Abstract
The tumor microenvironment has an important role in cancer progression. Here we show that miR-148a is downregulated in 15 out of 16 samples (94%) of cancer-associated fibroblasts (CAFs) compared with matched normal tissue fibroblasts (NFs) established from patients with endometrial cancer. Laser-capture microdissection of stromal cells from normal tissue and endometrial cancer confirmed this observation. Treatment of cells with 5-aza-deoxycytidine stimulated the expression of miR-148a in the majority of CAFs implicating DNA methylation in the regulation of miR-148a expression. Investigation of miR-148a function in fibroblasts demonstrated that conditioned media (CM) from CAFs overexpressing miR-148a significantly impaired the migration of five endometrial cancer cell lines without affecting their growth rates in co-culture experiments. Among predicted miR-148a target genes are two WNT family members, WNT1 and WNT10B. Activation of the WNT/β-catenin pathway in CAFs was confirmed by microarray analysis of gene expression and increased activity of the SuperTOPFIash luciferase reporter. We found elevated levels of WNT10B protein in CAFs and its level decreased when miR-148a was re-introduced by lentiviral infection. The 3′-UTR of WNT10B, cloned downstream of luciferase cDNA, suppressed luciferase activity when co-expressed with miR-148a indicating that WNT10B is a direct target of miR-148a. In contrast to the effect of miR-148a, WNT10B stimulated migration of endometrial cancer cell lines. Our findings have defined a molecular mechanism in the tumor microenvironment that is a novel target for cancer therapy.
Keywords: cancer-associated fibroblasts, endometrial cancer, microRNA, miR-148a, WNT10B
INTRODUCTION
The tumor microenvironment is now recognized to have an important role in cancer progression. Advanced stages of neoplasia require the co-evolution and continuous cross-talk between cancer and stromal cells.1–3 During tumorigenesis, malignant cells accumulate a large number of chromosomal aberrations and epigenetic changes. However, their interactions with surrounding cells of the microenvironment provide additional critical factors that determine whether they remain dormant or develop into invasive and metastatic cancer cells.4,5
The tumor microenvironment consists of various nontransformed cells including fibroblasts, myofibroblasts, leukocytes, endothelial cells and bone marrow-derived progenitor cells. Cancer-associated fibroblasts (CAFs) are among the most abundant cells in the stroma of solid tumors and are characterized by diverse phenotypes. They support tumor progression through the secretion of growth factors, suppression of host immune responses and deposition of specialized extracellular matrix. Extensive infiltration of carcinomas with activated myofibroblasts, a type of CAF, is correlated with higher grades of malignancy and a poor prognosis.6,7 Importantly, CAFs preserve their tumor-promoting properties when cultured in vitro in the absence of direct contact with cancer cells.8–10 This property makes them a valuable tool to study the effects of the microenvironment on tumorigenesis.
Despite the extensive research demonstrating the role of the microenvironment in tumor progression, our understanding of the genes and pathways responsible for the formation of tumor-promoting stroma is still limited. Some genetic alterations have been reported in microdissected stroma from paraffin-fixed tissues, notably the loss of p53.11–13 However, it is not clear that the loss of p53 expression can induce differentiation of progenitor cells or resident fibroblasts into CAFs. Moreover, comprehensive analyses of isolated stromal cells from fresh-frozen tissues, including comparative genomic hybridization and single nucleotide polymorphism, failed to identify substantial genetic alterations in CAFs.14–16 This raises the possibility that changes in gene expression in CAFs may result from epigenetic modifications. Indeed, altered methylation patterns have been observed not only in tumor cells but also in stromal fibroblasts.17–19
Another mechanism that may contribute to the activation of fibroblasts and progenitor cells is post-transcriptional gene regulation by microRNAs. Because individual microRNAs regulate hundreds of genes, they could account for the dramatic changes in gene expression seen in CAFs. MicroRNAs are 19–25 nucleotide RNAs that control diverse biological processes.20,21 Usually, they bind to a complementary site in the 3′-UTR of their target transcripts, resulting in translational inhibition or messenger RNA degradation. Recent data clearly indicate that microRNAs function as oncogenes or tumor suppressors in different tumor types.22
We previously identified a microRNA signature in CAFs freshly isolated from human endometrial cancer, including several microRNAs that had been implicated in various stages of tumorigenesis.23 For example, the upregulated miR-146 and miR-424 have been associated with an angiogenic switch in a mouse RIP-tag model of pancreatic cancer. Downregulation of miR-31 in CAFs and the corresponding increased expression of homeobox gene SATB2 contributed to the migration and invasiveness of endometrial tumor cells.23 The downregulation of miR-148a, which we also detected in CAFs,23 has been recognized as a metastatic marker for a number of other tumors.24,25 A connection between inhibition of miR-148a expression and tumor metastasis was reinforced by a study that documented the silencing of this microRNA by gene methylation in several metastatic tumors (n = 207) and showed a strong correlation of silencing with lymph node-positive disease.26 Although several target genes of miR-148 have been identified, how its suppression promotes cancer metastasis remains a subject of investigation.27 In the present study, we further documented the downregulation of miR-148a in endometrial cancer CAFs, showed that methylation contributed to its suppression and established that WNT10B is a direct target of its activity. A decrease in miR-148a expression in CAFs resulted in an upregulation of secreted WNT10B protein, which stimulated the motility of endometrial cancer cells.
RESULTS
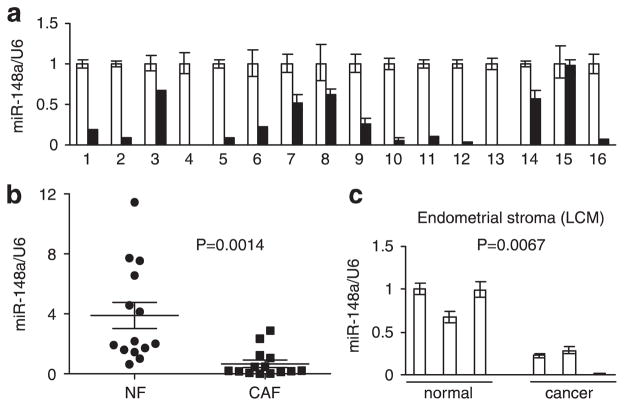
miR-148a expression is suppressed in cancer-associated fibroblasts The isolation and characterization of fibroblasts from endometrial cancer and matched normal endometrial tissue was described previously.23 Initially, microarray analysis identified 11 microRNAs that were differentially expressed in CAFs.23 Here, we focused on miR-148a because its downregulation has been associated with metastasis in a variety of settings.24–26 The contrast in miR-148a expression was further validated in 16 pairs of normal and cancer fibroblasts by quantitative real-time PCR (Figure 1a). The miR-148a expression level was downregulated in CAFs relative to normal tissue fibroblasts (NFs) in 15 out of 16 cases (94%). However, we noticed a large range of variability in miR-148a expression among NFs from individual patients. To see if expression of miR-148a in NFs and CAFs can be discriminated at the global level, we normalized the quantity of miR-148a transcript in all NFs and CAFs to the NF1 sample (patient 1) and plotted the two groups separately (Figure 1b). This analysis revealed a statistically significant downregulation of miR-148a in CAFs with P = 0.0014. To minimize the impact of cell propagation on plastic, we performed our experiments with early passages of cells, typically passage 3 to 6. However, there is a possibility that the expression of microRNA changes as a result of adaptation to cell culture conditions. Therefore, to confirm the lower expression of miR-148a in stromal cells of endometrial tumors, we obtained stromal cells from three normal endometrial tissues and three endometrial cancers using laser-capture microdissection. Expression of miR-148a was significantly higher in the stroma dissected from normal tissue than stroma from endometrial cancer (Figure 1c). We also microdissected cells from normal endometrial glands, endometrial stroma and myometrium, and found no significant differences in miR-148a expression among these compartments (Supplementary Figures S1a and S1 b). This reinforced the view that downregulation of miR-148a in cancer-associated stroma was specifically characteristic of malignant transformation.
Figure 1.
Expression of miR-148a in endometrial cells, (a) qRT-PCR of miR-148a expression in CAFs relative to matched NFs from 16 patients. White bars: NFs; black bars: CAFs. Results are presented as mean ± s.e.m. of triplicate measurements, (b) Distribution of miR-148a expression values in NF and CAFs. Same as in (a), but all samples normalized to NF1. Analysis shows mean values with 95% confidence levels, (c) Expression of miR-148a in laser capture microdissected endometrial stromal cells from normal and cancer patients. Results are means ± s.e.m. of triplicate measurements.
The miR-148a gene is located in a CpG-rich region of the genome, consistent with its methylation-dependent silencing in pancreatic cancer28 and in lymph node metastases of colon cancer, head and neck cancer and melanoma.26 Based on these observations, we tested the hypothesis that silencing of miR-148a in CAFs is mediated by promoter methylation. We analyzed miR-148a expression in 15 pairs of fibroblast cell lines after treatment with the DNA demethylating agent, 5-aza-2′-deoxycytidine (5azaC) and the histone deacetylase inhibitor, trichostatin A (TSA). The majority of CAFs (11 out of 15) showed increased expression of miR-148a after treatment with 5azaC + TSA relative to untreated cells (Supplementary Figure S2). However, bisulfite sequencing analysis indicated that CpG methylation of the miR-148a gene was not increased in CAFs (Supplementary Figure S3), implying that the effect of 5azaC + TSA was indirect. Moreover, methylation was not the only mechanism of miR-148 silencing because three CAF lines did not re-express miR-148a after treatment even though their miR-148a transcript level was low compared with the matched normal counterparts.
Restoration of miR-148a expression in CAFs reduced their chemoattractant properties
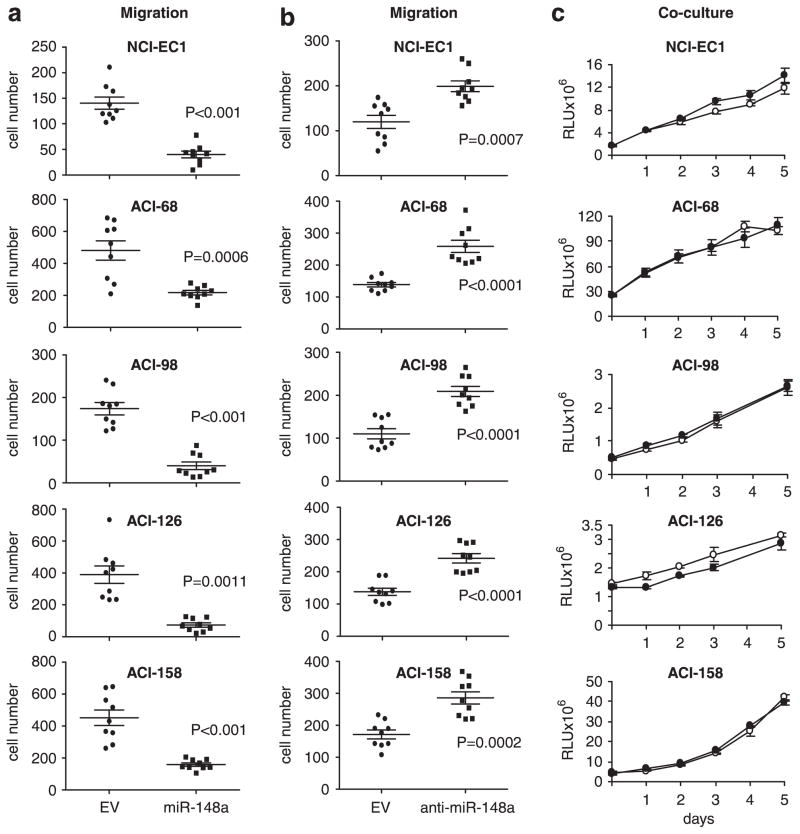
To study the function of miR-148a in fibroblasts, we used a lentiviral vector to re-introduce it into CAF cell lines having very low endogenous levels. The vector also encoded green fluorescent protein (GFP) and the Zeocin-resistance gene. As a control, CAF cells were infected in parallel with empty vector. After selection with antibiotic more than 95% of cells were GFP positive as measured by flow cytometry and fluorescent microscopy. The expression of miR-148a did not affect fibroblast morphology or growth (Supplementary Figures S4a and S4b). CM from these fibroblasts had a diminished chemoattractant effect on five different endometrial cancer cell lines compared with CM from the same fibroblast line expressing the empty vector (Figure 2a). We also used a reverse approach to inhibit miR-148a activity in normal fibroblasts with a lentiviral anti-miR-148a construct. Migration of the same endometrial tumor cells was increased when they were incubated with CM from fibroblasts in which miR-148a activity was disrupted (Figure 2b). In contrast, we did not observe any difference in growth of tumor cells when the five cancer cell lines were co-cultured with CAFs infected with the lentivirus encoding miR-148a vs. empty vector (Figure 2c). Moreover, there was no difference in the growth of the cancer cells when they were treated for 2 weeks with CM from CAFs expressing miR-148a vs empty vector (data not shown).
Figure 2.
Effect of miR-148a overexpression in fibroblasts on endometrial tumor cell motility and growth, (a) Transwell migration of five endometrial cancer cell lines towards CAFs expressing empty lentiviral vector (circles) or miR-148a (squares). Results are the means with 95% confidence interval of migrated cells/field. P-value was calculated using unpaired two-tailed t-test. All experiments were performed in triplicate, with three fields analyzed/replicate, and repeated at least two times, (b) Transwell migration of endometrial cancer cell lines towards NFs expressing empty lentiviral vector (circles) or anti-miR-148a (squares). The data are analyzed as in (a), (c) CAFs expressing empty lentiviral vector or miR-148a were co-cultured with luciferase-expressing endometrial cancer cell lines during 5-day time course. The luciferase activity was measured every day in three replicate experiments. White circles: CAF-EV; black circles: CAF-miR-148a. Results are means ± s.e.m.
miR-148a expression in CAFs decreased cancer cell invasion in matrigel culture
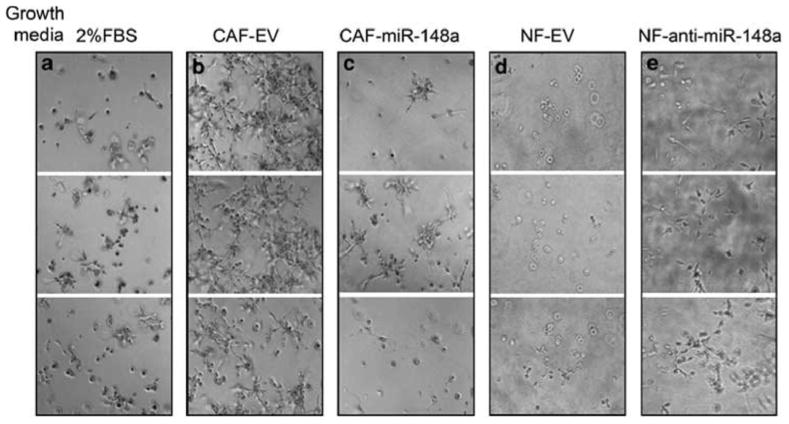
In vivo, cells are surrounded by extracellular matrix, which affects the cellular phenotype and homeostasis. Because such regulation may be lost when cells are cultured on plastic, we tested the ability of endometrial cancer cell lines to grow in a three-dimensional (3D) matrigel culture. The ACI-158 cell line showed robust growth in matrigel when supported with media containing 10% FBS (data not shown). However, only isolated colonies were detected after 5 days in culture when the serum concentration was reduced to 2% (Figure 3a). Cells incubated with CM from CAFs expressing empty vector (also containing 2% FBS) showed a distinctly invasive phenotype with long projections that created a branched morphology in matrigel (Figure 3b). The invasive phenotype was nearly eliminated when cells were incubated with CM from CAFs expressing miR-148a (Figure 3c). Furthermore, inhibition of miR-148a activity in NFs by anti-miR-148a induced tumor cell growth in matrigel (Figure 3e) compared with control NFs expressing the empty vector (Figure 3d). These results suggested that miR-148a suppressed the expression of one or more factors that promoted the invasive phenotype of ACI-158 cancer cells in matrigel culture.
Figure 3.
ACI-158 endometrial cancer cells in 3D matrigel culture. Cells were grown in growth factor-reduced matrigel and DMEM containing 2% FBS (a), CM from CAFs expressing empty vector (b) or miR-148a (c), or NFs expressing vector control (d) or anti-miR-148a (e) cultured in DMEM/2% FBS. Media were replaced every 2 days and images were taken from three different fields at day 5. Experiments were repeated four times and a representative experiment is shown.
WNT10B is a direct target of miR-148a in CAFs
Predicted targets for miR-148 include several growth factors that might regulate tumor progression, such as TGFα, M-CSF, KITL (stem cell factor) and two members of the WNT family, WNT1 and WNT10B. ELISA analysis revealed no differences in the levels of TGFα, M-CSF or KITL in the CM of CAFs overexpressing miR-148a vs. empty vector or in CM from NFs vs CAFs. We then screened previously obtained CAF and NF mRNA microarray data23 for evidence of WNT pathway activation using human fibroblast-specific WNT target genes identified by Klapholz-Brown et al.29 Among the genes differentially expressed by CAFs, we observed a significant enrichment in WNT-responsive genes (Figure 4a). The differential level of WNT target gene expression, as determined by SLEPR analysis of pathway enrichment,30 is illustrated by the heat map of individual fibroblasts cell lines used in microarray experiments (Figure 4b). The heat map shows that expression of these genes in CAF samples varies significantly relative to levels observed in NF samples. To confirm these results, we also used the significance analysis of microarrays method, which showed that almost all WNT-responsive genes (96%) were upregulated in CAFs (Supplementary Figure S5).
Figure 4.
WNT pathway activation in CAFs. (a) Top ranking pathways enriched in CAFs versus NFs using SLEPR method. The analysis was run with KEGG pathway annotation with inclusion of fibroblast-specific WNT-responsive genes obtained from Klapholz-Brown et al.29 103 permutations were performed. Permutated P-values: P-value of terms derived from permutated data; FDR-false discovery rates. FDR q values: FDR of terms derived from permutated data, (b) Heat map of WNT-responsive genes expressed in samples of CAFs and NFs. The rows of the heat map represent the WNT-responsive genes and the columns correspond to individual cell lines. The red color indicates enriched expression. (c) SuperTOPFIash reporter assay of CM from NFs vs CAFs. Paired samples from two patients were analyzed in luciferase reporter assay normalized either to Renilla-TK or SuperFopFlash (reporter with mutated TCF binding sites). Results are mean ± s.d. of triplicate measurements, (d) Western blot analysis of WNT10B in NFs and CAFs. Numbers below blots indicate WNT10B level in CAF normalized to β-actin and expressed relative to NF from the same patient, and miR-148a expression levels in CAFs relative to NF from Figure 1a.
To verify activation of WNT signaling in CAFs, we transfected different NFs and matching CAFs with a SuperTOPFIash reporter vector which measures activity in the canonical WNT/(β-catenin signaling pathway. SuperTOPFIash luciferase activity, normalized to either renilla-TK or the negative control SuperFOPFIash vector, was much more robust in CAFs than in NFs (Figure 4c). Western blot analysis of WNT10B and WNT1 expression in a panel of endometrial NFs and CAFs revealed greater amounts of WNT10B protein in the majority of CAFs than in the corresponding NFs (Figure 4d). Overall, a negative correlation was observed between WNT10B protein level and miR-148a expression. WNT1 expression was not detected in NFs or CAFs by quantitative RT–PCR or western blot analysis. Therefore, we focused our study on WNT10B.
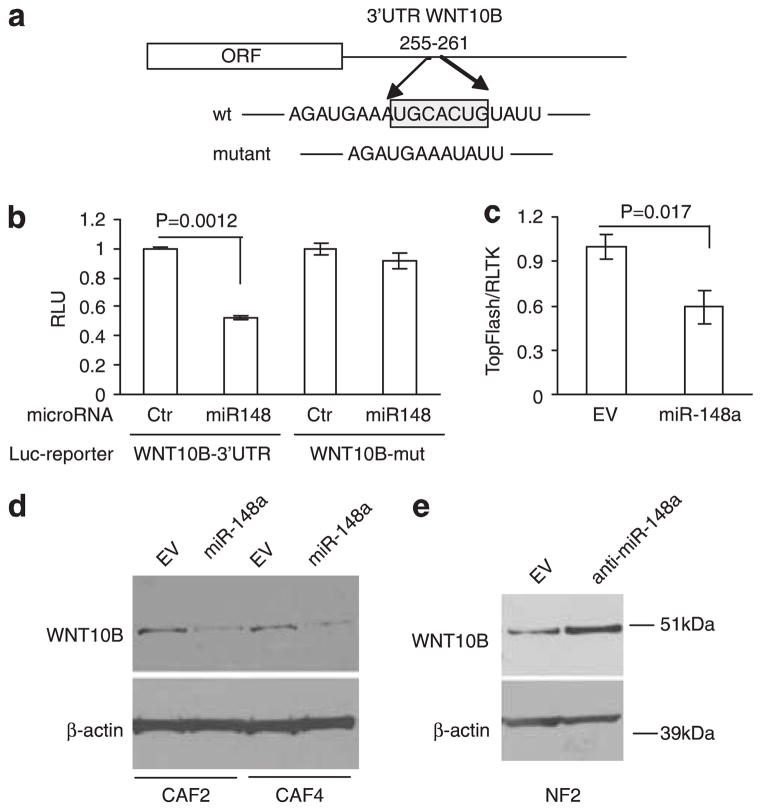
To prove that WNT10B is a direct target of miR-148a, we used a 3′-UTR sequence of WNT10B cloned into a reporter vector downstream of the luciferase complementary DNA (cDNA) (Figure 5a). Co-transfection of this construct with miR-148a or control microRNA into HeLa cells showed that miR-148a suppressed expression of the WNT10B reporter (Figure 5b). Mutation of the miR-148a binding site abolished the inhibitory effect of miR-148a on the reporter activity (Figure 5b). Consistent with these results, miR-148a decreased SuperTOPFIash reporter activity in CAFs (Figure 5c). Furthermore, miR-148a decreased the expression of WNT10B protein in CAFs (Figure 5d), and anti-miR-148a increased WNT10B protein in NF (Figure 5e), reinforcing the idea that WNT10B is a direct target of miR-148a in fibroblasts.
Figure 5.
WNT10B is a direct target of miR-148a in endometrial fibroblasts, (a) Schematic representation of WNT10B 3′UTR with putative miR-148a-binding site. The seed region is boxed and mutated reporter construct has seed region deleted, (b) Luciferase activity of HeLa cells co-transfected with reporter vector containing either wild-type or mutant WNT10B 3′-UTR and miR-148a or non-targeting control. Results are mean ± s.d. of triplicate measurements. (c) miR-148a suppresses SuperTopFlash reporter activity. CAFs stably expressing empty vector (EV) or lentiviral miR-148a construct were electroporated with the SuperTOPFIash and Renilla-TK. Luciferase activity was measured after 24 h. Results are mean ± s.d. of triplicate measurements, (d) Western blot analysis of WNT10B in CAF cell lysates after stable transfection with miR-148a construct or empty vector. Transfection and immunoblotting were performed with CAFs from two patients (CAF2 and CAF4). β-actin blot served as loading control, (e) Western blot analysis of WNT10B in NF cell lysates after stable transfection with anti-miR-148a construct or empty vector.
WNT10B stimulates migration of endometrial cancer cells
Because miR-148a expression in CAFs inhibited the chemoattractant activity of their CM (Figure 2a), we hypothesized that WNT10B might stimulate tumor cell motility. To address this idea, we overexpressed WNT10B or an empty plasmid in 293T cells and tested the CM from these cells in a transwell migration assay. The three endometrial cancer cell lines used in this assay showed a significantly stronger response towards WNT10B than control CM (Figure 6a). As the ACI-158 line showed the strongest response to WNT10B, we used it in the subsequent experiments. To confirm that endometrial cancer cell migration was due to WNT10B, we treated the CM from CAFs with anti-WNT10B antibody or control IgG to remove WNT10B from the medium. The ability of the antibody to immunoprecipitate WNT10B was verified by western blotting (Supplementary Figure S6). WNT10B immunodepletion diminished the activity of CM in a β-catenin stabilization assay (Figure 6b). Importantly, the WNT10B-depleted CAF CM also had markedly reduced activity in the ACI-158 endometrial cancer cell migration assay (Figure 6c).
Figure 6.
WNT10B stimulates migration of endometrial cancer cells. (a) Transwell migration assay of three endometrial cancer cell lines in response to CM from 293 T cells transfected with human WNT10B construct or empty vector control. Results are the means with 95% confidence interval of migrated cells/field. P-value was calculated using unpaired two-tailed t-test. (b) β-catenin stabilization assay following immunodepletion of WNT10B. ACI-158 cells were incubated for 3 h with untreated CAF CM (−), or CM treated with control IgG or anti-WNT10B. Wnt3a CM was used a positive control. Free (soluble) β-catenin obtained with a GST-Ecadherin pull-down protocol and β-catenin in whole cell lysates (total β-catenin) were detected by immunoblotting. (c) Transwell migration assay of ACI-158 cells towards CAF CM pre-treated with control IgG or anti-WNT10B. Assay was performed as described in (a), (d) Transwell migration assay of ACI-158 cells towards CM from CAF with stable expression of miR-148a and transient expression of WNT10B or empty vector, (e) Transwell migration assay of ACI-158 cells towards CM from NF with stable expression of anti-miR-148a immunodepleted with anti-WNT10B antibody or control IgG.
To strengthen the functional link between miR-148a and WNT10B, we tested the effect of ectopic miR-148a-resistant, WNT10B expression on chemoattractant activity of CM from CAFs transduced with miR-148a construct. The results presented in Figure 6d demonstrate that WNT10B stimulated endometrial cancer cell migration. Moreover, immunodepletion of WNT10B from the CM of NFs transduced with anti-miR-148a plasmid inhibited cell migration, indicating that WNT10B was a functionally important target of miR-148a (Figure 6e).
WNT10B has been reported to stimulate both β-catenin-dependent and -independent signaling pathways.31 To further dissect the mechanism of WNT10B-mediated cell migration, we pre-treated cells with the specific WNT/β-catenin pathway antagonist Dickkopf-1 (DKK1) or added the general WNT antagonist, secreted frizzled-related protein 1 (sFRP1) to the CM. As expected, both inhibited CAF CM activity in the β-catenin stabilization assay (Figure 7a). However, only sFRP1 decreased the motility of ACI-158 cells in response to CAF CM (Figure 7b), implying that non-canonical WNT signaling was responsible for tumor cell migration.
Figure 7.
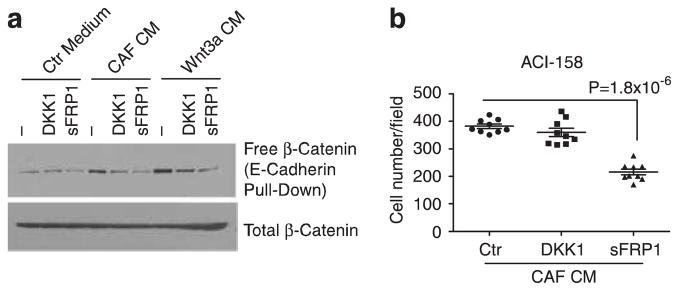
WNT10B stimulates tumor cell migration via non-canonical signaling, (a) β-catenin stabilization assay in ACI-158 cells treated with control or CAF CM or Wnt3a CM. Assay as described in (Figure 6b), with cells either pre-incubated with DKK1 (1 μg/ml) or sFRP1 (10 μg/ml) added to the CM. (b) Transwell migration assay of ACI-158 in the presence of DKK1 or sFRP1. Assay was performed as described in (Figure 6a) without addition of recombinant protein (Ctr) or with DKK1 (1 μg/ml) or sFRPI (10 μg/ml).
DISCUSSION
The present study was undertaken to investigate the significance of miR-148a silencing in endometrial CAFs. We demonstrated that CAF CM promoted the migration and invasive properties of endometrial cancer cells in a miR-148a-dependent manner. Previous work had indicated that miR-148a expression was decreased in various metastatic cancers.25,26,32 However, this is the first report to demonstrate that miR-148a silencing in CAFs contributed to tumor cell motility. We established that WNT10B is a direct target of miR-148a, and immunodepletion experiments revealed that WNT10B was responsible for much of the tumor cell chemoattractant activity in CAF CM. The association of cell motility with non-canonical Wnt signaling was consistent with several other studies concerning cell migration and metastasis.33–35 Our results suggest that miR-148a silencing and WNT10B derepression in CAFs may contribute to the metastasis of other tumors besides endometrial cancers. More generally, they emphasize a role for microRNA in regulating the characteristics of cells in the tumor microenvironment that influence cancer cell behavior.
There is precedent for a connection between WNT activity and endometrial cancer. Previously, we observed that the expression of sFRP1 and sFRP4 was suppressed in a subset of endometrial carcinomas.36 Subsequently, sFRP4 was identified as an endometrial stromal marker that inhibited cancer cell growth and was downregulated in CAFs and endometrial cancers.37 The current findings indicate that in addition to the reduced expression of these WNT antagonists, endometrial cancers are characterized by the upregulation of WNT10B in the tumor microenvironment. Moreover, oncogenic β-catenin mutations were reported in 15–40% of endometrial cancer patients,38–40 and 31 to 85% of well-differentiated endometrioid carcinomas contained nuclear β-catenin.41,42 Endometrial tumors also were associated with APC promoter hypermethylation and truncation mutations.40 These observations imply that Wnt/β-catenin signaling contributes to endometrial malignancy, although our data suggest that this pathway was not involved in paracrine-mediated tumor cell motility.
WNT10B has varying effects on cancer in other organs. Transgenic mice overexpressing WNT10B develop adenocarcinomas in the breast, and cell lines established from these tumors displayed anchorage-independent growth.43 However, WNT10B overexpression suppressed the growth of hepatocellular carcinoma cells In vitro and in vivo, although it promoted tumor growth and metastasis when co-expressed with FGF-2.31 WNT10B reportedly elicited a stronger chemotactic response from metastatic osteosarcoma cells than non-metastatic cells.44
The relevance of WNT regulation by miR-148a might extend beyond cancer and WNT10B. Inhibition of WNT10B expression is a key factor in the differentiation of pre-adipocytes into adipocytes.45 Recently, Qin et al.46 reported that miR-148a was among a set of miRs that were upregulated during adipogenesis. We speculate that this increase in miR-148a contributes to the silencing of WNT10B required for adipogenesis. As noted above, WNT1 is a potential target of miR-148a, but this was not germane to the present study because WNT1 was not expressed by endometrial CAFs. We verified that its expression is decreased by miR-148a in HeLa cells (unpublished observations, OA), indicating that WNT1 is a bona fide target of miR-148a activity. However, others have detected little or no regulation of WNT1 or WNT10B expression by miR148a or miR148b in gastric cancer cells,27,47 suggesting that inhibition is dependent on cellular context.
Besides its effect on adjacent cancer cells, the upregulation of WNT10B in CAFs might have a direct impact on the ‘activated’ fibroblast phenotype. WNT10B overexpression was documented in skin biopsy specimens from patients with systemic sclerosis and from mice with bleomycin-induced fibrosis.48 Transgenic mice expressing WNT10B were characterized by dermal fibrosis with fibroblast activation, myofibroblast accumulation and increased collagen deposition.48 These data suggest that WNT10B stimulates the differentiation of mesenchymal progenitor cells into activated fibroblasts and myofibroblasts, and maintains these populations during tumorigenesis.
In summary, we identified WNT10B as a direct target of miR-148a in CAFs from endometrial cancers and demonstrated that its upregulation in these cells increases tumor cell motility. Together with other factors regulated by miR-148a, this might be a general mechanism of metastasis in tumors with reduced miR-148a expression.
MATERIALS AND METHODS
Cell lines
All fibroblast cells were derived from patients undergoing operations for endometrial cancer and characterized as previously described.23 The primary fibroblast lines were tested for mycoplasma, the presence of fibroblast-specific markers (vimentin, FSP1 and FAP1), and were selected to have more than 90% cytokeratin-negative cells. The protocols for human tissues that were followed to obtain the fibroblast lines had been approved by the Institutional Review Boards of the University of Virginia and National Cancer Institute. Endometrial cancer cell lines were established by JIR and described in Nagendra et al.49 Tumor cell lines were tested for mycoplasma contamination and used after at least 30 passages. All cells were cultured in DMEM (high glucose) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and penicillin/streptomycin (Invitrogen) under standard culture conditions.
Plasmids
The lentiviral expression vector for miR-148a (pMIF-cGFP-Zeo-miR148a) and the control vector (pMIF-cGFP-Zeo), as well as the anti-miR-148a construct and its control (pGreenPuro Scramble Hairpin Control) were purchased from System Biosciences (SBI, Mountain View, CA, USA). A plasmid with the WNT10B 3′-UTR cloned into a reporter vector (pLightSwithch_3′UTR-WNT10B) and the control empty vector (pLightS-witch_3′UTR) were purchased from Switch Gear Genomics Inc. (Menlo Park, CA, USA). MiR-148a-binding sites in the reporter vector were mutated using QuickChange Site-Directed Mutagenesis Kit (Agilent Technologies Inc., Santa Clara, CA, USA), according to the manufacturer’s instructions. All constructs were verified by sequence analysis.
Real-time PCR
Total RNA was isolated from cells by TRIzol extraction (Invitrogen). TaqMan miRNA assays were used to quantify the level of mature miR-148a (probe 4373130, Applied Biosystems, Carlsbad, CA, USA). Briefly, 50 ng of total RNA were reverse-transcribed in 15 μl reaction according to manufacturer’s instructions. 1.33 μl of the resultant reaction was PCR-amplified in a total volume of 20 μl for 40 cycles using Applied Biosystems 7500 instrument. Small nuclear RNA RNU6B was used as a standard for normalization. All reactions were performed in triplicate.
Quantitative RT-PCR analysis of methylation-dependent miR-148a derepression was performed after fibroblasts were treated with 3 μM 5-aza-2′-deoxycytidine (5-azaC) (Sigma, St Louis, MO, USA) for 6 days with addition of fresh 5-azaC every other day. The last treatment was accompanied by addition of 150nM TSA (Sigma) and cells were collected 24 h later for microRNA analysis.
Transwell migration assay
5 × 104 fibroblasts expressing miR-148a or empty vector were seeded into 24-well plates in DMEM containing 2% FBS. In 2 days, 1–3 × 104 endometrial cancer cells in serum-free media were placed in Boyden chambers (8 μm, BD Biosciences, Bedford, MA, USA) and allowed to migrate towards the fibroblast-CM for 24 h. Cells were fixed and stained with Diff-Quick Stain Set (Dade Behring Inc., Deerfield, IL, USA). The cells that migrated through the pores of the membrane were photographed and quantified using Image J software. All experiments were done in triplicate and three images were processed per each membrane.
The experiments with ACI-158 cells described in Figures 6b–e and Figure 7b were performed with serum-free CM from CAFs or NFs collected after 48 h. Where indicated, CAF CM or Wnt3a CM (collected from L929 cells overexpressing recombinant Wnt3a, ATCC) was pre-incubated with 10 μg/ml of purified recombinant sFRP1 protein50 at 4°C for 30 min and used as chemoattractant for ACI-158 cell migration. To inhibit the canonical WNT/β-catenin pathway by DKK1, ACI-158 cells were trypsinized, washed, counted and incubated with 1 μg/ml of DKK1 (R&D Systems, Minneapolis, MN, USA) in serum-free medium for 30 min at room temperature. Then 5 × 104 cells were placed in Boyden chambers for migration towards CAF CM or Wnt3a CM.
Co-culture of endometrial cancer cells with fibroblasts
A total of 3 × 104 fibroblasts and 1 × 104 endometrial cancer cells stably transfected with a luciferase cDNA were seeded in triplicate in DMEM supplemented with 5% FBS into 12-well plates. The tumor cell growth was monitored by measuring luciferase activity using a Luciferase Assay System (Promega Corporation, Madison, Wl, USA). For the long-term co-culture experiment, only endometrial cancer cell lines were seeded on the plates and allowed to grow in the CM collected from fibroblasts expressing miR-148a or empty vector control. The CM was replaced twice a week for two weeks.
3D culture of endometrial cancer cells
Growth factor-reduced matrigel (BD Biosciences) was thawed on ice, and 50 μl were used to coat wells in pre-chilled 96-well plates. The matrigel was allowed to solidify at 37 °C for 30 min. Cells were trypsinized, counted and resuspended in DMEM-supplemented with 2 FBS and 4% matrigel. A total of 2 × 103 cells were plated on top of the matrigel and allowed to attach overnight at 37 °C. Next morning, the medium was replaced with 80 μl of DMEM/2% FBS, CM from CAFs that expressed the empty vector control or miR-148a and had been cultured for 2 days in DMEM/2% FBS, or with CM from NFs that expressed empty vector or anti-miR-148a and had been similarly cultured. Media were replaced every other day and cells were imaged on day 5–6.
Microarray pathway enrichment analysis
All mRNA chips were normalized using GCRMA in Partek Genomic Suite (www.partek.com). The data after normalization were subjected to pathway analysis using a Sample-Level Enrichment-Based Pathway Ranking (SLEPR) method.30
All the described procedures for the SLEPR method are part of the WPS program and can be downloaded from WPS website (http://www.abcc.ncifcrf.gov/wps/wps_index.php). The inclusion of sample-level differentiated genes was performed using the two-sided MADe method. Computation of the enrichment scores for each sample-level differentiated gene using a Fisher’s exact test was performed based on a 2 × 2 contingency table, and all results were ranked based on the P-value. To determine the statistical significance of the ranking for the gene, a permutation P-value was calculated from 103 permutations. The false discovery rates (FDR) q values were also computed from permutated data.
WNT10B immunodepletion and western blotting
10 μg of anti-WNT10B antibody (H-70, Santa Cruz Biotechnology Biotechnology, Santa Cruz, CA, USA) or control rabbit IgG (sc-2027, Santa Cruz) were added to 1.5 ml serum-free CM and rotated overnight at 4°C. The immune complexes were collected with 40 μl of A/G beads (Santa Cruz Biotechnology). After centrifugation, another 40 μl aliquot of A/G beads was incubated with the supernatant for 1 h, followed by centrifugation. The resultant supernatant was tested in the transwell migration assay.
Western blotting was carried out as described.23 The membrane was probed with anti-WNT10B antibody (Santa Cruz Biotechnology Inc.) or anti-β-actin antibody (Sigma).
WNT10B 3′-UTR luciferase reporter assay
105 HeLa cells were first transfected with miR-148 mimic (Thermo Scientific, Lafayette, CO, USA) or non-silencing control using HiPerfect Reagent (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. Final concentration of microRNA was 50 nM. Next day, cells were transfected with 0.2 μg of luciferase reporter vector and 0.1 μg of pCMV-lacZ using Effectene Transfection Reagent (Qiagen). After 24 h, cells were lysed in Passive Lysis Buffer and 20 μl were used to measure luciferase activity with Luciferase Assay System (Promega Corporation) and 50 μl were used to measure β-galactosidase activity with the β-Gal Assay Kit (Invitrogen). The assay was performed in triplicate and repeated three times.
SuperTOPFIash reporter assay
A total of 105 fibroblasts were seeded in 12-well plates and, next day, transfected with 0.2 μg of pSuperTOPFIash or pSuperFOPFIash and 0.05 μg RL-TK, using Effectene reagent (Qiagen), according to the manufacturer’s instructions. Dual luciferase Assay (Promega Corporation) was performed after 48 h. Alternatively, 105 CAFs with stable lentiviral expression of miR-148a or vector control were electroporated with 0.3 μg of pSuperTOPFIash and 0.1 μg of pRL-TK using Neon Transfection system (Invitrogen). Briefly, cells were trypsinized, washed, resuspended in R-buffer, mixed with DNA and electroporated in 10 μl tip with 2 pulses at 1400v and pulse width 20 ms. All transfections were performed in triplicate.
β-Catenin stabilization assay
A total of 0.5 × 106 ACI-158 cells per well were seeded into 6-well plates. Next day, the medium was replaced with serum-free medium to reduce the background levels of endogenous β-catenin. After 20–24 h, cells were pre-incubated with 1 μg/ml DKK1 (R&D Systems) for 30 min at 37 °C and then treated for 3 h with equal volume of control medium vs. CAF CM or Wnt3a CM. For sFRP1 treatment, CAF CM or Wnt3a CM was pre-incubated with 10 μg/ml of purified recombinant sFRP1 protein,50 at 4°C, for 30 min, and then added to serum-starved ACI-158 cells for 3h. β-catenin was precipitated with GST-E cadherin and detected as previously described.33
Statistical analysis
Statistical analysis for transwell migration experiments was performed with GraphPad Prism5 software that showed mean values with 95% confidence interval. The P-values were calculated using Student’s two-tailed t-test. The TaqMan data were obtained using ΔΔCt method and presented as mean fold change ± s.e.m.
Supplementary Material
Acknowledgments
We thank Julie Oliver, Tracy Litzi, and Shelley Hoover for help with laser capture microdissection, Dr Xin Wei Wang (NCI) for the WNT10B expression vector and Dr Randall Moon (University of Washington) for the SuperTOPFIash and SuperFOPFIash constructs. This work was supported by the Intramural Research Program of National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.natu.re.com/onc)
References
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their micro-environment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 4.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardone A, Tolino A, Zarcone R, Borruto Caracciolo G, Tartaglia E. Prognostic value of desmoplastic reaction and lymphocytic infiltration in the management of breast cancer. Panminerva Med. 1997;39:174–177. [PubMed] [Google Scholar]
- 7.Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci USA. 2006;103:14842–14847. doi: 10.1073/pnas.0606857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Kiaris H, Chatzistamou I, Trimis G, Frangou-Plemmenou M, Pafiti-Kondi A, Kalofoutis A. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65:1627–1630. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 13.Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 14.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet. 2008;40:650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter K, Omura N, Hong SM, Griffith M, Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7:882–888. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiegl H, Millinger S, Goebel G, Muller-Holzner E, Marth C, Laird PW, et al. Breast cancer DNA methylation profiles in cancer cells and tumor stroma: association with HER-2/neu status in primary breast cancer. Cancer Res. 2006;66:29–33. doi: 10.1158/0008-5472.CAN-05-2508. [DOI] [PubMed] [Google Scholar]
- 18.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Gonda TA, Gamble MV, Salas M, Seshan V, Tu S, et al. Global hypo-methylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Di Leva G, Croce CM. Roles of small RNAs in tumor formation. Trends Mol Med. 2010;16:257–267. doi: 10.1016/j.molmed.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aprelikova O, Yu X, Palla J, Wei BR, John S, Yi M, et al. The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle. 2010;9:4387–4398. doi: 10.4161/cc.9.21.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson P, Lu J, Zhang H, Shai A, Chun MG, Wang Y, et al. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009;23:2152–2165. doi: 10.1101/gad.1820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 26.Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-Cespedes M, Blanco D, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, et al. MicroRNA-148a Suppresses Tumor Cell Invasion and Metastasis by Downregulating ROCK1 in Gastric Cancer. Clin Cancer Res. 2011;12:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 28.Hanoun N, Delpu Y, Suriawinata AA, Bournet B, Bureau C, Selves J, et al. The silencing of microRNA 148a production by DNA hypermethylation is an early event in pancreatic carcinogenesis. Clin Chem. 2010;56:1107–1118. doi: 10.1373/clinchem.2010.144709. [DOI] [PubMed] [Google Scholar]
- 29.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2:e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi M, Stephens RMSLEPR. a sample-level enrichment-based pathway ranking method – seeking biological themes through pathway-level consistency. PLoS One. 2008;3:e3288. doi: 10.1371/journal.pone.0003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshikawa H, Matsubara K, Zhou X, Okamura S, Kubo T, Murase Y, et al. WNT10B functional dualism: beta-catenin/Tcf-dependent growth promotion or independent suppression with deregulated expression in cancer. Mol Biol Cell. 2007;18:4292–4303. doi: 10.1091/mbc.E06-10-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tchernitsa O, Kasajima A, Schafer R, Kuban RJ, Ungethum U, Gyorffy B, et al. Systematic evaluation of the miRNA-ome and its downstream effects on mRNA expression identifies gastric cancer progression. J Pathol. 2010;222:310–319. doi: 10.1002/path.2759. [DOI] [PubMed] [Google Scholar]
- 33.Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, et al. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005;280:777–786. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi A, Yamamoto H. Tumor formation due to abnormalities in the beta-catenin-independent pathway of Wnt signaling. Cancer Sci. 2008;99:202–208. doi: 10.1111/j.1349-7006.2007.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risinger JI, Maxwell GL, Chandramouli GV, Aprelikova O, Litzi T, Umar A, et al. Gene expression profiling of microsatellite unstable and microsatellite stable endometrial cancers indicates distinct pathways of aberrant signaling. Cancer Res. 2005;65:5031–5037. doi: 10.1158/0008-5472.CAN-04-0850. [DOI] [PubMed] [Google Scholar]
- 37.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 38.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 39.Konopka B, Janiec-Jankowska A, Czapczak D, Paszko Z, Bidzinski M, Olszewski W, et al. Molecular genetic defects in endometrial carcinomas: microsatellite instability, PTEN and beta-catenin (CTNNB1) genes mutations. J Cancer Res Clin Oncol. 2007;133:361–371. doi: 10.1007/s00432-006-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreno-Bueno G, Hardisson D, Sanchez C, Sarrio D, Cassia R, Garcia-Rostan G, et al. Abnormalities of the APC/beta-catenin pathway in endometrial cancer. Oncogene. 2002;21:7981–7990. doi: 10.1038/sj.onc.1205924. [DOI] [PubMed] [Google Scholar]
- 41.Saegusa M, Okayasu I. Frequent nuclear beta-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol. 2001;194:59–67. doi: 10.1002/path.856. [DOI] [PubMed] [Google Scholar]
- 42.Scholten AN, Creutzberg CL, van den Broek U, Noordijk EM, Smit VT. Nuclear beta-catenin is a molecular feature of type I endometrial carcinoma. J Pathol. 2003;201:460–465. doi: 10.1002/path.1402. [DOI] [PubMed] [Google Scholar]
- 43.Lane TF, Leder P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene. 1997;15:2133–2144. doi: 10.1038/sj.onc.1201593. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Fallen S, Abaan HO, Hayran M, Gonzalez C, Wodajo F, et al. Wnt10b induces chemotaxis of osteosarcoma and correlates with reduced survival. Pediatr Blood Cancer. 2008;51:349–355. doi: 10.1002/pbc.21595. [DOI] [PubMed] [Google Scholar]
- 45.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 46.Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, et al. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics. 2010;11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song YX, Yue ZY, Wang ZN, Xu YY, Luo Y, Xu HM, et al. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol Cancer. 2011;10:1. doi: 10.1186/1476-4598-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: A novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagendra DC, Burke J, 3rd, Maxwell GL, Risinger JI. PPP2R1A mutations are common in the serous type of endometrial cancer. Mol Carcinog. doi: 10.1002/mc.20850. e-pub ahead of priant 31 August 2011. [DOI] [PubMed] [Google Scholar]
- 50.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, et al. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.