Abstract
Caspase 8 is required not only for death receptor-mediated apoptosis but also for lymphocyte activation in the immune system. FLIP(L), the long-splice form of c-FLIP, is one of the specific substrates for caspase 8, and increased expression of FLIP(L) promotes activation of the NF-κB signaling pathway. The synthetic caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk) markedly blocked NF-κB activation induced by overexpression of FLIP(L). FLIP(L) is specifically processed by caspase 8 into N-terminal FLIP(p43) and C-terminal FLIP(p12). Only FLIP(p43) was able to induce NF-κB activation as efficiently as FLIP(L), and FLIP(p43)-induced NF-κB activation became insensitive to zVAD-fmk. In caspase 8-deficient cells, FLIP(p43) provoked NF-κB activation only when procaspase 8 or caspase 8(p43) was complemented. FLIP(p43)-induced NF-κB activation was profoundly blocked by the dominant-negative TRAF2. Moreover, endogenous TRAF2 interacted specifically with FLIP(p43), and the formation of the FLIP(p43)-caspase 8-TRAF2 tertiary complex was a prerequisite to induction of NF-κB activation. zVAD-fmk prevented the recruitment of TRAF2 into the death-inducing signaling complex. Thus, our present results demonstrate that FLIP(p43) processed by caspase 8 specifically interacts with TRAF2 and subsequently induces activation of the NF-κB signaling pathway.
Death receptors belong to the tumor necrosis factor (TNF) receptor superfamily and play an essential role in the regulation of lymphocyte homeostasis (4, 23, 30, 33). In addition to the extracellular cysteine-rich domains, death receptors are characterized by the intracellular sequence known as the death domain (DD), which is critical for apoptosis signaling (30, 33). Upon engagement with corresponding ligands, death receptors are oligomerized to recruit the adapter proteins FADD or TRADD via the homotypic DD interaction. To transmit apoptosis signals, the death effector domain (DED) of FADD recruits procaspase 8, which contains two DED and a caspase domain consisting of the large subunit (p20) and the small subunit (p10). Dimerization of procaspase 8 facilitates its self-processing and subsequent generation of active heterotetramers (p20/p10)2 (3, 10). The active caspase 8 initiates apoptosis by the cleavage of downstream substrates such as procaspase 3 (8, 36).
c-FLIP is an important modulator that renders many types of cells resistant to death receptor-mediated apoptosis (24, 43, 45). At least two splice variants are expressed in a variety of cells. The full-length form (55 kDa), FLIP(L), contains two DED and a catalytically inactive caspase-like domain and shows overall homology to procaspase 8, while the shorter form (26 kDa), FLIP(S), has tandem DED followed by a C-terminal short additional sequence. Both FLIP(L) and FLIP(S) are recruited into the death-inducing signaling complex (DISC) and block death receptor-mediated apoptosis (25, 38). FLIP(S) totally prevents self-processing of procaspase 8 in the DISC, whereas FLIP(L) allows the first cleavage of procaspase 8 (25). FLIP(L) itself is cleaved at Asp-376 by caspase 8 in the DISC to generate N-terminal FLIP(p43) and C-terminal FLIP(p12) (25, 32, 38, 40). Under physiological conditions, FLIP(L) appears to act as an activator of procaspase 8 in the DISC by facilitating self-processing of procaspase 8 (5, 32). However, this processing does not result in inducing apoptosis but leads to the formation of the membrane-restricted active heterodimers probably able to cleave limited substrates (32). Thus, FLIP(L) and FLIP(S) exert different inhibitory activities at least in blocking procaspase 8 activation.
Caspase 8 is required not only for death receptor-mediated apoptosis but also for T-cell proliferation. In mice deficient for FADD or overexpressing dominant-negative (DN) FADD in the T-cell lineage, in addition to the defect in apoptosis induction, peripheral T cells and thymocytes do not proliferate upon mitogenic stimulation (34, 47, 49, 50). It has recently been reported that the serine phosphorylation site of FADD at the C-terminal region is responsible for proliferative activity but not for cell death-inducing activity (15). These results clearly demonstrate that the death receptor signaling pathway using FADD is essential for T-cell proliferation. In agreement with these studies, we and others have shown that Fas ligand (FasL) or anti-Fas antibodies can costimulate human T cells (2, 21) and that synthetic peptide-based caspase inhibitors block proliferation and interleukin-2 (IL-2) production of human T cells (1, 21). These observations indicate that death receptor-mediated caspase activation is required for T-cell proliferation. Recently, the essential role of caspase 8 in T-cell proliferation has been clearly demonstrated by two independent studies (7, 37). Human individual homozygous caspase 8 mutations manifest not only defective lymphocyte apoptosis, but also defects in the activation of T cells, B cells, and NK cells, which leads to immunodeficiency (7). On the other hand, mice lacking caspase 8 in the T-cell lineage show impaired T-cell responses upon antigen stimulation and a marked decrease in the number of peripheral T cells despite the normal development of thymocytes (37). Thus, in contrast to mutations in the Fas/FasL system that lead to autoimmune disorders (33), caspase 8 mutations oppositely lead to immunodeficiency.
Recently, we have shown that mice expressing increased amounts of FLIP(L) in the T-cell compartment exhibit augmented proliferative responses of mature T cells with suboptimal doses of antigen stimulation but neither the accumulation of abnormal T cells nor the protection toward activation-induced cell death (27). Thus, these data suggest that FLIP(L) mainly modulates T-cell proliferation by decreasing the T-cell receptor (TCR) signaling threshold but not T-cell apoptosis in vivo. Consistent with these observations, FLIP(L) promotes activation of the mitogen-activated protein kinase ERK and the transcription factor NF-κB upon antigen stimulation in T cells in parallel with the enhanced production of IL-2 (19). Thus, FLIP(L) appears to divert death receptor-mediated apoptotic signals into proliferative signals by its capacity to promote the ERK and NF-κB signaling pathways.
The NF-κB signaling pathway is regulated by the cytosolic-nuclear shuttling and modulation of the transcriptional activity (12). The adapter protein TRAF2 and the kinase RIP play essential roles in NF-κB activation via TNF-R1 (20, 26, 41, 48). TRAF2 recruits the IκB kinase (IKK) complex to TNF-R1, whereas RIP is required for activation of the IKK complex (9). The IKK complex phosphorylates IκB that forms and sequesters the NF-κB dimers in the cytosol. Immediately after phosphorylation, IκB undergoes ubiquitination and subsequent degradation by the 26S proteasome, which allows the translocation of the NF-κB dimers to the nucleus, where it activates a variety of target genes. Yeast two-hybrid screening and coimmunoprecipitation studies in mammalian cells have revealed that FLIP(L) interacts with TRAF1, TRAF2, and RIP (19, 40).
Overexpression of FLIP(L) brings about activation of the NF-κB signaling pathway (6, 14, 19). It has been reported that FLIP(L)-induced NF-κB activation is sensitive to caspase inhibitors, although caspase 8 induces NF-κB activation independently of its proteolytic activity (6, 14). In addition, FADD initiates the NF-κB signaling pathway even in the presence of the caspase inhibitors (14). Thus, among the DED protein family (44), only FLIP(L) might require caspase 8 activity to trigger activation of the NF-κB signaling pathway. Since FLIP(L) is specifically cleaved by caspase 8 in the DISC and has the capacity to promote T-cell proliferation, we have hypothesized that the cleaved forms of FLIP(L) by caspase 8 are responsible for proliferative signals and that NF-κB activation is one of the likely downstream signaling pathways involved in T-cell proliferation. In the present paper, we have investigated why caspase activation is required for NF-κB activation induced by FLIP(L). We have found that FLIP(p43), but not FLIP(p12), induces activation of the NF-κB signaling pathway independently of caspase activation. Moreover, NF-κB activation induced by FLIP(p43) was markedly prevented by DN-TRAF2, and only FLIP(p43) specifically bound to endogenous TRAF2. Thus, our present results clearly demonstrate that FLIP(L) processing into FLIP(p43) by caspase 8 facilitates specific interaction with TRAF2 and subsequently induces activation of the NF-κB signaling pathway.
MATERIALS AND METHODS
Cells, expression vectors, and reagents.
Human embryonic kidney (HEK) 293 cells and 293T cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; JRH Biosciences, Lenexa, Kans.) and a penicillin-streptomycin-neomycin antibiotic mixture (Invitrogen). The FLIP(L) stably transfected Raji cells (RFL23) (16) were maintained in RPMI 1640 medium supplemented with 10% (vol/vol) heat-inactivated FCS and the penicillin-streptomycin-neomycin antibiotic mixture. Expression vectors for human FLIP(L), FLIP(S), FLIP(C), and human procaspase 8 were described previously (16). Human FLIP(p43), FLIP(p12), FLIP(233-376), FLIP(D376A), human caspase 8(p43), and catalytically inactive mouse procaspase 8 were generated by PCR amplification and subcloned into PCR3 (Invitrogen)-based expression vectors containing N-terminal FLAG tag or vesicular stomatitis virus (VSV) tag. The expression vectors for RIP, DN-TRAF2, DN-TRAF6, and DN-IKKβ were described before (31, 42). The caspase inhibitor benzyloxycarbonyl-Val-AlaAsp(OMe)-fluoromethylketone (zVAD-fmk) and the proteasome inhibitor MG-132 were purchased from the Peptide Institute, Inc. (Osaka, Japan).
NF-κB activation assay.
Twenty-four-well culture plates (0.6 ml per well) were seeded with 293 (2 × 105 cells) and 293T (0.5 × 105 to 1 × 105 cells) cells the day before transfection, and the cells were transfected by the calcium phosphate method with various expression vectors, together with the NF-κB-driven luciferase reporter plasmid (200 ng) and the β-galactosidase plasmid (100 ng). The cells were washed with phosphate-buffered saline (PBS) 16 h after transfection and lysed with 30 μl of the lysis buffer (25 mM Tris-HCl [pH 7.8], 1% Triton X-100) followed by centrifugation (10,000 × g, 5 min) to sediment insoluble materials. Cell lysates (5 to 10 μl) were mixed with 50 μl of the luciferase assay mixture (25 mM Tris-phosphate [pH 7.8], 9 mM MgCl2, 15% glycerol, 0.75% bovine serum albumin, 0.25 mM luciferin [Promega, Madison, Wis.], 0.8 mM ATP, 1 mM dithiothreitol [DTT]), and relative light units were measured with a Lumister K-100 luminometer (Hammatsu Photonics, Hamamatsu, Japan). β-Galactosidase activity was determined in cell lysates (10 to 20 μl) mixed with Z buffer (100 mM sodium phosphate [pH 7.5], 10 mM KCl, 1 mM MgSO4, 40 mM 2-mercaptoethanol) containing 2.2 mM o-nitrophenyl-β-d-galactopyranoside (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The reaction mixtures were incubated at 37°C until a yellow color developed, and A405 was measured with the model 550 microplate reader (Bio-Rad Laboratories, Inc., Hercules, Calif.). These values were used to normalize transfection efficiency.
Western blotting.
Cells were washed with PBS and lysed with lysis buffer (50 mM Tris-HCl [pH 7.8], 1% Triton X-100, 2 mM DTT, 2 mM sodium vanadate, and Complete protease inhibitor cocktail from Roche Diagnostics, Mannheim, Germany) followed by centrifugation (10,000 × g, 5 min). Cell lysates (30 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Hybond-ECL nitrocellulose membrane (Amersham Biosciences, Piscataway, N.J.). Membrane filters were first incubated with blocking buffer (PBS, 0.5% Tween 20, 5% skim milk) and then incubated first with antibodies in blocking buffer followed by horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) or anti-rabbit IgG antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Immunoreactive proteins were visualized by ECL detection reagents (Amersham Biosciences). The following antibodies were used: actin, C4 (Chemicon International, Inc., Temecula, Calif.); procaspase 8, 5F7 (Medical and Biological Laboratories Co., Ltd., Nagoya, Japan); FADD, Clone 1 (BD Biosciences, San Jose, Calif.); FLAG, M2 (Sigma Chemicals Co., St. Louis, Mo.); FLIP, Dave II (Alexis, San Diego, Calif.); TRAF2, C20 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.); and VSV, P5D4 (Sigma).
DISC analysis.
RFL23 cells (4 × 107 cells) were treated with 2-μg/ml FasL (39) and 2-μg/ml anti-FLAG antibody M2 for the indicated times. The cells were quickly cooled down by adding 5 volumes of ice-cold PBS and lysed with 1 ml of the DISC lysis buffer (0.2% Nonidet P-40, 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM sodium vanadate, 10% glycerol, protease inhibitor cocktail). After repeated centrifugation (10,000 × g, 5 min), postnuclear lysates were precleared with Sepharose 6B (Sigma) for 90 min and then incubated with protein G-Sepharose (Amersham Biosciences) for 3 h. Sepharose beads were washed four times with the DISC lysis buffer. Immunoprecipitates and cell lysates were analyzed by Western blotting.
Immunoprecipitation.
Sixty-millimeter-diameter culture plates (3 ml per plate) were seeded with 293 cells (1.5 × 106 cells) and 293T cells (5 × 105 cells) on the day before transfection and transfected by the calcium phosphate method with various expression vectors. The cells were harvested 16 h after transfection, washed with PBS, and lysed with 200 μl of the lysis buffer (0.2% Nonidet P-40, 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM sodium vanadate, protease inhibitor cocktail) or DISC lysis buffer. After repeated centrifugation (10,000 × g, 5 min), postnuclear lysates were precleared with Sepharose 6B for 1 h and then incubated with anti-FLAG M2 agarose (Sigma) for 3 h. Agarose beads were washed four times with the lysis buffer or the DISC lysis buffer. Immunoprecipitates and cell lysates were analyzed by Western blotting.
RESULTS
The caspase inhibitor blocks NF-κB activation induced by FLIP(L).
Overexpression of FLIP(L) initiates activation of the NF-κB signaling pathway (6, 14, 19). To address if caspase activation is required for NF-κB activation induced by FLIP(L), HEK 293T cells were transiently transfected with FLIP(L) in the presence or the absence of zVAD-fmk for 16 h. RIP was used as the positive control to induce NF-κB activation. Overexpression of RIP resulted in a 20-fold increase in the NF-κB-driven luciferase activity compared with spontaneous activity (Fig. 1A). zVAD-fmk was unable to inhibit RIP-induced NF-κB activation (Fig. 1A), whereas RIP processing into the N-terminal 36-kDa fragment (RIPn) was markedly prevented by zVAD-fmk (Fig. 1C). This processing is mediated by caspase 8, since it has been reported that caspase 8 specifically cleaves RIP at Asp-324 (22, 29, 31). As previously observed (6, 14, 19), FLIP(L) strongly induced NF-κB activation, and this activation was inhibited by zVAD-fmk in a dose-dependent manner (50% inhibitory concentration, 3 μM) (Fig. 1A and B). FLIP(L)-induced NF-κB activation was also blocked by coexpression of the cowpox viral protein CrmA that specifically inhibits caspases (data not shown) (6, 14). In FLIP(L)-transfected cells, FLIP(L) was processed into the N-terminal 43-kDa fragment detectable by anti-FLAG antibody, and zVAD-fmk completely abrogated FLIP(L) processing (Fig. 1C). Caspase 8 specifically cleaves Asp-376 in the caspase-like domain of FLIP(L), generating N-terminal FLIP(p43) and C-terminal FLIP(p12) (Fig. 1D). Thus, these results suggest that caspase 8 activity is required for NF-κB activation induced by FLIP(L).
FIG. 1.
FLIP(L)-induced NF-κB activation is inhibited by the caspase inhibitor. (A and B) 293T cells were transfected with RIP (100 ng per well) or FLIP(L) (100 ng per well) together with κB-luciferase (200 ng per well) and β-galactosidase (100 ng per well) in the presence (+) or absence (−) of 20 μM zVAD-fmk (A). 293T cells were transfected with FLIP(L) (100 ng per well) together with κB-luciferase (200 ng per well) and β-galactosidase (100 ng per well) in the presence of serial dilutions of zVAD-fmk (B). κB-luciferase activity was determined 16 h after transfection and normalized on the basis of β-galactosidase activity. The results represent the mean ± standard deviation of triplicate cultures. (C) 293T cells were transfected with FLAG-RIP (1 μg per plate) or FLAG-FLIP(L) (1 μg per plate) in the presence (+) or absence (−) of 20 μM zVAD-fmk. Expression of RIP and FLIP(L) was analyzed 16 h after transfection by Western blotting with anti-FLAG antibody. (D) Structures of FLIP(L), FLIP(p43), and FLIP(p12) are shown. FLIP(L) is cleaved into N-terminal FLIP(p43) and C-terminal FLIP(p12) at Asp-376 by caspase 8.
The caspase inhibitor does not affect NF-κB activation induced by FLIP(p43).
To investigate if FLIP(L) processing into FLIP(p43) is a prerequisite for NF-κB activation, 293T cells were transfected with FLIP(p43), FLIP(p12), or FLIP(D376A) where the caspase 8 cleavage site Asp-376 was replaced by an alanine residue. Unlike the previous report showing that FLIP(1-435) only weakly activates the NF-κB-driven luciferase activity (14), we found that FLIP(p43) still induces NF-κB activation in a dose-dependent manner and that it exhibited activity similar to that of FLIP(L) (Fig. 2A). FLIP(D376A) significantly induced NF-κB activation (Fig. 2A). Since FLIP(D376A) and FLIP(p43) were equivalently expressed in cells transfected with the same amounts of DNA (Fig. 2B), FLIP(D376A) appears to exert a lower NF-κB-inducing activity, especially at higher DNA concentrations. In contrast, FLIP(p12) was totally inactive to induce NF-κB activation (Fig. 2C). To further address whether caspase activity is required for FLIP(p43)-induced NF-κB activation, 293T cells were transfected with FLIP(p43) in the presence or the absence of zVAD-fmk. Of note, FLIP(p43)-induced NF-κB activation became insensitive to zVAD-fmk (Fig. 2D), while FLIP(D376A)-induced NF-κB activation was still sensitive to zVAD-fmk (Fig. 2E). These results suggest that FLIP(p43) is able to induce NF-κB activation independently of the caspase 8 activity.
FIG. 2.
NF-κB activation induced by FLIP(p43) is insensitive to the caspase inhibitor. (A) 293T cells were transfected with indicated amounts of FLIP(L), FLIP(p43) or FLIP(D376A). NF-κB activation was determined 16 h after transfection. The results represent the mean ± standard deviation of triplicate cultures. (B) 293T cells were transfected with FLAG-FLIP(L), FLIP(p43), or FLIP(D376A) (each at 1 μg per plate). Cell lysates were prepared 16 h after transfection and analyzed by Western blotting with anti-FLAG antibody. (C to E) 293T cells were transfected with FLIP(L), FLIP(p43), or FLIP(p12) (100 ng per well) (C). 293T cells were transfected with FLIP(p43) (100 ng per well) (D) or FLIP(D376A) (100 ng per well) (E) in the presence (+) or absence (−) of 20 μM zVAD-fmk. NF-κB activation was determined 16 h after transfection. The results represent the mean ± standard deviation of triplicate cultures.
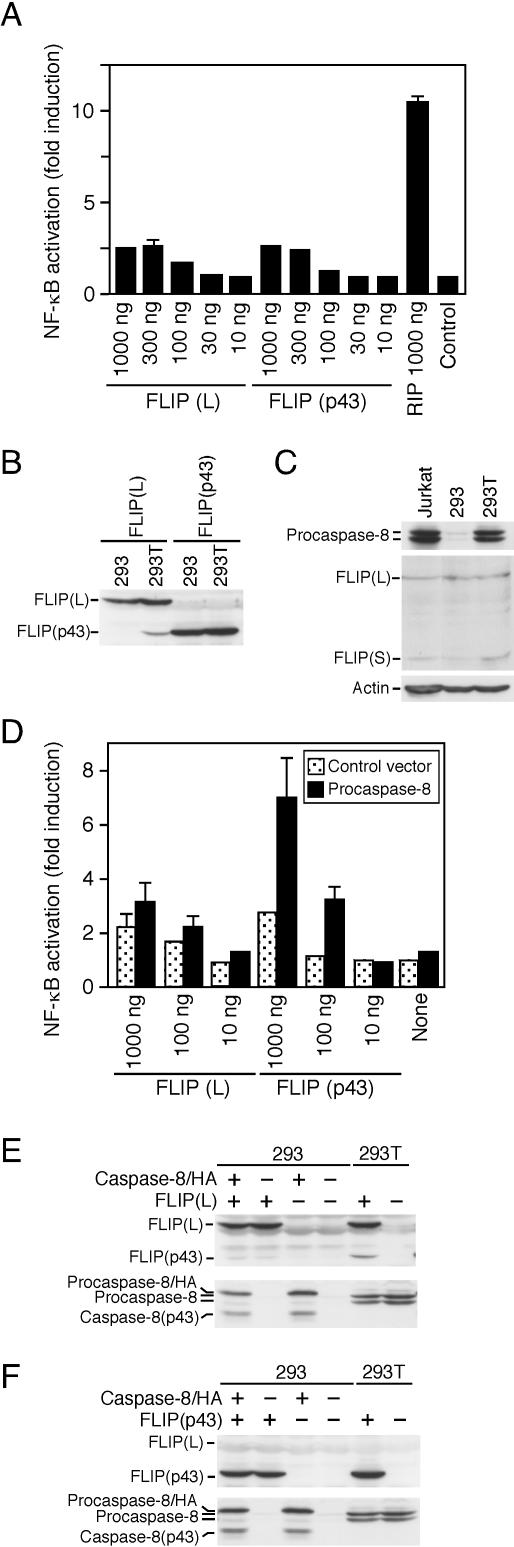
Caspase 8 is required for NF-κB activation induced by FLIP(p43).
293T cells were introduced with the simian virus 40 (SV40) T antigen, which allows the efficient replication of plasmid vectors encoding the SV40 origin. In transient transfections using expression vectors such as PCR3, 293T cells produce a larger amount of exogenous proteins than parental 293 cells. In 293 cells, FLIP(L) and FLIP(p43) only poorly initiated the NF-κB signaling pathway even when 10-fold amounts of DNA were used for transfection, although RIP strongly provoked NF-κB activation (Fig. 3A). The inability to induce NF-κB activation is not due to the insufficient expression of FLIP(L) and FLIP(p43), since they were equally expressed between 293 and 293T cells when fivefold more plasmid vectors were used for 293 cells (Fig. 3B). Nevertheless, in contrast to 293T cells, FLIP(L) was not processed into FLIP(p43) in 293 cells regardless of the same expression levels between two types of cells (Fig. 3B). Likewise, RIP was also unprocessed in 293 cells (data not shown). Since FLIP(L) and RIP are specifically cleaved by caspase 8, these data suggest that caspase 8 is deficient or inactivated in 293 cells. Despite the equal expression of actin, procaspase 8 was barely detectable in 293 cells (Fig. 3C). In contrast, 293T cells expressed procaspase 8 at levels comparable to that of Jurkat T cells highly sensitive to Fas-mediated apoptosis (Fig. 3C). Similar to Jurkat T cells, 293 and 293T cells did not express large amounts of FLIP(L) and FLIP(S) sufficient to block Fas-mediated apoptosis (Fig. 3C).
FIG. 3.
Caspase 8 is required for NF-κB activation induced by FLIP(p43). (A) 293 cells were transfected with the indicated amounts of FLIP(L), FLIP(p43), or RIP. NF-κB activation was determined 16 h after transfection. The results represent the mean ± standard deviation of triplicate cultures. (B) 293 cells and 293T cells were transfected with FLAG-FLIP(L) or FLAG-FLIP(p43) (5 μg per plate for 293 cells and 1 μg per plate for 293T cells, respectively). Cell lysates were prepared 16 h after transfection and analyzed by Western blotting with anti-FLAG antibody. (C) Expression of procaspase 8, c-FLIP, and actin was analyzed by Western blotting. (D) 293 cells were transfected with the indicated amounts of FLIP(L) or FLIP(p43), together with or without (control) procaspase 8 (100 ng per well). NF-κB activation was determined 16 h after transfection. The results represent the mean ± standard deviation of triplicate cultures. (E and F) 293 cells were transfected with (+) or without (−) FLAG-FLIP(L) (5 μg per plate) (E) or FLAG-FLIP(p43) (5 μg per plate) (F), together with (+) or without (−) procaspase 8-HA (0.5 μg per plate). In comparison, 293T cells were transfected with (+) or without (−) FLAG-FLIP(L) or FLAG-FLIP(p43) (each at 1 μg per plate). Cell lysates were prepared 16 h after transfection and analyzed by Western blotting with anti-FLAG and anti-caspase 8 antibodies.
To obtain further evidence for the requirement of procaspase 8 in NF-κB activation induced by FLIP(L), procaspase 8 was complemented at the endogenous level in 293 cells. Procaspase 8 alone significantly induced NF-κB activation under conditions in which it did not trigger substantial apoptosis (6) or even when its enzymatic activity was inactivated by zVAD-fmk or mutation of active-center cysteine in 293 cells (data not shown). Hence, the DNA concentration that did not induce NF-κB activation was titrated and used for the complementation of procaspase 8. As expected, FLIP(p43) strongly induced NF-κB activation only when procaspase 8 was cotransfected (Fig. 3D). In procaspase 8-transfected 293 cells, a portion of procaspase 8 was cleaved into caspase 8(p43) without induction of apoptosis, irrespective of the presence of FLIP(p43), which inhibits self-processing of procaspase 8 (Fig. 3F), suggesting that caspase 8(p43) is produced by autoprocessing of the procaspase 8 homodimers. However, contrary to our prediction, FLIP(L) exerted an only marginal activity to initiate NF-κB activation in cells cotransfected with procaspase 8 (Fig. 3D). This is probably due to the fact that FLIP(L) was inefficiently processed into FLIP(p43) by transfected procaspase 8 (Fig. 3E). The human procaspase 8-hemagglutinin (HA) construct used here appears to have a weak intrinsic proteolytic activity for autoprocessing and for the cleavage of other substrates, such as FLIP(L).
The heterodimers of both FLIP(p43)/caspase 8 and FLIP(p43)/caspase 8(p43) are able to induce NF-κB activation.
The results presented above suggest that either procaspase 8 or caspase 8(p43) is indispensable for NF-κB activation induced by FLIP(p43). In the presence of caspase 8(p43), FLIP(p43) was able to induce a 10-fold activation of the NF-κB signaling pathway (Fig. 4A). In contrast, FLIP(D376A) was totally inactive even when procaspase 8 or caspase 8(p43) was cotransfected (Fig. 4A). zVAD-fmk did not perturb FLIP(p43)-induced NF-κB activation in 293 cells cotransfected with procaspase 8 or caspase 8(p43) (Fig. 4B). Moreover, FLIP(p43) strongly induced NF-κB activation when catalytically inactive procaspase 8 was cotransfected (Fig. 4C). Thus, these data indicate that either procaspase 8 or caspase 8(p43), but not their proteolytic activity, is required for FLIP(p43)-induced NF-κB activation. To examine if FLIP(p43) is able to interact with procaspase 8 and caspase 8(p43), 293 cells were transfected with VSV-FLIP(p43), together with FLAG-procaspase 8 or FLAG-caspase 8(p43), and postnuclear lysates were immunoprecipitated with anti-FLAG antibody. The comparable amounts of FLIP(p43) were pulled down with procaspase 8 and caspase 8(p43) (Fig. 4D). Taken together, these data suggest that the heterodimers of both FLIP(p43)/procaspase 8 and FLIP(p43)/caspase 8(p43) are able to induce NF-κB activation and that caspase 8 proteolytic activity is no longer required for the downstream signaling pathway of NF-κB activation after FLIP(L) is processed into FLIP(p43).
FIG. 4.
The heterodimers of FLIP(p43)/procaspase 8 and FLIP(p43)/caspase 8(p43) are able to induce NF-κB activation. (A to C) 293 cells were transfected with FLIP(p43) or FLIP(D376A) (each at 1 μg per well), together with procaspase 8 (100 ng per well) (black bars) or caspase 8(p43) (50 ng per well) (gray bars) (A). 293 cells were transfected with FLIP(p43) (1 μg per well) together with procaspase 8 (100 ng per well) or caspase 8(p43) (50 ng per well) in the presence (+) or absence (−) of 20 μM zVAD-fmk (B). 293 cells were transfected with or without FLIP(p43) (0.5 μg per well), together with (black bars) or without (hatched bars) catalytically inactive procaspase 8 (25 ng per well) (C). NF-κB activation was determined 16 h after transfection. The results represent the mean ± standard deviation of triplicate cultures. (D) 293 cells were transfected with FLAG-procaspase 8 or FLAG-caspase 8(p43) (1 μg per plate) together with VSV-FLIP(p43) (1 μg per plate). Cell lysates were prepared 16 h after transfection and immunoprecipitated with anti-FLAG antibody. The immunoprecipitates and cell lysates were analyzed by Western blotting using anti-VSV and anti-FLAG antibodies.
DN-TRAF2 markedly prevents NF-κB activation induced by FLIP(p43).
NF-κB activation depends on adapter proteins such as the TRAF family of proteins and the IκB kinase complex containing IΚΚα, IKKβ, and IKKγ (12). In our earlier paper (19), we have shown that FLIP(L)-induced NF-κB activation is profoundly inhibited by DN-IKKβ and partially by DN-TRAF2. In contrast to FLIP(L), DN-TRAF2 profoundly inhibited FLIP(p43)-induced NF-κB activation in a dose-dependent manner (Fig. 5A and B). The inhibitory activity of DN-TRAF2 on FLIP(p43)-induced NF-κB activation was as strong as that of DN-IKKβ (Fig. 5C). Thus, TRAF2 appears to be required for FLIP(p43)-induced NF-κB activation. In contrast, FLIP(D376A)-induced NF-κB activation was only marginally inhibited by DN-TRAF2 (Fig. 5D), suggesting that TRAF2 is dispensable for NF-κB activation induced by FLIP(D376A).
FIG. 5.
DN-TRAF2 prevents NF-κB activation induced by FLIP(p43). (A and B) 293T cells were transfected with FLIP(p43) (100 ng per well) together with indicated amounts of DN-TRAF2 for 16 h. NF-κB activation represents the mean ± standard deviation of triplicate cultures (A). Expression of DN-TRAF2 and endogenous TRAF2 was analyzed by Western blotting with anti-TRAF2 antibody (B). (C and D) 293T cells were transfected with FLIP(p43) (100 ng per well) together with DN-TRAF2 or DN-IKKβ (500 ng per well) (C). 293T cells were transfected with FLIP(D376A) (100 ng per well) together with indicated amounts of DN-TRAF2 (D). NF-κB activation was determined 16 h after transfection. The results represent the mean ± standard deviation of triplicate cultures.
TRAF2 specifically interacts with FLIP(p43).
To investigate the TRAF2 interaction in mammalian cells, 293T cells were transfected with FLAG-tagged constructs, and immunoprecipitates with anti-FLAG beads were probed with anti-TRAF2 antibody to detect endogenous TRAF2. zVAD-fmk was included in FLIP(L)-transfected cells to block its processing into FLIP(p43). Although these constructs were equally expressed, endogenous TRAF2 interacted strongly with FLIP(p43) but only weakly with FLIP(L) (Fig. 6A). Neither procaspase 8 nor caspase 8(p43) seems to bind to TRAF2 (Fig. 6A). Moreover, compared with FLIP(p43), no discernible association was observed with FLIP(S), FLIP(233-480), FLIP(233-376), or FLIP(p12) (Fig. 6B). The specific interaction of FLIP(p43) with TRAF2 was also observed with 293 cells that do not express procaspase 8 (data not shown). To address if TRAF2 forms a complex with caspase 8 via binding to FLIP(p43), 293T cells were transfected with FLAG-procaspase 8 together with either VSV-FLIP(L) or VSV-FLIP(p43), and anti-FLAG beads were used for immunoprecipitation (Fig. 6C). Although transfected FLIP(L) was readily processed into FLIP(p43) and similar amounts of FLIP(L) and FLIP(p43) were present in cell lysates, only FLIP(p43) was pulled down with procaspase 8 and caspase 8(p43) (Fig. 6C). Since FLIP(L) and FLIP(p43) were equivalently immunoprecipitated with procaspase 8 in the presence of zVAD-fmk (Fig. 6D), it seems likely that FLIP(L) and procaspase 8 have a high affinity, but FLIP(L) is very quickly cleaved into FLIP(p43). Endogenous TRAF2 was immunoprecipitated with procaspase 8 and caspase 8(p43) only when FLIP(p43) was present in the cell (Fig. 6C). Thus, these data demonstrate that TRAF2 forms the tertiary complex with FLIP(p43) and caspase 8.
FIG. 6.
TRAF2 specifically interacts with FLIP(p43). (A) 293T cells were transfected with FLAG-procaspase 8, FLAG-caspase 8(p43), FLAG-FLIP(L), or FLAG-FLIP(p43) (each at 1 μg per plate). zVAD-fmk (20 μM) was included in cells transfected with FLAG-FLIP(L). Cell lysates were prepared 16 h after transfection and immunoprecipitated with anti-FLAG antibody. The immunoprecipitates and cell lysates were analyzed by Western blotting with anti-TRAF2 and anti-FLAG antibodies. (B) 293T cells were transfected with various FLIP constructs (each 1 μg per plate). The proteasome inhibitor MG-132 (10 μM) was included in cells transfected with FLIP(p12) for the last 2 h. Cell lysates were prepared 16 h after transfection and immunoprecipitated with anti-FLAG antibody. The immunoprecipitates and cell lysates were analyzed by Western blotting with anti-TRAF2 and anti-FLAG antibodies. (C) 293T cells were transfected with (+) or without (−) FLAG-procaspase 8 (1 μg per plate) together with (+) or without (−) VSV-FLIP(L) or VSV-FLIP(p43) (each at 1 μg per plate). Cell lysates were prepared 16 h after transfection and immunoprecipitated with anti-FLAG antibody. The immunoprecipates and cell lysates were analyzed by Western blotting with anti-caspase 8, anti-FLIP, and anti-TRAF2 antibodies. (D) 293T cells were transfected with (+) or without (−) FLAG-procaspase 8 (1 μg per plate) together with (+) or without (−) VSV-FLIP(L) or VSV-FLIP(p43) (each at 1 μg per plate) in the presence of zVAD-fmk (20 μM). Cell lysates were prepared 16 h after transfection and immunoprecipitated with anti-FLAG antibody. The immunoprecipitates and cell lysates were analyzed by Western blotting with anti-caspase 8 and anti-FLIP antibodies. (E) RFL23 cells were preincubated with (+) or without (−) 100 μM zVAD-fmk for 30 min and then treated with (+) or without (−) cross-linked FasL (2 μg/ml) for 30 min. The DISC and cell lysates were analyzed by Western blotting with anti-FADD, anti-caspase 8, anti-FLIP, and anti-TRAF2 antibodies.
As shown in our earlier paper (19), TRAF2 was recruited in the DISC upon Fas ligation in Jurkat T cells and Raji cells, and it was much more pronounced in FLIP(L)-transfected cells. To test the possibility that caspase 8-dependent cleavage of FLIP(L) is a prerequisite for the recruitment of TRAF2 into the DISC, FLIP(L)-transfectant Raji cells (RFL23) were preincubated with zVAD-fmk and then treated with cross-linked FasL for 30 min (Fig. 6E). In the DISC, nearly all FLIP(L) was processed into FLIP(p43), and a large part of procaspase 8 underwent self-processing to convert into caspase 8(p43), accompanied by significant recruitment of TRAF2 (Fig. 6E). zVAD-fmk strongly inhibited both the processing of procaspase 8 and FLIP(L) and TRAF2 recruitment (Fig. 6E).
DISCUSSION
FLIP(L) is able to bring about strong activation of the NF-κB signaling pathway (6, 14, 19). In contrast to other NF-κB inducers such as RIP or caspase 8, NF-κB activation induced by FLIP(L) is potently inhibited by zVAD-fmk or Crm A (Fig. 1A and B) (6, 14). These findings suggest that caspase activity is required for NF-κB activation induced by FLIP(L). In the present paper, we have investigated the molecular mechanism of how caspase activation contributes to FLIP(L)-induced activation of the NF-κB signaling pathway. Strikingly, the N-terminal fragment FLIP(p43) processed by caspase 8 induced NF-κB activation even in the presence of zVAD-fmk. Thus, caspase 8 activity appears to be required for FLIP(L) cleavage, but not for its downstream events leading to the NF-κB signaling pathway. Moreover, endogenous TRAF2 interacted specifically with FLIP(p43), and DN-TRAF2 abolished FLIP(p43)-induced NF-κB activation. Our present results clearly demonstrate that TRAF2 is an essential adapter protein involved in FLIP(p43)-induced NF-κB activation.
Yeast two-hybrid screening has revealed that the caspase-like domain of FLIP(L) binds to TRAF1 and TRAF2 (40). FLIP(L) also interacts with TRAF1 and TRAF2 when overexpressed in mammalian cells (19, 40). However, the present work has clearly shown that endogenous TRAF2 interacts highly selectively with FLIP(p43) but poorly with FLIP(L). Thus, it is likely that, although FLIP(L) binds to TRAF2 only weakly or transiently, FLIP(p43) forms a more stable complex with TRAF2. The observation that TRAF2 failed to interact with FLIP(S), FLIP(C), FLIP(233-376), and FLIP(p12) suggests that the overall structure of FLIP(p43) is necessary for its stable association with TRAF2 and that the C-terminal region of FLIP(L) interferes with TRAF2 binding to FLIP(L).
By analyzing mice deficient for TRAF2 or overexpressing DN-TRAF2, it was initially concluded that TRAF2 is essential for JNK activation but not NF-κB activation (26, 48). However, NF-κB activation is severely impaired in mice lacking TRAF2 and TRAF5 (41), demonstrating that TRAF2 and TRAF5 play redundant roles in NF-κB activation. In contrast to TRAF2, TRAF5 failed to associate with FLIP(L) even when overexpressed in 293T cells under conditions in which the interaction between TRAF2 and FLIP(L) was readily detectable (19). Thus, TRAF2, but not TRAF5, is likely to be the main adapter protein that elicits NF-κB activation downstream of FLIP(p43). TRAF2 recruits the IKK complex to TNF-R1 upon TNF treatment, whereas RIP is responsible for activation of IKK complex (9). Since we have shown that RIP is also recruited into the DISC upon Fas ligation in a FLIP-dependent fashion (19), it is possible that FLIP(p43)-recruited TRAF2 collaborates with RIP to induce NF-κB activation in response to stimulation of death receptors such as Fas.
Contrary to our prediction, overexpression of FLIP(D376A) still induced NF-κB activation. However, in contrast to FLIP(p43), FLIP(D376A)-induced NF-κB activation was markedly blocked by caspase inhibitors. Autoprocessing of procaspase 8 into caspase 8(p43) was dispensable for NF-κB activation induced by FLIP(D376A), since cotransfection of FLIP(D376A) and caspase 8(p43) failed to induce NF-κB activation in 293 cells. These observations suggest that caspase 8-cleavable substrates are involved in NF-κB activation induced by FLIP(D376A). DN-TRAF2 abolished NF-κB activation induced by FLIP(p43), whereas it failed to block FLIP(D376A)-induced NF-κB activation, clearly indicating that FLIP(D376A) induces NF-κB activation independently of TRAF2. At present, the molecular basis for NF-κB activation induced by FLIP(D376A) remains unclear. Since FLIP(L) is always processed by caspase 8 rapidly after its recruitment to the DISC, and FLIP(L) is apparently absent in the DISC (19, 25, 38), it seems unlikely that uncleaved FLIP(L) transmits NF-κB activation in the DISC. However, it is possible that FLIP(L) contributes to NF-κB activation without caspase 8-dependent cleavage outside the death receptor signaling pathway.
Caspase 8 plays an essential role in T-cell proliferation. Murine T cells either lacking FADD or overexpressing DN-FADD do not proliferate in response to TCR signals (34, 47, 49, 50). Consistent with this observation, synthetic caspase blockers such as zVAD-fmk inhibit CD3-induced proliferation of human mature T cells (1, 21). These observations, coupled with the findings of recent studies using mice lacking caspase 8 in the T-cell lineage (37) and homozygous caspase 8 deficiency in humans (7), have clearly indicated that caspase 8 activation mediated by death receptors plays an essential role in T-cell proliferation. FLIP(L) is the first substrate that is cleaved by caspase 8 in the DISC. Mature T cells from FLIP(L)-transgenic mice proliferate more efficiently than wild-type T cells in response to suboptimal TCR stimulation (27), suggesting that FLIP(L) modulates T-cell proliferation by decreasing the threshold of the TCR signaling. Thus, these studies provide further evidence that FLIP(L) processing by caspase 8 plays a critical role in T-cell proliferation.
In mice overexpressing DN-TRAF2, mature T cells exhibit compromised proliferation upon TCR stimulation (26). In contrast to TRAF2-deficient mice that manifest severe developmental defects of lymphocytes (48), TRAF1-deficient mice have normal lymphocyte development but exhibit enhanced T-cell proliferation (46). TRAF1 is specifically cleaved at Asp-163 and the C-terminal cleavage product blocks NF-κB activation (17, 28). Thus, unlike TRAF1, TRAF2 appears to be a positive regulator in T-cell proliferation. Taken together with our present observations, it might be postulated that caspase 8-dependent cleavage of FLIP(L) and following recruitment of TRAF2 into FLIP(p43) is fundamental for proliferative responses in mature T cells.
It has been shown that the N-terminal cleaved fragment of FLIP(L) is recruited more preferentially than caspase 8 in the DISC (5, 19, 38). In cells expressing FLIP(L) insufficient to block apoptosis, the procaspase 8 homodimers are formed within the DISC at the plasma membrane, allowing the generation of the active heterotetramers by autoprocessing and their subsequent release into the cytosol. In contrast, in cells expressing a sufficient amount of FLIP(L) to prevent apoptosis, FLIP(L) is dominantly incorporated into the DISC and processed into FLIP(p43) after dimerization with caspase 8 (5, 19, 38). The FLIP(p43)/caspase 8 heterodimers are enzymatically active to cleave local substrates, but inactive to induce apoptosis, and stick to the membrane-anchored DISC for the sustained period (32). Upon Fas ligation, FLIP(L) was processed into FLIP(p43) by caspase 8 in the DISC, accompanied by the recruitment of TRAF2 (Fig. 6E) (19). Moreover, TRAF2 formed the tertiary complex with FLIP(p43) and caspase 8. Thus, in response to death receptor stimulation, FLIP(L) appears to be a major protein that determines either cell death or survival, depending on its expression levels, and if cells are committed to survive, FLIP(L) is able to transmit proliferative signals by TRAF2 after being cleaved into FLIP(p43) in the DISC.
Upon TCR stimulation, Jurkat T cells overexpressing FLIP(L) exhibited increased IκB phosphorylation and its subsequent degradation (19). Moreover, mouse blast T cells possessed a decreased amount of IκB in FLIP(L)-transgenic mice (19). Therefore, NF-κB activation might be one of the major signaling pathways modulated by FLIP(L) in T cells. Since part of FLIP(L) was processed into FLIP(p43) even in freshly isolated mature T cells in FLIP(L)-transgenic mice (27), it is possible that the NF-κB signaling pathway is constitutively activated at the basal level without further TCR stimulation, which might contribute to maintain the long-term survival and responsiveness of T cells.
It has been reported that T cells lacking caspase 8 or expressing DN-FADD manifest unimpaired NF-κB activation upon TCR stimulation (35, 37). These observations have suggested that NF-κB activation upon antigen stimulation is independent of caspase 8. Recently, it has been shown that NF-κB activation via TCR is mainly activated by the Carma-1/CARD11 pathway (11, 13, 18). However, TCR signals can induce the TNF family of proteins such as FasL, and FasL can even costimulate human mature T cells (21). Based on these findings, we speculate that T cells might utilize the death receptor pathway to augment NF-κB activation, especially when TCR stimulation alone is insufficient to induce proliferative responses. It might be also possible that the caspase 8-FLIP(L) pathway is dominantly involved in the induction of the NF-κB signaling pathway upon TCR stimulation in certain subpopulations of mature T cells expressing high levels of FLIP(L).
In conclusion, caspase 8 processes FLIP(L) into FLIP(p43) and induces the recruitment of TRAF2 to FLIP(p43), leading to activation of the NF-κB signaling pathway. As the specific substrate of caspase 8, FLIP(L) appears to act as the molecular switch between cell death and cell growth by preventing generation of the active caspase 8 as well as transmitting proliferative signals such as the NF-κB signaling pathway. Our present results provide one possible molecular mechanism that accounts for why caspase 8 is required for T-cell proliferation.
Acknowledgments
We thank Ralph C. Budd for critical reading of the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and a research grant from the Kato Memorial Bioscience Foundation.
REFERENCES
- 1.Alam, A., L. Y. Cohen, S. Aouad, and R. P. Sékaly. 1999. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 190:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderson, M. R., R. J. Armitage, E. Maraskovsky, T. W. Tough, E. Roux, K. Schooley, F. Ramsdell, and D. H. Lynch. 1993. Fas transduces activation signals in normal human T lymphocytes. J. Exp. Med. 178:2231-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 4.Budd, R. C. 2002. Death receptors couple to both cell proliferation and apoptosis. J. Clin. Investig. 109:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, D. W., Z. Xing, Y. Pan, A. Algeciras-Schimnich, B. C. Barnhart, S. Yaish-Ohad, M. E. Peter, and X. Yang. 2002. c-FLIPL is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 21:3704-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary, P. M., M. T. Eby, A. Jasmin, A. Kumar, L. Liu, and L. Hood. 2000. Activation of the NF-κB pathway by caspase 8 and its homologs. Oncogene 19:4451-4460. [DOI] [PubMed] [Google Scholar]
- 7.Chun, H. J., L. Zheng, M. Ahmad, J. Wang, C. K. Speirs, R. M. Siegel, J. K. Dale, J. Puck, J. Davis, C. G. Hall, S. Skoda-Smith, T. P. Atkinson, S. E. Straus, and M. J. Lenardo. 2002. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419:395-399. [DOI] [PubMed] [Google Scholar]
- 8.Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551-1570. [DOI] [PubMed] [Google Scholar]
- 9.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. G. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 10.Donepudi, M., A. M. Sweeney, C. Briand, and M. G. Grütter. 2003. Insights into the regulatory mechanism for caspase-8 activation. Mol. Cell 11:543-549. [DOI] [PubMed] [Google Scholar]
- 11.Gaide, O., B. Favier, D. F. Legler, D. Bonnet, B. Brissoni, S. Valitutti, C. Bron, J. Tschopp, and M. Thome. 2002. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat. Immunol. 3:836-843. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 13.Hara, H., T, Wada, C. Bakal, I. Kozieradzki, S. Suzuki, N. Suzuki, M. Nghiem, E. K. Griffiths, C. Krawczyk, B. Bauer, F. D'Acquisto, S. Ghosh, W. C. Yeh, G. Baier, R. Rottapel, and J. M. Penninger. 2003. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18:763-775. [DOI] [PubMed] [Google Scholar]
- 14.Hu, W. H., H. Johnson, and H. B. Shu. 2000. Activation of NF-κB by FADD, Casper, and caspase-8. J. Biol. Chem. 275:10838-10844. [DOI] [PubMed] [Google Scholar]
- 15.Hua, Z. C., S. J. Sohn, C. Kang, D. Cado, and A. Winoto. 2003. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity 18:513-521. [DOI] [PubMed] [Google Scholar]
- 16.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schröter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 17.Irmler, M., V. Steiner, C. Ruegg, H. Wajant, and J. Tschopp. 2000. Caspase-induced inactivation of the anti-apoptotic TRAF1 during Fas ligand-mediated apoptosis. FEBS Lett. 468:129-133. [DOI] [PubMed] [Google Scholar]
- 18.Jun, J. E., L. E. Wilson, C. G. Vinuesa, S. Lesage, M. Blery, L. A. Miosge, M. C. Cook, E. M. Kucharska, H. Hara, J. M. Penninger, H. Domashenz, N. A. Hong, R. J. Glynne, K. A. Nelms, and C. C. Goodnow. 2003. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18:751-762. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka, T., R. C. Budd, N. Holler, M. Thome, F. Martinon, M. Irmler, K. Burns, M. Hahne, N. Kennedy, K. Kovacsovics, and J. Tschopp. 2000. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr. Biol. 10:640-648. [DOI] [PubMed] [Google Scholar]
- 20.Kelliher, M. A., S. Grimm, Y. Ishida, F. Kuo, B. Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8:297-303. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, N. J., T. Kataoka, J. Tschopp, and R. C. Budd. 1999. Caspase activation is required for T cell proliferation. J. Exp. Med. 190:1891-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. W., E. J. Choi, and C. O. Joe. 2000. Activation of death-inducing signaling complex (DISC) by pro-apoptotic C-terminal fragment of RIP. Oncogene 19:4491-4499. [DOI] [PubMed] [Google Scholar]
- 23.Krammer, P. H. 2000. CD95's deadly mission in the immune system. Nature 407:789-795. [DOI] [PubMed] [Google Scholar]
- 24.Krueger, A., S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21:8247-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger, A., I. Schmitz, S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276:20633-20640. [DOI] [PubMed] [Google Scholar]
- 26.Lee, S. Y., A. Reichlin, A. Santana, K. A. Sokol, M. C. Nussenzweig, and Y. Choi. 1997. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity 7:703-713. [DOI] [PubMed] [Google Scholar]
- 27.Lens, S. M. A., T. Kataoka, K. A. Fortner, A. Tinel, I. Ferrero, R. H. MacDonald, M. Hahne, F. Beermann, A. Attinger, H. A. Orbea, R. C. Budd, and J. Tschopp. 2002. The caspase 8 inhibitor c-FLIPL modulates T-cell receptor-induced proliferation but not activation-induced cell death of lymphocytes. Mol. Cell. Biol. 22:5419-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leo, E., Q. L. Deveraux, C. Buchholtz, K. Welsh, S. Matsuzawa, H. R. Stennicke, G. S. Salvesen, and J. C. Reed. 2001. TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-α-induced apoptosis. J. Biol. Chem. 276:8087-8093. [DOI] [PubMed] [Google Scholar]
- 29.Lin, Y., A. Devin, Y. Rodriguez, and Z. G. Liu. 1999. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13:2514-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 31.Martinon, F., N. Holler, C. Richard, and J. Tschopp. 2000. Activation of a pro-apoptotic amplification loop through inhibition of NF-κB-dependent survival signals by caspase-mediated inactivation of RIP. FEBS Lett. 468:134-136. [DOI] [PubMed] [Google Scholar]
- 32.Micheau, O., M. Thome, P. Schneider, N. Holler, J. Tschopp, D. W. Nicholson, C. Briand, and M. G. Grütter. 2002. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 277:45162-45171. [DOI] [PubMed] [Google Scholar]
- 33.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 34.Newton, K., A. W. Harris, M. L. Bath, K. G. C. Smith, and A. Strasser. 1998. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 17:706-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton, K., C. Kurts, A. W. Harris, and A. Strasser. 2001. Effects of a dominant interfering mutant of FADD on signal transduction in activated T cells. Curr. Biol. 11:273-276. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson, D. W. 1999. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6:1028-1042. [DOI] [PubMed] [Google Scholar]
- 37.Salmena, L., B. Lemmers, A. Hakem, E. Matysiak-Zablocki, K. Murakami, P. Y. B. Au, D. M. Berry, L. Tamblyn, A. Shehabeldin, E. Migon, A. Wakeham, D. Bouchard, W. C. Yeh, J. C. McGlade, P. S. Ohashi, and R. Hakem. 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541-1548. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, P., N. Holler, J. L. Bodmer, M. Hahne, K. Frei, A. Fontana, and J. Tschopp. 1998. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu, H. B., D. R. Halpin, and D. V. Goeddel. 1997. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity 6:751-763. [DOI] [PubMed] [Google Scholar]
- 41.Tada, K., T. Okazaki, S. Sakon, T. Kobarai, K. Kurosawa, S. Yamaoka, H. Hashimoto, T. W. Mak, H. Yagita, K. Okumura, W. C. Yeh, and H. Nakano. 2001. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-κB activation and protection from cell death. J. Biol. Chem. 276:36530-36534. [DOI] [PubMed] [Google Scholar]
- 42.Thome, M., F. Martinon, K. Hofmann, V. Rubio, V. Steiner, P. Schneider, C. Mattmann, and J. Tschopp. 1999. Equine herpesvirus-2 E10 gene product, but not its cellular homologue, activates NF-κB transcription factor and c-Jun N-terminal kinase. J. Biol. Chem. 274:9962-9968. [DOI] [PubMed] [Google Scholar]
- 43.Thome, M., and J. Tschopp. 2001. Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 1:50-58. [DOI] [PubMed] [Google Scholar]
- 44.Tibbetts, M. D., L. Zheng, and M. J. Lenardo. 2003. The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 4:404-409. [DOI] [PubMed] [Google Scholar]
- 45.Tschopp, J., M. Irmler, and M. Thome. 1998. Inhibition of Fas death signals by FLIPs. Curr. Opin. Immunol. 10:552-558. [DOI] [PubMed] [Google Scholar]
- 46.Tsitsikov, E. N., D. Laouini, I. F. Dunn, T. Y. Sannikova, L. Davidson, F. W. Alt, and R. S. Geha. 2001. TRAF1 is a negative regulator of TNF signaling: enhanced TNF signaling in TRAF1-deficient mice. Immunity 15:647-657. [DOI] [PubMed] [Google Scholar]
- 47.Walsh, C. M., B. G. Wen, A. M. Chinnaiyan, K. O'Rourke, V. M. Dixit, and S. M. Hedrick. 1998. A role for FADD in T cell activation and development. Immunity 8:439-449. [DOI] [PubMed] [Google Scholar]
- 48.Yeh, W. C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, J., D. Cado, A. Chen, N. H. Kabra, and A. Winoto. 1998. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296-300. [DOI] [PubMed] [Google Scholar]
- 50.Zörnig, M., A. O. Hueber, and G. Evan. 1998. p53-dependent impairment of T cell proliferation in FADD dominant-negative transgenic mice. Curr. Biol. 8:467-470. [DOI] [PubMed] [Google Scholar]