Abstract
Purpose
To evaluate macular thickness profiles using spectral-domain optical coherence tomography (SDOCT) and image segmentation in patients with chronic exposure to hydroxychloroquine.
Methods
This study included 8 patients with chronic exposure to hydroxychloroquine (Group 1) and 8 controls (Group 2). Group 1 patients had no clinically-evident retinal toxicity. All subjects underwent SDOCT imaging of the macula. An image segmentation technique was used to measure thickness of 6 retinal layers at 200 µm intervals. A mixed-effects model was used for multivariate analysis.
Results
By measuring total retinal thickness either at the central macular (2800 µm in diameter), the perifoveal region 1200-µm-width ring surrounding the central macula), or the overall macular area (5200 µm in diameter), there were no significant differences in the thickness between Groups 1 and 2. On an image segmentation analysis, selective thinning of the inner plexiform + ganglion cell layers (p=0.021) was observed only in the perifoveal area of the patients in Group 1 compared to that of Group 2 by using the mixed-effects model analysis.
Conclusions
Our results suggest that chronic exposure to hydroxychloroquine is associated with thinning of the perifoveal inner retinal layers, especially in the ganglion cell and inner plexiform layers, even in the absence of functional or structural clinical changes involving the photoreceptor or retinal pigment epithelial cell layers. This may be a contributing factor as the reason most patients who have early detectable signs of drug toxicity present with paracentral or pericentral scotomas.
Keywords: hydroxychloroquine, perifoveal, parafoveal, ganglion cell, retinal toxicity, OCT
Introduction
Hydroxychloroquine, an anti-malarial medication, is useful in treating several forms of malaria as well as various rheumatologic diseases, such as rheumatoid arthritis (RA),1 systemic lupus erythematosus (SLE) and dermatologic conditions.2 Retinal toxicity is a well-known adverse effect associated with the use of chloroquine and hydroxychloroquine.3 Although retinal toxicity is currently reported infrequently,4, 5 a careful monitoring for potential drug toxicity is necessary because it may lead to potentially severe and irreversible visual loss.
Retinal toxicity may be clinically evident as pigment mottling within the macular area or present with a characteristic bull’s eye maculopathy.6 The affected individuals may or may not observe visual field defects which can occur prior to fundus changes.7 Patients with macular pathology may experience a deficiency in their color vision. In later stages of toxicity, atrophy of the retinal pigment epithelium (RPE) and neurosensory retina can spread centrifugally and become visible over the entire fundus. Retinal toxicity is generally irreversible and may be progressive despite discontinuation of medication.8 Early detection and prompt discontinuation of medication may reverse retinal toxicity at an initial stage.6 Multifocal electroretinography (mfERG) may be useful to detect the reduction of retinal cone function at an early stage of toxicity.9 However, it is a relatively inconvenient and not always readily available test.
The mechanism of retinal toxicity is not yet well-understood. Clinical characteristics, including pigmentary changes in the macular area, may have led some clinicians into believing that photoreceptors were primarily involved. Nonetheless, previous studies in animals with chronic exposure to chloroquine showed that the first histopathological changes were detected in the retinal ganglion cells.10, 11 Using spectral-domain optical coherence tomography (SDOCT), our recent study in humans showed that thinning of the peripapillary retinal nerve fiber layer (RNFL) and retinal ganglion cell axons were consistently observed in patients with fundus changes due to drug toxicity.12 However, such thinning was not generally detected in those who had chronic exposure to hydroxychloroquine without fundus changes. Furthermore, a 7×7-mm macular scan analysis showed that the inner retina (RNFL + ganglion cell layer (GCL) + inner plexiform layer (IPL)) was selectively thin in those who had chronic exposure without clinically-evident toxicity, compared to controls.
Since patients with initial stages of hydroxychloroquine retinal toxicity often present with partial paracentral or complete pericentral ring scotomas,6, 7 we hypothesized that retinal anatomical changes may be observed initially in the perifoveal area. This study expanded upon an initial study by more specifically identifying which region of the macula and cellular levels in the inner retina were selectively thin. Using an image segmentation technique,13 we were able to define retinal structures into 6 separate cellular levels compared to 2 levels in our previous study. In addition, we were able to evaluate retinal thickness in selected areas of the macula. The ability to more comprehensively stratify cellular layers allowed us to better identify which retinal layers were more specifically affected in our previously evaluated cohort of patients.12 This study can provide a more comprehensive insight into the retinal structural changes that occur in hydroxychloroquine retinal toxicity, which in turn, may lead to earlier detection with the use of more sensitive screening procedures.
Material and Methods
Patients
Included in this study were 8 female patients (16 eyes) with a history for chronic use of hydroxychloroquine for at least 5 years (Group 1), and 8 (16 eyes) visually-normal, age-similar, race-matched female controls (Group 2). The study was conducted in the Electrophysiology and Inherited Retinal Disease unit at the Illinois Eye and Ear Infirmary. Three patients were prospectively recruited from the Electrophysiology unit, and three patients attended a comprehensive eye clinic at the University of Illinois at Chicago. Two additional patients participated after obtaining a telephone invitation. All patients were examined by two authors (SP and GAF). This study followed the tenets of the Declaration of Helsinki and was approved by an institutional review board at the University of Illinois. Informed consent was obtained from all participants.
Exclusion criteria included known optic nerve diseases or anomalies, glaucoma or glaucoma suspects, known retinal diseases, uveitis, intraocular pressure higher than 20 mmHg or a previous history of ocular hypertension, refractive error of more than ± 6 D sphere or ± 3 D cylinder, previous intraocular or refractive surgery, and media opacity that precluded a high-quality OCT examination.
Ocular Examination and Psychophysical Tests
All participants underwent a comprehensive ocular examination, including best-corrected visual acuity (BCVA) measurement using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart (The LighthouseTM, Long Island City, NY), slit-lamp biomicroscopy, Goldmann applanation tonometry, color vision testing using Ishihara Psuedoisochromatic Plates (Kanehara Shuppan Co.,Ltd, Tokyo, Japan), and dilated fundus examination. The patients in Group 1 underwent visual field testing using the Humphrey 10-2 program (Zeiss Humphrey Systems, Dublin, CA) and evaluation by mfERG (VERIS, Electro-Diagnostic Imaging, San Mateo, CA). The protocol for the mfERG technique was previously described.14 Data collection included date of birth, race, gender, ocular and medical history, duration of drug exposure, dosages, as well as previous and current body weight.
Scan Acquisition and Image Segmentation
SDOCT imaging was performed using Optovue technology (RTVue Model-RT100 version 3.5; Optovue Inc., Fremont, CA). Internal fixation was used to facilitate OCT image acquisition. Macular scans were performed using the Radial Lines protocol, which provided 12, 6-mm scans centered at the fovea. Scan acquisition time required for each of the Radial Lines scans was 0.27 seconds. The scans had a depth resolution of 3 µm/pixel and spatial resolution of 6 µm/pixel.
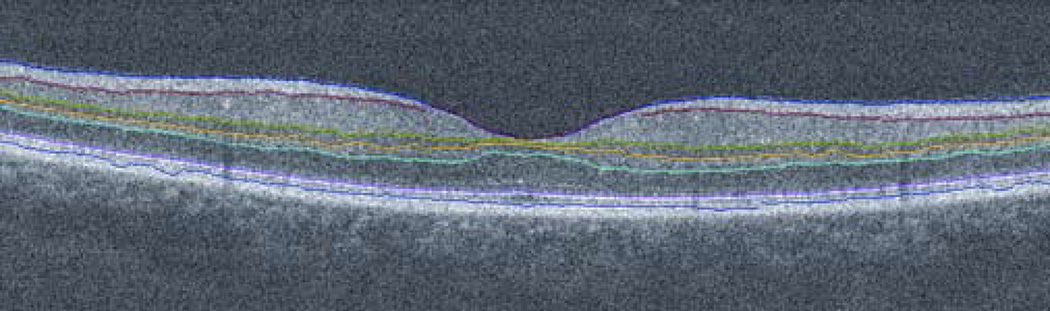
Horizontal and vertical SDOCT images from the Radial Lines scans were exported in TIF format. Automated image segmentation and analysis were performed using a dedicated software program developed in Matlab (The Mathworks Inc, Natick, Massachusetts, USA) and previously described.12 The program enabled the measurement of the thickness profiles for 6 retinal layers (Fig.1): RNFL, ganglion cell and inner plexiform layers (GCL+IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer and photoreceptor inner segments (ONL+PIS), and photoreceptor outer segments (POS). Thickness measurements were averaged at 200 µm intervals along 6 mm lengths of the Radial Lines scans.
FIGURE 1.
An example of a vertical scan through the foveal center in a control subject, displaying a retinal layer segmentation technique applied to spectral-domain optical coherence tomography (SDOCT) image. The retina is segmented with 7 boundary lines into 6 layers, including retinal nerve fiber layer (RNFL), ganglion cell + inner plexiform layers (GCL+IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer + photoreceptor inner segments (ONL+PIS), and photoreceptor outer segments (POS), respectively (from top to bottom).
Data Analysis
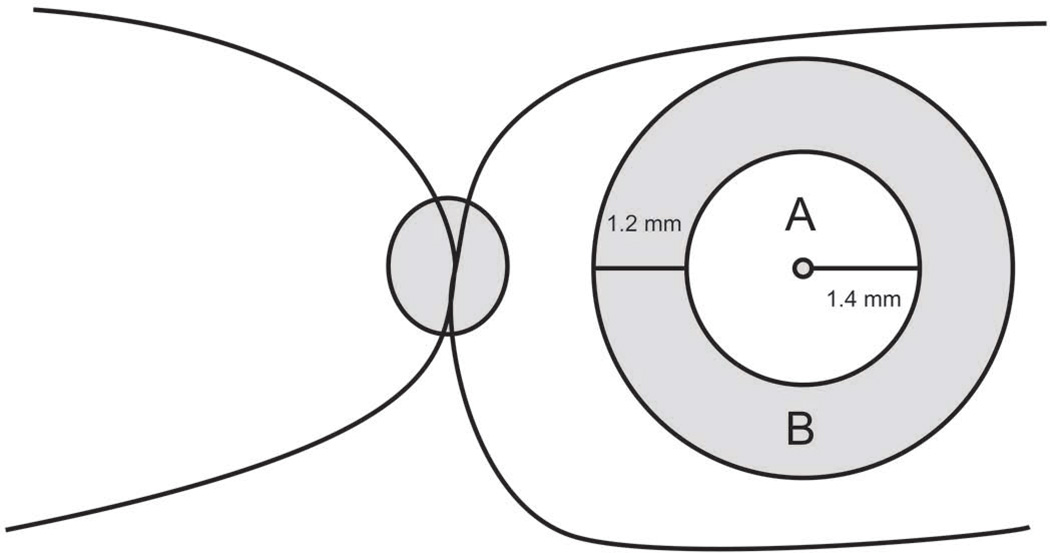
In each scan, the foveal center was identified as the location on the thickness profile corresponding to the minimum retinal thickness (or thinnest retina). Thickness in central, perifoveal, and overall macular areas (Fig.2) was calculated by averaging 15, 14, and 27 measurements on thickness profiles, respectively, from both vertical and horizontal scans. The central macular area was 2800 µm in diameter (approximately 10 degrees), centered at the foveal center. The perifoveal area was defined as a 1200-µm-width ring surrounding the central macular area. The overall macular area was 5200 µm in diameter, centered at the foveal center. Thickness measurements in the central macular and perifoveal areas obtained from images in Group 1 (patients) were compared to those obtained in Group 2 (controls). Mixed-effects models were employed to compare all layers between the two groups simultaneously, while accounting for potential interpersonal correlations. Statistical significance was accepted at p < 0.05.
FIGURE 2.
Cartoon shows the central macular area (A) and perifoveal area (B), as referred to in this study.
Results
Demographic Characteristics
The mean age in Groups 1 and 2 were 54.9 ± 11.0 (range, 35–68 years) and 53.7 ± 10.5 years (range, 34–70 years), respectively. There were no statistically significant differences in ages between the groups (p = 0.836). In each group, 6 and 2 subjects were Caucasian and African American, respectively.
Patients in Group 1 were exposed to hydroxychloroquine from 6 to 35 years (median, 10 years). Six patients in Group 1 had a diagnosis of SLE, one had RA, and one had juvenile idiopathic arthritis (JIA). Maximum daily doses of hydroxychloroquine ranged from 3.14–9.26 mg/kg/day (median, 6.56 mg/kg/day). Total accumulative doses ranged from 792–2,628 gm (median, 1,651 gm).
Ocular Examination and Psychophysical Tests
All subjects had normal anterior segment and fundus examinations. Corneal verticillata was not observed in any of the participants. Both groups had a mean logMAR BCVA of 0.0 (equivalent to 20/20). Intraocular pressure ranged from 12 to 18 mmHg. Each subject in both groups had normal color vision screening (21 out of 21 Ishihara plates). Humphrey 10-2 visual field testing results were normal in all Group 1 patients. All patients in Group 1 underwent mfERG testing; however, reliable results were not obtained in two patients due to technical issues during recordings. Of the remaining 6 patients, 5 had normal mfERG results in all 6 rings compared to a visually-normal age-similar population, while one had a reduction in amplitude in 2 of the 6 rings for the right eye and in 5 of the 6 rings for the left eye.
Image Segmentation Analysis
Table 1 shows the mean thickness measurements for each of 6 retinal layers and total retinal thickness in the central macular, perifoveal and overall macular areas, in Groups 1 (patients) and 2 (controls). There were no significant differences in thickness measurements of each layer in the central and overall macular areas. However, there was a statistically significant reduction in thickness measurements of the GCL+IPL (p = 0.021) only in the perifoveal area using mixed-effect models.
TABLE 1.
Mean Retinal Thickness Measurements in Each Retinal Layer in Group 1 (With Exposure to Hydroxychloroquine) Compared to Those of Group 2 (Controls)
| Macular Area/Layers |
Group 1 (Mean ± SD, µm) |
Group 2 (Mean ± SD, µm) |
P value^ |
|---|---|---|---|
| Central | |||
| - NFL | 15.76 ± 3.22 | 16.93 ± 2.23 | 0.562 |
| - GCL+ IPL | 73.00 ± 6.01 | 73.38 ± 8.93 | 0.849 |
| - INL | 28.95 ± 4.00 | 29.24 ± 3.16 | 0.884 |
| - OPL | 30.89 ± 6.80 | 32.80 ± 1.94 | 0.347 |
| - ONL+ PIS | 91.27 ± 9.07 | 90.60 ± 3.23 | 0.739 |
| - POS | 34.95 ± 3.47 | 33.42 ± 2.75 | 0.451 |
| - Total | 274.81 ± 17.61 | 276.38 ± 12.73 | 0.838 |
| Perifoveal | |||
| - NFL | 34.95 ± 4.89 | 37.83 ± 5.25 | 0.097 |
| - GCL+ IPL | 70.02 ± 4.41 | 74.26 ± 5.46 | 0.021* |
| - INL | 30.66 ± 2.48 | 32.84 ± 2.38 | 0.202 |
| - OPL | 27.41 ± 5.54 | 28.26 ± 1.94 | 0.608 |
| - ONL+ PIS | 77.57 ± 8.38 | 75.20 ± 3.02 | 0.166 |
| - POS | 33.46 ± 2.76 | 33.07 ± 3.16 | 0.816 |
| - Total | 274.06 ± 12.41 | 281.46 ± 5.69 | 0.132 |
| Overall | |||
| - NFL | 24.54 ± 3.57 | 26.50 ± 3.12 | 0.232 |
| - GCL+ IPL | 70.28 ± 4.55 | 72.47 ± 5.83 | 0.185 |
| - INL | 29.41 ± 2.90 | 30.57 ± 2.38 | 0.474 |
| - OPL | 29.09 ± 5.61 | 30.39 ± 1.50 | 0.420 |
| - ONL+ PIS | 84.96 ± 8.30 | 83.39 ± 2.62 | 0.333 |
| - POS | 34.28 ± 2.85 | 33.38 ± 2.66 | 0.574 |
| - Total | 272.57 ± 13.74 | 276.70 ± 6.11 | 0.441 |
NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PIS, photoreceptor inner segments; POS, photoreceptor outer segments
From mixed-effects models
Statistically significant
Discussion
In 1978, Rosenthal et al reported that histopathological changes of inner and outer retinal structures could be observed in rhesus monkeys with chronic exposure to chloroquine, even in the absence of clinically evident retinal changes on fundus photography, fluorescein angiography or electroretinography. The earliest pathological change was an accumulation of cytoplasmic granules in ganglion cells, which was followed by ganglion cell degeneration with shrunken cells and pyknotic irregular nuclei. At later stages, degeneration of photoreceptor and RPE cells was subsequently observed.10 A previous histopathological study in a human eye with chronic exposure to chloroquine demonstrated cytoplasmic inclusion bodies most prominently in the ganglion cells, but also some accumulation in IPL, INL and RPE cells. Only minimal photoreceptor cell loss was detected.15 Our recent SDOCT study also showed that thinning of inner retinal structures may be observed prior to clinically detectable structural and functional changes.12
Characteristic signs of retinal toxicity related to the use of chloroquine or hydroxychloroquine include paracentral or pericentral scotomas and a bull’s eye maculopathy, shown as bilateral pigmentary changes of the macula with relative sparing of the central fovea. The mechanism to explain these clinical signs remains unclear. There has been initial speculation that cone photoreceptors, which are most dense in the macular region, are primarily involved in the course of toxicity. However, retention of central visual acuity and preservation of color vision in some patients who have a bull’s eye maculopathy7 are inconsistent with this hypothesis. Our image segmentation results suggest that ganglion cells, and possibly also bipolar cells, are initially affected. It is known that not only cones are most dense in the macular area, but also that several layers of ganglion cells are present outside the foveal center. We speculate that hydroxychloroquine may accumulate in ganglion cells throughout the retina; however, significant SDOCT changes were detected in the perifoveal area where ganglion cells are most populated. This may explain why most of the patients with early toxicity present with paracentral or pericentral scotomas, even though a bull’s eye maculopathy may be absent. With progression of toxicity, reduction in mfERG amplitudes and a bull’s eye maculopathy may become apparent. Additionally, an impairment of central visual field sensitivity, visual acuity and color vision are clinically detectable due to subsequent degeneration of photoreceptor and RPE cells. Kellner and coworkers demonstrated that parafoveal RPE loss was observed in two patients with chloroquine retinopathy using fundus autofluorescence.16
Interestingly, image segmentation results did not show significant RNFL thinning in the perifoveal area. This is consistent with our previous observation that peripapillary RNFL thinning is absent in the patients who have chronic hydroxychloroquine exposure without fundus changes, but may be present in those with fundus changes related to drug toxicity.12 This observation may imply that RNFL thinning follows significant ganglion cell degeneration.
Thinning of the GCL and a decrease in visual functions should theoretically occur in concurrence with or follow degenerative loss of ganglion cells. The degree of ganglion cell degeneration that is sufficient to produce clinical symptoms or a detectable abnormality on psychophysical testing is essentially unknown. In glaucoma, retinal structural changes may precede clinically detectable functional abnormalities.17 Similarly, inner retinal thinning was observed, although none of the patients in our study had apparent Humphrey visual field defects at this stage. Further studies are warranted to establish the time course for development of retinal structural and functional changes in patients with hydroxychloroquine retinal toxicity.
We realize that there is a theoretical possibility that SLE and other rheumatological diseases, themselves, may have an impact on retinal structures. Nonetheless, we excluded any patients with other abnormal fundus findings, including perivascular sheathing, retinal hemorrhages and exudates. A more ideal control group might have been patients, with the same rheumatological diagnoses for the same period of time, who did not have a history of hydroxychloroquine exposure. However, in the absence of glaucoma or retinal vascular disease, we would not have anticipated inner retinal thinning solely from rheumatological diseases.
Since we aimed to detect an initial change of retinal microstructures related to chronic use of hydroxychloroquine, included in this study are only patients without symptoms and signs of hydroxychloroquine retinal toxicity. Several reports previously described abnormal OCT findings, including parafoveal thinning18,19 and abnormalities in the parafoveal photoreceptor inner segment/outer segment junction19 in patients with chloroquine or hydroxychloroquine retinal toxicity. However, those patients with noticeable OCT changes had either clinical symptoms, visual loss, visual field defects, color vision deficiency or abnormal mfERG findings. In this study, all subjects had a normal inner segment/outer segment junction, and we were unable to differentiate perifoveal inner retinal thinning in SDOCT images between patients and controls by eye.
In summary, the use of SDOCT technology and image segmentation algorithms enhances the ability to detect early thickness abnormalities in retinal layers. We believe that the development of higher-resolution imaging technique with automated segmentation protocols will be useful to detect initial change of perifoveal ganglion cell layers. Since clinically detectable signs of toxicity, including visual field defects, color vision deficiency or fundus changes, are usually irreversible once they occur, longitudinal monitoring of perifoveal inner retinal thickness may have clinical relevance to detect earlier structural changes from hydroxychloroquine or chloroquine retinal toxicity prior to clinically evident functional impairment.
What was known before.
- Peripapillary retinal nerve fiber layer thinning was consistently seen in patients who presented with retinal lesions compatible with antimalarial toxicity. - Selective thinning of the inner retina in the 7 × 7 mm posterior pole was observed in those without clinically-apparent fundus changes.
What this study adds
- Since patients with initial stages of hydroxychloroquine retinal toxicity often present with partially paracentral or complete pericentral ring scotomas, we hypothesized that retinal anatomical changes may be initially observed in the perifoveal area. - We were able to define retinal structures into 6 separate cellular levels by using an image segmentation technique. In addition, we were able to evaluate retinal thickness in selected areas of the macula. - Using spectral-domain OCT, only the ganglion cell layer and inner plexiform layer complex in the perifoveal area was affected in the patients with hydroxychloroquine exposure compared to controls.
Acknowledgement
We are thankful to Ms.Patricia Grant-Jordan from the Department of Ophthalmology, University of Illinois for her technical support on a multifocal electroretinographic procedure.
Support: Foundation Fighting Blindness, Owings Mills, Maryland, and Grant Healthcare Foundation, Chicago, Illinois (GAF); NIH core grant EY01792; an unrestricted departmental grant from Research to Prevent Blindness; NIH grant EY014275, department of Veterans’ Administration, and Research to Prevent Blindness Senior Scientific Investigator Award (MS).
Footnotes
The authors have no financial or proprietary interest in any of the products or techniques mentioned in this article.
References
- 1.Rynes RI. Hydroxychloroquine treatment of rheumatoid arthritis. Am J Med. 1988;85:18–22. doi: 10.1016/0002-9343(88)90357-9. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DJ. The use of chloroquine and hydroxychloroquine for non-infectious conditions other than rheumatoid arthritis or lupus: a critical review. Lupus. 1996;5(Suppl 1):S59–S64. [PubMed] [Google Scholar]
- 3.Butler I. Retinopathy following the use of chloroquine and allied substances. Ophthalmologica. 1965;149:204–208. doi: 10.1159/000304767. [DOI] [PubMed] [Google Scholar]
- 4.Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997;40:1482–1486. doi: 10.1002/art.1780400817. [DOI] [PubMed] [Google Scholar]
- 5.Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003;110:1321–1326. doi: 10.1016/S0161-6420(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol. 1967;12:415–447. [PubMed] [Google Scholar]
- 7.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377–1382. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 8.Easterbrook M. Long-term course of antimalarial maculopathy after cessation of treatment. Can J Ophthalmol. 1992;27:237–239. [PubMed] [Google Scholar]
- 9.Penrose PJ, Tzekov RT, Sutter EE, et al. Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquine. Retina. 2003;23:503–512. doi: 10.1097/00006982-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978;17:1158–1175. [PubMed] [Google Scholar]
- 11.Hallberg A, Naeser P, Andersson A. Effects of long-term chloroquine exposure on the phospholipid metabolism in retina and pigment epithelium of the mouse. Acta Ophthalmol (Copenh) 1990;68:125–130. doi: 10.1111/j.1755-3768.1990.tb01892.x. [DOI] [PubMed] [Google Scholar]
- 12.Pasadhika S, Fishman GA. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye. doi: 10.1038/eye.2009.65. (In press) [DOI] [PubMed] [Google Scholar]
- 13.Bagci AM, Shahidi M, Ansari R, Blair M, Blair NP, Zelkha R. Thickness profiles of retinal layers by optical coherence tomography image segmentation. Am J Ophthalmol. 2008;146:679–687. doi: 10.1016/j.ajo.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vajaranant TS, Seiple W, Szlyk JP, Fishman GA. Detection using the multifocal electroretinogram of mosaic retinal dysfunction in carriers of X-linked retinitis pigmentosa. Ophthalmology. 2002;109:560–568. doi: 10.1016/s0161-6420(01)00984-8. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey MS, Fine BS. Chloroquine toxicity in the human eye. Histopathologic observations by electron microscopy. Am J Ophthalmol. 1972;73:229–235. doi: 10.1016/0002-9394(72)90137-7. [DOI] [PubMed] [Google Scholar]
- 16.Kellner U, Kellner S, Weinitz S. Chloroquine retinopathy: lipofuscin- and melanin-related fundus autofluorescence, optical coherence tomography and multifocal electroretinography. Doc Ophthalmol. 2008;116:119–127. doi: 10.1007/s10633-007-9105-6. [DOI] [PubMed] [Google Scholar]
- 17.Girkin CA. Relationship between structure of optic nerve/nerve fiber layer and functional measurements in glaucoma. Curr Opin Ophthalmol. 2004;15:96–101. doi: 10.1097/00055735-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Korah S, Kuriakose T. Optical coherence tomography in a patient with chloroquine-induced maculopathy. Indian J Ophthalmol. 2008;56:511–513. doi: 10.4103/0301-4738.43379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Padilla JA, Hedges TR, Monson B, et al. High-speed ultra-high-resolution optical coherence tomography findings in hydroxychloroquine retinopathy. Arch Ophthalmol. 2007;125:775–780. doi: 10.1001/archopht.125.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]