Abstract
IL-17A is produced from Th17 cells, and is involved in many autoimmune and inflammatory diseases. The IL-13 receptor (IL-13R) has not previously been reported to be functionally expressed on T cells; however, we found that purified BALB/c CD4+ cells polarized to Th17 with TGF-β, IL-6, and IL-23 have increased mRNA and protein expression of IL-13Rα1 and mRNA expression of IL-4Rα compared to Th0, Th1, or Th2 polarized cells. The addition of IL-13 at Th17 polarization negatively regulated IL-17A and IL-21 expression, and reduced the number of CD4+ T cells producing IL-17A. Further, adding IL-13 at the time of Th17 cell restimulation attenuated IL-17A expression. CD4+ Th17 polarized cells from IL-4 KO mice also had IL-13-induced inhibition of IL-17A production, but this was not observed in IL-4R KO and STAT6 KO mice. Addition of IL-13 at polarization increased IL-13R expression in WT Th17 cells. Further, IL-13 administration during Th17 polarization downregulated ROR-γT, the transcription required for Th17 development; increased STAT6 phosphorylation, and upregulated GATA3, the transcription factor activated during the development of Th2 cells. This IL-13-mediated effect was specific to Th17 cells as IL-13 neither decreased IFN-γ expression by Th1 cells nor affected Th2 cell production of IL-4. Collectively, we have shown that Th17 cells express a functional IL-13R and that IL-13 negatively regulates IL-17A and IL-21 production by decreasing ROR-γT expression and while increasing phosphorylation of STAT6 and GATA3 expression. Therefore, therapeutic intervention inhibiting IL-13 production could have adverse consequences by upregulating Th17 inflammation in certain disease states.
Keywords: T cells, cytokines, cytokine receptors
Introduction
Th17 cells are a recently described class of CD4+ T cells that have a distinct lineage from Th1 and Th2 cells (1,2). These cells are known to be involved in autoimmune and inflammatory disorders, such as the experimental allergic encephalitis (EAE) model of multiple sclerosis and rheumatoid arthritis (reviewed in (3)). Th17 cells are also critical for the clearance of extracellular pathogens, such as the Gram-negative bacteria Klebsiella pneumoniae (4) or Mycoplasma pulmonis (5). Differentiation of Th17 cells requires the cytokines TGF-β and IL-6 or IL-21 in the mouse, (6,7) and IL-6 and IL-1β in the humans (8). Until recently, it was also believed that IL-23, a member of the IL-12 cytokine family, was also required for Th17 differentiation. However, studies utilizing IL-23 KO mice have shown CD4+ cells are capable of differentiating into Th17 cells in the absence of IL-23, but that IL-23 is required for Th17 proliferation and sustainability (8, 9). Th17 cell differentiation and proliferation is negatively regulated by the Th2 cytokine IL-4 and the Th1 cytokine IFN-γ (1). Polarizing Th17 cells from CD4+ splenocytes cultured in the presence of anti-IFN-γ and anti-IL-4 increases IL-17A production from Th17 cells (1).
Recently, in an in vivo model of EAE, Kleinscheck and colleagues reported that exogenous IL-25 administration induced the production of the Th2 cytokine IL-13, which then negatively regulated Th17 responses (9). IL-13 effects intracellular signaling via the IL-13R which is composed of the heterodimer IL-13Rα1 and IL-4Rα. While T cells have been shown to express IL-4Rα, IL-13Rα1 has never been described to be present on murine CD4+ T lymphocytes (9,10). Therefore, Kleinscheck and colleagues speculated that in the EAE model IL-13 might act by inhibiting dendritic cell function, and this was supported by their observation that IL-13 blocked dendritic cell production of the Th17-promoting factors IL-1β, IL-6, and IL-23 (9). However, an alternative hypothesis is that CD4+ Th17 cells, in contrast to Th1 and Th2 cells, express IL-13R and that IL-13 can negatively regulate CD4+ Th17 cytokine expression directly through this receptor. We tested this hypothesis by polarizing purified naïve CD4+ cells in the presence of cytokines that promote differentiation to Th17 cells, and show for the first time that Th17 cells express a functional IL-13R, and IL-13 inhibits the total number of CD4+ Th17 cells producing IL-17A in WT BALB/c mice. Further, we show that IL-13 decreases polarized Th17 cell production of the Th17 cytokine IL-21 and a transcription factor that is critical for Th17 development, ROR-γT. IL-13 also increases STAT6 phosphorylation and GATA3 expression in Th17 polarized T cells. Collectively, these data demonstrate that IL-13Rα1 is expressed on Th17 cells and that the IL-13R is functional. These results suggest that inhibiting IL-13 expression or function may upregulate Th17 inflammation in certain disease states.
Materials and Methods
Mice
Pathogen-free 8 to 10-week old female BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). IL-4 KO, IL-4Rα KO, and STAT6 KO BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, ME), and breeding colonies were established. In caring for the animals the investigators adhered to the revised 1996 Guide for the Care and Use of Laboratory Animals prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council.
T cell isolation and Th17 cell polarization, activation, and restimulation
CD4+ T cells were purified from the spleens of mice as previously described (11). T cells were activated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) (BD Biosciences, San Diego, CA) in 96-well plates for 4 days. T cells were differentiated into Th17 cells by adding rmIL-23 (10 ng/ml), rhTGF-β (5 ng/ml), rmIL-6 (20 ng/ml), anti-IFN-γ (10 μg/ml) and anti-IL-4 (10 μg/ml). Th1 and Th2 cells were differentiated as previously described (1). In select cultures, rmIL-4 (1–10ng/ml) or rmIL-13 (1–10 ng/ml) was also added. In other experiments involving Th17 cells, anti-IL-13 (0 – 10 μg/ml) or goat IgG isotype control was used instead of anti-IL-4. To stimulate effector cytokine production, the cells were restimulated with anti-CD3 for 24h in the presence of rmIL-4 or rmIL-13 (0 ng/ml to 10 ng/ml). All antibodies and rmIL-23, rmIL-4, rmIL-13, and rmIL-12 were purchased from R & D systems (Minneapolis, MN). rmIL-6 and rmTGF- β were purchased from PeproTech, Inc. (Rocky Hill, NJ).
Quantitative PCR
A 2-step real-time PCR assay using SYBR green mix (BioRad, Hercules, CA) was used to detect IL-13Rα1, IL-4Rα, common γ chain, RORγT, and GATA3 as previously described (11). Primer sequences were as follows: IL-13Rα1: F 5′TTCCAGTCTTTGTCGCAGTG, R 5′ CAGGATCAGGAATTGGAGGA 3′; IL-4Rα: F 5′ GGTGGAGTCCAGTCCACACT 3′; R 5′ CTGTGATCAAGCATCGCTGT 3′; common γ chain: F 5′ TGCCTAGTGTGGATGAGCTG 3′; R 5′ CAGGCTGGCTCCATTTACTC 3′; RORγT: F 5′ GCG GCT TTC AGG CTT CAT GGA G 3′; R 5′R GGG CGC TGA GGA AGT GGG AAA A 3′; GATA3: F 5′ GGCGAGATGGTACCGGGCACTA 3′; R 5′ CCCCATTAGCGTTCCTCCTCCAGA 3′.
ELISAs
Protein levels of cytokines from cell culture supernatant were measured by commercially available Duoset or Quantikine ELISA kits (R & D Systems) following the manufacturer’s instructions.
Flow cytometry and intracellular staining
Four days after polarization, Th0 and Th17 CD4+ T cells were harvested and restimulated with PMA and ionomycin for 5 hours in the presence of Golgi-stop (BD Biosciences) at 37°C, 5% CO2. Cells were blocked with anti-FcR Ab 2.4G2 (BD Biosciences) and surface stained with PE-Cy7 conjugated anti-CD4 (BD Biosciences). For intracellular staining, cells were permeabolized with Cytofix/cytoperm (BD Biosciences), washed thoroughly, and stained with PE-conjugated anti-IL-17A (BD Biosciences). Cells were analyzed using a LSR II flow cytometer (BD Biosciences), and data were analyzed using Flow Jo 7.2 software.
Immunoblotting
Cells were lysed using RIPA buffer and total protein was extracted and resolved by 4–20% SDS-PAGE gel, transferred to a nitrocellulose membrane (BioRad, Hercules, CA), and probed with phospho-STAT6 (Cell Signaling, Beverly, MA), total STAT6 (Cell Signaling), IL-13Rα1 (Santa Cruz Biotechnology, Santa Cruz, CA), normal goat IgG isotype control (Santa Cruz Biotechnology) or actin (Santa Cruz Biotechnology). Signals were amplified and visualized with horse radish peroxidase-conjugated secondary antibody (BioRad) and chemiluminescence solution (Pierce, Rockford, IL).
Statistical analyses
Data is presented as mean ± SEM. Data was analyzed with ANOVA followed by the Tukey posthoc test using GraphPad Prism 4 with values being considered significant when p < 0.05.
Results
IL-13R expression is increased on Th17 polarized cells, but not Th1 or Th2 cells
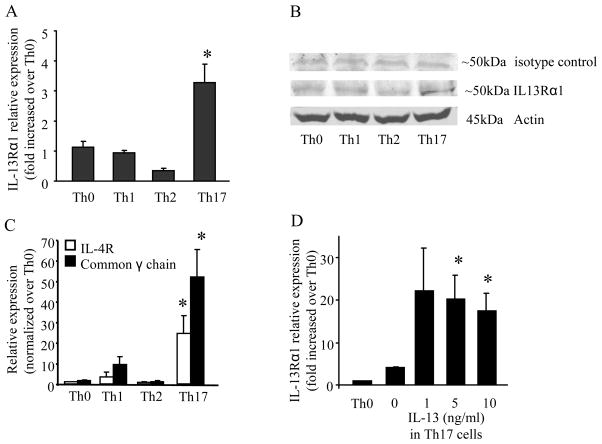
IL-13Rα1 has been reported to not be expressed on the surface of T cells (9,10). As IL-13 negatively regulated Th17 responses in the EAE model (9), we hypothesized that Th17 cells expressed IL-13Rα1. Cells were polarized to Th1, Th2, or Th17 cells and total RNA was collected 4 days after polarization and examined for IL-13Rα1 expression by real-time PCR. Th17 cells had a significant increase in IL-13Rα1 relative expression when compared to naïve T cells (Th0), Th1, and Th2 cells (Figure 1A). Th17 cells also showed an increase in IL-13Rα1 protein expression (Figure 1B) that was not seen in Th0, Th1 or Th2 cells. Th17 cells also had a significant increase in the mRNA expression of IL-4Rα and the common-γ chain, the heterodimeric components of the IL-4 receptor (Figure 1C). The full-length decoy soluble receptor, IL13Rα2 was undetectable in Th17 cells by real-time PCR (data not shown). Finally, increasing concentrations of IL-13 in Th17 polarized cells caused a significant increase in IL-13Rα1 mRNA expression (Figure 1D).
Figure 1.
IL-13Rα1 expression is increased in Th17 polarized T cells. Total RNA or protein was isolated from naïve (Th0), Th1, Th2, or Th17 cells 4 days after polarization. (A) Real-time PCR was performed for IL-13Rα1. (B) Western blot for IL-13Rα1 (blot is representative of 3 individual blots). (C) Real-time PCR was performed for IL-4Rα and the common γ chain mRNA expression. (D) IL-13Rα1 mRNA expression in Th17 cells polarized in the presence of IL-13. For all real-time PCR experiments, data is normalized to GAPDH and relative expression is compared to Th0 cells. Data is compiled from 3 experiments for panels A, C, and D; n=5, * p < 0.05 compared to Th0 cells in (A) and (C) and compared to Th17 cells (no IL-13) in (D), ANOVA.
IL-13 attenuates IL-17A production from Th17 differentiated cells
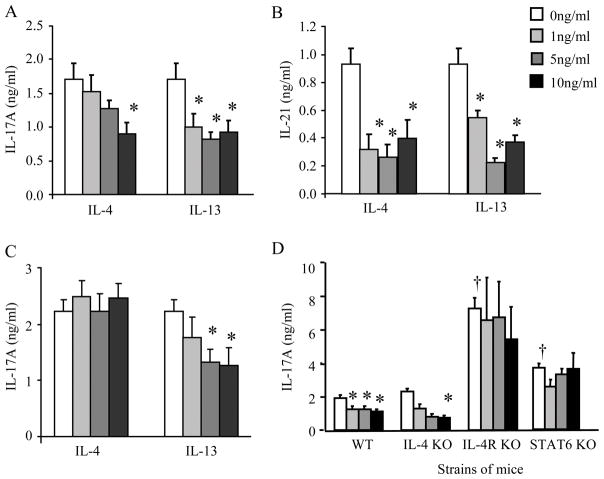
Based on the increased relative expression of IL-13Rα1 on CD4+ purified Th17 polarized cells, we hypothesized that IL-13 would directly decrease IL-17A production by these cells. T cells were polarized to Th17 cells in the presence of IL-13 or IL-4 and IL-17A protein production was measured in cultured supernatants 4 days after polarization. As previously reported, IL-4 (10 ng/ml) attenuated IL-17A protein production (1); however, IL-13 also significantly attenuated IL-17A protein production (Figure 2A). Similar to IL-17A, protein expression of IL-21, an autocrine cytokine produced by Th17 cells, was also attenuated in the presence of IL-4 or IL-13 (Figure 2B).
Figure 2.
IL-13 attenuates IL-17A production from Th17 differentiated T cells in WT and IL-4 KO mice but not IL-4R KO and STAT6 KO mice. IL-4 or IL-13 (0 – 10 ng/ml) was added at the time of Th17 polarization and cultured supernatants were collected 4 days after polarization, and examined for IL-17A (panel A) and IL-21 (panel B) protein production. (C) T cells were polarized to Th17 cells and restimulated with plate-bound anti-CD3 in the presence of IL-4 or IL-13 (0 – 10 ng/ml) and IL-17A protein production was measured 24 h after restimulation. (D) WT, IL-4 KO, IL-4R KO, and STAT6 KO CD4+ T cells were polarized to Th17 cells in the presence of IL-13 and IL-17A protein production was measured 4 days after polarization. Data is compiled from 3 separate experiments, n = 6–14, * p < 0.05 compared to Th17 cells (no IL-13) from respective strain of mice; † p< 0.05 compared to Th17 cells (no IL-13) from WT mice, ANOVA.
CD4+ cells were also polarized to Th17 cells (in the absence of IL-4 or IL-13) for 4 days and then restimulated with anti-CD3 in the presence of IL-4 or IL-13. Restimulation of Th17 in the presence of IL-4 has been previously shown to have no effect on IL-17A protein production (1), and we found the same result (Figure 2C). However, the higher concentrations of IL-13 (5 – 10 ng/ml) were able to significantly inhibit IL-17A protein production (Figure 2C). Taken together these data suggest that IL-13, as well as the previously reported IL-4, negatively regulated IL-17A protein production from Th17 cells at the time of polarization. In contrast to IL-4, IL-13 attenuated IL-17A protein production at restimulation.
Th17 polarized cells were also cultured from IL-4 KO, IL-4R KO, and STAT6 KO mice. IL-4 KO mice showed an IL-13-dependent attenuation of IL-17A protein production (Figure 2D). IL-4R KO and STAT6 KO mice had a significant increase in IL-17A protein production compared to WT mice in Th17 polarized cells without IL-13. However, IL-4R KO and STAT6 KO mice did not have an IL-13-mediated decrease in IL-17A protein production (Figure 2D). These data demonstrate that IL-13 attenuates IL-17A protein expression from Th17 cells in an IL-4R-signaling specific manner.
IL-13 decreases the percentage of CD4+ polarized Th17 cells producing IL-17A
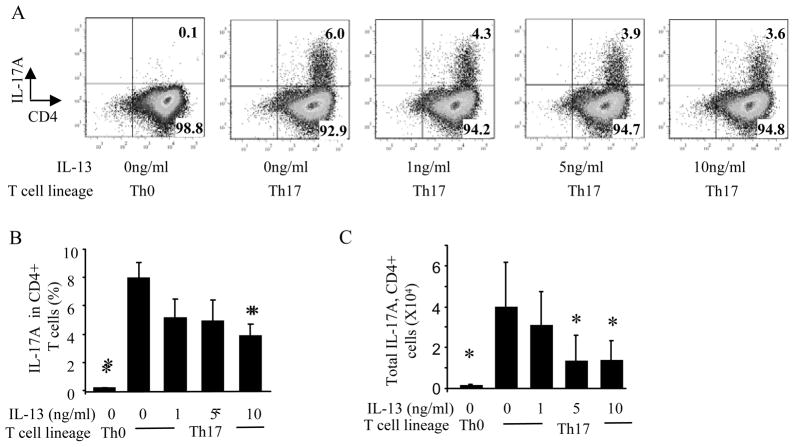
Th17 cells were restimulated with PMA and ionomycin in the presence of Golgi-stop and IL-17A intracellular staining was analyzed on CD4+ cells within the lymphocyte gate. In the presence of IL-13, there was a decrease in the percentage of Th17 polarized CD4+ T cells producing IL-17A (Figure 3A and B), and also a decrease in the total number of CD4+ T cells producing IL-17A (Figure 3C). However, neither the mean fluorescent intensity of IL-17A nor the total number of CD4+ T cells were altered with the addition of IL-13 (data not shown), suggesting that IL-13 decreases the number of Th17 cells present, but not the amount of IL-17A produced from each Th17 cell.
Figure 3.
IL-13 decreases the number of IL-17A producing CD4+ Th17 polarized T cells. T cells were restimulated with PMA and ionomycin in the presence of Golgi-stop and stained with PE-Cy7 conjugated anti-CD4 and PE conjugated anti-IL-17A antibodies. (A) Dots plots of IL-17A producing T cells in naïve and Th17 polarized T cells in the presence of IL-13 (0–10ng/ml). (B) Quantification of the percentage of IL-17A producing CD4 T cells. (C) Total number of IL-17A producing CD4+ cells. Dot plots are representative of 3 independent experiments, n=4–6,* p<0.05 compared to Th17 cells (no IL-13), ANOVA.
Anti-IL-13 augments IL-17A production from Th17 differentiated T cells
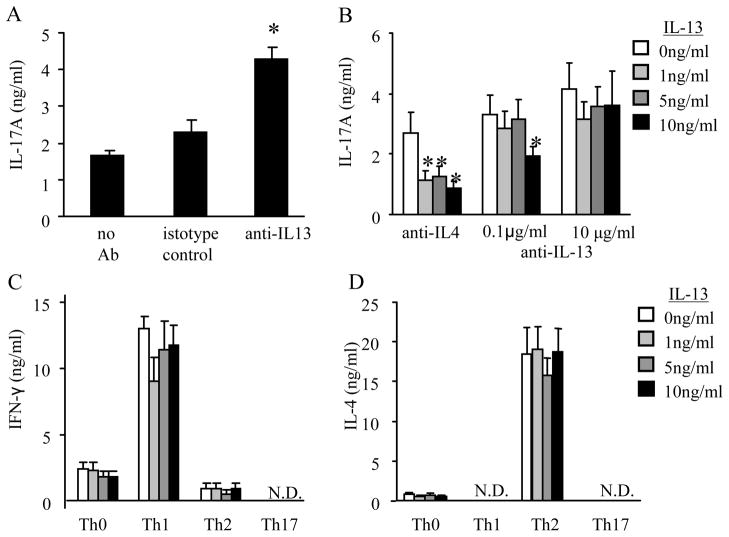
As previously reported, anti-IL-4 (10 μg/ml) increases Th17 differentiation (1). Since IL-13 added at the time of Th17 polarization inhibited IL-17A protein production, we hypothesized that the administration of an anti-IL-13 antibody at the time of Th17 polarization augments IL-17A protein secretion. Compared to isotype control antibody, anti-IL-13 (10 μg/ml) added during Th17 polarization, in conjunction with the Th17 polarizing cytokines listed above and anti-IFN-γ, increased IL-17A protein production (Figure 4A).
Figure 4.
IL-13 specifically inhibits IL-17A production from Th17 polarized cells and does not regulate cytokine production in Th1 or Th2 polarized cells. (A–B) Th17 cells were polarized cells using anti-IL-13 (0–10μg/ml), anti-IL-4 (10μg/ml), isotype control, or no antibody (no anti-IL-13 or isotype control) and examined for IL-17A protein production. (C and D) Th1 or Th2 polarized T cells were measured for IFN-γ and IL-4 protein production, respectively. N.D. cytokine values were below the limit of detection for the ELISA. Data is compiled from 3 independent experiments. (A) n=6–10, * p < 0.05 compared to Th17 cells without anti-IL-13 (no antibody) (B–D) n=6, * p < 0.05 compared to T cells without IL-13 from respective group.
To determine if the attenuation of IL-17A was specific to the recombinant IL-13 protein, CD4+ T cells were polarized to Th17 cells in the presence of IL-13 and either anti-IL-4 or increasing concentrations of anti-IL-13 (0.1 – 10 μg/ml). Using anti-IL-13 in combination with IL-13 decreased IL-17A protein production at the lower antibody concentration but not the higher antibody concentration (Figure 4B). These data suggest that IL-13 specifically attenuates cytokines from Th17 polarized cells.
IL-13 regulates cytokine production by Th17 cells, but not Th1 or Th2 cells
To determine if IL-13 regulated cytokine production from Th1 and Th2 cells, T cells were polarized to Th1, Th2, or Th17 cells in the presence of IL-13. Four days after these polarizations, cultured supernatants were collected and examined for IFN-γ, IL-4, and IL-17A protein. As previously noted in Figure 2, IL-13 added at the time of polarization attenuated IL-17A protein production in the Th17 cells, and IL-17A was undetectable in Th1 or Th2 polarized cells (data not shown). IFN-γ protein production was only observed in Th1 polarized cells and was not affected by the presence of IL-13 (Figure 4C). IL-4 protein production was only detected in Th2 cells and IL-13 had no effect on IL-4 protein expression (Figure 4D). Th17 polarized cells did not have detectable protein levels of IFN-γ and IL-4. These data show that IL-13 inhibits IL-17A protein production but has no effect on the production of IFN-γ in Th1 cells or IL-4 secretion in Th2 cells. This data correlates with IL-13Rα1 expression occurring on Th17 cells (Figure 1A).
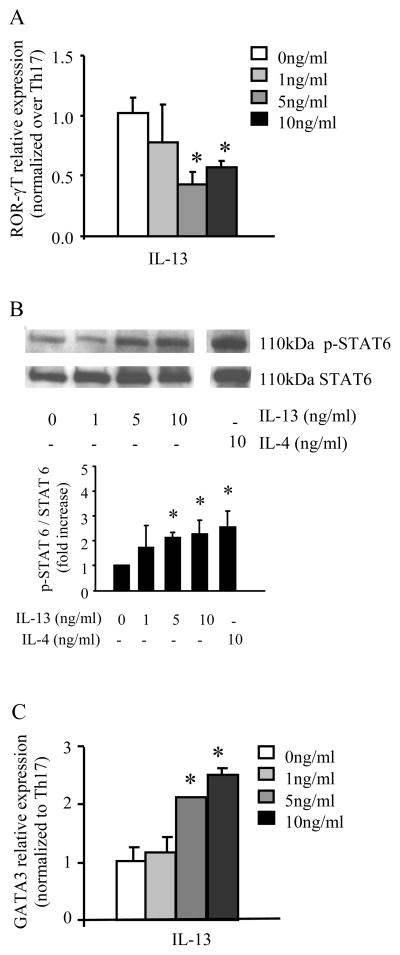
IL-13 decreases ROR-γT expression while increasing STAT6 phosphorylation and GATA3 expression on Th17 polarized T cells
IL-13 reduced Th17 cytokine production and since ROR-γT is an essential transcription factor for the development of the Th17 lineage, we hypothesized that IL-13 administered during Th17 polarizing conditions decreases ROR-γT expression. We found that polarized Th17 CD4+ cells exposed to IL-13 have decreased ROR-γT mRNA expression (Figure 5A), and IL-13-mediated attenuation of Th17 cell cytokine production paralleled this decrease in ROR-γT mRNA expression.
Figure 5.
IL-13 signals through IL-13R decreases in ROR-γT relative expression and increases in STAT6 phosphorylation and GATA3 relative expression. (A) ROR-γT mRNA expression with each sample normalized to GAPDH and relative expression compared to Th17 cells polarized without IL-13 present. (B) Total protein was harvested 3 days after polarization and phospho-STAT6 and total STAT6 levels were determined. Blot is representative of 3 separate experiments with densitometry is shown graphically below blot. (A) GATA3 mRNA expression with each sample normalized to GAPDH and relative expression compared to Th17 cells polarized without IL-13 present. Data is compiled from three different experiments, n=3–5, * p<0.05 compared to Th17 cells (no IL-13), ANOVA.
We further hypothesized that since IL-13Rα1 signaling is mediated through STAT6, IL-13 administration during Th17 polarizing conditions upregulates phosphorylated STAT6 in this setting. In human carcinoma cell lines, IL-13 binds to the IL-13Rα1 portion of the IL-13 heterodimer receptor with intermediate affinity (10) and causes activation of Tyk2, JAK1 and JAK3 leading to the phosphorylation of the transcription factor STAT6 at the Tyr-641 residue (12–14). Polarized T cells were collected 3 days after polarization in the presence of IL-13 and total protein was assayed for STAT6 Tyr-641 phosphorylation and total STAT6 by immunoblotting in the setting of increasing concentrations of IL-13. STAT6 phosphorylation was increased in a concentration dependent manner in IL-13-treated Th17 cells (Figure 5B), but STAT6 phosphorylation was not altered in similarly-treated Th1 or Th2 cells (data not shown). These data support previous reports by Andrews and colleagues that STAT6 activation and translocation is maintained 72 hours after stimulation (15). We further hypothesized that since IL-13 is able to successfully signal through STAT6 in CD4+ Th17 cells that express the functional IL-13R, there would be an increase in the expression of the Th2 transcription factor GATA3. Indeed, as shown in Figure 5C, GATA3 expression was increased in Th17 polarized T cells exposed to IL-13.
Discussion
Our data demonstrate that IL-13R is functionally expressed by Th17 polarized CD4+ T cells. Th17 cells had a three-fold increase in IL-13Rα1 mRNA expression as quantified by real time PCR compared and IL-13Rα1 expression was seen in Th17 cells and not in naïve (Th0), Th1, or Th2 cells. There was also a greater than 5-fold increase in the mRNA expression of the other component of the IL-13R, IL-4Rα, by real time PCR in Th17 cells compared to Th0, Th1, and Th2 cells. We clearly show that the IL-13R is functional in our system as IL-13 negatively regulates Th17 cell production of IL-17A at the polarization and restimulation stages, and IL-21 production at the Th17 polarization stage. This is in contrast to IL-4 which only downregulated Th17 cytokine production at the polarization phase and not at restimulation, as has also been shown by Harrington and colleagues (1). We further show that IL-13 decreases the number of IL-17A producing CD4+ cells, but not the amount of IL-17A produced by each cell. IL-13Rα1 expression was only increased on Th17 polarized cells, and not Th1 or Th2 polarized cells. This provides an explanation as to why IL-13 had no effect on cytokine production in Th1 and Th2 polarized T cells, but attenuated Th17 cytokine secretion in Th17 cells. In addition, the presence of IL-13 during Th17 polarization increased the phosphorylation of STAT6 and the mRNA expression of the Th2 transcription factor GATA3 in these cells, which has not been reported in either Th1 or Th2 CD4+ cells.
The IL-13-mediated attenuation of IL-17A production also paralleled a decrease in IL-21 production and ROR-γT relative mRNA expression. ROR-γT is the transcription factor responsible for Th17 proliferation and sustainability (16) and expression of ROR-γT is increased in the presence of IL-21 (7). IL-21 and ROR-γT cause an increase in IL-23R expression on the T cells (7,17,18), and IL-23 has shown to be essential in sustaining Th17 cells (7). Therefore, the observed decreases in IL-17A production could potentially be caused by decreased IL-21 production and ROR-γT expression leading to decreased IL-23R expression on Th17 cells. However, more studies will need to be conducted to confirm this hypothesis.
Although it remains to be formally demonstrated in this article, it seems likely that human Th17 cells will behave similar to mouse Th17 cells. Therefore, since IL-13 inhibits IL-17A production, therapeutic interventions that block IL-13 activity might have unanticipated effects in disease states that are driven by Th17-mediated inflammation. IL-13 has been identified as a potential therapeutic target in allergic diseases, such as asthma, as this cytokine is recognized as a central mediator of mucus production, airway responsiveness, and lymphocyte infiltration in animal models (12,19). Molecules that block IL-13 activity, either through antibody neutralization or by soluble receptor, result in reduced airway responsiveness, mucus hyperplasia, inflammation, and chemokine production, such as CCL11, CCL5 and KC, in mice or monkeys (19–22). Therefore, based on the results of our study, the use of IL-13 inhibitors in these disease states may have the unintended consequence of upregulating Th17 cytokine production. Increased production of IL-17A could potentially cause induction or exacerbation of autoimmune diseases, such as the EAE model or Crohn’s disease, as mouse models of these diseases are critically dependent on IL-17A (reviewed in (3)). On the other hand, diseases that cause increases in IL-13 levels might lead to the attenuation of Th17 cytokine production. In this setting, IL-13 mediated downregulation of IL-17A could increase disease severity from extracellular pathogens, such as Klebsiella pneumoniae (4) or Mycoplasma pulmonis (5), that require IL-17 to resolve the infection.
In summary, we show that Th17 polarized T cells express a functional IL-13R and provide a mechanism by which IL-13, an abundantly produced Th2 cytokine, negatively regulates IL-17A production.
Abbreviations
- N.D
not detectable
- ROR
retinoic acid-related
Footnotes
This work was supported by the NIH: F32HL091653, R01 HL090664, R01AI070672, R01AI054660, R01HL069449, R01AI059108, and GM 015431.
References
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, I, Ivanov I, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 8.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 9.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, Blumenschein WM, McClanahan T, Brombacher F, Hurst SD, Kastelein RA, Cua DJ. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, Paul WE. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W, Blackwell TS, Goleniewska K, O’Neal JF, FitzGerald GA, Lucitt M, Breyer RM, Peebles RS., Jr Prostaglandin I2 analogs inhibit Th1 and Th2 effector cytokine production by CD4 T cells. J Leukoc Biol. 2007;81:809–817. doi: 10.1189/jlb.0606375. [DOI] [PubMed] [Google Scholar]
- 12.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Murata T, Noguchi PD, Puri RK. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–2978. [PubMed] [Google Scholar]
- 15.Andrews RP, Ericksen MB, Cunningham CM, Daines MO, Hershey GK. Analysis of the life cycle of stat6. Continuous cycling of STAT6 is required for IL-4 signaling. J Biol Chem. 2002;277:36563–36569. doi: 10.1074/jbc.M200986200. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 18.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 19.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 20.Bree A, Schlerman FJ, Wadanoli M, Tchistiakova L, Marquette K, Tan XY, Jacobson BA, Widom A, Cook TA, Wood N, Vunnum S, Krykbaev R, Xu X, Donaldson DD, Goldman SJ, Sypek J, Kasaian MT. IL-13 blockade reduces lung inflammation after Ascaris suum challenge in cynomolgus monkeys. J Allergy Clin Immunol. 2007;119:1251–1257. doi: 10.1016/j.jaci.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Kumar RK, Herbert C, Webb DC, Li L, Foster PS. Effects of anticytokine therapy in a mouse model of chronic asthma. Am J Respir Crit Care Med. 2004;170:1043–1048. doi: 10.1164/rccm.200405-681OC. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Volk A, Petley T, Emmell E, Giles-Komar J, Shang X, Li J, Das AM, Shealy D, Griswold DE, Li L. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–232. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]