Abstract
Hydroxyl radicals are widely implicated in the oxidation of carbohydrates in biological and industrial processes and are often responsible for their structural modification resulting in functional damage. In this study, the radical depolymerization of the polysaccharide hyaluronan was studied in a reaction with hydroxyl radicals generated by Fenton Chemistry. A simple method for isolation and identification of the resulting non-sulfated oligosaccharide products of oxidative depolymerization was established. Hyaluronan oligosaccharides were analyzed using ion-pairing reversed phase high performance liquid chromotography coupled with tandem electrospray mass spectrometry. The sequence of saturated hyaluronan oligosaccharides having even- and odd-numbers of saccharide units, afforded through oxidative depolymerization, were identified. This study represents a simple, effective ‘fingerprinting’ protocol for detecting the damage done to hyaluronan by oxidative radicals. This study should help reveal the potential biological outcome of reactive-oxygen radical-mediated depolymerization of hyaluronan.
Keywords: Hyaluronan, oligosaccharides, free radical depolymerization, mass spectrometry
1. Introduction
There are numerous diseases that involve reactive oxygen species (ROS) at the onset and/or in later stages of disease, including arthritic conditions and inflammatory diseases of joints (Rees, Kennett, Whitelock, & Davies, 2008; Sampson, Rochester, Freundlich, & Elias, 1992). Of the more than 100 arthritic diseases, osteoarthritis (OA) and rheumatoid arthritis (RA) are the most common chronic conditions affecting elderly populations. Although the etiology and pathogenesis of RA are as yet not completely understood, a progressive degradation of polymeric carbohydrates, including hyaluronan, a main component of joints, is observed during the course of the disease (Henrotin, Bruckner, & Pujol, 2003). The observed reduction of hyaluronan molecular weight in the synovial fluid of patients suffering from RA has motivated researchers to study the hyaluronan degradation by reactive oxygen (Halliwell, 1982; Kvam, Fragonas, Degrassi, Kvam, Matulova, Pollesello, Zanetti, & Vittur, 1995).
Hyaluronan is a negatively charged high molecular weight glycosaminoglycan, with repeating disaccharide units consisting of D-glucuronic acid (GlcA) and D-N-acetylglucosamine (GlcNAc) through alternating β-1,3, β-1,4 linkages (Fraser, Laurent, & Laurent, 1997). Hyaluronan is ubiquitously distributed in the extracellular matrix and is a component of the basement membrane of normal lungs, joints, and vitreous fluid, functions in water homeostasis, plasma protein distribution and transportation, joint lubrication, and matrix structure. The vast majority of hyaluronan is produced by fibroblasts and to a lesser degree by smooth muscle cells (Fraser, Laurent, & Laurent, 1997). Hyaluronan is also used as pharmaceutical excipient (hydrogel formation, drug attachment) as well as drug (wound healing, anti-inflammation). In vivo, at sites of inflammation, high molecular weight hyaluronan (Mr 2–6×106) can be depolymerized to lower molecular weight (Mr 2×105) hyaluronan and to hyaluronan oligosaccharides by oxygen radicals and through enzymatic degradation by hyaluronidase, β-glucuronidase, and β-hexosaminidase (Henrotin, Bruckner, & Pujol, 2003). Inflammatory cytokines can also stimulate pulmonary fibroblasts to produce increased amounts of hyaluronan fragments (Sampson, Rochester, Freundlich & Elias, 1992). In its high molecular weight form, hyaluronan is believed to play a homeostatic role. However, in the setting of tissue destruction, high molecular weight hyaluronan is broken down into its lower molecular weight components that possess the ability to induce inflammatory gene expression (Scheibner, Lutz, Boodoo, Fenton, Powell, & Horton, 2006)
The sequences of oligosaccharide products have been studied through the complex process of size chromatographic column separation, desalting and MS analysis (Yang, Yu, Zhao, Jiao, Ren, & Chai, 2009). Anion exchange-high performance liquid chromatography (HPLC), although conventionally used to separate GAG oligosaccharides, is difficult to interface with electrospray ionization (ESI)-mass spectrometry (MS) since it uses mobile phases containing high concentrations of nonvolatile salts required to elute the anionic analytes (Volpi, & Linhardt, 2010). For a rapid determination of complex mixtures of hyaluronan oligosaccharides from digests, without the further derivatization steps, analytical separation is often required followed by detection and identification by MS. On-line, reversed-phase ion-paring (RPIP)-HPLC-ESI-MS analysis has enabled the separation and simultaneous characterization of a number of different GAG-derived oligosaccharide mixtures obtained by controlled enzymatic depolymerization of heparan sulfate, chondroitin sulfate and keratan sulfate GAGs without extensive sample purification (Volpi, & Linhardt, 2010). RPIP-HPLC, using tributylamine ion-pairing reagent affords a volatile mobile phase, providing excellent chromatographic resolution and MS compatibility. Hyaluronan oligosaccharides, resulting from enzymatic degradation with hyaluronate lyase, have been characterized by liquid chromatography (LC)-ESI-tandem MS and by matrix-assisted laser description ionization (MALDI)-time of flight (TOF) mass spectrometry (Kühn, Raith, Sauerland & Neubert, 2003; Volpi, 2007). Hyaluronan lyase is an endo-β-hexosaminidase, producing even-numbered hyaluronan oligosaccharide of structure 4-deoxy-α-L-threo-hax-4-enopyranosyl uronic acid (ΔUA)-(1→3)-[β-D-GlcNAc-(1→4)-β-D-Glc A]n-(1→3)-D-GlcNAc (where n = 0,1,2,…). These products are accompanied by very minor amounts of odd-numbered oligomers of structures β-D-GlcNAc-(1→4)[-β-D-GlcA(1→4) -β-D-GlcNAc]n and ΔUA-(1→3)-[β-D-GlcNAc-(1→4)-β-D-GlcA]n. On-line analysis can eliminate a time-consuming fraction collection step, and greatly enhances the analysis and structural characterization of complex mixtures of GAG-derived oligosaccharides. Furthermore, the application of this analytical approach also allows the observation of minor products arising from the natural sequence.
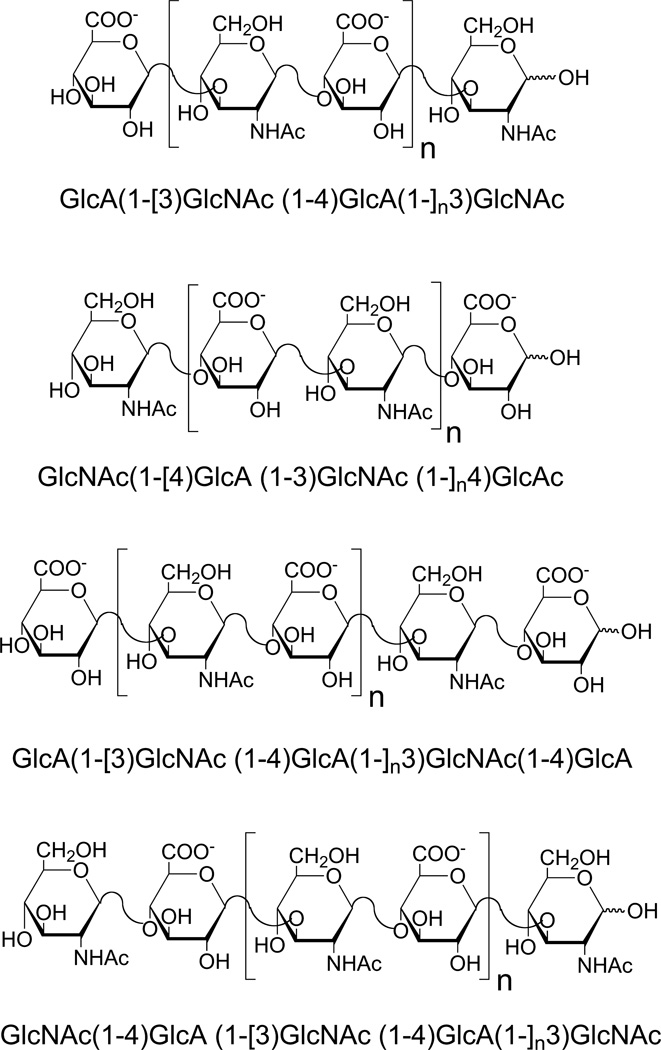
The hydroxyl radical is the most reactive of the ROS. Electron paramagnetic resonance (EPR) and nuclear magnetic spectroscopy (NMR) have been used for the analysis of hyaluronan fragments produced by hydroxyl radical-induced depolymerization reactions (Šoltes, Kogan, Schiller, Stankovká, &Arnhold, 2006). Results show random hydrogen atom abstraction at all the ring C-H bonds within glucuronic acid, as well as at all sites except the N-acetyl group and C2 position within the N-acetylglucosamine unit. Results of EPR spectroscopic studies support the hypothesis that the hyaluronan strand cleavage may be due to β-cleavage of the radicals formed either at C1 of the monosaccharide ring at C3 of the N-acetylglucosamine, or at C4 of the glucuronic acid ring. Radical deplymerization products were much more complex than enzymatic digestion products, with even-numbered and odd-numbered fragments (Fig. 1), making their analysis more difficult.
Fig. 1.
Structure of typical oligosaccharides produced by free radical depolymerization. Four structures are shown. The first two have an even number of saccharide residues terminating with different saccharides at their reducing and nonreducing ends. The second two have an odd number of saccharide residues terminating with different saccharides at their reducing and nonreducing ends. The number n = 0, 1, 2, 3, etc.
In this paper, we describe a new method using on-line RPIP-HPLC-ESI-tandem MS for the measurement of hyaluronan fragments derived from both lyase digestion and free radical depolymerization. This analytical approach provides an oligosaccharide map together with sequence information of the hyaluronan after free radical treatment.
2. Materials and methods
2.1 Reagents
Hyaluronan sodium salt from Streptococcus zooepidemicus was generously provided by Professor Toshihiko Toida (Chiba University, Japan). Hydrogen peroxide (H2O2), iron (II) sulfate heptahydrate (FeSO4· 7H2O), sodium borodeuteride (NaBD4) (98 atom % D), boric acid and chondroitin lyase AC2 were from Sigma-Aldrich. Cation exchange resin AG50W-X8 (H form) was from Bio-Rad.
2.2 Partially enzymatic digestion of hyaluronan
Pure hyaluronan (10 mg in 500 μL water) was partially digested by chondroitin lyase AC2 (EC 4.2.2.5, 50 mU) at 37° C for 3 h, after which the reaction was stopped by heating to 100° C for 3 min and large molecules were removed by 50 vol. % ethanol precipitation. The supernatant was concentrated by rotary evaporation and freeze-dried. Hyaluronan can be recovered and purified from cultured cells or tissues by proteolysis and filtration using mini-strong anion exchange spin column was washed with 8 M urea buffer (2% CHAPS, pH 8.3) to remove peptides, and sequentially eluted with aqueous NaCl washes (Zhao, Yang, Datta, Gasmili, Zhang & Linhardt).
2.3 Free radical depolymerization of hyaluronan
Hyaluronan (10 mg in 500 μL Na2HPO4(50 mM)) was mixed with 5 μL ferrous sulfate (0.1 M) and 15 μL H2O2 (30%) to start the free radical depolymerization at 60° C for 6 h. The solution was cooled to room temperature and centrifuged at 10,000 × g and the supernatant was recovered and adjusted to pH 8.0 with 0.5 M NH4HCO3. Residual H2O2 was removed by vacuum evaporation at 45° C and freeze-dried two times from water. The depolymerized hyaluronan mixture was dissolved into elution buffer for RPIP–HPLC–ESI-MS analysis.
2.4 Oligosaccharide reduction
NaBD4 reagent (20 μL of 0.05 M NaBD4 in 0.01 M NaOH) was added to the freeze-dried hyaluronan oligosaccharide (typically 20 μg), and the reduction carried out overnight at 4°C as previously described (Yang, Yu, Zhao, Jiao, Ren, & Chai, 2009). The reaction solution was then neutralized to pH 7 with a solution of AcOH/H2O (1:1) to destroy borohydride before passing through a 100-μL mini-column packed with cation exchange resin (AG50W-X8, H+ form). After loading the sample on the column, the column was washed by 500-μL water and the eluent solution was collected and freeze-dried. Boric acid was removed by repeated co-evaporation with MeOH.
2.5 LC-CID MS/MS analysis of hyaluronan oligosaccharides
RPIP-HPLC separation of hyaluronan oligosaccharides was performed on a Poroshell 120 EC-C18 column (3.0 × 150 mm, 2.7 μm, Agilent) according to Volpi & Linhardt (2010). Eluent A: water/acetonitrile (85:15, v/v), Eluent B: water/acetonitrile (35:65, v/v). Both eluents contained 12 mM TrBA and 38 mM NH4OAc with pH adjusted to 6.5 with HOAc. The freeze-dried depolymerized hyaluronan mixture was dissolved into elution buffer A for RPIP-HPLC-ESI-MS analysis. A gradient of solution A for 10 min followed by a linear gradient from 10 to 40 min (0–50% solution B) was used at a flow rate of 100 μL/min for oligosaccharide separation.
The column effluent entered the source of the ES-MS for continuous detection by MS. The ES-MS was carried on Agilent 1200 LC/MSD instrument (Agilent Technologies, Wilmington, DE, USA) equipped with a 6300 ion trap and a binary pump. The electrospray interface was set in negative ionization mode with a skimmer potential of 40.0 V, a capillary exit of −50.0 V, and a source temperature of 350° C to obtain the maximum abundance of the ions in a full-scan spectrum (200–1700 Da). Nitrogen (8 L/min, 40 psi) was used as a drying and nebulizing gas. For (collisionally induced dissociation (CID)-tandem MS product-ion scanning, argon was used as the collision gas at a pressure of 1.7 bar and the collision energy was adjusted between 0.1–0.7 for optimal sequence information.
3. Results and discussion
3.1 RPIP-HPLC separation of hyaluronan-derived oligosaccharides
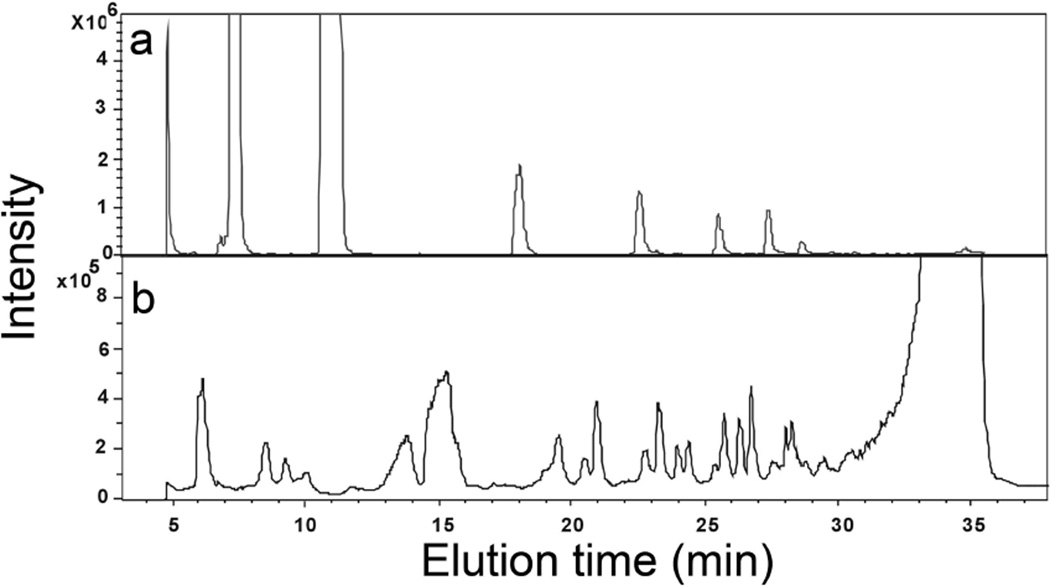
Hyaluronan was partially depolymerized (40%) by controlled digestion with chondroitin lyase AC2 (chondroitin lyase AC2 is known to act endolytically on hyaluronan (Linhardt, 2001)). RPIP-HPLC-ESI-MS was applied to the separation and characterization of unsaturated oligosaccharides from 2-mer (disaccharide) to 30-mer. These hyaluronan oligosaccharides were composed of repeating β-1,4-D-glucuronic acid (GlcA) and β-1,3-D-N-acetylglucosamine (GlcNAc) disaccharide unit, with a non-reducing terminal ΔUA residue. Extensive experiments were undertaken to optimize the HPLC separation of both small oligosaccharides (2–6-mers) and large oligosaccharides (10–30-mers) from enzymatic digestion mixture in order to obtain a peak for each oligosaccharide present (Fig. 2A and Table 1).
Fig. 2.
Total ion chromatogram (TIC) of hyaluronan oligosaccharides analyzed in the negative-ion mode and separated by RPIP-HPLC. a. oligosaccharides obtained by partial treatment with chondroitin lyase AC2 and b. oligosaccharides obtained from free radical depolymerization.
Table 1.
Negative-ion ESI-MS of oligosaccharide obtained from enzymatic digestion and radical depolymerization of hyaluronan.
| Found ions (charge) |
Calculated Mol Weight (Da) |
Sequences | Theoretical Weight (Da) |
Ion strength (M) |
Retention time (min) |
|
|---|---|---|---|---|---|---|
| dp | Assignment | |||||
| Even number | ||||||
| 377.9 | 378.9 | 2 | ΔGlcA-GlcNAc | 379.1 | 7.2 | |
| 757.0 | 758.0 | 4 | ΔGlcA-GlcNAc-GlcA-GlcNAc | 758.2 | 11.2 | |
| 567.6 | 1137.2 | 6 | ΔGlcA-GlcNAc-(GlcA-GlcNAc)2 | 1137.3 | 18.1 | |
| 756.9 | 1515.8 | 8 | ΔGlcA-GlcNAc-(GlcA-GlcNAc)3 | 1516.4 | 23.0 | |
| 946.7 | 1895.4 | 10 | ΔGlcA-GlcNAc-(GlcA-GlcNAc)4 | 1895.6 | 25.8 | |
| 395.9 (−1) | 396.9 | 2 | GlcA-GlcNAc | 397.1 | 1.8 × 104 | 6.1 |
| 775.1 (−1) | 776.1 | 4 | GlcA-GlcNAc-GlcA-GlcNAc | 776.2 | 7.2 × 104 | 8.4 |
| 1154.1(−1) | 1155.1 | 6 | GlcA-(GlcNAc-GlcA)2-GlcNAc | 1155.3 | 3.0 × 104 | 13.8 |
| 576.4 (−2) | 1154.8 | 5.6 × 104 | ||||
| 766.0(−2) | 1534.0 | 8 | GlcA-(GlcNAc-GlcA)3-GlcNAc | 1534.5 | 6.1 × 104 | 19.8 |
| 955.9(−2) | 1913.8 | 10 | GlcA-(GlcNAc-GlcA)4-GlcNAc | 1913.6 | 7.2 × 104 | 23.9 |
| Odd number | ||||||
| 598.9 (−1) | 599.9 | 3 | GlcNAc-GlcA-GlcNAc | 600.2 | 7.0 × 104 | 6.1 |
| 571.9 (−1) | 572.9 | 3 | GlcA-GlcNAc-GlcA | 573.1 | 1.3 × 105 | 8.6 |
| 978.0 (−1) | 979.0 | 5 | GlcNAc-GlcA-GlcNAc-GlcA-GlcNAc | 979.3 | 1.2 × 104 | 8.6 |
| 951.0(−1) | 952.4 | 5 | GlcA-GlcNAc-GlcA-GlcNAc-GlcA | 952.4 | 5.2 × 104 | 15.3 |
| 474.2(−2) | 952.0 | 2.2 × 104 | ||||
| 677.9 (−2) | 1357.8 | 7 | GlcNAc-(GlcA-GlcNAc)2-GlcA-GlcNAc | 1358.4 | 5.2 × 104 | 13.8 |
| 664.4 (−2) | 1330.8 | 7 | GlcA-(GlcNAc-GlcA)2-GlcNAc-GlcA | 1331.4 | 1.1 × 105 | 20.9 |
| 867.8 (−2) | 1737.6 | 9 | GlcNAc-(GlcA-GlcNAc)3-GlcA-GlcNAc | 1737.5 | 4.0 × 104 | 19.6 |
Controlled, partial depolymerization of hyaluronan by hydroxyl radical generated within Fenton Chemistry was next monitored by RPIP-HPLC-ESI-MS. The total ion chromatography (TIC) showed that hyaluronan oligosaccharides obtained by free radical depolymerization were much more complex and negative-ion ESIMS unambiguously identified that each peak contained two or three oligosaccharides (Fig. 2B). The identification of a series of the new ions, resulting from the depolymerization of hyaluronan, is summarized in Table 1. No oligosaccharides containing a non-reducing terminal ΔUA residue were observed in the free radical depolymerization products. The smallest oligomer observed in this mixture was disaccharide with m/z 395.9 (−1). A series of negatively charged species of various m/z ratios were observed for the smaller hyaluronan oligosaccharides (2-mer to 5-mer) present mainly as [M-H]−1 anions and the 6-mer to 10-mer were observed predominantly at a charged state of -2. Saturated hyaluronan oligosaccharides from 2-mer to 10-mer were observed and identified, having both an even and odd number of saccharide residues. However there was no introduction of additional oxygen atoms in the saturated oligosaccharides found in the radical depolymerization products. Negative-ion ESIMS unambiguously identified two types of odd-numbered oligosaccharides with monosaccharide compositions of GlcA-(GlcNAc-GlcA)n and GlcNAc-(GlcA-GlcNAc)n (Fig. 1). However, the sequences of even-numbered hyaluronan oligosaccharides could not be confirmed and further CID MS/MS spectra were needed to differentiate the isomeric structures.
The comparison of the retention time of unsaturated oligosaccharides derived from enzymatic digestion and the saturated oligosaccharides derived from free radical depolymerization (Fig. 2 and Table 1) showed that even-numbered unsaturated oligosaccharides (containing a ΔUA their non-reducing terminals) from enzymatic digestion eluted at 7.2, 11.0, 18.0, 23.0, 25.8, 29.0 min. In contrast, saturated even-number oligosaccharides from free radical depolymerization eluted earlier at 6.1, 8.4, 13.8, 19.8 and 23.9. The odd-number saturated oligosaccharides trisaccharide, pentasaccharide and nonasaccharide with GlcNAc at the reducing end was always eluted before the odd-numbered saturated oligosaccharides with GlcA at the reducing end. The results showed that RPIP-HPLC was effective means for separating hyaluronan oligosaccharides with the same degree of polymerization having different sequences.
3.2 Sequence determination of unsaturated even-numbered hyaluronan oligosaccharides
Negative-ion ESI CID-tandem MS was initially performed on molecular-ions having various charge-states, including singly- doubly- and triply- charged ions, as precursors. Product-ion spectra of singly-charged ions were for further study as the other precursors did not produce fragments that were more structurally informative (Yang, et al., 2009). Even-number oligosaccharides obtained from enzyme digestion or free radical depolymerization of hyaluronan were used to investigate the fragmentation pattern, and the principles established were then applied to sequence determination of oligosaccharides with an odd-number of residues.
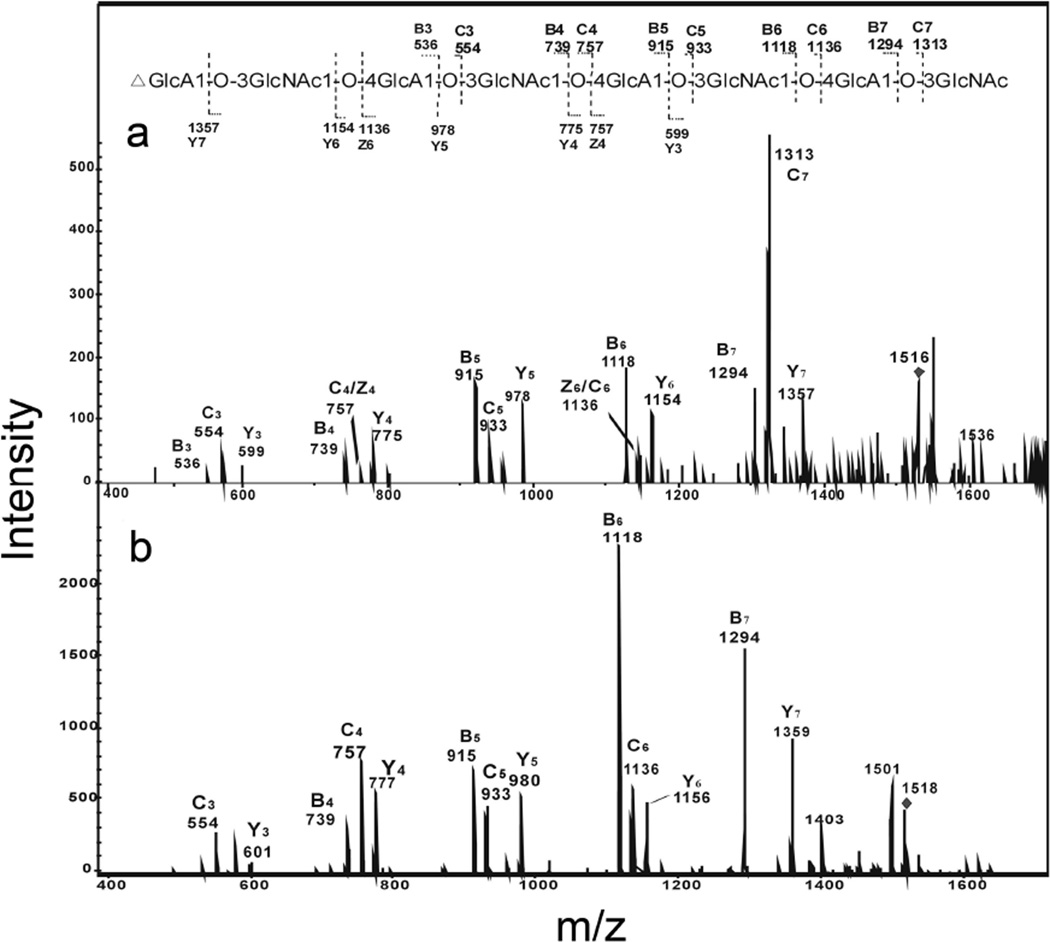
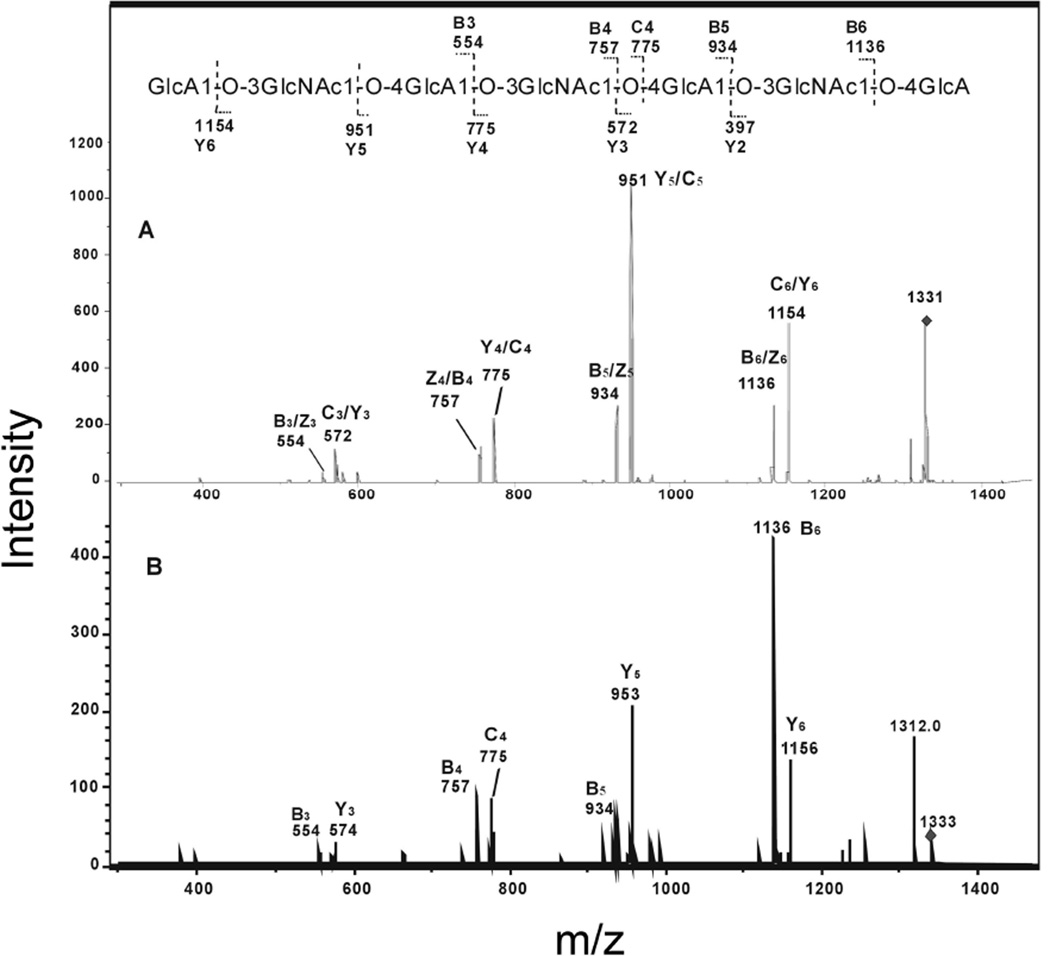
The product-ion spectrum of the deprotonated molecule ion [M - H]− (m/z 1516) of the unsaturated octasaccharide obtained from enzymatic digestion produced extensive glycosidic bond leavage (Fig. 3A). Enzymatic digestion products from hyaluronan were reduced and the product-ion spectrum of the alditol (Fig. 3B) was obtained to differentiate fragment-ions with identical masses arising from glycosidic cleavages at both termini. The reducing terminal fragment-ions showed an increase of 2 Da from the reduction. Only five glycosidic ions, m/z 599, 775, 978, 1154 and 1357 showed 2 Da shift and these were assigned as Y3, Y4, Y5, Y6 and Y7 (Fig. 3 A and B). Fragment-ions m/z 757 and 1136 were confirmed to be C4 and C6, not Z-fragment-ions, because its fragment-ions were unchanged in the product-ion spectrum of its alditol after reduction. Clearly the sequence of unsaturated oligosaccharide can be readily derived from the product-ion spectrum of [M - H]−, with the B-/C-ion doublets and Y-fragment formed at each residue, except the residue at the non-reducing terminal. No cross-ring fragmentation was observed for the hyaluronan oligosaccharides.
Fig. 3.
Negative-ion ESI-CID-tandem mass spectra product-ion spectra of unsaturated octasaccharide obtained from the enzymatic digestion of hyaluronan. A. [M-H]−1 of free radical and B. [M-H]−1 of the reduced alditol. The sequence together with the glycosidic oxygens is shown along with the proposed fragmentation.
3.3 Sequence determination of saturated even-numbered hyaluronan oligosaccharides
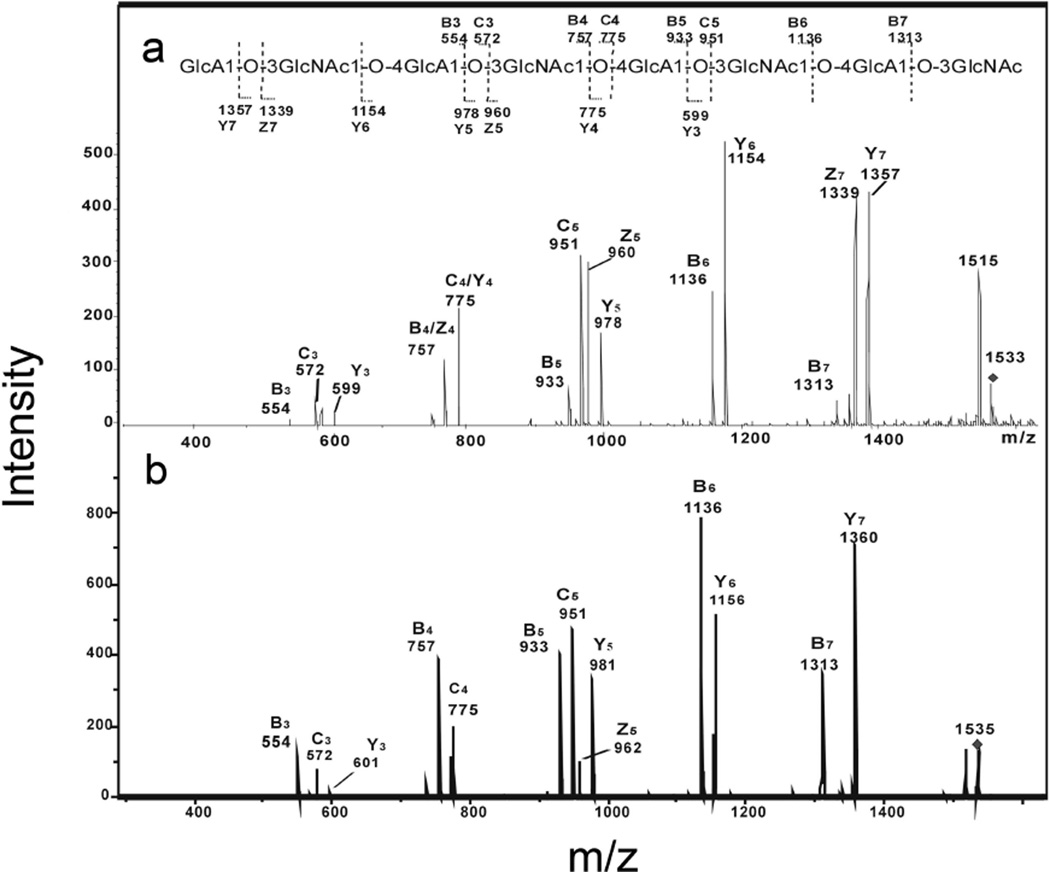
Saturated even-numbered oligosaccharides (Table 1) were obtained from hydroxyl radical depolymerization of hyaluronan. Negative-ion ESI-MS unambiguously identified their even-number monosaccharide compositions to be (GlcA-GlcNAc)n (n = 2–5). ESI-CID tandum MS analysis was used to investigate the fragmentation pattern of saturated oligosaccharides and identify the sequence of isomeric tetrasaccharide, hexasaccharide, and octasaccharide having either GlcNAc or GlcA at the non-reducing terminus. As shown in the ESI-CID tandem MS analysis of saturated octasaccharide, the spectrum of saturated octasaccharide featured extensive B-ions and C-ions together some prominent Y-ions, which quite similar with the unsaturated octasaccharide afforded on enzymatic digestion (Fig. 4). Ions at m/z 599, 978 and 1357 especially from oligosaccharides with GlcNAc termini showed an increase of 2 Da from NaBD4 reduction, while ions at m/z 554, 572, 933 and 951, especially from oligosaccharides with GlcA termini remained unchanged. The sequence of saturated octasaccharide could be unambiguously confirmed to contain four GlcA-GlcNAc repeat units, with a GlcNAc residue at the reducing end. The same sequences were derived from the ESI-CID tandem MS analysis of saturated tetrasaccharide and hexasaccharide obtained by free radical depolymerization contained a GlcNAc at the reducing end (data not shown).
Fig. 4.
Negative-ion ESI-CID product-ion tandem mass spectra of saturated octasaccharide (8-mer) from radical depolymerization of hyaluronan. a. [M-H]−1 of free radical and b. [M-H]−1 of the reduced alditol. The sequence together with the glycosidic oxygens is shown indicating the proposed fragmentation.
3.4 Sequence determination of unusual odd-numbered hyaluronan oligosaccharides from radical depolymerization
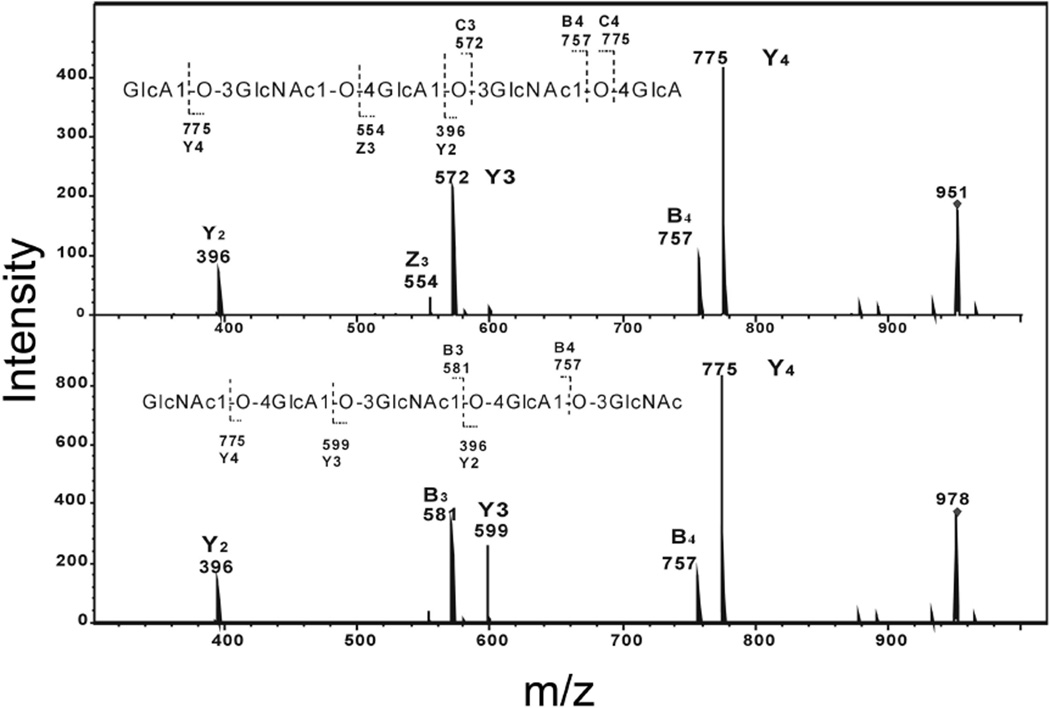
Saturated odd-numbered oligosaccharides with GlcA and GlcNAc at the reducing ends were both obtained from free radical treatment of hyluronan (Table 1). Due to the symmetrical nature of the sequence of odd-numbered oligosaccharides, the ESI-CID tandem MS analysis of saturated trisaccharide, pentasaccharide and nonasaccharide showed Y-ions and C-ions at the same m/z values, and so it was for B-ions and Z-ions. The spectrum of heptasaccharide GlcNAc-(GlcA-GlcNAc)3 with m/z 1331 (Fig. 5) featured extensive B-ions and C-ions together with some prominent Y-ions, the latter assignment was confirmed by the product-ion spectrum of its alditol after reduction. We next compared the CID-ESI-tandem mass spectrum of pentasaccharide ions with m/z 951 (GlcA at the reducing end) and m/z 978 (GlcNAc at the reducing end) (Fig. 6). Although both kind of pentasaccahrides had the same Y2-ions, B4-ions and Y4-ions, based on the analysis of the spectrum of their aditol, the ions at m/z 554 and at 572 having major contributions from Z3, Y3 of the pentasaccharide with GlcA at the reducing end, while the ions at m/z 581 and 599 were confirmed to be B3 and Y3 of pentasaccharide with GlcNAc at the reducing end, which can help differentiate the sequence of pentasaccharide.
Fig. 5.
Negative-ion ESI-CID product-ion tandem mass spectra of saturated heptasaccharide (7-mer) from radical depolymerization of hyaluronan. a. [M-H]−1 of free radical and b. [M-H]−1 of the reduced alditol. The sequence together with the glycosidic oxygens is shown indicating the proposed fragmentation.
Fig. 6.
Comparison of negative-ion ESI-CID product-ion tandem mass spectra of two saturated pentsaccharides (5-mer) obtained from radical depolymerization of hyaluronan. a. Pentsaccharide GlcA-GlcNAc-GlcA-GlcNAc-GlcA with m/z 951.0 and b. Pentsaccharide GlcNAc-GlcA-GlcNAc-GlcA-GlcNAc with m/z 977.9. The sequence together with the glycosidic oxygens is shown indicating the proposed fragmentation.
4. Conclusions
The hyaluronan oligosaccharides obtained by free radical depolymerization are much more complex than that of enzymatic products containing nearly equal amounts of both even- and odd- numbered saturated oligosaccharides. RPIP-HPLC effectively separated hyaluronan oligosaccharides obtained by radical depolymerization with the same degree of polymerization but having different sequences. Negative-ion ESI-MS unambiguously identified the odd-numbered and even-numbered saturated oligosaccharides and two different kinds of odd-numbered saturated fragments were observed having either GlcA or GlcNAc at the reducing end. As hyaluronan are linear polysaccharides, it is possible to derive the oligosaccharide sequence information directly from the glycosidic cleavage fragmentation in the product ion spectra. However, no cross-ring fragmentation was observed for the hyaluronan oligosaccharides investigated. We have also demonstrated that negative-ion ESI- CID-tandem MS using the free acid molecular species as precursors to permit complete sequence assignment from the extensive B-ion and C-ion doublets and Y-ions. Isomeric saturated odd-numbered oligosaccharides could also be readily differentiated as illustrated with pentasaccharide, with a GlcNAc at the reducing end.
Table 2.
Fragment-ions observed in the product-ion spectra of [M - H]− as precursors
| samples | [M-H]−1 | B3 | C3 | B4 | C4 | B5 | C5 | B6 | C6 | B7 | C7 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | Z3 | Z5 | Z7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unsaturated | ||||||||||||||||||||
| 4-mer GlcNAc | 757 | 554 | 396 | 599 | ||||||||||||||||
| 6-mer GlcNAc | 1136 | 554 | 757 | 933 | 396 | |||||||||||||||
| 8-mer GlcNAc | 1516 | 536 | 554 | 739 | 757 | 915 | 933 | 1118 | 1136 | 1294 | 1313 | 599 | 775 | 978 | 1154 | 1357 | ||||
| Saturated | ||||||||||||||||||||
| 4-mer GlcNAc | 775 | 572 | 396 | 599 | 581 | |||||||||||||||
| 6-mer GlcNAc | 1154 | 572 | 757 | 775 | 951 | 396 | 599 | 775 | 978 | 581 | ||||||||||
| 8-mer GlcNAc | 1532 | 554 | 572 | 757 | 775 | 933 | 951 | 1136 | 1313 | 599 | 775 | 978 | 1154 | 1357 | 581 | 960 | 1339 | |||
| 3-mer GlcNAc | 599 | 396 | ||||||||||||||||||
| 3-mer GlcA | 572 | 396 | ||||||||||||||||||
| 5-mer GlcNAc | 978 | 581 | 757 | 396 | 599 | 775 | ||||||||||||||
| 5-mer GlcA | 951 | 757 | 396 | 572 | 775 | 554 | ||||||||||||||
| 7-mer GlcA | 1331 | 554 | 572 | 757 | 775 | 934 | 1136 | 396 | 572 | 775 | 951 | 1154 |
Highlights.
Hyaluronan is an unsulfated glycosaminoglycan
Hyaluronan can be broken down enzymatically into even numbered oligosaccharides number
Depolymerization of hyaluronan using reactive oxygen species is possible
Controlled depolymerization can afford hyaluronan oligosaccharides for mass spectral analysis
Reactive oxygen species breakdown of hyaluronan offers an insight into its breakdown in disease
Acknowledgements
This work was supported by grants from the National Institutes of Heath GM38060, HL096972, ES020903 to RJL and by grants from the China Scholarship Council and Natural Science Foundation of China (No.30800858) and the Shandong Natural Science Foundation (No. ZR2010CQ020).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- Henrotin YE, Bruckner P, Pujol JPL. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–757. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Production of superoxide, hydrogen peroxide and hydroxyl radicals by phagocytic cells: a cause of chronic inflammatory disease. Cell Biology International Reports. 1982;6:529–542. doi: 10.1016/0309-1651(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Hawkins CL, Davies MJ. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radical Biolological Medicine. 1996;21:275–290. doi: 10.1016/0891-5849(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Henrotin YE, Bruckner P, Pujol JPL. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–745. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- Kühn AV, Raith K, Sauerland V, Neubert RHH. Quantification of hyaluronic acid fragments in pharmaceutical formulations using LC-/ESI-/MS. Journal of Pharmaceutical and Biomedical Analysis. 2003;30:1531–1537. doi: 10.1016/s0731-7085(02)00544-7. [DOI] [PubMed] [Google Scholar]
- Kvam BJ, Fragonas E, Degrassi A, Kvam C, Matulova M, Pollesello P, Zanetti F, Vittur F. Oxygen-derived free radical (ODFR) action on hyaluronan (HA), on two HA ester derivatives, and on the metabolism of articular chondrocytes. Expermental Cell Research. 1995;218:79–86. doi: 10.1006/excr.1995.1133. [DOI] [PubMed] [Google Scholar]
- Linhardt RJ. Analysis of glycosaminoglycans with polysaccharide lyases. Current Protocols in Molecular Bology. 2001;17:1317–1332. doi: 10.1002/0471142727.mb1713bs48. [DOI] [PubMed] [Google Scholar]
- Rees MD, Kennett EC, Whitelock JM, Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radical Biology & Medicine. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Šoltes L, Kogan MG, Schiller J, Stankovká M, Arnhold J. Degradative Action of Reactive Oxygen Species on Hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR21. Journal of Immunology. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Sampson P, Rochester C, Freundlich B, Elias J. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production: evidence for cytokine-regulated hyaluronan (hyaluronic acid) degradation and human lung fibroblast-derived hyaluronidase. Journal of Clinical Investigation. 1992;90:1492–1503. doi: 10.1172/JCI116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi N, Linhardt RJ. High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides. Nature Protocols. 2010;5:993–1004. doi: 10.1038/nprot.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi N. On-line HPLC/ESI-MS separation and characterization of hyaluronan oligosaccharides from 2-mers to 40-mers. Analytical Chemistry. 2007;79:6390–6397. doi: 10.1021/ac070837d. [DOI] [PubMed] [Google Scholar]
- Yang B, Yu GL, Zhao X, Jiao GL, Ren SM, Chai WG. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS Journal. 2009;276:2125–2137. doi: 10.1111/j.1742-4658.2009.06947.x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang B, Dutta P, Gasmili L, Zhang F, Linhardt RJ. Cell-based microscale isolation of glycoaminoglycans for glycomics study. Journal of Carbohydrate Chemistry. 2012;31:420–435. doi: 10.1080/07328303.2012.658126. [DOI] [PMC free article] [PubMed] [Google Scholar]