Abstract
In Type 1 diabetes (T1D), reactive oxygen species (ROS) and pro-inflammatory cytokines produced by macrophages and other innate immune cells destroy pancreatic β-cells while promoting autoreactive T cell maturation. Superoxide-deficient Non-Obese Diabetic mice (NOD.Ncf1m1J) are resistant to spontaneous diabetes, revealing the integral role of ROS-signaling in T1D. Here, we evaluate the innate immune activation state of bone marrow-derived macrophages (BM-Mϕ) from NOD and NOD.Ncf1m1J mice after poly(I:C)-induced Toll-like receptor 3 (TLR3) signaling. We show that ROS synthesis is required for efficient activation of the NF-κB signaling pathway and concomitant expression of TLR3 and the cognate adaptor molecule, TRIF. Poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ exhibited a 2- and 10-fold decrease in TNF-α and IFN-β pro-inflammatory cytokine synthesis, respectively, in contrast to NOD BM-Mϕ. Optimal expression of IFN-α/β is not solely dependent on superoxide synthesis, but requires p47phox to function in a NOX-independent manner to mediate Type I interferon synthesis. Interestingly, MHC-II I-Ag7 expression necessary for CD4 T cell activation is increased 2-fold relative to NOD, implicating a role for superoxide in I-Ag7 down-regulation. These findings suggest that defective innate immune pattern-recognition receptor activation and subsequent decrease in TNF-α and IFN-β pro-inflammatory cytokine synthesis necessary for autoreactive T cell maturation, may contribute to the T1D protection observed in NOD.Ncf1m1J mice.
Keywords: Innate immunity, Type 1 diabetes, NOD mouse, Macrophage, Superoxide, poly(I:C), TLR3 signaling
Introduction
The immune system has evolved to take advantage of reactive oxygen species (ROS) as intra- and inter-cellular signaling molecules to mediate regulation of redox-dependent metabolic processes [1, 2]. Upon microbial infection, neutrophils and antigen presenting cells (APC) generate ROS as a frontline defense to efficiently contain and destroy pathogens [2]. Moreover, the activation process of naïve T cells [3, 4] requires three signals consisting of 1) T cell receptor (TCR) interactions with a peptide bound to the major histocompatibility complex (MHC), 2) ligation of co-stimulatory molecules, and 3) the induction of ROS-dependent signaling pathways to provoke the synthesis of pro-inflammatory cytokines to elicit efficient initiation, enhancement, and maintenance of antigen-driven adaptive immune responses [5-7]. In Type 1 diabetes (T1D), macrophages chiefly comprise early infiltrating immune cells of pancreatic islets [8] and directly contribute to the destruction of insulin-secreting β-cells by generating ROS and pro-inflammatory cytokines via the activation of redox-sensitive signaling pathways [9, 10].
Superoxide is specifically generated in response to stimulation by the multimeric NADPH-oxidase (NOX) enzyme [11]. Upon activation, the cytoplasmic p47phox subunit facilitates coordinated assembly of the NOX complex [12, 13] and the production of O2.− at the membrane surface [11]. A spontaneous mutation in the Ncf1 gene encoding the p47phox subunit was shown to inhibit O2.− production in a dominant negative manner [12] and has been widely utilized as a model for studying the effects of O2.− deficiency in other autoimmune diseases including rheumatoid arthritis and experimental autoimmune encephalomyelitis [14, 15]. We recently demonstrated the importance of O2.− deficiency in the context of T1D by introgressing the Ncf1m1J mutation into the Non-Obese Diabetic (NOD) mouse [16]. NOD.Ncf1m1J mice were highly resistant to spontaneous T1D that was partially explained by a decreased Th1 cytokine profile and a concomitant increased Th17 cytokine response. Studies using the NOD.Ncf1m1J mouse demonstrate the importance of ROS-dependent signaling in controlling T helper lineage commitment and autoimmune disease development and progression [16]. In this report we describe the mechanistic defects elicited by the absence of ROS-dependent signaling on innate immune activation in NOD.Ncf1m1J bone marrow-derived macrophages (BM-Mϕ).
Activation of innate immune cells is mediated by specialized intracellular or membrane localized pattern recognition receptors expressed by APC. Among these, Toll-like receptors (TLR) are highly efficient in initiating immune responses upon recognition of specific microbial-associated molecular patterns [17, 18]. The expression and localization of TLR are regulated by ROS [19] and evidence suggests an association of dysregulated TLR activation with the risk [20, 21], development [21-23], and progression of T1D [24]. TLR3 activation requires the adaptor molecule TRIF to further transduce antiviral immune responses into the NF-κB and IRF signaling pathways. The NF-κB pathway is activated through canonical IKKα/β kinases that phosphorylate the inhibitory protein IκBα. Following phosphorylation, IκB-α is degraded, allowing for the nuclear localization of the NF-κB p50/p65 transcription factor and the stimulation of pro-inflammatory cytokines and expression of various chemokines [25]. TRIF signaling through IKKε and TBK1 kinases will also induce IRF3-dependent transcriptional activation of type I interferons and other antiviral genes [26].
Here, we show that the TLR3-dependent innate immune response in superoxide-deficient NOD BM-Mϕ results in decreased expression of TNF-α and IFN-β pro-inflammatory cytokines (signal 3), but increased expression of MHC-II molecules (signal 1) in comparison to superoxide-competent NOD BM-Mϕ. Mechanistically, NOD.Ncf1m1J BM-Mϕ display defects in TLR3 signaling as evidenced by decreased TLR3, adaptor molecule TRIF and phospho-IκB-α expression after poly(I:C) stimulation. Our data suggest that in the absence of ROS synthesis, a concomitant dysregulated innate immune and acute anti-viral response contribute to the unique T1D resistance exhibited by NOD.Ncf1m1J mice.
Materials and Methods
Mice
NOD/ShiLtJ (NOD) and NOD.Ncf1m1J [27] mice were bred and housed at the Research Support Building of the University of Alabama at Birmingham, under pathogen free conditions and observing IACUC approved mouse protocols. Age- (8-16 weeks) and sex-matched mice were used in all experiments.
Differentiation and stimulation of bone marrow-derived macrophages
Bone marrow hematopoietic stem cells were isolated from the femurs and tibias of mice and differentiated into Mϕ [28]. Cells were stimulated for different time intervals with 25μg/ml of the TLR3 ligand polyinosinic-polycytidylic acid (poly(I:C)) Low Molecular Weight dsRNA synthetic analog (InvivoGen), 1mU/mL of xanthine oxidase (Sigma), or with 34-68μM of MnTE-2-PyP5+ (manganese (III) mesotetrakis (di-N-diethylimidazole) porphyrin), a potent SOD mimetic [29, 30] generously obtained from Dr. James Crapo (National Jewish Hospital, Denver, CO).
Oxidation of luminol to detect superoxide synthesis from bone marrow-derived macrophages
Since luminol is cell permeable and readily oxidized by superoxide and hydrogen peroxide resulting in chemiluminescence, it can be used to detect NOX activity with NOD and NOD.Ncf1m1J bone marrow-derived macrophages as previously described [31, 32]. Briefly, differentiated bone marrow-derived macrophages were plated onto a 96-well round bottom plate at 5×104 cells in phenol red-free HBSS with 200μM luminol (Sigma) and 0.32 Units/mL of horseradish peroxidase (Sigma) in a total volume of 200μL. Luminescence was quantified using a SpectraMax L Luminescence microplate reader and was recorded every 2 minutes for 1 hour.
ELISA and Bio-Plex Multiplex assays
Cytokine and chemokine expression were measured in the supernatants of untreated or poly(I:C)-treated APC. TNF-α was detected with a DuoSet ELISA kit (R&D Systems) and IFN-α/β with the Verikine Mouse Interferon-α/β ELISA kits (PBL Interferon Source) according to the manufacturer’s instructions. ELISA plates were read on a Synergy 2 microplate reader (BioTek) using the Gen5 software. Multi-plex cytokine expression profiling was performed using the Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad). Data acquisition and quantification was conducted with the Bio-Plex Manager version 3.0 software.
Flow cytometry
Prior to staining, Fcγ receptors were blocked with Fc block (eBiosciences) for 10′ at 4°C and then incubated with fluorochrome-conjugated antibodies specific for CD80, CD86, F4/80, CD11c, CD40, OX-6 and CD11b (eBiosciences) for 30′ at 4°C. Cells were collected on a FACSCalibur (BD Biosciences) and analyzed with FlowJo (8.8.6) software (Tree Star, Inc.).
Protein extraction and Western immunoblotting
Whole cell lysates and nuclear extracts were prepared as described previously [28, 30]. For Western immunoblotting, 25 to 40 μg of whole cell lysates or 5 μg of nuclear extracts from untreated or poly(I:C)-treated BM-Mϕ were separated on a 10% PAGE, transferred to PVDF membranes (Bio-Rad) and blocked with 5% non-fat milk in TBST for 1 hr at room temperature. The membranes were then incubated overnight with antibodies against TLR3, IRF3, NF-κB p50 (Santa Cruz); TRIF (IMGENEX); phospho-IκB-α (S32/S36), IκB-α (Cell Signaling); or β-actin (Sigma). After incubation with appropriate HRP-conjugated secondary antibodies, membranes were developed with ECLplus substrate (GE). Images were acquired and quantitated with the Bio-Rad Image Lab software (version 3.0).
Quantitative RT-PCR
RNA was isolated from unstimulated and poly(I:C)-stimulated BM-Mϕ with TRIzol (Invitrogen) and cDNA prepared by SuperScript III (Invitrogen) according to the manufacturer’s protocol. The generated cDNA was amplified on a Roche LightCycler 480 instrument by quantitative PCR using the following TaqMan Gene Expression Assays (AB Applied Biosystems): Isg15 (Mm01705338_s1); Tlr3 (Mm00628112_m1); Ifna2 (Mm00833961_s1); Ticam1 (Mm00844508_s1); Ifnb1(Mm00439552_s1); Ciita (Mm00482914_m1); Gapdh (Mm99999915_g1). The relative gene expression levels were calculated with the 2−ΔΔCt method, Gapdh was used as an endogenous control gene for normalization and the unstimulated samples were used as calibrator controls and set as 1.
Statistical analysis
Data were analyzed using GraphPad Prism Version 5.0 statistical software. Determination of the difference between mean values for each experimental group was assessed using the 2-tailed Student’s t test, with p < 0.05 considered significant. All experiments were performed at least three separate times with data obtained in triplicate wells in each experiment.
Results
NOD.Ncf1m1J BM-Mϕ are attenuated in ROS synthesis after poly(I:C) stimulation
To determine if innate immune activation upon TLR3 engagement resulted in an increase in superoxide synthesis, NOD and NOD.Ncf1m1J BM-Mϕ were stimulated with poly(I:C) in the presence of luminol. Oxidation of luminol and a concomitant increase in luminescence has been utilized previously to demonstrate NOX-dependent superoxide synthesis in phagocytic and non-phagocytic cells [31, 33]. A rapid increase in luminol oxidation was observed in NOD BM-Mϕ within 2 minutes (data not shown) and peaked at 8 minutes (Figure 1) after poly(I:C) treatment. NOD BM-Mϕ exhibited a 5-fold increase in chemiluminescent arbitrary units after poly(I:C) stimulation in comparison to NOD.Ncf1m1J BM-Mϕ. In contrast, oxidation of luminol was significantly decreased and barely above background levels in NOD.Ncf1m1J BM-Mϕ after poly(I:C) treatment (Figure 1) in all time points assayed (data not shown). These results demonstrate that stimulation of the TLR3 signaling pathway in BM-Mϕ will initiate a rapid burst of superoxide generation to effectively mediate innate immune responses.
Figure 1. Poly(I:C) stimulation of NOD BM-Mϕ results in a rapid increase in superoxide generation.
Luminol-oxidized chemiluminescence of 5×104 NOD and NOD.Ncf1m1J BM-Mϕ was observed kinetically at 2 minute intervals for an hour after 25μg/mL poly(I:C) stimulation. NOD BM-Mϕ exhibit a 5-fold increase in luminescent arbitrary units at the 8-minute time point in contrast to NOD.Ncf1m1J BM-Mϕ. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample. *** p < 0.001 versus poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ.
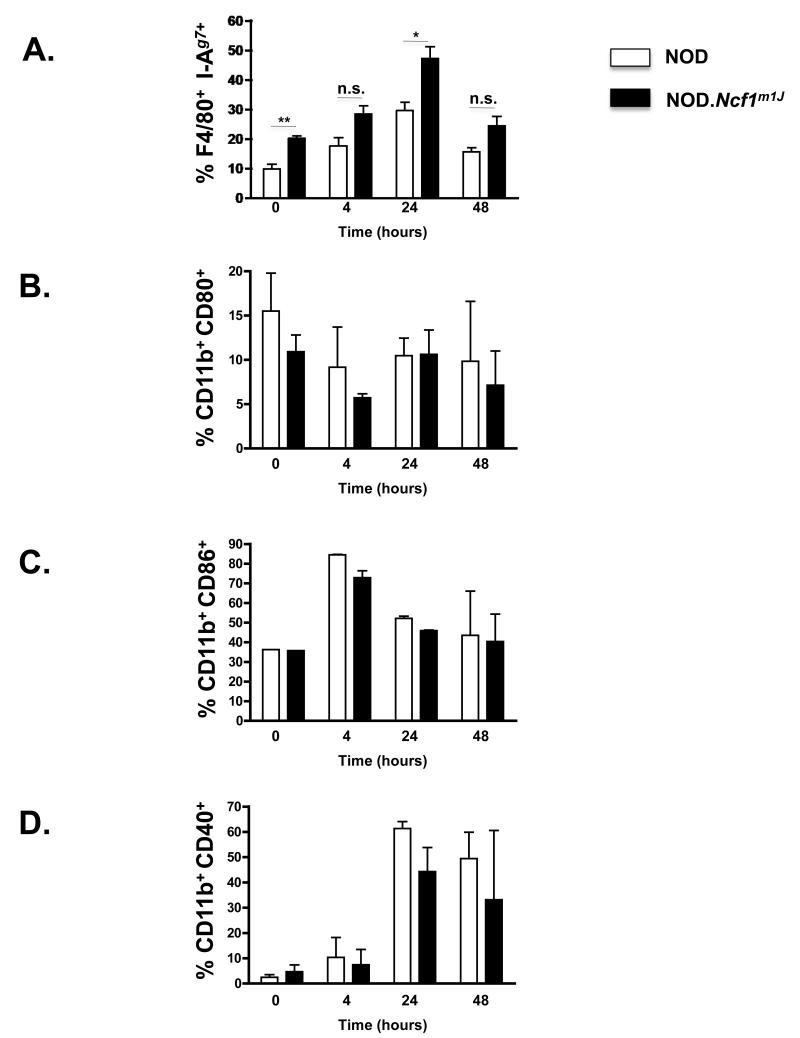
MHC-II I-Ag7 expression is dysregulated in NOD.Ncf1m1J BM-Mϕ
Upon TLR stimulation, APC maturation occurs by up-regulating the expression of MHC-II (Signal 1) and co-stimulatory molecules (Signal 2) to efficiently activate naive T cells to become effector cells [25, 34]. To investigate whether the lack of superoxide in NOD.Ncf1m1J mice affected BM-Mϕ maturation, flow cytometric surface expression levels of the NOD MHC-II molecule I-Ag7 (OX-6) and CD40, CD80 and CD86 co-stimulatory molecules were analyzed at different time points after poly(I:C) stimulation (Figure 1). The kinetics of MHC-II I-Ag7, CD40, CD80 and CD86 expression was similar in both NOD and NOD.Ncf1m1J BM-Mϕ after poly(I:C) stimulation. Expression of MHC-II I-Ag7 (Figure 2A) increased relative to baseline and peaked at 24 hours. Interestingly, MHC-II I-Ag7 expression in both unstimulated and poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ was significantly higher than NOD BM-Mϕ (Figure 2A, ** p < 0.005) and exhibited 2-fold higher expression level of I-Ag7 at the 24 hr time point (* p < 0.05). CD80 (Figure 2B), CD86 (Figure 2C), and CD40 (Figure 2D). In contrast to MHC-II I-Ag7, no significant difference in CD80 (Figure 2B), CD86 (Figure 2C), or CD40 (Figure 2D) expression was observed between poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ at all time points assayed. These results indicate that superoxide is not required for inducing efficient co-stimulatory molecule expression, but the absence of superoxide results in dysregulated MHC-II I-Ag7 expression in both unstimulated conditions and after poly(I:C) treatment in NOD BM-Mϕ.
Figure 2. Superoxide is required for down-regulation of MHC-II I-Ag7 (signal 1) on NOD.Ncf1m1J BM-Mϕ.
Flow cytometric surface expression of F4/80 - MHC-II I-Ag7 (A), CD11b - CD80 (B), CD11b - CD86 (C), or CD11b - CD40 (D) double positive cells after poly(I:C) stimulation for 0, 4, 24, and 48 hr. Bar graphs representing the average percent of double positive cells in 3 independent experiments. ** p < 0.005, * p < 0.05, and n.s. – not significant versus poly(I:C)-stimulated NOD BM-Mϕ.
Decreased production of pro-inflammatory cytokines (signal 3) after poly(I:C) stimulation in NOD.Ncf1m1J BM-Mϕ
In order to further investigate the effects of superoxide on innate immune activation, pro-inflammatory cytokine synthesis from poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ were analyzed by ELISA and Bio-Plex multiplex assays. Expression of TNF-α, an NF-κB-dependent pro-inflammatory cytokine and a key mediator in the pathogenesis of T1D [35], showed a 3-fold decrease in NOD.Ncf1m1J BM-Mϕ relative to NOD (Figure 3A; p < 0.005). In addition, other NF-κB-dependent pro-inflammatory cytokines such as IL-6 (Figure 3B; p = 0.311), IL-1β (Figure 3C; p = 0.192), and G-CSF (Figure 3D; p = 0.06) also exhibited a decrease, but the differences in expression between NOD and NOD.Ncf1m1J BM-Mϕ were not statistically significant. The expression of IFN-α was reduced from 94.78 ± 8.6 pg/ml in NOD BM-Mϕ to 66.19 ± 0.7 pg/ml in NOD.Ncf1m1J BM-Mϕ (Figure 3E; p = 0.087) after poly(I:C) stimulation for 24 hr. Interestingly, IFN-β expression was severely affected by superoxide deficiency displaying a 9-fold reduction in NOD.Ncf1m1J compared to NOD BM-Mϕ (Figure 3F; p < 0.005). To corroborate the decrease in IFN-α/β protein expression in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ, the effects of this dsRNA synthetic analog on Ifna2 and Ifnb1 mRNA accumulation was evaluated. The expression of Ifna2 was highly induced after poly(I:C) stimulation, but no differences in Ifna2 mRNA accumulation were observed between NOD and NOD.Ncf1m1J BM-Mϕ (Figure 3G; p = 0.683). Similar to observations at the protein level, gene expression of Ifnb1 was reduced by 12-fold in NOD.Ncf1m1J compared to NOD BM-Mϕ (Figure 3H; p < 0.05). The decrease in TNF-α and type I interferon synthesis in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ suggest that superoxide is necessary for efficient anti-viral responses. To confirm this observation, expression of Isg15, an IFN-stimulated gene typically upregulated in response to viral infections [36] was examined in poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ. Following an 8 hour incubation, poly(I:C) stimulated a 70-fold increase in the accumulation of Isg15 mRNA in NOD BM-Mϕ, while Isg15 accumulation was reduced by 7-fold in NOD.Ncf1m1J BM-Mϕ (Figure 3I; p < 0.05). The reduced expression of IRF3- and NF-κB-induced pro-inflammatory cytokines in NOD.Ncf1m1J BM-Mϕ suggests that both downstream TLR3-dependent signaling pathways, NF-κB and IRF3, require superoxide for optimal activation.
Figure 3. Poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ exhibit a decrease in pro-inflammatory cytokine expression.
Supernatants from NOD and NOD.Ncf1m1J BM-Mϕ stimulated with 25 μg/ml of poly(I:C) for 24 hr were assayed for TNF-α (A), IL-1β (B), IL-6 (C), G-CSF (D), IFN-α (E), and IFN-β (F) cytokine expression by ELISA (TNF-α, IFN-α, IFN-β) or with a Bio-plex Multiplex kit (IL-1β, IL-6, G-CSF). qRT-PCR expression analysis of Ifna2 (G), Ifnb1 (H), and Isg15 (I) genes in NOD and NOD.Ncf1m1J BM-Mϕ after 8 hr stimulation with poly(I:C). Gene expression was calculated using the 2−ΔΔCt method and is represented as fold-change relative to the non-stimulated cells used as calibrator samples and arbitrarily set to 1, and Gapdh was used as an endogenous normalization control. Plotted data represent averages of 3 independent experiments, done in triplicates ** p < 0.005, * p < 0.05, and n.s. – not significant versus poly(I:C)-stimulated NOD BM-Mϕ.
Exogenous addition of xanthine oxidase rescues NF-κB-dependent pro-inflammatory cytokines, but not IRF3-dependent Type I interferons
To demonstrate that the absence of superoxide production in NOD.Ncf1m1J macrophages was primarily responsible for the decrease in pro-inflammatory cytokine and Type I interferon responses, we exogenously added superoxide to poly(I:C)-stimulated NOD.Ncf1m1J macrophages via xanthine oxidase (XO) addition. As demonstrated in Figure 4, addition of 1mU/mL XO rescued TNF-α (Figure 4A) synthesis in NOD.Ncf1m1J macrophages to NOD levels at all time points assayed (4, 8, and 24 hours; data only shown for 24 hour time point) when subtracted from XO treatment alone. The levels of TNF-α produced by 1mU/mL XO treatment alone were consistently equal and less than poly(I:C)-stimulation alone at all time points assayed (data not shown) in both NOD and NOD.Ncf1m1J macrophages. Interestingly, IFN-α/β (Figure 4B for IFN-β; data not shown for IFN-α) synthesis in poly(I:C)- and XO-treated NOD.Ncf1m1J macrophages was not restored to NOD levels, but exhibited a further decrease in both Type I interferons. Suppression of IFN-α/β synthesis by exogenous XO addition was also observed with poly(I:C)-stimulated NOD macrophages (Figure 4B; data not shown for IFN-α). IFN-β levels decreased 2-fold when poly(I:C)-stimulated NOD macrophages were treated with 1mU/mL of XO (Figure 4B). These results demonstrate that NOX and superoxide synthesis have a divergent role in both NF-κB-dependent pro-inflammatory cytokines and IRF3-dependent Type I interferons. Exogenous addition of superoxide can rescue the inability of NOD.Ncf1m1J macrophages to efficiently generate NF-κB-dependent innate immune pro-inflammatory cytokines upon poly(I:C) stimulation, but not IRF3-dependent Type I interferons in NOD.Ncf1m1J macrophages.
Figure 4. Exogenous xanthine oxidase (XO) can restore NF-κB-dependent pro-inflammatory cytokines, but not Type I interferon synthesis in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ.
Supernatants from NOD and NOD.Ncf1m1J BM-Mϕ stimulated with 25 μg/ml of poly(I:C) with or without 1mU/mL of xanthine oxidase for 24 hr were assayed for TNF-α (A) and IFN-β (B) cytokine expression by ELISA. Plotted data represent averages of 3 independent experiments, done in triplicates *** p < 0.001, * p < 0.05, and n.s. – not significant versus poly(I:C)-stimulated NOD BM-Mϕ or NOD.Ncf1m1J BM-Mϕ.
Scavenging of superoxide decreases TNF-α and IFN-β synthesis in poly(I:C)-stimulated NOD BM-Mϕ
Since NF-κB-dependent pro-inflammatory cytokine synthesis was efficiently restored in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ with XO addition (Figure 4A), the reciprocal experiment was performed to determine if scavenging of superoxide with poly(I:C)-stimulated NOD BM-Mϕ would exhibit a decrease in innate immune cytokine responses similar to NOD.Ncf1m1J BM-Mϕ. By using a SOD mimetic [29, 30] to scavenge superoxide synthesis from poly(I:C)-stimulated NOD BM-Mϕ, a 6-fold decrease in TNF-α (Figure 5A) and 5-fold decrease in IFN-β (Figure 5B) synthesis were observed. The levels of TNF-α produced from SOD mimetic- and poly(I:C)-treated NOD BM-Mϕ were similar to poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ alone (Figure 5A). Dissipation of superoxide further decreased TNF-α and IFN-β levels in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ by 2- and 5-fold, respectively (Figure 5). These observations demonstrate the importance of superoxide synthesis to mediate efficient pro-inflammatory cytokine and Type I interferon production in macrophages after poly(I:C) stimulation.
Figure 5. Scavenging of superoxide with a superoxide dismutase (SOD) mimetic inhibits TNF-α and IFN-β synthesis in NOD and NOD.Ncf1m1J BM-Mϕ after poly(I:C) stimulation.
Supernatants from NOD and NOD.Ncf1m1J BM-Mϕ stimulated with 25 μg/ml of poly(I:C) with or without 68μM SOD mimetic for 24 hr were assayed for TNF-α (A) and IFN-β (B) cytokine expression by ELISA. Plotted data represent averages of 3 independent experiments, done in triplicates * p < 0.05 versus poly(I:C)-stimulated NOD BM-Mϕ or NOD.Ncf1m1J BM-Mϕ.
Defective NF-κB and TLR3 signaling pathways in NOD.Ncf1m1J BM-Mϕ
To define the molecular mechanism underlying the deficiencies observed in the production of pro-inflammatory cytokines in NOD.Ncf1m1J BM-Mϕ, we analyzed the expression status of TLR3 and its cognate adaptor molecule TRIF [18] at both the transcriptional and protein expression levels. Expression of Tlr3 (Figure 6A) and Ticam1 (Figure 6B), gene products of TLR3 and TRIF, respectively, decreased 3- and 2-fold in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ compared to NOD after stimulation for 4 (data not shown) and 8 hours. Consistent with mRNA accumulation, TLR3 and TRIF protein levels (Figure 6C) exhibited a concomitant 2-fold decrease in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ as compared to NOD BM-Mϕ (Figure 6D) after stimulation for 24 hours.
Figure 6. NOD.Ncf1m1J BM-Mϕ exhibit a decrease in mRNA accumulation and protein expression levels of TLR3 and TRIF after poly(I:C)-stimulation.
qRT-PCR was performed on mRNA isolated from NOD and NOD.Ncf1m1J BM-Mϕ untreated or treated with poly(I:C) for 8 hours to assess the expression of Tlr3 (A) and the TRIF gene, Ticam1 (B). Western blot analysis of whole cell lysates from untreated or 25 μg/ml poly(I:C)-treated NOD and NOD.Ncf1m1J BM-Mϕ for 24 hours. Membranes were probed for TLR3, TRIF or β-actin as a loading control (C). Densitometry graph of TLR3 and TRIF protein expression as a ratio with β-actin with stimulated NOD BM-Mϕ arbitrarily set as 1. The results are representative of three independent experiments.
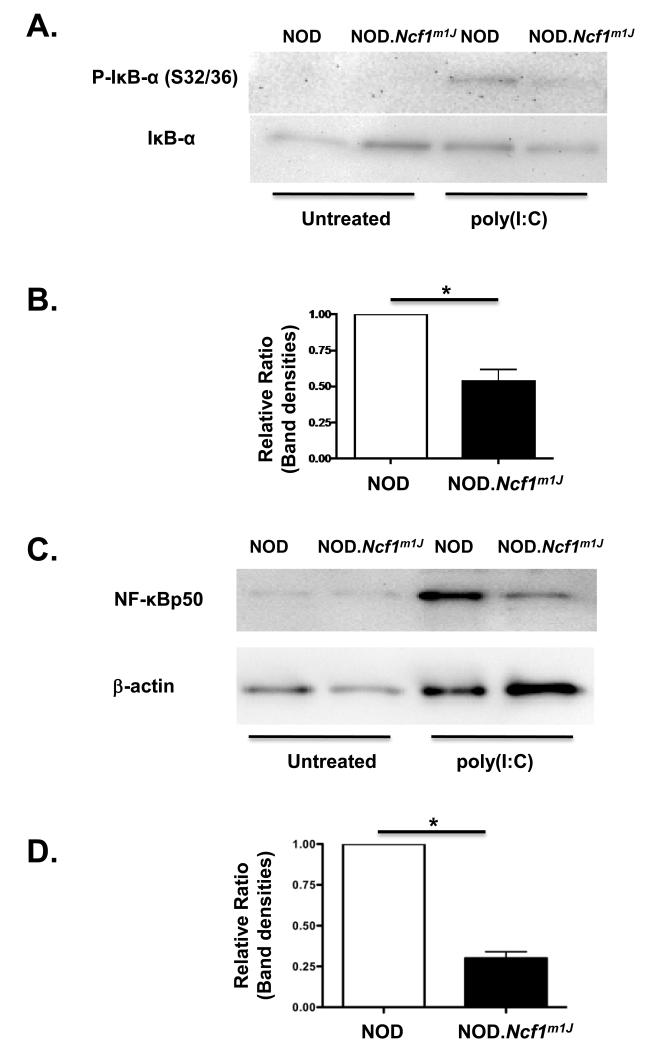
The decrease in TLR3 expression in poly(I:C)-stimulated NOD.Ncf1m1J macrophages may be partially mediated by dysregulation of the NF-κB signaling pathway. In the cytoplasm of resting cells, IκB-α complexes with the NF-κB p50/p65 heterodimer to prevent activation. Upon stimulation, IκB-α undergoes phosphorylation and degradation to permit NF-κB p50/p65 nuclear translocation and the induction of NF-κB-dependent gene transcription [37, 38]. Since the murine Tlr3 promoter contains several κB binding sites [39], a short time course for NF-κB activation in poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ was examined. To determine if transcriptional (Figure 6A) and translational (Figure 6C) downregulation of TLR3 in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ may be due to defects in NF-κB activation, the phosphorylation status of IκB-α (S32/S36) was examined by immunoblotting (Figure 7A). As shown in Figure 7B, densitometry analysis of phospho-IκB-α (S32/S36) expression was reduced 2-fold in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ as compared to NOD, further demonstrating that the absence of superoxide alters NF-κB signaling pathway activation. To further corroborate that NOD.Ncf1m1J BM-Mϕ were defective in NF-κB activation and initiating efficient innate immune responses, purified nuclear extracts from NOD and NOD.Ncf1m1J BM-Mϕ were probed by immunoblotting for the presence of NF-κB p50 after treating with poly(I:C) for 60′. As shown in Figure 7C, nuclear translocation of NF-κB p50 was observed in stimulated NOD BM-Mϕ, but densitometry analysis of NF-κB p50 levels in NOD.Ncf1m1J BM-Mϕ was decreased 3-fold (Figure 7D).
Figure 7. Impaired NF-κB activation in NOD.Ncf1m1J BM-Mϕ after poly(I:C)-stimulation.
(A) Representative Western blot of phospho-IκB-α (S32/S36) and total IκB-α from NOD and NOD.Ncf1m1J BM-Mϕ untreated or treated with 25 μg/ml of poly(I:C) for 30′. (B) Densitometry bar graph of the average of 3 independent experiments of phospho-IκB-α (S32/S36) expression in poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ. The phospho-IκB-α (S32/S36) expression level in stimulated NOD BM-Mϕ was arbitrarily set as 1. (C) Representative Western blot of NF-κB p50 and actin from NOD and NOD.Ncf1m1J BM-Mϕ nuclear extracts after 25 μg/ml poly(I:C) treatment for 60′. (D) Densitometry bar graph of the average of 3 independent experiments of NF-κB p50 expression in poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ. NF-κB p50 expression level in stimulated NOD BM-Mϕ was arbitrarily set as 1. The results are representative of three independent experiments. * p < 0.05 versus poly(I:C)-stimulated NOD BM-Mϕ.
Discussion
Efficient activation of immune responses requires three APC-derived “activation signals”, including antigen presentation of MHC-peptide complexes to the TCR (signal 1) [40, 41], molecular interactions between APC and T cell co-stimulatory molecules (signal 2) [42-44], and the production of ROS and pro-inflammatory cytokines/chemokines (signal 3) [4, 5, 45]. In this process, ROS synthesis by Mϕ and dendritic cells, is a critically important step given that key signaling pathways involved in innate immune-derived pro-inflammatory cytokine/chemokine responses, such as NF-κB, MAPK, or AP-1, are regulated by cellular redox balance [30, 46]. We have recently shown that NOD mice unable to generate NOX-induced superoxide, NOD.Ncf1m1J, were resistant to the development of diabetes with an underlying deficiency in the activation of the diabetogenic Th1 adaptive immune response [16]. In the present study, we define the importance of superoxide deficiency in innate immune activation of NOD.Ncf1m1J BM-Mϕ in response to TLR3-induced signaling.
Mϕ are important innate immune cells involved in the pathogenesis of T1D, illustrated by the resistance to T1D displayed by NOD mice depleted of Mϕ [8]. In addition, the majority of infiltrating leukocytes within the pancreas in the early stages of insulitis are activated Mϕ which produce ROS, reactive nitrogen species (RNS), and pro-inflammatory cytokines to directly destroy insulin-producing β-cells and maintain a noxious inflammatory environment [8, 47-49]. Moreover, NOD Mϕ exhibit other key defects likely to contribute to T1D pathogenesis, such as the inability to properly clear apoptotic cells [50] and to effectively present antigen [51].
The activation of Mϕ and other APC depends on pattern recognition receptors expressed intracellularly or on the cell surface [25]. Among these, endosomally-localized TLR3, TLR7, and TLR9 specifically recognize dsRNA, ssRNA, and unmethylated DNA, respectively [18]. Upon ligation, these receptors are capable of inducing type I IFN responses which have potent antimicrobial effects [52, 53]. In addition to their beneficial role in host defense mechanisms, TLR7 and TLR9 have been associated with induction of inflammatory responses due to inappropriate recognition of self-nucleic acids or nucleoprotein immunocomplexes released by apoptotic or necrotic cells in systemic lupus erythematosus or psoriasis [52, 54]. The TLR3 signaling pathway, however, has been implicated in the etiology of autoimmune diabetes in genetically predisposed individuals or animal models [55-58]. Viral infections are proposed environmental triggers of T1D and a wealth of literature supporting this paradigm has been reported [59-64]. The pleiotropic effects of TLR3-induced type I IFN-α/β generate an “anti-viral state” by inducing the transcription of genes involved in viral degradation, activation of natural killer cells, and the enhancement of MHC-I expression for efficient CD8 T cell activation [65]. Inappropriate and/or sustained activation of the TLR3 signaling pathway may also promote the transition to a pathological autoimmune response and development of T1D in genetically susceptible individuals [66-68].
Here, we observed defects in macrophage activation and a marked dysregulation of TLR3- and NF-κB-dependent signaling events in NOD.Ncf1m1J BM-Mϕ that may partially explain the unique T1D resistance in NOD.Ncf1m1J mice. Specifically, (1) inefficient synthesis of TNF-α and IFN-β in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ; and (2) decreased activation of TLR3 and NF-κB signaling pathways. NOD.Ncf1m1J BM-Mϕ exhibited significant decreases in TNF-α and IFN-β expression, key cytokines linking innate and adaptive immune responses in T1D [35] and T1D development in NOD mice [69, 70]. Interestingly, we did not observe significant differences in Ifna2 or IFN-α expression between poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ even though this is a critical cytokine in the pathogenesis of T1D [66, 68, 71]. Attenuated expression of innate immune-derived pro-inflammatory cytokines (signal 3) in NOD.Ncf1m1J mice may result in inefficient synergy with T cell adaptive immune Th1 cytokine responses and subsequently, T1D resistance [16].
The decrease in poly(I:C)-stimulated signaling in NOD.Ncf1m1J BM-Mϕ appears to be partially due to a marked decreased in TLR3 expression at both the mRNA and protein levels in comparison to NOD BM-Mϕ. The 5′ proximal promoter region of the Tlr3 gene contains one AP-1 and two NF-κB consensus-binding sites [39]. Both redox-sensitive transcription factors are activated by ROS [72] and the inability of NOD.Ncf1m1J BM-Mϕ to generate superoxide [16] may impair efficient transcriptional induction of Tlr3 expression. Impairment of Tlr3 expression may be partially mediated by inefficient NF-κB signaling in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ. This was evident with Western blot analysis of phospho-IκB-α (S32/S36) as poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ exhibited a 2-fold decrease in NF-κB activation in comparison to NOD BM-Mϕ. Concomitant with a decrease in IκB-α phosphorylation, a decrease in nuclear translocation of NF-κB p50 was also observed in poly(I:C)-stimulated NOD.Ncf1m1J versus NOD BM-Mϕ. The decrease in TLR3 protein expression may hamper the recruitment of the adaptor molecule, TRIF, leading to downstream TLR3 signaling defects and lowered synthesis of type I interferons. We demonstrated previously that LPS-stimulated NOD.Ncf1m1J BM-Mϕ also resulted in a decrease in TNF-α, IL-1β, and IL-12p70 pro-inflammatory cytokine synthesis, suggesting that perturbations with the TLR4 signaling pathway also exist in NOD.Ncf1m1J BM-Mϕ [16].
Defective innate immune responses in NOD.Ncf1m1J BM-Mϕ after poly(I:C) stimulation appears to arise not only from the absence of superoxide synthesis, inefficient NF-κB activation, and dysregulated TLR3 expression, but the absence of the p47phox NOX subunit may also negatively affect additional signaling pathways associated with Type I interferon synthesis. Evidence for this stems from the inability of exogenous superoxide to rescue IFN-α/β synthesis in poly(I:C)-stimulated NOD.Ncf1m1J BM-Mϕ, but was able to restore NF-κB-dependent pro-inflammatory cytokines such as TNF-α. Addition of superoxide via xanthine oxidase inhibited IFN-α/β levels further in both poly(I:C)-stimulated NOD and NOD.Ncf1m1J BM-Mϕ demonstrating that efficient synthesis of Type I interferons is redox-sensitive. Previous reports have also shown that mutations in Ncf1 can affect innate immune responses independent of superoxide synthesis. Richter, et al demonstrated that the TLR9/MyD88 signaling pathway and expression of IL-12p70 in dendritic cells was negatively regulated by p47phox independent of NOX2 activation [73]. Interestingly, Takeshita, et al showed that p47phox physically interacts with TNF-associated factor (TRAF) 4 to bind TRAF6 and TRIF to suppress TLR9 signaling and IFN-β expression [74]. Consistent with these observations, our work demonstrates the importance of both superoxide synthesis and NOX protein function to regulate innate immune responses in macrophages after poly(I:C) stimulation for Type I interferon synthesis. Future experiments will determine if p47phox in NOD BM-Mϕ will physically interact with downstream TLR3 signaling intermediates and adaptor molecules such as TRAF6, TRAF4, and TBK1 for optimal IFN-α/β production.
In contrast to TNF-α and IFN-β pro-inflammatory cytokine synthesis, MHC-II I-Ag7 expression increased 2-fold in NOD.Ncf1m1J BM-Mϕ relative to NOD BM-Mϕ, suggesting that redox balance may also negatively impact the expression of MHC-II I-Ag7 and the subsequent activation of effector and regulatory CD4 T cells. The transcriptional regulation of MHC-II I-Ag7 is highly complex, involving chromatin modifying enzymes [75], redox-sensitive transcription factor binding sites including AP-1, and the involvement of the class II transactivator (CIITA) to facilitate the coordinated interaction of transcription factors/co-factors in an enhanceosome complex for optimal MHC-II expression [76]. Interestingly, transcriptional analysis of the gene encoding CIITA, Ciita, was moderately upregulated in NOD.Ncf1m1J BM-Mϕ relative to NOD BM-Mϕ (data not shown), suggesting that additional post-transcriptional mechanisms may underlie the marked increase in MHC-II I-Ag7 expression. Lack of NOX-derived ROS may downregulate negative regulators of MHC-II such as E3 ubiquitin ligases that target MHC-II to lysosomes for degradation [77] or histone deacetylases 1 or 2 known to bind to CIITA inhibiting its interaction with the enhanceosome or target CIITA for degradation, respectively [78].
In addition to TLR3, viral dsRNA is also detected by cytosolic sensors of dsRNA including dsRNA-dependent protein kinase R (PKR), retinoic acid-inducible gene I (RIG-I), and melanoma differentiation-associated gene 5 (MDA5) to efficiently induce the synthesis of type I interferons and anti-viral responses [79, 80]. The role of ROS in mediating the activation of these innate immune pattern recognition receptors is understudied, but a recent manuscript demonstrated that NOX2-derived ROS synthesis is also necessary for mediating RIG-I-dependent anti-viral responses and IFN-β synthesis [81]. Anti-viral responses can also be initiated by a TLR3-independent mechanism consisting of MAPK pathway activation. Steer, et al. demonstrated that TLR3-deficient macrophages induced cyclooxygenase-2 and pro-inflammatory prostaglandin production via p38MAPK and JNK pathway activation in response to poly(I:C)- or encephalomyocarditis viral-stimulation [82]. Additionally, PKR-deficient macrophages in response to poly(I:C)-treatment were still capable of IL-1β and inducible nitric oxide synthase (iNOS) expression due to the activation of the ERK pathway [83]. Future studies will examine if TLR3-independent signaling pathways involved in anti-viral responses such as MAPK, PKR, RIG-I, and MDA5 are regulated by ROS synthesis and if they also exhibit altered innate immune responses in NOD.Ncf1m1J BM-Mϕ.
Interestingly, the incidence of spontaneous diabetes was not affected in TLR3-deficient NOD mice [84] and discrepancies exist with induced TLR3 signaling and T1D. Poly(I:C)-treated rodent models of T1D have demonstrated protection [71, 85], but also susceptibility to disease [86, 87]. These differences may be partially due to different model systems, genetic backgrounds, and poly(I:C) dose. Nevertheless, we have gained additional insight on the effects of ROS-mediated activation of innate immune responses in T1D. NOD.Ncf1m1J BM-Mϕ exhibited defects in TLR3/TRIF and NF-κB signaling, with concomitant decreases in pro-inflammatory cytokine synthesis and dysregulated MHC-II I-Ag7 expression that may partially explain the unique T1D-resistance exhibited by NOD.Ncf1m1J mice [16, 88].
Acknowledgments
We wish to thank Lindsey E. Padgett and Drs. Sasanka Ramanadham and Jon Piganelli for careful reading of this manuscript and Dr. Guangjie Cheng for help with the luminol-based oxidation assay.
Grant Support: This work was supported by an American Diabetes Association Junior Faculty Award (1-09-JF-54) and a P30 Pilot Feasibility award from the UAB Comprehensive Diabetes Center/Diabetes Research Training Center (HMT). The following core facilities were used to generate data for the manuscript: Animal Resources Program (G20RR025858, Sam Cartner, DVM, PhD), the Comprehensive Arthritis, Musculoskeletal, and Autoimmunity Center: Analytic and Preparative Cytometry Facility (P30 AR48311, John D. Mountz, MD, PhD), and the Comprehensive Arthritis, Musculoskeletal, and Autoimmunity Center: Epitope Recognition Immunoreagent Core (P30 AR48311, Mary Ann Accavitti-Loper, PhD). No author of this paper has a conflict of interest, including financial interests, relationships, and/or affiliations relevant to the subject matter or materials included in this manuscript.
List of Abbreviations
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- T1D
Type 1 diabetes
- NOD
Non-Obese Diabetic
- BM-Mϕ
bone marrow-derived macrophages
- MHC
Major Histocompatibility Complex
- NOX
NADPH oxidase
- Ncf1
neutrophil cytosolic factor 1
- TLR
Toll-like receptor
- poly(I:C)
polyinosinic-polycytidylic acid
- TRIF
Toll/lL-1 receptor domain-containing adapter inducing interferon-beta
- NF-κB
nuclear factor κB
- IκB-α
NF-κB inhibitory protein α
- IKKα/β/ε
IκB kinase α/β/ε
- TBK1
TANK-binding kinase 1
- IRF3
interferon regulatory factor 3
- IFN-α/β
interferon-α/β
- TNF-α
Tumor necrosis factor-α
- PKR
dsRNA-dependent protein kinase R
- RIG-I
retinoic acid-inducible gene I
- MDA5
melanoma differentiation-associated gene 5
- iNOS
inducible nitric oxide synthase
- XO
xanthine oxidase
- SOD
superoxide dismutase
- TRAF
TNF-associated factor
References
- [1].D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- [2].Medzhitov R, Janeway CA., Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- [3].Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- [4].Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- [5].Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- [6].Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- [7].Sklavos MM, Tse HM, Piganelli JD. Redox modulation inhibits CD8 T cell effector function. Free Radic Biol Med. 2008;45:1477–1486. doi: 10.1016/j.freeradbiomed.2008.08.023. [DOI] [PubMed] [Google Scholar]
- [8].Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gusdon AMC, John A. Mathews, Clayton E. Type 1 diabetes: Islet inflammation - the contribution of cytokines and beta cells. Drug Discovery Today: Disease Mechanisms. 2006:3. [Google Scholar]
- [10].Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- [11].Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- [12].Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+ J Leukoc Biol. 2000;67:210–215. doi: 10.1002/jlb.67.2.210. [DOI] [PubMed] [Google Scholar]
- [13].Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hultqvist M, Holmdahl R. Ncf1 (p47phox) polymorphism determines oxidative burst and the severity of arthritis in rats and mice. Cell Immunol. 2005;233:97–101. doi: 10.1016/j.cellimm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [15].Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tse HM, Thayer TC, Steele C, Cuda CM, Morel L, Piganelli JD, Mathews CE. NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J Immunol. 2010;185:5247–5258. doi: 10.4049/jimmunol.1001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaisho T, Akira S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol Scand. 2001;59:124–130. doi: 10.1080/000163501750266701. [DOI] [PubMed] [Google Scholar]
- [18].Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- [19].Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meyers AJ, Shah RR, Gottlieb PA, Zipris D. Altered Toll-like receptor signaling pathways in human type 1 diabetes. J Mol Med. 2010;88:1221–1231. doi: 10.1007/s00109-010-0666-6. [DOI] [PubMed] [Google Scholar]
- [21].Park Y, Park S, Yoo E, Kim D, Shin H. Association of the polymorphism for Toll-like receptor 2 with type 1 diabetes susceptibility. Ann N Y Acad Sci. 2004;1037:170–174. doi: 10.1196/annals.1337.028. [DOI] [PubMed] [Google Scholar]
- [22].Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein H, Hong SC, McInerney MF. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int Immunol. 2006;18:1101–1113. doi: 10.1093/intimm/dxl045. [DOI] [PubMed] [Google Scholar]
- [23].Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- [25].Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- [26].Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [27].Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, Shi W, Zheng SG. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3(+) Treg. Eur J Immunol. 2009 doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tse HM, Josephy SI, Chan ED, Fouts D, Cooper AM. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J Immunol. 2002;168:825–833. doi: 10.4049/jimmunol.168.2.825. [DOI] [PubMed] [Google Scholar]
- [29].Batinic-Haberle I, Reboucas JS, Spasojevich I. Superoxide Dismutase Mimics: Chemistry, Pharmacology and Therapeutic Potential. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- [31].Cheng G, Salerno JC, Cao Z, Pagano PJ, Lambeth JD. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic Biol Med. 2008;45:1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dahlgren C, Follin P, Johansson A, Lock R, Orselius K. Localization of the luminol-dependent chemiluminescence reaction in human granulocytes. Journal of bioluminescence and chemiluminescence. 1989;4:263–266. doi: 10.1002/bio.1170040137. [DOI] [PubMed] [Google Scholar]
- [33].Johansson A, Dahlgren C. Characterization of the luminol-amplified light-generating reaction induced in human monocytes. J Leukoc Biol. 1989;45:444–451. doi: 10.1002/jlb.45.5.444. [DOI] [PubMed] [Google Scholar]
- [34].Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J Immunol. 2001;166:2444–2450. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- [35].Cantor J, Haskins K. Effector function of diabetogenic CD4 Th1 T cell clones: a central role for TNF-alpha. J Immunol. 2005;175:7738–7745. doi: 10.4049/jimmunol.175.11.7738. [DOI] [PubMed] [Google Scholar]
- [36].Lenschow DJ, Giannakopoulos NV, Gunn LJ, Johnston C, O’Guin AK, Schmidt RE, Levine B, Virgin H. W. t. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79:13974–13983. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- [38].Moynagh PN. The NF-kappaB pathway. J Cell Sci. 2005;118:4589–4592. doi: 10.1242/jcs.02579. [DOI] [PubMed] [Google Scholar]
- [39].Heinz S, Haehnel V, Karaghiosoff M, Schwarzfischer L, Muller M, Krause SW, Rehli M. Species-specific regulation of Toll-like receptor 3 genes in men and mice. J Biol Chem. 2003;278:21502–21509. doi: 10.1074/jbc.M301476200. [DOI] [PubMed] [Google Scholar]
- [40].Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- [41].Hayes SM, Li L, Love PE. TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- [42].Slavik JM, Hutchcroft JE, Bierer BE. CD28/CTLA-4 and CD80/CD86 families: signaling and function. Immunol Res. 1999;19:1–24. doi: 10.1007/BF02786473. [DOI] [PubMed] [Google Scholar]
- [43].Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- [44].Mackey MF, Barth RJ, Jr., Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- [45].Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- [46].Haddad JJ, Safieh-Garabedian B, Saade NE, Lauterbach R. Inhibition of glutathione-related enzymes augments LPS-mediated cytokine biosynthesis: involvement of an IkappaB/NF-kappaB-sensitive pathway in the alveolar epithelium. Int Immunopharmacol. 2002;2:1567–1583. doi: 10.1016/s1567-5769(02)00117-0. [DOI] [PubMed] [Google Scholar]
- [47].Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760–5767. doi: 10.4049/jimmunol.179.9.5760. [DOI] [PubMed] [Google Scholar]
- [48].Rosmalen JG, Martin T, Dobbs C, Voerman JS, Drexhage HA, Haskins K, Leenen PJ. Subsets of macrophages and dendritic cells in nonobese diabetic mouse pancreatic inflammatory infiltrates: correlation with the development of diabetes. Lab Invest. 2000;80:23–30. doi: 10.1038/labinvest.3780004. [DOI] [PubMed] [Google Scholar]
- [49].Ho E, Bray TM. Antioxidants, NFkappaB activation, and diabetogenesis. Proc Soc Exp Biol Med. 1999;222:205–213. doi: 10.1046/j.1525-1373.1999.d01-137.x. [DOI] [PubMed] [Google Scholar]
- [50].Maree AF, Komba M, Dyck C, Labecki M, Finegood DT, Edelstein-Keshet L. Quantifying macrophage defects in type 1 diabetes. J Theor Biol. 2005;233:533–551. doi: 10.1016/j.jtbi.2004.10.030. [DOI] [PubMed] [Google Scholar]
- [51].Piganelli JD, Martin T, Haskins K. Splenic macrophages from the NOD mouse are defective in the ability to present antigen. Diabetes. 1998;47:1212–1218. doi: 10.2337/diab.47.8.1212. [DOI] [PubMed] [Google Scholar]
- [52].Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- [53].Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [54].Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- [55].Shibasaki S, Imagawa A, Tauriainen S, Iino M, Oikarinen M, Abiru H, Tamaki K, Seino H, Nishi K, Takase I, Okada Y, Uno S, Murase-Mishiba Y, Terasaki J, Makino H, Shimomura I, Hyoty H, Hanafusa T. Expression of toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–219. doi: 10.1507/endocrj.k09e-291. [DOI] [PubMed] [Google Scholar]
- [56].Pirie FJ, Pegoraro R, Motala AA, Rauff S, Rom L, Govender T, Esterhuizen TM. Toll-like receptor 3 gene polymorphisms in South African Blacks with type 1 diabetes. Tissue Antigens. 2005;66:125–130. doi: 10.1111/j.1399-0039.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- [57].Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA. TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J Immunol. 2005;174:131–142. doi: 10.4049/jimmunol.174.1.131. [DOI] [PubMed] [Google Scholar]
- [58].Wen L, Peng J, Li Z, Wong FS. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J Immunol. 2004;172:3173–3180. doi: 10.4049/jimmunol.172.5.3173. [DOI] [PubMed] [Google Scholar]
- [59].Gamble DR, Kinsley ML, FitzGerald MG, Bolton R, Taylor KW. Viral antibodies in diabetes mellitus. Br Med J. 1969;3:627–630. doi: 10.1136/bmj.3.5671.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gamble DR, Taylor KW, Cumming H. Coxsackie viruses and diabetes mellitus. Br Med J. 1973;4:260–262. doi: 10.1136/bmj.4.5887.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- [62].Yoon JW, Jun HS. Viruses cause type 1 diabetes in animals. Ann N Y Acad Sci. 2006;1079:138–146. doi: 10.1196/annals.1375.021. [DOI] [PubMed] [Google Scholar]
- [63].Hyoty H, Taylor KW. The role of viruses in human diabetes. Diabetologia. 2002;45:1353–1361. doi: 10.1007/s00125-002-0852-3. [DOI] [PubMed] [Google Scholar]
- [64].Tracy S, Drescher KM, Jackson JD, Kim K, Kono K. Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev Med Virol. 2010;20:106–116. doi: 10.1002/rmv.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- [66].Devendra D, Jasinski J, Melanitou E, Nakayama M, Li M, Hensley B, Paronen J, Moriyama H, Miao D, Eisenbarth GS, Liu E. Interferon-alpha as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes. 2005;54:2549–2556. doi: 10.2337/diabetes.54.9.2549. [DOI] [PubMed] [Google Scholar]
- [67].Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- [68].Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Alba A, Puertas MC, Carrillo J, Planas R, Ampudia R, Pastor X, Bosch F, Pujol-Borrell R, Verdaguer J, Vives-Pi M. IFN beta accelerates autoimmune type 1 diabetes in nonobese diabetic mice and breaks the tolerance to beta cells in nondiabetes-prone mice. J Immunol. 2004;173:6667–6675. doi: 10.4049/jimmunol.173.11.6667. [DOI] [PubMed] [Google Scholar]
- [70].Kado S, Miyamoto J, Komatsu N, Iwaki Y, Ozaki H, Taguchi H, Kure M, Sarashina G, Watanabe T, Katsura Y, Nemoto Y, Noritake M, Matsuoka T. Type 1 diabetes mellitus caused by treatment with interferon-beta. Intern Med. 2000;39:146–149. doi: 10.2169/internalmedicine.39.146. [DOI] [PubMed] [Google Scholar]
- [71].Wong FS, Wen L. IFN-alpha can both protect against and promote the development of type 1 diabetes. Ann N Y Acad Sci. 2008;1150:187–189. doi: 10.1196/annals.1447.031. [DOI] [PubMed] [Google Scholar]
- [72].Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- [73].Richter C, Juan MH, Will J, Brandes RP, Kalinke U, Akira S, Pfeilschifter JM, Hultqvist M, Holmdahl R, Radeke HH. Ncf1 provides a reactive oxygen species-independent negative feedback regulation of TLR9-induced IL-12p70 in murine dendritic cells. J Immunol. 2009;182:4183–4191. doi: 10.4049/jimmunol.0800795. [DOI] [PubMed] [Google Scholar]
- [74].Takeshita F, Ishii KJ, Kobiyama K, Kojima Y, Coban C, Sasaki S, Ishii N, Klinman DM, Okuda K, Akira S, Suzuki K. TRAF4 acts as a silencer in TLR-mediated signaling through the association with TRAF6 and TRIF. Eur J Immunol. 2005;35:2477–2485. doi: 10.1002/eji.200526151. [DOI] [PubMed] [Google Scholar]
- [75].Zika E, Ting JP. Epigenetic control of MHC-II: interplay between CIITA and histone-modifying enzymes. Curr Opin Immunol. 2005;17:58–64. doi: 10.1016/j.coi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [76].Harari O, Liao JK. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des. 2004;10:893–898. doi: 10.2174/1381612043452893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ishido S, Goto E, Matsuki Y, Ohmura-Hoshino M. E3 ubiquitin ligases for MHC molecules. Curr Opin Immunol. 2009;21:78–83. doi: 10.1016/j.coi.2009.01.002. [DOI] [PubMed] [Google Scholar]
- [78].Choi NM, Majumder P, Boss JM. Regulation of major histocompatibility complex class II genes. Curr Opin Immunol. 2011;23:81–87. doi: 10.1016/j.coi.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- [80].Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- [81].Soucy-Faulkner A, Mukawera E, Fink K, Martel A, Jouan L, Nzengue Y, Lamarre D, Vande Velde C, Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Steer SA, Moran JM, Christmann BS, Maggi LB, Jr., Corbett JA. Role of MAPK in the regulation of double-stranded RNA- and encephalomyocarditis virus-induced cyclooxygenase-2 expression by macrophages. J Immunol. 2006;177:3413–3420. doi: 10.4049/jimmunol.177.5.3413. [DOI] [PubMed] [Google Scholar]
- [83].Maggi LB, Jr., Moran JM, Buller RM, Corbett JA. ERK activation is required for double-stranded RNA- and virus-induced interleukin-1 expression by macrophages. J Biol Chem. 2003;278:16683–16689. doi: 10.1074/jbc.M211744200. [DOI] [PubMed] [Google Scholar]
- [84].Wong FS, Hu C, Zhang L, Du W, Alexopoulou L, Flavell RA, Wen L. The role of Toll-like receptors 3 and 9 in the development of autoimmune diabetes in NOD mice. Ann N Y Acad Sci. 2008;1150:146–148. doi: 10.1196/annals.1447.039. [DOI] [PubMed] [Google Scholar]
- [85].Serreze DV, Hamaguchi K, Leiter EH. Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun. 1989;2:759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- [86].Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N. Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol. 2004;110:134–144. doi: 10.1016/j.clim.2003.09.014. [DOI] [PubMed] [Google Scholar]
- [87].Horwitz MS, Ilic A, Fine C, Rodriguez E, Sarvetnick N. Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J Clin Invest. 2002;109:79–87. doi: 10.1172/JCI11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Thayer TC, Delano M, Liu C, Chen J, Padgett LE, Tse HM, Annamali M, Piganelli JD, Moldawer LL, Mathews CE. Superoxide Production by Macrophages and T Cells Is Critical for the Induction of Autoreactivity and Type 1 Diabetes. Diabetes. 2011 doi: 10.2337/db10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]