Abstract
Chemotherapy has been known to cause severe side effects, including fatigue. While the mechanisms for chemotherapy induced fatigue (CIF) are likely to be multi-factorial in origin, it is thought that inflammation and anemia may play a role. The purpose of this study was to examine the effect of chemotherapy on fatigue in mice, and further, to begin to determine if inflammation and anemia may contribute to this response. For experiment 1, C57BL/6 mice were assigned to: vehicle (PBS), low (20 mg/kg), medium (40 mg/kg), or high (60 mg/kg) doses of 5-fluorouracil (5-FU). Voluntary physical activity (PA) was measured throughout the treatment period (day 1–5) as well as during the recovery period (day 6–14). In experiment 2, we examined the effects of 5-FU (60 mg/kg) on the inflammatory mediator MCP-1 and on markers of anemia (RBC, Hct and Hb). Finally, using MCP-1−/− mice we examined the role of MCP-1 on CIF (experiment 3). 5-FU reduced voluntary PA in a dose response manner (p < 0.05). Plasma MCP-1 was increased following 5-FU treatment on both days 5 (p = 0.10) and 14 (p < 0.05). In addition, RBCs, Hct and Hb were reduced with 5-FU on days 5 and 14 (p < 0.05). Both C57BL/6 and MCP-1−/− mice saw similar decrements in PA through the duration of the treatment period (days 1–5), however the MCP-1−/− mice recovered much earlier than wildtype mice. This study provides evidence of the dose response effect of a standard chemotherapy agent on fatigue and demonstrates a potential role of MCP-1 and presumably inflammation, and anemia.
Keywords: Cancer, Inflammation, MCP-1, Fatigue, Chemotherapy
1. Introduction
The most commonly reported symptom among cancer sufferers is fatigue. The National Comprehensive Cancer Network (NCCN) has defined cancer related fatigue (CRF) as an “unusual, persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.” CRF is thought to affect more than 70% of cancer patients with some assessments as high as 80–99% for those who are currently undergoing treatment (Jean-Pierre et al., 2007). This type of fatigue may drastically affect quality of life (QOL) (Curt, 2000; Mock, 2001), self-care capabilities (Jean-Pierre et al., 2007; Macquart-Moulin et al., 1999), desire to continue treatment (Macquart-Moulin et al., 1999) and consequently, overall survival. Even with the prevalence of CRF so high, little research has been done to elucidate the underlying mechanisms. This is not surprising given the complexity of this phenomenon; both cancer itself and cancer treatments like chemotherapy can cause fatigue but the relative contribution of each is currently unknown.
While it is generally well accepted that chemotherapy can contribute to fatigue in cancer patients, the mechanisms for this have not yet been fully elucidated (Kornblith et al., 2011). However, several hypotheses have been postulated and include: central serotonin dysregulation, HPA axis dysfunction, circadian disruption, depression, anemia, and central and peripheral inflammation (Ryan et al., 2007; Jager et al., 2008; Morrow et al., 2002; Dantzer et al., 2012). Arguably, the strongest of these are inflammation and anemia. Indeed, fatigue in cancer patients has been associated with an increase in inflammatory proteins, specifically in those patients who have undergone chemotherapy (Pusztai and Mendoza, 2004; Dantzer et al., 2012). For example, Wang et al. (2010) found that IL-6 and TNF-αR1 were positively associated with fatigue in patients completing concurrent chemoradiation therapy. This same association was observed for the Nf-κB pathway in breast cancer survivors (Bower et al., 2011). In addition, cancer patients suffering from fatigue have increased plasma levels of IL-6 and in some cases TNF-α (Jager et al., 2008). Inflammatory cytokines have also been linked to anemia (Jager et al., 2008); inflammation has been reported to increase apoptosis in erythrocytes and reduce erythrocyte production (Jager et al., 2008; Birgegård et al., 2005). Further, chemotherapy itself, and specifically 5-FU, can directly damage bone marrow cells resulting in inhibition of RBC production (Akagi et al., 2008; Hilton et al., 2009; Soares et al., 2010).
The purpose of this investigation was to examine the effects of chemotherapy on fatigue in mice as measured by voluntary wheel running activity, and further, to begin to determine if inflammation and anemia may contribute to this response. Because both cancer and cancer treatments are known to cause fatigue, we examined chemotherapy without cancer to establish the specific effects of chemotherapy alone on fatigue in mice. This study design is consistent with previous work by Wood et al. (2006) and Ray et al. (2011) that reported a reduction in wheel-running activity following the chemotherapy drugs etoposide and paclitaxel, respectively. The chemotherapy treatment used in the current study was 5-fluorouracil (5-FU), the most common chemotherapy treatment for colorectal cancer in the United States. While 5-FU has been the standard of care for more than 40 years, it has been associated with fatigue in humans. However, there are no experimental studies done in mice to confirm a causal relationship.
We used monocyte chemoattractant protein 1 (MCP-1) as a marker of inflammation. MCP-1 has been shown to attract monocytes to sites of inflammation and to stimulate their maturation into macrophages, primary mediators of inflammatory processes (Goede et al., 1999). Further, MCP-1 is often expressed in correlation with IL-1β and TNF-α, cytokines associated with fatigue in cancer patients (Soria et al., 2011). We hypothesized that 5-FU would decrease voluntary physical activity in mice in a dose response manner and this would be associated with an increase in circulating MCP-1 and markers of anemia. In addition, we hypothesized that the effects of 5-FU on fatigue would be alleviated in MCP-1 knockout mice, further confirming a potential causal link between inflammation and chemotherapy induced fatigue (CIF).
2. Methods
2.1. Mice
Male and female C57BL/6 wild-type mice and MCP-1−/− mice (C57BL/6 background) were bred for use in our laboratory at the animal research facility at the University of South Carolina. All experimentation was performed on 6 week old mice and using equal numbers of male and female mice in each experimental group. Mice were maintained on a 12:12 light–dark cycle in a low-stress environment (22 °C, 50% humidity, low noise) and given food and water ad libitum. The University’s Institutional Animal Care and Use Committee approved all aspects of this experimental protocol.
2.2. 5-FU treatment
For experiment 1, 5-FU (Sigma Chemical Co., St. Louis MO) was dissolved in phosphate buffered saline (PBS), pH 7.4, at one of three different doses; 2.0 mg/10 mL (20 mg/kg), 4.0 mg/10 mL (40 mg/kg), and 6.0 mg/10 mL (60 mg/kg). For experiments 2 and 3, only the 60 mg/kg dose was used. PBS alone served as the vehicle injection. All treatments were sterile filtered and stored at 4 °C for no more than 7 days. Mice were weighed immediately prior to injection and were given their respective 5-FU treatment (20, 40 or 60 mg/kg) in proportion to their body weight through intraperitoneal (i.p.) injection. After the collection of baseline voluntary physical activity data, 5-FU (20, 40 or 60 mg/kg) or vehicle injections were given once per day for 5 consecutive days (treatment period) and voluntary activity was continually monitored 24 h per day through 10 days post final injection (days 1–14).
2.3. Experiment 1: dose response of 5-FU on physical activity measures
2.3.1. Voluntary physical activity
Mice were assigned to one of four treatment groups as follows: Vehicle (PBS), 20 mg/kg (low), 40 mg/kg (med), or 60 mg/kg (high) (n = 12/group; n = 6 male and n = 6 female). All mice were removed from their home cages at 6 weeks of age and housed individually in cages containing a running wheel (VitalView, Mini Mitter Co., Inc.). Animals were allowed to acclimate to the wheels for a period of 7 days (previous data in our laboratory has shown that it takes approximately 7 days for mice to become acclimated to the activity wheels (Carmichael et al., 2006). Following the acclimation period, voluntary activity (distance run, time on the wheel and peak speed) was recorded for a period of 3 days to establish a baseline value. Voluntary activity was assessed for total distance (Distance), time on wheel (Time), and peak speed (Speed) as calculated using the following equations: Distance = (No. of wheel rotations during a 2 min interval) × [circumference of the running wheel (0.7581 m)]; Time = [(No. of 2 min intervals where wheel rotations were >0) × 2]; Speed = (95th percentile of rotations during a given time interval) × [circumference of wheel (0.7581 m)/2]. Data is presented as the sum of all 2 min intervals collected over the 12 h active dark cycle and expressed as a percentage of baseline (average of data collected for 3 days before treatment).
2.3.2. Grip strength assessment
Additional age-matched mice (n = 8/group; n = 4 males and n = 4 females) were used to evaluate the effects of 5-FU treatment on grip strength. Grip strength measurements were taken once per day for three days prior to injection to establish a baseline value, and then again at day 5 and day 14 following 5-FU treatment. Briefly, holding the mice by the tail, the front and back feet were allowed to grip the grate. Mice were then pulled from the grate, generating a force that was measured by the force transducer. Five measurements were taken consecutively, with 2 min rest between sets until a total of 15 measurements were taken for each mouse. The averages of the 15 measurements were used in the data analysis.
2.4. Experiment 2: effect of 5-FU on inflammatory mediators & anemia markers
2.4.1. Tissue collection
Mice were housed 4–5 per cage and were given 5-FU treatment (60 mg/kg) or vehicle (PBS) injections once per day for 5 days (n = 8/group). Mice were either sacrificed 12 h post final injection (day 5) or 10 days post final injection (day 14). Vehicle (PBS) mice were sacrificed on day 5. Mice were euthanized by isoflurane overdose and blood was collected from the inferior vena cava.
2.4.2. Anemia markers
A complete blood count was performed using the VetScan HMT (Abaxis, Union City, CA) for determination of RBCs, Hct and Hb. Briefly, 100uL of whole blood was placed in an EDTA microtube and analyzed on the VetScan HMT according to manufacturer’s instructions.
2.4.3. Plasma MCP-1
Blood was centrifuged for collection of plasma and stored at −80 °C until analysis for MCP-1 using an ELISA (R and D Systems, Minneapolis MN). The assay was performed according to manufacturer’s instructions. All samples were run in duplicate.
2.5. Experiment 3: role of MCP-1 on voluntary physical activity following 5-FU
C57BL/6 wild-type mice and MCP-1−/− mice were randomly assigned to either 5-FU (60 mg/kg) or vehicle (PBS) treatment as follows: WT-PBS, WT-60, MCP-1−/−-PBS or MCP-1−/−-60 (n = 11–12/group). Voluntary activity was assessed using the same protocol as described above in experiment 1. Briefly, mice were housed individually in cages containing a running wheel. Mice were allowed to acclimate to the wheel for a period of 7 days followed by the collection of baseline data (3 consecutive days). Each mouse was then given their treatment via i.p. injection once per day for 5 days and voluntary activity was assessed for an additional 10 days.
2.6. Statistics
Data was analyzed with commercial software (SigmaStat, Chicago, IL and SPSS, Chicago, IL). The voluntary activity data was analyzed by two-way repeated measures ANOVA (Time X Group) for experiment 1, and a three-way repeated measures ANOVA was used for experiment 3 (Time X Strain X Treatment). Post hoc analysis using a Bonferroni test was used to determine differences among groups on each day. One-way ANOVA tests were used to detect differences between groups for MCP-1 and for anemia markers (RBCs, Hct & Hb) in experiment 2. Statistical significance was set with an alpha value of p < 0.05. Data are presented as mean (±SEM).
3. Results
3.1. Experiment 1
3.1.1. Dose response of 5-FU on physical activity measures
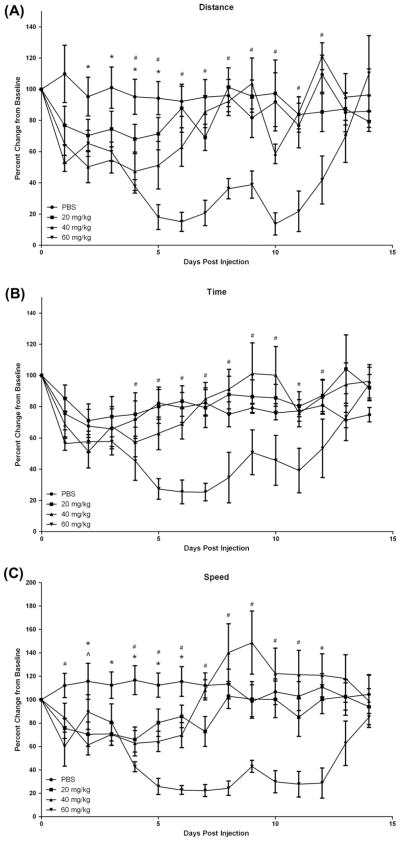
In experiment 1, we examined the influence of 5-FU on voluntary physical activity in mice. Given that there were no differences in absolute values at baseline between any of the groups, the data is presented as percent change from baseline (Davis et al., 2007). Voluntary physical activity (distance, time on the wheel and peak speed) was decreased in a dose dependent manner following treatment with 5-FU. A main effect of time was found (p = 0.01) as well as a significant interaction between treatment groups and time (p = 0.04). Specifically, for total distance, mice that received the 60 mg/kg dose ran significantly less than baseline levels on days 5–12 (p < 0.05) and less than vehicle mice on days 4–12 (p < 0.05) (Fig. 1). Similarly, treatment with the 40 mg/kg dose reduced total distance run beginning on day 4 (p < 0.05), but these values returned to baseline by day 8. This group was also significantly lower than vehicle but only on days 2–5 (p < 0.05). While distance in the 20 mg/kg group was reduced by 15–25% this was not different from baseline or the vehicle group. Time on the wheel was significantly different from baseline only in the 60 mg/kg group (p < 0.05); the 60 mg/kg group spent less time on the wheel on days 5–11 compared to baseline and on days 5–12 compared to vehicle (p < 0.05). There were no significant differences in time on the wheel for the 40 and 20 mg/kg doses; however, the vehicle group was reduced to 75% of baseline levels, which may have diminished any effects of these doses of 5-FU on this parameter. For peak speed all three doses of 5-FU were reduced from baseline, although the duration of this effect was dose dependent; the 60 mg/kg group ran slower on days 4–13 (p < 0.05), the 40 mg/kg dose on days 4–7 (p < 0.05) and the 20 mg/kg dose on days 4–5 (p < 0.05).
Fig. 1.
Voluntary wheel cage activity for 3 doses of 5-fluorouracil chemotherapy (20, 40 and 60 mg/kg) as compared to vehicle injection (PBS). Data are presented as percent change from baseline (mean ± SE). *p < 0.05 between 40 mg/kg dose and PBS, ^p < 0.05 between 20 mg/kg dose and PBS, #p < 0.05 between 60 mg/kg dose and PBS (A) Change from baseline in average distance run per night (B) Change from baseline in average time run per night (C) Change from baseline in average speed per night.
3.1.2. Grip strength
To evaluate the effects of 5-FU on peripheral muscle fatigue, an additional group of mice was used. Grip strength was measured (N/g body weight) prior to the 5-FU (60 mg/kg) injection regime (1.59 ± 0.05 N/g), at 5 days (1.60 ± 0.09 N/g) and at 14 days (1.64 ± 0.02 N/g). No significant differences in strength were found.
3.2. Experiment 2: effect of 5-FU on inflammatory mediators & anemia markers
3.2.1. Anemia markers
Mice receiving 5-FU (60 mg/kg) treatment were sacrificed 12 h post final injection (day 5) or 10 days post final injection (day 14). Vehicle (PBS) mice were sacrificed on day 5. Blood was taken from the inferior vena cava and analyzed for anemia markers (RBCs, Hct & Hb) using a VetScan (Abaxis, Union City, CA) (Table 1). RBC count was significantly reduced at 5 days (7.38 ± 0.15, p = 0.01) and at 14 days (6.04 ± 0.46, p < 0.002) following 5-FU when compared to vehicle (8.38 ± 0.19). Similar effects were seen for Hct and Hb. Specifically, Hct was reduced at 5 days (11.58 ± 0.23) and 14 days (9.23 ± 0.75) compared to vehicle (13.12 ± 0.75). And Hb levels were 35.7 ± 1.64 and 29.7 ± 2.68 on days 5 and 14, respectively, and these were both different from vehicle (40.58 ± 0.76). For all anemia markers (RBCs, Hct and Hb) the 14 day post-5FU values were significantly lower than 5 day values (p < 0.05).
Table 1.
CBC panel and plasma MCP-1 following 60 mg/kg dose of 5-fluorouracil chemotherapy at 5 days and 14 days post initial injection as compared to placebo.
| RBC | Hb | Hct | MCP-1 | |
|---|---|---|---|---|
| PBS | 8.38 ± 0.19 | 40.58 ± 0.76 | 13.12 ± 0.24 | 21.7 ± 2.54 |
| 5 Day | 7.38 ± 0.15* | 35.74 ± 1.06* | 11.58 ± 0.23* | 32.1 ± 2.75 |
| 14 Day | 6.04 ± 0.46*,# | 29.7 ± 2.68*,# | 9.23 ± .75*,# | 47.3 ± 8.0$ |
p < 0.002 between vehicle and 5, 14 D.
p < 0.01 between 5 and 14 D.
p < 0.05 between vehicle and 14 D.
3.2.2. Circulating MCP-1
MCP-1 concentration was measured in the plasma of mice at 5 and 14 days following 5-FU (60 mg/kg) treatment using an ELISA (Table 1). MCP-1 was elevated at 5 days (32.1 ± 2.75) and 14 days (47.3 ± 8.0) following 5-FU treatment as compared to vehicle (21.7 ± 2.54), however, this reached statistical significance at day 14 only (p < 0.05).
3.3. Experiment 3
3.3.1. Role of MCP-1 on voluntary physical activity following 5-FU
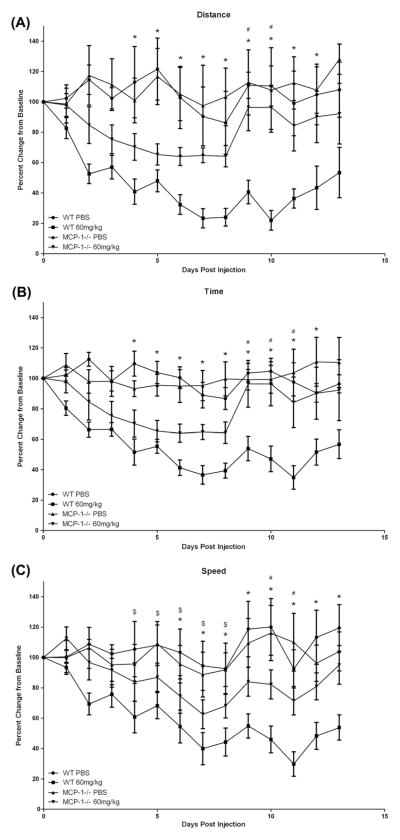
Based on the findings of experiments 1 and 2, we next sought to examine the more specific role of MCP-1 on CIF using MCP-1−/− mice. We used voluntary wheel running activity as our fatigue paradigm and the 60 mg/kg dose of 5-FU to induce fatigue. As there were no differences in absolute values between the groups at baseline, the data is presented as percent change from baseline (Davis et al., 2007). There was a significant time × strain × treatment interaction for each of the three measures (p < 0.05). Additionally, a significant treatment × time interaction was also found for distance, time and speed (p < 0.01) and a strain × treatment interaction (p < 0.05). In general, in each of the three measures of physical activity (distance, time on the wheel and peak speed), the MCP-1−/− mice returned to baseline activity levels before the WT mice. For distance, the WT-60 group ran significantly less than the WT-PBS group (p < 0.05) on days 4–13 but this effect was negated in MCP-1 deficient mice; MCP-1−/−-60 returned to WT levels by day 8 and these mice ran significantly more than WT-60 on days 9 and 10 (p < 0.05) (Fig. 2). For the WT-60 group, average time spent running per night was significantly lower (p < 0.05) than the WT-PBS group from days 4 to 14 and this effect was reduced in MCP-1 knockout mice. In the MCP-1−/−-60 group, time spent on the wheel was lower than MCP-1−/−-PBS on days 4–8 only and these mice spent significantly more time on the wheel than the WT-60 group on days 9–11 (p < 0.05). Similarly, for average nightly speed, the WT-60 group was significantly lower than WTPBS on days 7–14 and MCP-1 deficiency blunted this response; the MCP-1−/−-60 group recovered to baseline by day 8 (p < 0.05).
Fig. 2.
Voluntary wheel cage activity for C57BL/6 and MCP-1−/− mice following treatment with 5-fluororacil. Data are presented as percent change from baseline (mean ± SE). *p < 0.05 between WT-60 and WT-PBS, #p < 0.05 between WT-60 and MCP-1−/−-60, $p < 0.05 between MCP-1−/−-60 and MCP-PBS. (A) Change from baseline in average distance run per night. (B) Change from baseline in average time run per night. (C) Change from baseline in average speed per night.
4. Discussion
Fatigue has been described as the most debilitating symptom of cancer. It has been reported to affect more than 70% of cancer patients with some evaluations as high as 99% for patients currently undergoing treatment (Jean-Pierre et al., 2007). Cancer associated fatigue is undoubtedly a complex phenomenon that is influenced by both the cancer itself and cancer treatments like chemotherapy. However, the relative contribution of each is currently unknown and therefore it is not surprising that the underlying mechanisms have not yet been elucidated. We examined the effect of chemotherapy alone on fatigue in mice, and secondly sought to begin to determine if MCP-1 and presumably inflammation, and anemia may contribute to this fatigue. We show here for the first time that 5-FU, a standard chemotherapy drug, can decrease voluntary wheel running activity in mice in a dose dependent manner. Chemotherapy was also associated with an increase in circulating MCP-1 as well as markers of anemia. In order to explore a more causal relationship between MCP-1 and CIF, we treated MCP-1−/− mice with 5-FU and monitored voluntary physical activity; we report the novel finding that MCP-1 deficient mice recover from 5-FU more rapidly than wildtype mice. It is noteworthy that this fatigue occurred independent of cancer, as both chemotherapy and cancer itself have been implicated as causative agents of fatigue. However, we sought to examine the effects of 5-FU alone in order to determine the contribution of chemotherapy. Although exact dosage and administration can vary depending on the specific cancer, typical chemotherapy treatment is given several days at a time over the course of weeks or months and the fatigue experienced by cancer patients can last as long as or longer than the treatment duration (weeks/months after the completion of chemotherapy). Therefore, this model in which chemotherapy was given for 5 consecutive days, and causing fatigue that was persistent for several days after the treatment, mimics both the treatment regime and resulting symptoms that have been reported in the clinical literature.
Decreases in voluntary wheel running have been widely used as a measure of fatigue in animals (Wood et al., 2006; Carmichael et al., 2006; Ottenweller et al., 1998). It has been demonstrated that C57BL/6 mice have a high level of motivation to run; given free access to a wheel, mice will cover 3–7 km each night. The changes in voluntary activity measured in the current study indicate that 5-FU chemotherapy causes fatigue in mice in a dose–response manner. In general, both the 40 and 60 mg/kg 5-FU groups had significantly decreased voluntary activity (distance, time on the wheel & speed) compared to the vehicle group, whereas mice given the lowest dose of 5-FU (20 mg/kg) showed a reduction in peak speed only. In addition, the recovery response was influenced by 5-FU dose; the 40 mg/kg group returned to baseline levels of distance run per night by day 8 whereas the 60 mg/kg group did not quite reach baseline levels by the end of the experiment (day 14). There were some unexpected observations such as the increased running speed in the 40 mg/kg group during days 8–10, which is not unusual in studies like this where many factors can affect running behavior from time to time. The observed non-significant decrease in time spent on the wheel in the vehicle group is also not unusual and is most likely due to the behavioral disruption induced by the daily injections (Wood et al., 2006).
While the mechanisms responsible for CIF are likely to be multifactoral in origin, it has been hypothesized that inflammation may play a necessary role. In order to begin to test this hypothesis in our model, we examined circulating MCP-1 levels as an indicator of systemic inflammation using the 60 mg/kg dose only. MCP-1 was examined as it is essential for the recruitment of macrophages and is a key regulator in cancer-related inflammation (Balkwill and Mantovani, 2012). In addition, it is elevated in conjunction with pro-inflammatory cytokines in cancer patients and is correlated with disease severity in breast cancer (Soria et al., 2011; Steiner and Murphy, 2012). Further, recent data from our group shows that MCP-1 deficiency is associated with decreased inflammation and reduced polyp number in a genetic mouse model of colon cancer (McClellan et al., 2012) and others have reported that MCP-1 knockout mice have significant reductions in IL-1β and TNF-α expression in the brain following LPS administration (Thompson et al., 2008). In this model of CIF, plasma MCP-1 was significantly elevated at day 14. Our findings are supported by a recent in vitro study reporting an increased expression of IL-1β, IL-6 and TNF-α in peritoneal macrophages treated with the drug (Elsea et al., 2008). Similarly, plasma levels of these cytokines were increased in a rat model following 5-FU administration (Logan, 2008). On the contrary, Ray et al. (2011) examined the effects of Taxol on non-tumor bearing mice and found no increase in circulating cytokines, including MCP-1. However, there was also no significant effect of Taxol on markers of anemia, which may suggest that the symptom burden experienced from chemotherapy may be unique to each treatment. It is important to note that the increase in MCP-1 in our study occurred at a time when voluntary activity was returning to baseline levels undermining implications of a direct link between fatigue and inflammation in this model. However, the delay in systemic elevation of MCP-1 may be explained by accumulating tissue damage as others have shown that administration of 5-FU causes damage and inflammation in the intestines (Chang et al., 2012), bone marrow (Akagi et al., 2008; Hilton et al., 2009; Soares et al., 2010) and the central nervous system (Han et al., 2008). Further, only two time-points (days 5 and 14) were examined in this study, which prevents any understanding of an association between inflammation and fatigue that may have occurred at times other than these.
To further explore a role of MCP-1 on fatigue following 5-FU administration, we next repeated the voluntary activity experiment using MCP-1−/− mice and the 60 mg/kg dose. While the two groups were not significantly different from each other through the duration of the treatment (days 1–5), the MCP-1−/− mice did recover much quicker than the wild-type mice for distance, time and speed run per night. It is reasonable to conclude that while MCP-1 is not the sole cause of CIF, it does play a role in the recovery from treatment. Because MCP-1 has been shown to be a key regulator of inflammation, this data adds to the growing body of literature implicating a role of inflammation in the development of CIF.
In order to begin to decipher the mechanisms of fatigue in this model, we measured muscle specific strength using a grip strength test at baseline, day 5 and day 14. Our findings indicate that 5-FU chemotherapy did not influence maximal contractile force. Therefore, the decrease in voluntary running is not likely due to decreased muscular strength. However, a decrease in muscular endurance, which was not measured, could have been a factor. There is some evidence that 5-FU may be causing mitochondrial damage (Barclay et al., 2001), which could lead to reduced muscular endurance and fatigue, however this is mainly found in cardiac muscle and has yet to be studied in skeletal muscle tissue or in relation to exercise tolerance. Anemia and the resultant decrease in oxygen carrying capacity, which is a common side effect of chemotherapy occurring is approximately 40% of cancer patients, could also lead to a decrease in muscular endurance. Our results of a lower hemoglobin and hematocrit at 5 and 14 days post 5-FU treatment is consistent with other reports that show damage to bone marrow cells and decreased RBC production following 5-FU treatment (Akagi et al., 2008; Hilton et al., 2009; Soares et al., 2010). However, it is important to note that while mice receiving the 40 and 60 mg/kg dose of 5-FU return to near baseline levels of physical activity by day 14, their red blood cell counts actually worsened as the animals’ physical activity recovered. Because the CBC panel was only measured at days 5 and 14, we cannot match the timecourse of fatigue to anemia, which is a limitation of this model. Additionally, we were not able to measure markers of anemia in the MCP-1−/− mice and cannot conclude whether the changes in MCP-1 were directly related to the development of anemia. This suggests that while anemia may be playing a role in fatigue during treatment, it is not the sole contributor and further research is needed to fully elucidate this relationship. Finally, it is important to note that central nervous system inflammation has been strongly linked to sickness behavior and symptoms of fatigue (Carmichael et al., 2006; Dantzer et al., 2012; Maes 2012). This phenomenon has been specifically observed in cancer patients, especially among those recovering from chemotherapy or chemoradiation treatment (Wood et al., 2006; Dantzer et al., 2012), further supporting the hypothesis that CIF may be linked to an increase in central and peripheral inflammation. Clearly, at this time, further research is needed to fully elucidate the specific mechanisms.
This is the first report of the effects of 5-FU on fatigue in a controlled experimental mouse model. Given that both cancer and cancer treatments can cause fatigue, we established the effects of 5-FU chemotherapy on voluntary physical activity independent of any fatigue that may be associated with cancer itself. In summary, our findings show that the 60 mg/kg dose of 5-FU most accurately mirrored the effects on fatigue seen in humans. In addition, chemotherapy was associated with an increase in circulating MCP- 1 as well as anemia. Our findings provide strong support for further development of this mouse model for use in understanding the mechanisms of CIF and further, for the development of potential treatment options to alleviate this widespread condition.
References
- Akagi T, Saitoh T, O’Kelly J, Akira S, Gombart AF, Koeffler HP. Impaired response to GM-CSF and G-CSF, and enhanced apoptosis in C/EBPbeta-deficient hematopoietic cells. Blood. 2008;111 (6):2999–3004. doi: 10.1182/blood-2007-04-087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22 (1):33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Barclay BJ, DeHaan CL, Hennig UG, Iavorovska O, von Borstel RW, von Borstel RC. A rapid assay for mitochondrial DNA damage and respiratory chain inhibition in the yeast Saccharomyces cerevisiae. Environ Mol Mutagen. 2001;38 (2–3):153–158. doi: 10.1002/em.1066. [DOI] [PubMed] [Google Scholar]
- Birgegård G, Aparo M, Bokemeyer C, Dicato M, Drings P, Hornedo J, Krzakowski M, Ludwig H, Pecorelli S, Schmoll H, Schneider M, Schrijvers D, Shasha D, Van Belle S. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68 (1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. (Epub 2010 Sep 18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer EP, Ghaffar A. Role of brain IL-1beta on fatigue after exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1344–R1348. doi: 10.1152/ajpregu.00141.2006. (Epub 2006 Jun 15) [DOI] [PubMed] [Google Scholar]
- Chang CT, Ho TY, Lin H, Liang JA, Huang HC, Li CC, Lo HY, Wu SL, Huang YF, Hsiang CY. 5-fluorouracil induced intestinal mucositis via nuclear factor-κb activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One. 2012;7(3):e31808. doi: 10.1371/journal.pone.0031808. (Epub 2012 Mar 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curt G. Impact of fatigue on quality of life in oncology patients. Semin Hematol. 2000;37 (4 Suppl 6):14–17. doi: 10.1016/s0037-1963(00)90063-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9(7):414–426. doi: 10.1038/nrclinonc.2012.88. http://dx.doi.org/10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Murphy EA, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, Gangemi JD, Ghaffar A, Mayer EP. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2168–R2173. doi: 10.1152/ajpregu.00858.2006. (Epub 2007 Mar 1) [DOI] [PubMed] [Google Scholar]
- Elsea CR, Roberts DA, Druker BJ, Wood LJ. Inhibition of p38 MAPK suppresses inflammatory cytokine induction by etoposide, 5-fluorouracil, and doxorubicin without affecting tumoricidal activity. PLoS One. 2008;3 (6):e2355. doi: 10.1371/journal.pone.0002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goede V, Brogelli L, Ziche M, Augustin HG. Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer. 1999;82:765– 770. doi: 10.1002/(sici)1097-0215(19990827)82:5<765::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Pröschel M, Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J Biol. 2008;7(4):12. doi: 10.1186/jbiol69. (Epub 2008 Apr 22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DJ, Kile B, Alexander WS. Mutational inhibition of c-Myb or p300 ameliorates treatment-induced thrombocytopenia. Blood. 2009;133 (22):5599–5604. doi: 10.1182/blood-2008-12-195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A, Sleijfer S, van der Rijt CC. The pathogenesis of cancer related fatigue: could increased activity of pro-inflammatory cytokines be the common denominator? Eur J Cancer. 2008;44 (2):175–181. doi: 10.1016/j.ejca.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist. 2007;12(Suppl 1):11–21. doi: 10.1634/theoncologist.12-S1-11. (Review) [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Lan L, Archer L, Partridge A, Kimmick G, Hudis C, Winer E, Casey R, Bennett S, Cohen HJ, Muss HB. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol. 2011;29(8):1022–1028. doi: 10.1200/JCO.2010.29.9859. (Epub 2011 Feb 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DM. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther. 2008;7(7):1139–1145. doi: 10.4161/cbt.7.7.6207. (Epub 2008 Apr 29) [DOI] [PubMed] [Google Scholar]
- Macquart-Moulin G, Viens P, Genre D, Bouscary ML, Resbeut M, Gravis G, Camerlo J, Maraninchi D, Moatti JP. Concomitant chemoradiotherapy for patients with nonmetastatic breast carcinoma: side effects, quality of life, and organization. Cancer. 1999;85 (10):2190–2199. [PubMed] [Google Scholar]
- Maes M, Twisk FN, Ringel K. Nflammatory and cell-mediated immune biomarkers in myalgic encephalomyelitis/chronic fatigue syndrome and depression: inflammatory markers are higher in myalgic encephalomyelitis/chronic fatigue syndrome than in depression. Psychother Psychosom. 2012;81(5):286–295. doi: 10.1159/000336803. (Epub 2012 Jul 20) [DOI] [PubMed] [Google Scholar]
- McClellan JL, Davis JM, Steiner JL, Enos RT, Jung SH, Carson JA, Pena MM, Carnevale KA, Berger FG, Murphy EA. Linking tumor associated macrophages, inflammation, and intestinal tumorigenesis: Role of MCP-1. Am J Physiol Gastrointest Liver Physiol. 2012 Sep 27; doi: 10.1152/ajpgi.00252.2012. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock V. Fatigue management: evidence and guidelines for practice. Cancer. 2001;92:1699–1707. doi: 10.1002/1097-0142(20010915)92:6+<1699::aid-cncr1500>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Andrews P, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10 (5):389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- Ottenweller JE, Natelson BH, Gause WC, Carroll KK, Beldowicz D, Zhou XD, LaManca JJ. Mouse running activity is lowered by Brucella abortus treatment: a potential model to studychronic fatigue. Physiol Behav. 1998;63 (5):795–801. doi: 10.1016/s0031-9384(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25 (3):94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comp Med. 2011;61 (2):119–130. [PMC free article] [PubMed] [Google Scholar]
- Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12 (Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- Soares PM, Lima-Junior R, Mota JM, Justino PF, Brito GA, Ribeiro RA, Cunha FQ, Souza MH. Role of platelet-activating factor in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Cancer Chemother Pharmacol. 2010:14. doi: 10.1007/s00280-010-1540-5. [DOI] [PubMed] [Google Scholar]
- Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich- Rivkin T, Weitzenfeld P, Meshel T, Shabtai E, Gutman M, Ben-Baruch A. Inflammatory mediators in breast cancer: coordinated expression of TNFα& IL-1b with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer. 2011;12 (11):130. doi: 10.1186/1471-2407-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Murphy EA. Importance of chemokine (CC-motif) ligand 2 in breast cancer. Int J Biol Markers. 2012;31(0) doi: 10.5301/JBM.2012.9345. http://dx.doi.org/10.5301/JBM.2012.9345 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Thompson WL, Karpus WJ, Van Eldik LJ. MCP-1-deficient mice show reduced neuroinflammatory responses and increased peripheral inflammatory responses to peripheral endotoxin insult. J Neuroinflammation. 2008;15 (5):35. doi: 10.1186/1742-2094-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Mobley GM, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24(6):968–974. doi: 10.1016/j.bbi.2010.03.009. (Epub 2010 Mar 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LJ, Nail L, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol Res Nurs. 2006;8 (2):157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]