subjects’ approval from the investigators’ university and from all enrolment sites.
Design
The study design was a three-group randomised clinical trial. Women were randomised to reflexology, lay foot manipulation or conventional care control group. Interviewers were blinded to patient group assignment. Patients in the reflexology and lay foot manipulation groups were blinded to their group assignment.
Data collection
All groups were interviewed at home via telephone at three time points: baseline and prior to randomisation, and again at week 5 (immediately post-intervention) and week 11 (6 weeks post-intervention). Interviewers entered outcome data electronically into the study database. Patients’ charts were reviewed at the end of the study (post-week 11) to provide data on metastasis, types of treatment, treatment interruptions, comorbid conditions, and dates of cancer and metastasis diagnosis.
Randomisation
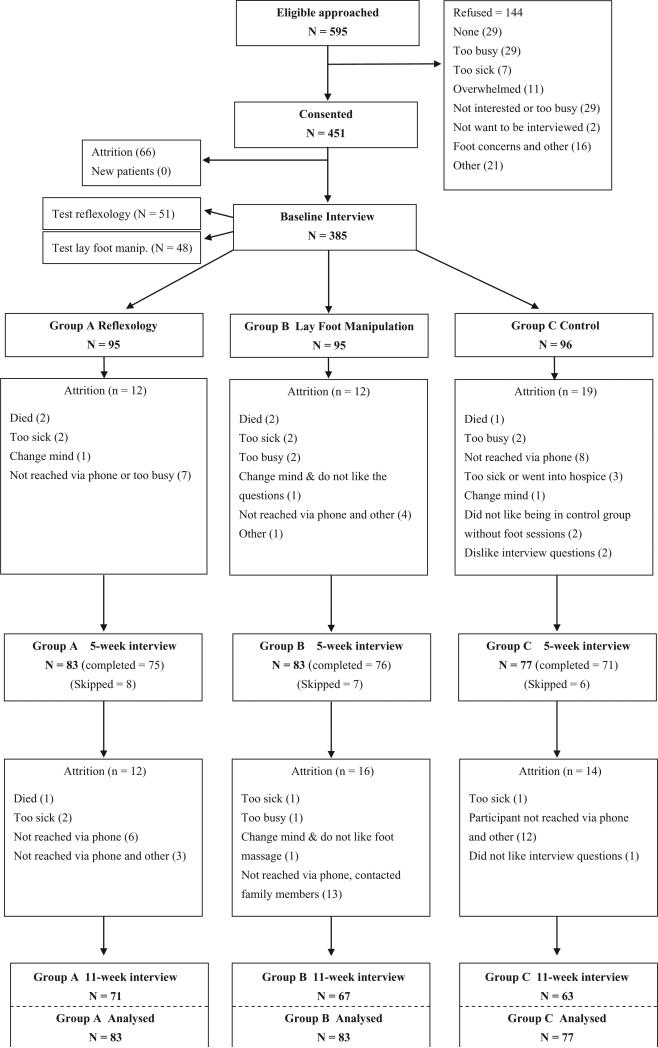
Following consent and baseline data collection, women were randomised using the computerised minimisation technique (Scott et al. 2002; McEntegart 2003). Recruitment site, levels of pain and fatigue, and goal of therapy were used as balancing factors to ensure the creation of equivalent groups at baseline. Pain and fatigue variables were dichotomised into low and high levels according to published cut-offs (Cleeland 1990; Mendoza et al. 1999), and the goal of therapy was at four levels (curative, maintenance, palliative and uncertain). Women were randomised to reflexology Group A (n = 95), the lay foot manipulation Group B (n = 95) and the conventional care control Group C (n = 96). Two test groups were also randomised, as the protocol was established, and were included in this analysis; there was a test reflexology (n = 51) and test lay foot manipulation (n = 48) (see Fig. 1). Nurse recruiters and healthcare providers at the sites were not involved in the randomisation. Concealment was achieved by running a computer program for randomisation at the central location of the investigators’ university.
Figure 1.
Flow of the participants throughout the trial.
Outcome measures
Medical Outcomes Study Short Form 36 (MOS SF-36), physical functioning subscale (Ware et al. 1993)
The physical functioning subscale of SF-36 has 10 items. The total scores range from 0 to 100 with higher scores reflecting better functioning. The scale has established content and construct validity, as well as internal consistency reliability for the subscales (0.78 to 0.93) with 100% scaling success and substantial clinical validity. The physical functioning subscale measures limitations in vigorous activities (such as an aerobic exercise programme), moderate activities (such as vacuuming), lifting groceries, climbing several flights of stairs, climbing one flight of stairs, bending (kneeling, stooping), walking one block, walking several blocks, walking more than one mile and bathing or dressing oneself (Ware et al. 1993).
Brief Pain Inventory-Short Form (Cleeland & Sloan 2010)
This instrument includes four items measuring the severity of pain in the last 24 h on a scale of 0–10, plus seven additional items measuring the extent to which pain interferes with life activities. Cronbach's alpha reliability ranges from 0.77 to 0.91.
Brief Fatigue Inventory (Mendoza et al. 1999)
This instrument consists of nine fatigue items, scored on a 0 to 10 point scale by the patient, and has an alpha coefficient exceeding 0.95.
The Functional Assessment of Cancer Therapy-Breast Scale-Version 4 (FACT-B) (Cella & Bonomi 1994)
This instrument covers five areas of HRQOL: physical, emotional, social, functional and other breast cancer-specific concerns. Test–retest reliability ranges from 0.82 to 0.92 with cancer patients. In this study, specific symptom items (nausea, shortness of breath and sleep), subscales and total scores were evaluated.
The State Anxiety Scale (Spielberger et al. 1983)
The State Anxiety scale consists of 20 items, scored on a 1 to 4 point scale, and has an alpha coefficient of 0.93.
Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff & Locke 1986)
The CES-D measures the state of a person's depressive symptomatology, and has a Cronbach's alpha ranging from 0.84 to 0.90. There are four subscales within this 20-item measure.
Long-Term Quality of Life Instrument (LTQL) spirituality subscale (Wyatt & Friedman-Donze 2003)
The 11-item spiritual/philosophical views of life subscale is rated on a 5-point scale, and reliability for this subscale is 0.87 with a sample of 188 female cancer survivors.
Demographics
Patient demographic characteristics included age, race, employment, education and marital status. They were obtained during the intake interview. Stage of cancer and whether it was recurrent and/or metastatic were obtained from the medical record audit at the end of each woman's participation in the study. Other measures obtained from medical records included time in months since the diagnosis of cancer and since the diagnosis of distant metastasis (for those diagnosed), type of treatment (chemotherapy with or without concurrent hormonal therapy versus hormonal therapy alone) and data on treatment interruptions such as dose delays or dose reductions.
Analysis
Descriptive statistics for the patient characteristics and outcomes at intake were obtained. Chi-squared and t-tests were used to compare patient characteristics at baseline for those with and without distant metastasis. The interview data for symptoms, physical functioning and spirituality were analysed using linear mixed effects (LME) models. These models generalise classical analysis of repeated measures and allow for data missing at random, structured covariance matrix and time-varying covariates. Patient characteristics at baseline that were found to differ according to metastatic status were included as covariates. In addition, time since cancer diagnosis and type of treatment (chemotherapy with and without hormonal therapy versus hormonal therapy alone) were included to control for their effects on HRQOL outcomes. The inclusion of the reflexology trial group variable adjusted for the effects of reflexology or lay foot manipulation interventions that patients received during the trial. These covariance adjustments were in place to control for the variation in outcomes due to sources other than distant metastatic versus local/regional metastatic disease. The least square (LS) means of the outcomes according to metastatic status were derived from the LME models. Since LME models incorporate not only complete cases (with all three completed interviews), but those who completed at least one interview, the LS means provide a comprehensive summary of the averaged values of outcomes over time, while adjusting for the important covariates listed above. To further understand factors associated with HRQOL for distant metastatic patients, their outcomes were related to the same set of covariates as in the previous analysis that included the total sample, with the addition of the time since metastasis diagnosis variable.
RESULTS
Research Question 1
Are there differences in characteristics (socio-demographics and comorbid conditions) between women with distant metastatic disease and those with local/regional metastatic disease?
Descriptive statistics for patients’ socio-demographic characteristics and the number of comorbid conditions are presented in Table 1. Those with distant metastasis were older, had fewer comorbid conditions, and a larger proportion were not employed. The presence of stages I and II in Table 1 is explained by the fact that some medical records showed the stage at the time of original diagnosis. Later, the cancer recurred or metastasised; however, women were not restaged. Due to this lack of restaging, the difference in stage of cancer was significant. However, no differences between those with and without distant metastasis were found on other demographic characteristics. There was also no difference in recurrence rates: 25% (n = 22) of women with the regional/local metastasis had a recurrent breast cancer, compared to 31% (n = 105) of women with distant metastasis (P > 0.05).
Table 1.
Total sample: descriptive statistics for socio-demographic characteristics and number of comorbid conditions of study participants at baseline
| Total sample (n = 385) n (%) | Local/regional metastasis (n = 87) n (%) | Distant metastasis (n = 298) n (%) | P-value | |
|---|---|---|---|---|
| Race | ||||
| Caucasian/White | 321 (83) | 76 (86) | 246 (83) | 0.5485 |
| Other | 53 (14) | 9 (10) | 44 (15) | |
| Employment | ||||
| Employed | 134 (35) | 40 (46) | 94 (32) | 0.0259* |
| Not employed | 249 (65) | 46 (53) | 203 (68) | |
| Education | ||||
| High school or less | 103 (27) | 19 (22) | 84 (28) | 0.4647 |
| Some college or more | 279 (72) | 67 (77) | 212 (71) | |
| Marital status | ||||
| Married/living with partner | 246 (64) | 56 (64) | 190 (64) | 0.4031 |
| Not married | 135 (35) | 29 (33) | 106 (36) | |
| Stage of cancer | ||||
| I | 20 (5) | 8 (9) | 12 (4) | <0.0001** |
| II | 52 (14) | 6 (7) | 46 (16) | |
| III | 125 (32) | 70 (80) | 55 (19) | |
| IV | 176 (46) | 0 (0) | 176 (59) | |
| Recurrent disease | ||||
| Yes | 127 (33) | 22 (25) | 105 (35) | 0.1885 |
| No | 247 (64) | 63 (72) | 184 (62) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 55.7 (11.1) | 52 (9.6) | 56.8 (11.2) | 0.0004** |
| Comorbid conditions | 0.81 (1.07) | 1.1 (1.24) | 0.7 (1.01) | 0.0057** |
P < 0.05
P < 0.01.
Research Question 2
Are there difference in physical functioning and/or symptoms reported at three time points between women with distant metastatic disease and those with local/regional metastatic disease?
Table 2 presents descriptive statistics of the outcomes for three time points for those with and without distant metastasis. As shown in Table 2, the differences between those with and without distant metastasis did not vary over time. This observation from descriptive statistics was confirmed by insignificance of the metastatic status by time interaction in the LME for the outcomes at three time points.
Table 2.
Total sample: descriptive statistics for health-related quality of life of distant metastasis and local/regional metastasis at intake, 5 and 11 weeks
| Local/regional metastasis, mean (SD) |
Distant metastasis, mean (SD) |
|||||
|---|---|---|---|---|---|---|
| Outcome | Intake (n = 87) | 5 weeks (n = 74) | 11 weeks (n = 66) | Intake (n = 298) | 5 weeks (n = 231) | 11 weeks (n = 204) |
| Physical function | 60.5 (26.2) | 61.8 (26.7) | 64.1 (28.5) | 53.6 (27.5) | 55.8 (27.3) | 54.5 (27.1) |
| CES-D | 17.3 (11.9) | 15.2 (11.5) | 11.3 (8.1) | 14.7 (10.3) | 13.6 (10) | 13.6 (10.3) |
| Anxiety | 38.6 (13.8) | 35.5 (13.3) | 34.3 (11.4) | 35.8 (11.9) | 35.1 (11.3) | 34.6 (11.1) |
| Pain (BPI) | 3.2 (3.1) | 3.1 (3.0) | 2.8 (2.9) | 3.9 (3.1) | 3.9 (3.1) | 3.5 (3.1) |
| Fatigue (BFI) | 5.4 (2.9) | 5.5 (2.9) | 4.8 (3.0) | 5.6 (2.9) | 5.6 (2.9) | 5.4 (3.0) |
| Shortness of breath (FACT-B item) | 3.1 (1.2) | 3.1 (1.1) | 3.3 (1.0) | 3.0 (1.2) | 3.1 (1.1) | 3.0 (1.1) |
| Nausea (FACT-B item) | 2.9 (1.4) | 3.6 (0.8) | 3.7 (0.7) | 3.2 (1.1) | 3.4 (1.0) | 3.4 (1.0) |
| Sleep (FACT-B item) | 2.2 (1.2) | 2.3 (1.1) | 2.4 (1.1) | 2.3 (1.2) | 2.4 (1.1) | 2.4 (1.1) |
| LTQL spiritual subscale | 10.4 (7.0) | 10.4 (7.8) | 9.6 (6.1) | 10.1 (6.6) | 10.1 (6.7) | 10.1 (6.8) |
CES-D, Center for Epidemiologic Studies Depression Scale; BPI, Brief Pain Inventory; BFI, Brief Fatigue Inventory; FACT-B, Functional Assessment of Cancer Therapy-Breast Scale-Version 4; LTQL, Long-Term Quality of Life Instrument.
Thus, Table 3 presents the average difference between groups over time as summarised by LS means based on all available longitudinal data. After adjusting for age, comorbidity, type of treatment and time since cancer diagnosis, those with distant metastatic breast cancer had worse physical functioning (P < 0.01) and more pain (P < 0.01); however, no differences over time were found on fatigue, nausea, shortness of breath, sleep quality, anxiety, depressive symptoms or spirituality. In addition, the per cent of patients with CES-D scores of 16 or higher did not differ according to metastatic status or over time, with 44% of all patients falling above this screening cut-off at intake (data not in Tables). Also considered were interruptions in treatment. This analysis identified that 14% of the patients with distant metastasis had treatment interruptions (dose delays or dose reductions) during the 11-week study period, compared to 9% of the patients in the local/regional metastatic group (data not in Tables).
Table 3.
Total sample: longitudinal comparison of patients with distant metastasis to local/regional metastasis at intake, 5 and 11 weeks: LS means adjusted for age, number of comorbid conditions, type of treatment and time since cancer diagnosis
| Distant metastasis |
Local/regional metastasis |
||||
|---|---|---|---|---|---|
| Outcome | LS means | SE | LS means | SE | P-value |
| Physical functioning | 55.8905 | 2.2104 | 65.4952 | 3.0910 | 0.0041** |
| CES-D | 13.8382 | 0.7839 | 13.7540 | 1.0925 | 0.9430 |
| Anxiety | 36.7698 | 0.9140 | 37.3993 | 1.2748 | 0.6466 |
| Pain (BPI) | 3.7527 | 0.2267 | 2.6102 | 0.3158 | 0.0009** |
| Fatigue (BFI) | 5.3299 | 0.2097 | 4.9785 | 0.2920 | 0.2652 |
| Shortness of breath (FACT-B item) | 3.0018 | 0.0851 | 3.2046 | 0.1186 | 0.1129 |
| Nausea (FACT-B item) | 3.4052 | 0.0702 | 3.4445 | 0.0976 | 0.7092 |
| Sleep (FACT-B item) | 2.3246 | 0.0895 | 2.3118 | 0.1249 | 0.9245 |
| LTQL spiritual subscale | 10.4960 | 0.5561 | 10.8035 | 0.7773 | 0.7129 |
P < 0.01.
LS, least square; CES-D, Center for Epidemiologic Studies Depression Scale; BPI, Brief Pain Inventory; BFI, Brief Fatigue Inventory; FACT-B, Functional Assessment of Cancer Therapy-Breast Scale-Version 4; LTQL, Long-Term Quality of Life Instrument.
Research Question 3
Among those with distant metastatic disease, is there an association of physical functioning and symptoms with patient characteristics (age and comorbidity), time since metastasis diagnosis and type of treatment (chemotherapy with and without concurrent hormonal therapy versus hormonal therapy alone)?
Drawing upon the unique opportunity of having a large group of distant metastatic breast cancer patients available, additional analyses within this group were conducted to incorporate the covariates of age, comorbidity and medical treatment, as well as time since distant metastasis diagnosis. The summary of results in Table 4 indicates that over and above age, a larger number of comorbid conditions were associated with worse physical functioning, pain and shortness of breath. In addition, those with more comorbid conditions had higher depressive symptomatology. Chemotherapy as compared to hormonal therapy alone was associated with worse physical functioning. Time since their distant metastasis diagnosis was associated with worse shortness of breath.
Table 4.
Distant metastasis subgroup: effects of age, number of comorbid conditions, time since distant metastasis diagnosis and type of medical treatment on outcomes (n = 298)
| Outcome | Effects | Estimate | SE | P-value |
|---|---|---|---|---|
| Physical function | Age | –0.2181 | 0.1471 | 0.1394 |
| # of comorbid conditions | –6.3656 | 1.5834 | <0.0001** | |
| # of months since metastasis diagnosis | –0.0260 | 0.0513 | 0.6136 | |
| Chemotherapy compared to hormonal | –15.2854 | 5.0515 | 0.0027* | |
| CES-D | Age | –0.1160 | 0.0508 | 0.0233* |
| # of comorbid conditions | 1.2463 | 0.5466 | 0.0234* | |
| # of months since metastasis diagnosis | 0.0084 | 0.0176 | 0.6320 | |
| Chemotherapy compared to hormonal | 2.4462 | 1.7305 | 0.1587 | |
| Anxiety | Age | –0.1013 | 0.0591 | 0.0876 |
| # of comorbid conditions | 0.7564 | 0.6354 | 0.2349 | |
| # of months since metastasis diagnosis | –0.0005 | 0.0205 | 0.9820 | |
| Chemotherapy compared to hormonal | –0.1129 | 2.0158 | 0.9554 | |
| Pain (BPI) | Age | –0.0280 | 0.0154 | 0.0696 |
| # of comorbid conditions | 0.4164 | 0.1650 | 0.0122* | |
| # of months since metastasis diagnosis | 0.0092 | 0.0053 | 0.0853 | |
| Chemotherapy compared to hormonal | 0.8970 | 0.5227 | 0.0873 | |
| Fatigue (BFI) | Age | –0.0118 | 0.0138 | 0.3934 |
| # of comorbid conditions | 0.0954 | 0.1481 | 0.5200 | |
| # of months since metastasis diagnosis | 0.0010 | 0.0047 | 0.8274 | |
| Chemotherapy compared to hormonal | 0.6968 | 0.4675 | 0.1373 | |
| Shortness of breath (FACT-B item) | Age | –0.0097 | 0.0058 | 0.0950 |
| # of comorbid conditions | –0.1269 | 0.0625 | 0.0432* | |
| # of months since metastasis diagnosis | –0.0044 | 0.0020 | 0.0308* | |
| Chemotherapy compared to hormonal | –0.1933 | 0.1977 | 0.3291 | |
| Nausea (FACT-B item) | Age | 0.0109 | 0.0046 | 0.0180* |
| # of comorbid conditions | –0.0516 | 0.0492 | 0.2957 | |
| # of months since metastasis diagnosis | –0.0018 | 0.0016 | 0.2641 | |
| Chemotherapy compared to hormonal | –0.0571 | 0.1547 | 0.7126 | |
| Sleep (FACT-B item) | Age | 0.0113 | 0.0059 | 0.0554 |
| # of comorbid conditions | –0.0410 | 0.0632 | 0.5171 | |
| # of months since metastasis diagnosis | 0.0000 | 0.0020 | 0.9837 | |
| Chemotherapy compared to hormonal | –0.2008 | 0.1997 | 0.3156 | |
| LTQL – spiritual subscale | Age | 0.0665 | 0.0347 | 0.0560 |
| # of comorbid conditions | 0.2855 | 0.3729 | 0.4447 | |
| # of months since metastasis diagnosis | –0.0122 | 0.0121 | 0.3111 | |
| Chemotherapy compared to hormonal | –0.4388 | 1.1859 | 0.7117 |
P < 0.05
P < 0.01.
CES-D, Center for Epidemiologic Studies Depression Scale; BPI, Brief Pain Inventory; BFI, Brief Fatigue Inventory; FACT-B, Functional Assessment of Cancer Therapy-Breast Scale-Version 4; LTQL, Long-Term Quality of Life Instrument.
DISCUSSION
These secondary analyses shed light on the differences in HRQOL between those with and without distant metastasis, and factors associated with HRQOL outcomes among patients with advanced breast cancer. The findings presented in this paper inform future research that is focused on HRQOL for women with advanced breast cancer. Specifically, when a decision to include those with or without distant metastasis or both in a supportive care study is being made, it is important to understand the differences between these groups to formulate appropriate inclusion or exclusion criteria.
In terms of demographic variables, only three distinguished those with distant versus local/regional metastasis at baseline: being older, having fewer comorbid conditions and being unemployed. No comparable literature was located that mentioned these demographic variations between groups. Further, it is challenging to speculate on these findings. It is possible that the older women simply had more time to develop the distant metastases; however, concerning fewer comorbids, it seems counterintuitive to understand why women with more advanced disease have fewer. Being unemployed is more likely to accompany the finding of being older, perhaps at the age of retirement, and possibly more disabled from both age and the distant metastatic cancer.
Looking next at symptoms and functional status, at intake, women with distant metastasis had fewer depressive symptoms and less nausea, but more pain and lower functional status. Over time, the presence of pain and lower functioning persisted for the distant metastatic group, whereas there were no differences over time for fatigue, nausea, shortness of breath, sleep quality, anxiety, depressive symptoms or spirituality. The lower physical functioning may be a result of the continuous symptom of pain over time. It is interesting to note that the persistence of pain is not reported by other investigators, but the presence of lowered physical functioning was reported for a group of recurrent cancer patients when compared with primary non-metastatic and primary metastatic groups of patients (Siddiqi et al. 2009). Depressive symptoms appear to be more commonly reported among recurrent metastatic patients (Groenvold et al. 2007; Reyes-Gibby et al. 2012). A note of caution is needed with this comparison since few investigators have divided their sample into distant versus local/regional metastasis, but rather used groups such as recurrent metastasis, recurrence or primary metastasis. Thornton et al. (2008) did use similar terminology to that used in the present study, and reported higher fatigue, pain and emotional distress (depression and anxiety) among the local/regional metastatic group when compared to the distant metastatic group. The present study only corroborates the pain for distant metastatic patients, but not the depressive symptoms over time.
When examining the outcome for the distant metastatic group only, the results indicate that over and above age, a larger number of comorbid conditions were associated with worse physical functioning, pain and difficulty breathing. In addition, those with more comorbid conditions had higher depressive symptomatology. These functional status and symptom findings need to be pondered by investigators when designing studies for patients with distant metastatic disease, since such factors must be considered when selecting inclusion and exclusion criteria, variables to incorporate into a randomisation plan and variables worthy of tracking over time. As for the association between treatment interruptions and severity of symptoms, it remains unclear whether treatment interruption was a result of intolerable symptoms, or if symptom severity decreased due to a delay in the next dose or a reduction in chemotherapy. It is difficult to compare the present sample with the work of others due to the general term of metastatic disease being used, rather than distant versus local/regional. However, one team (Aranda et al. 2005) reported that no differences were detected when comparing demographic and disease characteristics among a sample of 105 women where the majority had metastatic disease in one to three sites beyond the breast, and therefore were most comparable to the present study. This difference in findings could be due to a greater exploration of disease characteristics in the present study, and access to nearly three times as many patients (n = 298). Also, there was a difference in focus for the two studies. The Aranda team was most interested in reporting on health information needs of these women, whereas the present study team was interested in variables that may need special consideration in future research as well as guiding the clinician.
The limitations of this study include a relatively small number of women (n = 22) who had both recurrent breast cancer and distant metastasis. Thus, we were unable to further delineate the differences in HRQOL according to cancer recurrence, as was done by Siddiqi et al. (2009) in a sample of patients with solid tumours. However, our sample is more homogeneous as it includes advanced breast cancer only. Also, due to multiple tests conducted in this secondary analysis, the findings have to be interpreted with caution.
Overall, for this entire sample, most of the limitations for women with advanced disease were physical, whereas it was surprising to note that the emotional and spiritual quality of life outcomes were no different according to metastatic status. Women with advanced disease are willing and emotionally capable of participation in research studies. Despite advanced breast cancer and the presence of metastasis, the majority of women did not reach the clinical screening cut-off on the CES-D. These findings may indicate that patients with advanced breast cancer have adapted emotionally and spiritually; however, the management of physical symptoms remains a priority for these patients.
ACKNOWLEDGEMENT OF FUNDING
National Cancer Institute Grant #RO1 CA104883-01A1.
Contributor Information
G. WYATT, College of Nursing, Michigan State University, East Lansing, Michigan, USA.
A. SIKORSKII, Department of Statistics and Probability, Michigan State University, East Lansing, Michigan, USA.
D. TAMKUS, College of Human Medicine, Michigan State University, East Lansing, Michigan, USA.
M. YOU, Department of Psychology, Michigan State University, East Lansing, Michigan, USA.
REFERENCES
- Aranda S, Schofield P, Weih L, Yates D, Milne D, Faulkner R, Voudouris N. Mapping the quality of life and unmet needs of urban women with metastatic breast cancer. European Journal of Cancer Care. 2005;14:211–222. doi: 10.1111/j.1365-2354.2005.00541.x. [DOI] [PubMed] [Google Scholar]
- Cella DF, Bonomi AE. Manual Functional Assessment of Cancer Therapy (FACT) Scales and the Functional Assessment of HIV Infection (FAHI) Scale. Version 3. Rush Cancer Institute; Chicago, IL, USA: 1994. [Google Scholar]
- Cleeland C, Sloan JA. Assessing the symptoms of cancer using patient-reported outcomes (ASCPRO): searching for standards. Journal of Pain and Symptom Management. 2010;36:1077–1085. doi: 10.1016/j.jpainsymman.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Assessment of pain in cancer: measurement issues. Advances in Pain Research and Therapy. 1990;16:47–55. [Google Scholar]
- Cohen M. Coping and emotional distress in primary and recurrent breast cancer patients. Journal of Clinical Psychology in Medical Settings. 2002;9:245–251. [Google Scholar]
- Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health-related quality of life. Journal of Nursing Scholarship. 2005;37:336–342. doi: 10.1111/j.1547-5069.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- Groenvold M, Petersen M, Idler E, Bjorner J, Fayers P, Mouridsen H. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Research and Treatment. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- McEntegart DJ. The pursuit of balance using stratified and dynamic randomization techniques: an overview. Drug Information Journal. 2003;37:293–308. [Google Scholar]
- Mendoza T, Wang S, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute Surveillance Epidemiology and End Results (SEER) Stat Fact Sheets: Breast [Online] 2011 Available at: http://seer.cancer.gov/statfacts/html/breast.html.
- Northouse LL, Mood D, Kershaw T, Schafenacker A, Mellon S, Walker J, Galvin E, Decker V. Quality of life of women with recurrent breast cancer and their family members. Journal of Clinical Oncology. 2002;20:4050–4064. doi: 10.1200/JCO.2002.02.054. [DOI] [PubMed] [Google Scholar]
- Pirovano M, Maltonia M, Nanni O, Mariani M, Indelli M, Zaninetta G, Peterella V, Barni S, Zecca E, Scarpi E, Labianca R, Amadori E, Piva L. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Journal of Pain and Symptom Management. 1999;17:231–239. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Locke BZ. The community mental health assessment survey and the CED-D scale. In: Weissman MM, Meyers JK, Ross CE, editors. Community Surveys of Psychiatric Disorders. Rutgers University Press; New Brunswick, NJ, USA: 1986. pp. 177–189. [Google Scholar]
- Reyes-Gibby C, Anderson KO, Morrow PK, Shete S, Hassan S. Depressive symptoms and health-related quality of life in breast cancer survivors. Journal of Women's Health. 2012;21:311–318. doi: 10.1089/jwh.2011.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: a review. Controlled Clinical Trials. 2002;23:662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Given C, Given B, Sikorskii A. Quality of life among patients with primary, metastatic and recurrent cancer. European Journal of Cancer Care. 2009;18:84–96. doi: 10.1111/j.1365-2354.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Mind Garden; Palo Alto, CA, USA: 1983. [Google Scholar]
- Thornton LM, Madlensky L, Flatt SW, Kaplan RM, Pierce JP. The impact of a second breast cancer diagnosis on health related quality of life. Breast Cancer Research and Treatment. 2005;92:25–33. doi: 10.1007/s10549-005-1411-7. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Carson WE. Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunology, Immunotherapy. 2008;57:1471–1481. doi: 10.1007/s00262-008-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhauer RP. A qualitative study of the experiences of women with metastatic breast cancer. Palliative and Supportive Care. 2008;6:249–258. doi: 10.1017/S1478951508000382. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston, MA, USA: 1993. [Google Scholar]
- Wilson IB, Cleary PD. Linking clinical variables with health related quality of life. A conceptual model of patient outcomes. Journal of the American Medical Association. 1995;273:59–65. [PubMed] [Google Scholar]
- World Cancer Research Fund International Top 5 Most Common Cancers Worldwide are Lung, Breast, Bowel, Stomach, and Prostate [Online] 2008 Available at: http://www.wcrf.org/cancer_statistics/cancer_facts/5-most-common-cancers.php.
- Wyatt G, Friedman-Donze L. The long-term quality of life (LTQL) instruments for female cancer survivors. In: Strickland O, Dilorio C, editors. Measurement of Nursing Outcomes: Self Care and Coping. 2nd edn Vol. 3. Springer Publishing Company; Berlin, Germany: 2003. pp. 216–226. [Google Scholar]