Abstract
We report the clinical course of a patient with severe infantile onset Pompe disease [cross-reactive immunologic material (CRIM) negative, R854X/R854X] who was diagnosed prenatally and received standard dosing of alglucosidase alfa (Myozyme®) enzyme replacement therapy (ERT) from day 10 of life until she passed away at the age of 3 years 9 months. In the immediate neonatal period there was cardiomegaly on chest X-ray, cardiac hypertrophy by echocardiogram, and development of a wide complex tachycardia. CRIM negative (CN) status was suspected based on her family history, and the available data at the time indicated that CN patients had limited survival even with ERT. However, given the opportunity for very early treatment, the treating provider and family elected to initiate treatment with ERT, without immune modulation. By 9 months of age echocardiogram was normal. Early motor development was within normal limits but by 2 years of age her developmental progress had slowed. She seroconverted by the 4th month of ERT, and anti-rhGAA antibody titers peaked at 25,600 in the 27th month. Immunomodulatory therapy was considered but declined by family. She acquired Influenza A at 2 years 6 months, which led to a prolonged hospitalization with invasive respiratory support, and placement of tracheostomy and gastrostomy tube. Her developmental progress ceased, and she died suddenly at home from a presumed cardiac event at age 3 years 9 months. The poor outcomes observed in CN patients have been attributed to the development of high sustained antibody titers. Although this CN patient’s anti-rhGAA response was elevated and sustained, it is unlike any of the 3 patterns that have been previously described: high titer CN, high titer CRIM positive (HTCP), and low titer CP (LTCP) patients. This patient’s clinical course, with achievement of 24 months of motor gains, 30 months of ventilator-free survival and 45 month survival, is like that of only a fraction of ERT treated CN patients, yet it is identical to other reported CN patients in its relentless progression and early fatality. The immunologic response (moderate sustained antibody titers) described here has not been previously reported and may have played a role in the overall pattern of developmental decline. In light of proposed universal newborn screening for Pompe disease, there is an urgent need for improved understanding of the interplay between immunologic responses to the only available treatment, ERT, and the relentless nature of this disease in CN patients.
Keywords: Glycogen storage disease type II, Pompe disease, Enzyme replacement therapies, Immunologic response, Antibodies
1. Introduction
Pompe disease (glycogen storage disease type II) is an autosomal recessive lysosomal storage disorder caused by a deficiency of acid α-glucosidase, resulting in glycogen accumulation in multiple cell types, but affecting skeletal and cardiac muscle most severely [1]. Varying levels of residual enzymatic function result in disease onset ranging from the neonatal period through adulthood and a continuum of disease phenotype. The infantile form is the most severe, and is characterized by cardiomyopathy, hypotonia, and respiratory insufficiency. Without treatment, death typically occurs secondary to cardiorespiratory failure within the first 1 to 2 years of life [1-3]. Since the introduction of enzyme replacement therapy (ERT) with alglucosidase alfa (rhGAA, Myozyme®), many infantile Pompe disease patients are living longer[4-6]. However, response to ERT is heterogeneous and several poor prognostic factors have been identified. Among infants, cross-reactive immunologic material (CRIM) negative status is strongly correlated with a poor outcome [7,8]. In a majority of CRIM-negative (CN) infants, continuous use of ERT achieves early clinical improvement followed by a resurgence of the natural progression of the underlying disease process. These CN infants develop high sustained anti-rhGAA IgG antibody titers (HSAT) in the range of 102, 400–409, 600. These very HSAT are believed to play a major role in these patients’ poor clinical outcomes [7,8]. Here, we describe a new pattern of immunologic response to ERT in a severely affected Pompe patient. This case is unique, as this CN patient never tolerized to ERT, but rather had a persistence of antibody titers throughout her life that were outside of the typical range previously observed in CN patients.
2. Subjects and methods
Dried blood spot (DBS) GAA enzyme assays and anti-rhGAA IgG antibody titer measurements were performed at Genzyme Corporation (Cambridge, MA). Determination of CRIM status, fibroblast and amniocyte GAA enzyme activity assays, and urinary glucose tetrasaccharide biomarker (Glc4) measurements were performed at the Duke University Hospital Biochemical Genetics Laboratory, as previously described [10]. GAA mutation analysis was performed at Cincinnati Children’s Hospital Medical Center.
3. Results
3.1. Clinical course
Based on a positive family history (three older brothers with a confirmed diagnosis of infantile onset Pompe disease, each of whom had died between the ages of 6 and 9 months secondary to cardiorespiratory failure, and prior to availability of ERT), prenatal diagnosis was achieved via biochemical analysis of amniocytes: enzymatic activity of GAA was 0 nmol/min/mg protein. In the immediate postnatal period, diagnostic confirmation was made based on deficient GAA activity in a dried blood spot, and later in skin fibroblasts. On day 10 of life, ERT with rhGAA was initiated at a dose of 20 mg/kg, administered IV every other week. Homozygosity for the R854X/R854X mutation was identified; confirming a severe genotype [7,11,12].
The patient received standard treatment with rhGAA until her death at the age of 3 years, 9 months. Her clinical course was complicated by a number of hospital admissions for asthma, pneumonia, and central line sepsis, but most notably, at age 2 years 6 months, she presented to an outlying hospital in cardiopulmonary arrest. Upon transfer to our institution, infection with Influenza A was confirmed and the patient was hospitalized for 5 weeks. This event was notable for the requirement of prolonged invasive ventilation and tracheostomy, and gastrostomy tube placement. Subsequent to this event, she was unable to regain her pre-hospitalization gross motor skills, and she showed some motor regression with time. At age 3 years and 9 months she passed away unexpectedly, at home, not associated with a known illness. Aggressive resuscitation measures at an outlying hospital were unsuccessful. The ED physician noted no evidence of pulmonary disease in the resuscitation attempts.
3.2. Cardiac status
At birth, a chest X-ray from an outlying hospital revealed cardiomegaly. Echocardiogram performed at day one of life showed a left ventricular mass index of 141.5 g/m2(upper limit of normal for infants, 64 g/m2[13]). She had normalization of her echocardiogram by 9 months of age. Regular echocardiograms continued to show ventricular mass within normal limits. Following her cardiopulmonary arrest associated with Influenza A infection, her echocardiogram revealed some left ventricular hypertrophy as well as mild pulmonary hypertension, but returned to baseline with normal interventricular septum thickness when repeated 3 months later. Holter monitor on the 4th day of life showed frequent wide complex tachycardia representing monomorphic VT or SVT with aberrant conduction. Her arrhythmias were successfully treated with amiodarone (prescribed during the first 4 months of life) and with propranolol (for the duration of her life). Holter monitor studies from 1 month of age to 3 years of age were normal. EKGs showed normal sinus rhythm with right atrial enlargement and biventricular hypertrophy by voltage criteria. At age 3 years and 9 months she was found unresponsive at home. Given this patient’s early history of cardiac arrhythmia, the lack of evidence for acute respiratory disease, and the belief that Pompe disease patients on ERT are at increased risk for arrhythmias [14], it is presumed that this patient’s sudden cardiac death resulted from arrhythmia.
3.3. Musculoskeletal, developmental and neurologic status
At birth, this patient exhibited mild, generalized hypotonia as well as left arm weakness secondary to shoulder dystocia. At 5½ months of age she was moving well against gravity and reaching for objects with both hands. Her tone was normal, she had good head control, and had no residual left arm weakness. At 12 months, she had myopathic facies, could sit well unsupported, scoot on her buttocks, stand and cruise. At 18 months, her neurological exam revealed myopathic facies with little facial expression, but good muscle tone and strength, with a normal toddler gait and age-appropriate fine motor skills; she could say several words. At 22–23 months of age, she was at the 90th percentile for a 19 month old on the Alberta Infant Motor Scale (AIMS). By age 2 years, her progress had slowed and there was some early evidence of decline: she had speech delay, a persistent cough, and was suspected to have a swallowing disorder. At 2 years, 4 months she exhibited low muscle tone and had some difficulty getting up from the floor, and walked with a wide-based gait. She experienced a cardiopulmonary arrest at age 2 years 6 months associated with Influenza-A infection. It is notable that following this event, brain MRI revealed no evidence of acute ischemia.
She could not be weaned from invasive ventilation, and required tracheotomy; because of inconsistent swallow she also required gastrostomy tube. She was unable to regain the ability to walk. On exam at 3 years of age she had very significant facial weakness with bilateral ptosis; she had good strength proximally and distally in her arms, but moved her legs little. Thereafter, she received physical therapy at home, and maintained the ability to sit unassisted, pull to a stand, and move a scooter with her feet. At this point she showed little developmental progress, and experienced some decline in functioning. Her expressive language skills remained delayed throughout her lifetime; she utilized of a number of signs and gestures, but did not use oral language after the tracheostomy. She passed her newborn hearing screen and there was never parental concern about her hearing or vision.
3.4. Urinary glucose tetrasaccharide biomarker (Glc4) response
The urinary biomarker Glc4has previously been shown to correlate with skeletal muscle glycogen content and the clinical response to treatment [10,15]. During this patient’s later clinical course, as motor decline was suspected, urinary Glc4 levels were obtained from 92 to 188 weeks on treatment (22 to 44 months of age) and were found to be markedly elevated (median: 56; range: 36 to 71 mmol/mol creatinine; n=4; control range <4.4), with the highest level observed 1 month before her demise. The marked elevation of Glc4 in this patient’s second and third year of treatment was comparable with levels reported for 6 patients (2 CN and 4 CP) treated at or before 6 months of age who made motor gains during the first year of treatment, but then suffered a clinical decline or plateau after 2 to 3 years of treatment [10]. Patients in the low titer CRIM positive (LTCP) group maintained lower Glc4 levels during years 2 and 3 (mean Glc4 value 29.5, n=10 and 34.6 mmol/mol creatinine, n=10, respectively) [8] than this patient (53.4 and 47.5 mmol/mol of creatine, respectively).
3.5. Immunologic response (anti-rhGAA antibody titers)
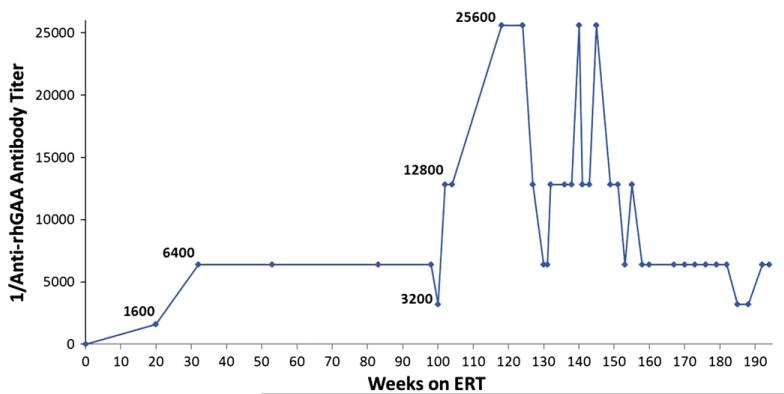
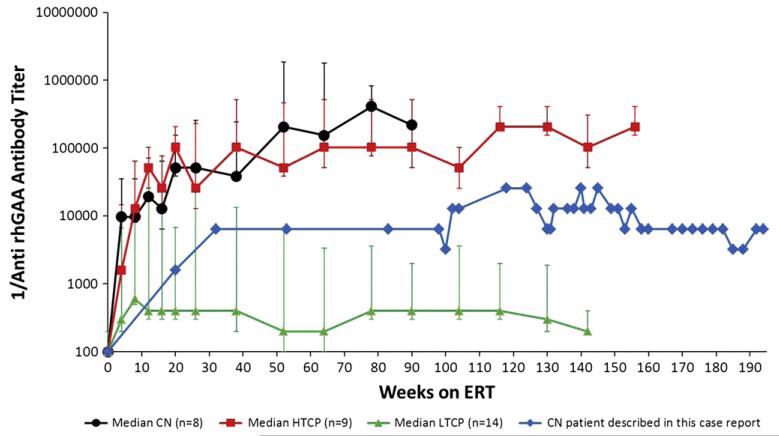
Fig. 1 shows anti-rhGAA IgG antibody titers seen in this patient at different time-points throughout her course. She seroconverted within the first 4 months following initiation of ERT, developing elevated antibody titers (1600). At age 2 years, she developed maximal titers of 25,600, which then declined and were sustained in the range of 6400 to 12,800. Titers remained moderately elevated (6400) during her final 9 months of life. Tolerization to ERT was never achieved. There was no evidence of neutralizing antibodies: fourteen serum time-points over the course of treatment, including the peak titer and last serum sample, were evaluated for the ability to inhibit enzyme uptake into cells and all were found to be negative. Of note, she received standard childhood vaccinations, including RSV prophylaxis during her first year of life, and testing of immunologic competence at 3 years, 7 months indicated that she had developed normal antibody responses to Streptococcus pneumoniae (protective responses to 12 of 14 IgG types) and Clostridium tetani, but remained in the unprotected range for Corynebacterium diphtheriae. Total IgG, IgA and IgM levels were in the normal to elevated range. Fig. 2 illustrates that the pattern of immunologic response seen in the CN patient described in this case report is different from the immunologic responses in previously described CN, HTCP and LTCP groups [8].
Fig. 1.
Patient anti-rhGAA IgG antibody titers over time.
Fig. 2.
Patient anti-rhGAA antibody titers compared with those from previously described CRIM negative (CN), high titer CRIM positive (HTCP) and low titer CRIM positive (LTCP) groups on log y-axis [8].
4. Discussion
The availability of ERT for Pompe disease has significantly altered the clinical progression of the disease for affected individuals. For affected infants, particularly those with CP status, ERT has been shown to be beneficial with prolonged ventilator-free survival and overall survival [7]. Recent studies have shown that CP patients have dichotomous immunologic responses which correspond to their clinical outcomes. High titer (<51,200) CP (HTCP) patients develop HSAT and experience poor clinical outcomes, not dissimilar to the CN group, whereas low titer CP (LTCP) patients typically tolerize to rhGAA, or have antibody titers ≤1600 at or beyond 52 weeks of ERT, and have the best clinical outcomes with continued motor gains [8]. Although the overwhelming majority of CN patients are either ventilator-dependent or deceased by age 27.1 months [7], a single case report has described a CN infant whose ventilator-free survival reached 42 months [9]. Of particular interest is the immunologic profile of the patient described in that report: there was treatment with a course of omalizumab (anti-IgE mAb) for IgE mediated anaphylactic episodes related to ERT, and the anti-rhGAA antibody titers remained very low (<800) [9]. Maintaining sustained benefit from ERT in patients who have developed, or are at-risk of developing HSAT, appears to require immune modulation [7,8,16].
The immunologic response of our CN infantile Pompe disease patient is atypical in that her antibody response was blunted in comparison to other reported CN patients, without the use of immunomodulatory therapies (Fig. 2). While this patient did not develop the very HSAT typical of a CN patient, she did have a sustained antibody titer (≥6400) and did not tolerize to continued ERT. Her clinical course of an extended period of motor gains and ventilator-free survival is at the upper limits of what has been described previously in rare CN patients [17], but follows the relentless pattern common to CN patients [7,8]. Like most of the CN patients with typical clinical courses and very HSAT, she did not test positive for neutralizing antibody (NAbs), which can either abrogate rhGAA uptake into target cells or the catalytic activity of the enzyme.
It may be the persistence of titers, sustained over a prolonged period of time above a certain threshold, rather than the absolute values of the titers, that determines clinical outcomes. Elegant studies in animal models show that persistently elevated antibody titers over time can sabotage the therapeutic activity of ERT [18,19]. Moderate antibody levels may be of sufficient magnitude to coat rhGAA molecules and mistarget them to off-target tissues such as those expressing FcR, rather than to mannose-6 phosphate (M-6P) receptor bearing tissues such as muscle [20].
This case describes a new pattern of immunologic response to ERT in a severely affected Pompe patient, and highlights the limitations of our current understanding about the specific role that a patient’s immunologic response will have on clinical outcome. It may be that blunted nature of this patient’s absolute antibody response allowed for a comparatively prolonged clinical course, yet the persistent nature of her significant antibody response determined her ultimate early demise. Although her specific immunologic response is likely to have contributed to her outcome, the limitations of a single case report preclude conclusive determinations about the factors that contributed to her clinical course. Non-immunologic factors may also have played a crucial role in her clinical course. Severe respiratory infections are known to be correlated with rapid clinical decline in CN patients; her response to Influenza A is different from LTCP patients who are able to recover from acute respiratory illness without requiring prolonged invasive ventilation (personal experience, PSK). Alternatively, it could be speculated that emergence of CNS disease, a complication that has been shown to develop in longer term CN survivors, could have contributed to the demise of this patient [17]. It is important to note that a specific cause of death was not determined.
Our report does suggest that early treatment is insufficient to protect a CN patient from the development of clinically relevant immunologic responses. In light of the proposed universal newborn screening for Pompe disease, and the corresponding potential for very early treatment, additional data from patients who have moderate, sustained antibody responses is needed in order to understand the role of immunologic responses in patient outcomes. The identification of interventions that can achieve immunotolerance to ERT may be necessary to substantially affect the outcomes for these severely affected patients.
References
- [1].Hirschhorn R, Reuser AJJ. Glycogen storage disease type II: acid a-glucosidase (acid maltase) deficiency. In: Valle D, Scriver CR, editors. Scriver’s OMMBID the Online Metabolic & Molecular Bases of Inherited Disease. McGraw-Hill, New York: 2009. [Google Scholar]
- [2].van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT, Bakker HD, Loonen MC, de Klerk JB, Reuser AJ, van der Ploeg AT. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- [3].Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- [4].Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, McDonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, De la Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- [5].Nicolino M, Byrne B, Wraith JE, Leslie N, Mandel H, Freyer DR, Arnold GL, Pivnick EK, Ottinger CJ, Robinson PH, Loo JC, Smitka M, Jardine P, Tato L, Chabrol B, McCandless S, Kimura S, Mehta L, Bali D, Skrinar A, Morgan C, Rangachari L, Corzo D, Kishnani PS. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet. Med. 2009;11:210–219. doi: 10.1097/GIM.0b013e31819d0996. [DOI] [PubMed] [Google Scholar]
- [6].Kishnani PS, Corzo D, Leslie ND, Gruskin D, Van der Ploeg A, Clancy JP, Parini R, Morin G, Beck M, Bauer MS, Jokic M, Tsai CE, Tsai BW, Morgan C, O’Meara T, Richards S, Tsao EC, Mandel H. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr. Res. 2009;66:329–335. doi: 10.1203/PDR.0b013e3181b24e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kishnani PS, Goldenberg PC, DeArmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Banugaria SG, Prater SN, Ng YK, Kobori JA, Finkel RS, Ladda RL, Chen YT, Rosenberg AS, Kishnani PS. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet. Med. 2011;13:729–736. doi: 10.1097/GIM.0b013e3182174703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rohrbach M, Klein A, Kohli-Wiesner A, Veraguth D, Scheer I, Balmer C, Lauener R, Baumgartner MR. CRIM-negative infantile Pompe disease: 42-month treatment outcome. J. Inherit. Metab. Dis. 2010;33:751–757. doi: 10.1007/s10545-010-9209-0. [DOI] [PubMed] [Google Scholar]
- [10].Young SP, Zhang H, Corzo D, Thurberg BL, Bali D, Kishnani PS, Millington DS. Long-term monitoring of patients with infantile-onset Pompe disease on enzyme replacement therapy using a urinary glucose tetrasaccharide biomarker. Genet. Med. 2009;11:536–541. doi: 10.1097/GIM.0b013e3181a87867. [DOI] [PubMed] [Google Scholar]
- [11].Hermans MM, de Graaff E, Kroos MA, Wisselaar HA, Willemsen R, Oostra BA, Reuser AJ. The conservative substitution Asp-645→Glu in lysosomal alpha-glucosidase affects transport and phosphorylation of the enzyme in an adult patient with glycogen-storage disease type II. Biochem. J. 1993;289(Pt 3):687–693. doi: 10.1042/bj2890687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, Herman GE, Amalfitano A, Thurberg BL, Richards S, Davison M, Corzo D, Chen YT. Chinese hamster ovary cell-derived recombinant human acid alpha-glucosidase in infantile-onset Pompe disease. J. Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vogel M, Staller W, Buhlmeyer K. Left ventricular myocardial mass determined by cross-sectional echocardiography in normal newborns, infants, and children. Pediatr. Cardiol. 1991;12:143–149. doi: 10.1007/BF02238520. [DOI] [PubMed] [Google Scholar]
- [14].McDowell R, Li JS, Benjamin DK, Jr., Morgan C, Becker A, Kishnani PS, Kanter RJ. Arrhythmias in patients receiving enzyme replacement therapy for infantile Pompe disease. Genet. Med. 2008;10:758–762. doi: 10.1097/GIM.0b013e318183722f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A, Amalfitano A, Chen YT, Koeberl DD. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol. Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- [16].Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N. Engl. J. Med. 2009;360:194–195. doi: 10.1056/NEJMc0806809. [DOI] [PubMed] [Google Scholar]
- [17].Rohrbach M, Klein A, Kohli-Wiesner A, Veraguth D, Scheer I, Balmer C, Lauener R, Baumgartner MR. CRIM-negative infantile Pompe disease: 42-month treatment outcome. J. Inherit. Metab. Dis. 2010;33:751–757. doi: 10.1007/s10545-010-9209-0. [DOI] [PubMed] [Google Scholar]
- [18].Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J. Clin. Invest. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brooks DA. Immune response to enzyme replacement therapy in lysosomal storage disorder patients and animal models. Mol. Genet. Metab. 1999;68:268–275. doi: 10.1006/mgme.1999.2894. [DOI] [PubMed] [Google Scholar]
- [20].Brooks DA, Kakavanos R, Hopwood JJ. Significance of immune response to enzyme-replacement therapy for patients with a lysosomal storage disorder. Trends Mol. Med. 2003;9:450–453. doi: 10.1016/j.molmed.2003.08.004. [DOI] [PubMed] [Google Scholar]