Abstract
Disruption of the serotonin system has been implicated in anxiety and depression and a related genetic variation has been identified that may predispose individuals for these illnesses. The relationship of a functional variation of the serotonin transporter promoter gene (5-HTTLPR) on serotonin transporter binding using in vivo imaging techniques have yielded inconsistent findings when comparing variants for short (s) and long (l) alleles. However, a significant 5-HTTLPR effect on receptor binding at the 5-HT1A receptor site has been reported in humans, suggesting the 5-HTTLPR polymorphism may play a role in serotonin (5-HT) function. Rhesus monkeys possess a 5-HTTLPR length polymorphism similar to humans and serve as an excellent model for studying the effects of this orthologous genetic variation on behaviors and neurochemical functions related to the 5-HT system. In this study, PET imaging of [18F]mefway was performed on 58 rhesus monkeys (33 l/l, 25 s-carriers) to examine the relation between 5-HT1A receptor-specific binding and 5-HTTLPR genotypes. Significantly lower 5-HT1A binding was found in s-carrier subjects throughout both cortical brain regions and the raphe nuclei. These results demonstrate that the underlying 5-HT neurochemical system is influenced by this functional polymorphism and illustrate the strong potential for extending the nonhuman primate model into investigating the role of this genetic variant on behavior and gene–environment interactions.
Introduction
Disruptions in the serotonin (5-HT) system, including biosynthesis, transmission, reuptake and degradation, are implicated in a wide variety of mood- and anxiety-related neuropsychiatric illnesses. The 5-HT transporter (5-HTT) clears serotonin from the synaptic cleft and thereby plays a major role in serotonergic neurotransmission. The gene encoding 5-HTT contains a functional length polymorphism in the promoter region (referred to as 5-HTTLPR) that is associated with the development of emotional traits and psychopathology. The role of allelic variation in 5-HTTLPR as a contributing factor to neuropsychiatric illness has been extensively studied. Such studies have strongly implicated 5-HTTLPR through behavioral association and neuroimaging studies of brain structure and function (for review, see Canli and Lesch, 2007). Imaging studies of neurochemical function in humans have focused on the baseline binding and expression of 5-HTT and have yielded inconsistent relations with 5-HTTLPR variants (Heinz et al., 2000; Shioe et al., 2003; Parsey et al., 2006; Praschak-Rieder et al., 2007; Reimold et al., 2007). Extending beyond the search for direct 5-HTTLPR allelic effects on 5-HTT expression, the 5-HT1A system has served as a candidate for altered function due to its role in serotonin regulation and its responsivity of receptor expression to selective serotonin reuptake inhibitor (SSRI) drugs. Using PET imaging of [11C]WAY100635 in a moderately large sample of healthy volunteers (n = 35), a significant reduction in 5-HT1A binding was revealed in carriers of the short 5-HTTLPR allele (David et al., 2005), thus providing intriguing evidence for 5-HTTLPR influence on serotonergic function.
The rhesus monkey (Macaca mulatta) serves as an excellent model for studying the influence of 5-HTTLPR allelic length on neurochemical endophenotypes. 5-HTTLPR in humans has an analogous biallelic length variation in the rhesus monkey (Lesch et al., 1996). In the same region, although not exactly the same location as the 5-HTTLPR polymorphism in humans, a 21 bp insertion/deletion variant is found in the rhesus monkey. Moreover, the ability to control the rearing environment has made the rhesus monkey an ideally suited model for studying 5-HTTLPR × environment interactions. For example, adverse early life events were found to interact with 5-HTTLPR allelic length to influence stress reactivity (Bennett et al., 2002; Barr et al., 2004b), neonatal response (Champoux et al., 2002), and alcohol sensitivity (Barr et al., 2004a). Behavioral studies with rhesus monkeys reported that s-carriers experiencing environmental challenge showed reduced cognitive flexibility (Izquierdo et al., 2007), neonatal irritability (Kraemer et al., 2008) and increased activation of the orbitofrontal cortex and the amygdala (Kalin et al., 2008). Similar studies that attempt to establish coupling between genetic and environmental influences are often not suitable for human subjects, making the rhesus monkey an invaluable resource for research in 5-HTTLPR × environment interactions.
In this paper, we examine the relation between 5-HTTLPR allelic length and 5-HT1A binding using a large cohort of rhesus monkeys with a close age range and similar rearing conditions. We used a highly selective 5-HT1A PET radioligand, [18F]mefway, with high resolution imaging. The rhesus monkey model was selected to minimize the environmental variability present in the general human population due to life experiences and conditions, with the goal of increasing the sensitivity for detecting gene–endophenotype relations.
Materials and Methods
Subjects.
Fifty-eight rhesus (Macaca mulatta) monkeys (36 females, 22 males; 10.4 ± 2.2 kg) were included. The age at the time of scanning was 14.5 ± 1.9 years. Genotyping for the rhesus 5-HTTLPR was based upon a modified protocol of Lesch et al. (1996) as previously reported by our group for some of the subjects in this cohort (Kraemer et al., 2008). Thirty-three subjects were l/l homozygous (21 females, 12 males) and 25 subjects (15 females, 10 males) were s-carriers, including s/s homozygous (n = 3) and l/s heterozygous (n = 23) subjects. Combining the s/s and s/l subjects within the s-carriers group is consistent with analysis methods for many human and rhesus 5HTTLPR studies due to the autosomal dominance of the 5HTTLPR s-allele (Lesch et al., 1996). The subjects were incorporated from two separate protocols involving the investigation of prenatal stress and prenatal alcohol exposure on development (see Schneider et al., 1997, 1999 for details). Briefly, prenatal stress consisted of daily exposure of the pregnant mother to three unpredictable horn blasts. Prenatal alcohol exposure consisted of the pregnant mother voluntarily consuming moderate levels of alcohol (0.6 mg/kg daily) during gestation. These offspring from the prenatal stress condition (7 l/l, 7 s-carriers) and prenatal alcohol condition (15 l/l, 11 s-carriers), along with controls (11 l/l, 7 s-carriers), were mother-reared for 6 months and housed in peer groups until 30 months of age and subsequently in pairs similar in age, sex, and prenatal condition. All experimental procedures involving the rhesus monkeys were approved by the Institutional Animal Care and Use Committee (IACUC).
PET measures.
The assay of 5-HT1A binding was performed using dynamic PET imaging of the 5-HT1A-specific antagonist radioligand [18F]mefway, which we have previously validated in rhesus monkeys (Wooten et al., 2011). The radiotracer was produced using previously published methods (Saigal et al., 2006) resulting in typical end of synthesis-specific activities of 150 GBq/μmol. The PET studies were conducted with an injected [18F]mefway activity of 59–130 MBq administered through a catheter placed in the saphenous vein in a volume of 3 ml of physiological saline. In preparation for the PET scans, the subjects were initially anesthetized with 10 mg/kg (i.m.) ketamine and subsequently maintained at ∼1.5% isoflurane throughout the course of the experiment. Vital signs including body temperature, breathing rate, heart rate, and oxygen saturation levels were monitored and recorded throughout the experiment. Atropine sulfate (0.27 mg) was given to reduce secretions. The subjects were positioned in the PET scanner using a custom built stereotaxic headholder with the head facing down. The PET scans were acquired on a Concorde Systems microPET P4 scanner (Tai et al., 2001). A 518 s transmission scan using a Co-57 rotating point source was first performed to correct for tissue attenuation and scatter of the annihilation radiation. The acquisition of the emission data was initiated with the 30 s bolus injection of [18F]mefway and continued for a duration of 90 min. Upon completion of the study, anesthesia was turned off and the monkey was returned to its cage when swallowing reflex was restored, and continuously monitored until fully alert.
Data processing.
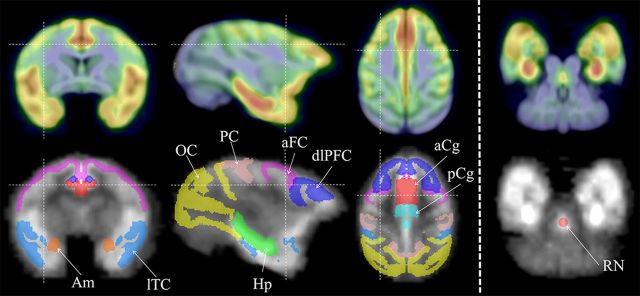
The dynamic PET data were binned into temporal frames and reconstructed using filtered back projection (0.5 cm−1 ramp filter) with corrections applied for dead time, scanner normalization, radiation attenuation, and scatter. The reconstructed PET time-series were 128 × 128 × 63 × 23 (x,y,z,t) voxels with spatial dimensions of 1.90 × 1.90 × 1.21 mm3. The time-series data for each subject were converted to a parametric image of the distribution volume ratio (DVR) to serve as a metric of [18F]mefway binding to the 5-HT1A receptor site. The DVR represents an index of receptor density (Bavail), apparent affinity (1/KD), and the ligand free fraction in the nondisplaceable tissue compartment (fND), as given by the relation: DVR = fNDBavail/KD + 1 (Innis et al., 2007). The cerebellar cortex was used as a reference region of negligible 5-HT1A-specific binding and the DVR was estimated using a multilinear reference tissue model (MRTM2) (Ichise et al., 2003). The DVR image volumes for each subject were spatially transformed into a standard space based upon the atlas of Paxinos et al. (2000). A rhesus monkey MRI atlas (McLaren et al., 2009) was used to create a [18F]mefway template and the DVR image volumes were transformed into the common space using the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/doc/). The spatially normalized DVR images were then smoothed with a 4 × 4 × 4 mm3 Gaussian filter to account for anatomical variation among subjects. Brain regions of interest (ROIs) were selected based upon the 5-HTTLPR regional findings reported by David et al. (2005), with each ROI consisting of subregions outlined according to atlas specifications (Saleem and Logothetis, 2007). Specifically, ROIs were extracted for the areas of the anterior cingulate gyrus (aCg, 1.4 cm3), posterior cingulate gyrus (pCg, 1.3 cm3), hippocampus (Hp, 1.6 cm3), amygdala (Am, 0.4 cm3), dorsal lateral prefrontal cortex (dlPFC, 4.2 cm3), parietal cortex (PC, 4.9 cm3), occipital cortex (OC, 16.1cm3), lateral temporal cortex (lTC, 7.0 cm3) and the agranular frontal cortex (aFC, 4.4 cm3). The placement of all ROIs was consistent across all subjects; however, for the raphe nuclei (RN), a fixed volume ROI (3 voxel diameter circle placed on 3 consecutive transaxial planes: 0.10 cm3) was centrally placed over the region of focal binding on the DVR images in native space to minimize variability in positioning over this small brain region. The location of the ROIs displayed on a [18F]mefway image are shown in Figure 1.
Figure 1.
5-HT1A binding distribution and region of interest definitions. Top row, [18F]Mefway binding displayed on MRI template in coronal, sagittal and two transaxial views. Bottom row, Regions of interest defined in template space for the amygdala (Am), lateral temporal cortex (lTC), occipital cortex (OC), parietal cortex (PC), hippocampus (Hp), agranular frontal cortex (aFC), dorsolateral prefrontal cortex (dlPFC), anterior cingulate gyrus (aCg), posterior cingulate gyrus (pCg), and raphe nuclei (RN). There was a slight overlap of some adjacent regions (∼1% of volume) due to spatial smoothing of the template masks.
Statistical analyses.
A multivariate ANOVA (MANOVA) model was used to determine the relationship between the 5-HTTLPR variant and the 5-HT1A binding (DVR) of the brain regions while adjusting for the effects of age, sex, and treatment. The multivariate response variable consisted of all of the brain ROIs under investigation and the predictors of interest were 5-HTTLPR, age, sex, and treatment group. The treatment groups consisted of subjects exposed to prenatal stress (n = 14), prenatal alcohol (n = 26), and age-matched controls (n = 18). In this paper, we report only on the 5-HTTLPR main effect results, while including the treatment groups in the model. The multivariate ANOVA tests the hypothesis that the vector of 5-HT1A DVR means in the l/l group is equal to the vector of means in the s-carrier group while adjusting for age, sex, and treatment. Pillai's trace is reported as the test statistic in our MANOVA in our analysis because it is the most robust statistical measure when there is unequal cell size as in this case (Hair et al., 2006). Analysis was performed using SAS (version 9.2) and R (version 2.13) (Everitt, 2007). Univariate analyses on specific brain regions were conducted using the same predictors as the multivariate model to follow up significant multivariate effects.
Results
Overall significant differences between the regional 5-HT1A DVRs of the two 5-HTTLPR groups (l/l homozygotes and s-carriers) were found in the model that contained only 5-HTTLPR status, Pillai's trace = 0.435 (p = 0.0025), and these overall differences were present after adjusting for age, sex, and treatment status (Pillai's trace: p = 0.002). As shown in Table 1, MANOVA also revealed a significant overall effect of age (Pillai's trace = 0.467, p = 0.001) as well as sex (Pillai's trace = 0.334, p = 0.039).
Table 1.
Multivariate effects
| Variable | Pillai's trace | F-statistic | df | Error df | Pr > Fa |
|---|---|---|---|---|---|
| 5-HTTLPR | 0.445 | 3.46 | 10 | 43 | 0.002 |
| Sex | 0.334 | 2.16 | 10 | 43 | 0.039 |
| Age | 0.467 | 3.77 | 10 | 43 | 0.001 |
| Treatment | 0.414 | 1.15 | 20 | 88 | 0.317 |
a p value associated with the F-statistic.
The reduced binding with age was not statistically significant in any of the univariate models; thus, the age effect represents an overall decline in 5-HT1A binding. In univariate analyses sex differences were significant in the amygdala (p = 0.013, females higher than males) and there was a trend toward the same sex differences in anterior cingulate (p = 0.051), hippocampus (p = 0.055), and aFC (p = 0.060), but they did not reach significance.
In the univariate tests to focus on the specific brain regions responsible for the significant effect 5-HTTLPR group, the s-carriers showed significantly lower binding then the l/l subjects in the following brain regions: raphe nucleus (p = 0.009), occipital (p = 0.031), parietal (p = 0.036). A trend toward lower [18F]mefway binding in the s-carriers was also found for the anterior cingulate (p = 0.059) and posterior cingulate (p = 0.058), but these did not reach significance (Table 2).
Table 2.
5-HT1A binding values (DVR) for each region by 5-HTTLPR (mean ± SD)
| 5-HTTLPR ROI | l/L (n = 33) | s-Carrier (n = 25) | Mean difference estimate (l/L − s-carrier) | p value |
|---|---|---|---|---|
| RN | 3.13 ± 0.43 | 2.87 ± 0.27 | 0.26 | 0.009* |
| aCg | 3.65 ± 0.80 | 3.28 ± 0.57 | 0.36 | 0.059 |
| pCg | 2.56 ± 0.49 | 2.32 ± 0.34 | 0.23 | 0.058 |
| OC | 1.28 ± 0.19 | 1.18 ± 0.14 | 0.10 | 0.031* |
| PC | 2.44 ± 0.51 | 2.17 ± 0.34 | 0.25 | 0.036* |
| lTC | 2.72 ± 0.47 | 2.60 ± 0.38 | 0.12 | 0.305 |
| dlPFC | 3.35 ± 0.74 | 3.11 ± 0.52 | 0.24 | 0.164 |
| aFC | 3.08 ± 0.65 | 2.84 ± 0.42 | 0.24 | 0.097 |
| Hp | 3.65 ± 0.55 | 3.67 ± 0.50 | −0.01 | 0.914 |
| Am | 3.30 ± 0.53 | 3.24 ± 0.33 | 0.06 | 0.585 |
p values are from the univariate analyses and test whether the difference between the l/L and s-carrier groups was significant (models were adjusted for sex, age, and treatment).
*Significant p values.
The assumption of multivariate normality was verified by examining residual plots of the model (i.e., χ2 plots for the multivariate normality of the error terms). No significant departures from the multivariate normal distributions were observed. One outlier was identified (female, l/l homozygote with elevated 5-HT1A binding) and the analysis was performed both including and excluding this subject. No differences in the significance of our results were observed when this observation was removed, therefore the animal was retained for the reported analyses. Within the s-carrier group, the three s/s homozygous subjects displayed no detectable trend toward elevated or decreased 5-HT1A binding compared with the l/s heterozygous subjects.
Discussion
The present findings that 5-HTTLPR allelic variations significantly alter the 5-HT1A receptor system in the rhesus monkey are consistent with a similar study in humans (David et al., 2005). In that retrospective human study, PET imaging of a 5-HT1A antagonist radioligand in a total of 35 healthy individuals revealed carriers of the short 5HTTLPR genotypes exhibited decreased 5-HT1A binding throughout 5-HT1A-expressing regions of the brain. As with our results, this decrease in binding was seen in all cortical brain regions as well as the raphe midbrain region. A more recent study in humans (n = 54), however, did not find an 5-HTTLPR association with 5-HT1A binding (Borg et al., 2009). Both these human studies describe similar methods, using [11C]WAY100635 in middle age subjects. However, the study with negative findings reported a much higher variation in 5-HT1A binding across the subjects (∼2-fold greater coefficient of variation) suggesting a more heterogeneous population or some unknown source of variance. It is also possible different processing methodologies might have contributed to large variabilities across subjects.
It has been proposed that the short allele genotype yields reduced transcriptional efficiency in expression of the 5-HT transporter (Collier et al., 1996; Lesch et al., 1996). This could in turn possibly result in elevated 5-HT tone and 5-HT1A receptor desensitization (David et al., 2005). Although a number of human (Jacobsen et al., 2000; Shioe et al., 2003; van Dyck et al., 2004) and rhesus (Christian et al., 2009; Jedema et al., 2010) PET neuroimaging studies do not support the notion that 5-HTT expression is compromised in s-carriers, we cannot fully dismiss this mechanistic component from the model of 5-HT system dysregulation associated with the s-carrier 5-HTTLPR genotype.
Radioligand imaging studies of 5-HTT binding in 5-HTTLPR variants have reported a metric of “binding potential” representing an index proportional to the receptor density (Bmax) and ligand-protein affinity (1/KDapp). Using this composite index, potential decreases in receptor density could be masked by matching increases in affinity, or accompanying decreases in endogenous 5-HT competition. With this limitation, the interpretation of the decreased 5-HT1A binding reported in s-carriers could be due to either elevated 5-HT tone or 5-HT1A downregulation. Additional studies and methodologies interrogating 5-HT function, such as measurements separating receptor density from affinity in presynaptic and postsynaptic receptors and 5-HT metabolism, will be required to understand the role of the 5-HTT and the mechanisms by which this decrease in 5-HT1A binding was achieved.
The rhesus 5-HTTLPR length polymorphism has been used extensively to study neurobehavioral functioning. Research on resiliency to stressful events or conditions in infant and juvenile rhesus monkeys has shown that carriers of the short allele demonstrate heightened physical aggression and HPA response, altered serotonin metabolism and regional brain metabolism (Bennett et al., 2002; Champoux et al., 2002; Barr et al., 2003, 2004b; Kalin et al., 2008), illustrating the strength of the rhesus monkey model for the study of gene × environment interactions (Suomi, 2006). Previous work from our laboratory using subjects included with the research reported herein found that prenatal alcohol-exposed carriers of the s-allele exhibited increased neonatal irritability and increased stress responses compared with l/l homozygotes independent of prenatal alcohol exposure (Kraemer et al., 2008). The statistical model for the present research included treatment groups to account for potential prenatal condition alterations, permitting us to focus on the effect of 5-HTTLPR allelic variations over and above any prenatal treatment main effects. We regard this research as a first step in understanding the effects of 5-HTTLPR variations on 5-HT function in the rhesus monkey model. Future analysis of this cohort will examine interactions between 5HTTLPR, 5-HT1A and prenatal treatment with behavioral measures.
A recent study from another laboratory evaluated 5-HT1A binding in rhesus monkeys as a function of 5-HTTLPR length polymorphisms, among other biomarker comparisons. Using a smaller sample size (n = 8) of all male subjects, Jedema et al. (2010) found no statistically significant difference in 5-HT1A binding between s-carriers and (l/l) homozygotes. However, consistent with our results, reduced 5-HT1A binding was observed in all reported cortical and midbrain regions (p < 0.116 using multivariate analysis). As suggested by the authors, their study may have been underpowered for detecting the allelic group differences. In our study, there was a 7% difference between groups when averaged over all regions of the 58 subjects. Using MRI T1 images of their cohort, Jedema et al. (2010) found statistically significant differences in cortical morphology in regional gray matter volume, with s-carriers having reduced volume in several brain regions that were implicated in measured cognitive tasks. A limitation of the present study is that we do not have MRI images available on this cohort. Therefore, it is conceivable that a thinner cortex in s-carriers would result in lower measured 5-HT1A binding due to image resolution (i.e., partial volume) effects even if 5-HT1A receptor density was preserved. To address this, in part, the PET DVR images were spatially smoothed after transforming to atlas space to minimize the variability of imperfect ROI placement over peak DVR values. Further, all cortical volumes consisted of large regional sampling resulting in lower [18F]mefway DVR values when compared with our previously published values using [18F]mefway (Wooten et al., 2011) and perhaps masking large differences in 5-HT1A binding in focal brain regions.
In conclusion, carriers of the 5-HTTLPR short allele were found to have significantly reduced binding at the 5-HT1A receptor sites, suggesting that 5-HTTLPR length variations play a role in the regulation of the 5-HT system. The rhesus model was used to provide a study cohort that minimizes variability potentially affecting the 5-HT system which is found in the human population, including psychosocial stress and substance abuse. These findings also confirm that 5-HT related alterations persist into adulthood, given the age of the rhesus subjects (14.5 years) were approximately equivalent to humans in their fourth decade of life, an age similar that of the study by David et al. (2005) in humans. An intriguing question is that of the developmental course of differences in 5-HT function between 5-HTTLPR variants and the possibility of developmentally vulnerable periods of 5-HT disruption.
Footnotes
This work was supported by NIH Grants AA017706, MH086014, AA12277, AA10079, and T32CA009206. Additional support was provided by NIH Grants S10RR015801, P30HD003352, and S10RR023033. We thank the following for their contribution to this research: Professor R. Jerry Nickles and Dr. Jonathan Engle for assistance with isotope production; Maxim Slesarev and Julie Larson and the staff at the Harlow Center for Biological Psychology at the University of Wisconsin for nonhuman primate handling; Andrew Higgins for data processing; and Professor Jim Holden and Professor Jogesh Mukherjee (University of California, Irvine) for technical discussions.
The authors declare no competing financial interests.
References
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003;27:812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004a;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004b;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Borg J, Henningsson S, Saijo T, Inoue M, Bah J, Westberg L, Lundberg J, Jovanovic H, Andrée B, Nordstrom AL, Halldin C, Eriksson E, Farde L. Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int J Neuropsychopharmacol. 2009;12:783–792. doi: 10.1017/S1461145708009759. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Christian BT, Fox AS, Oler JA, Vandehey NT, Murali D, Rogers J, Oakes TR, Shelton SE, Davidson RJ, Kalin NH. Serotonin transporter binding and genotype in the nonhuman primate brain using [C-11]DASB PET. Neuroimage. 2009;47:1230–1236. doi: 10.1016/j.neuroimage.2009.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier DA, Stöber G, Li T, Heils A, Catalano M, Di Bella D, Arranz MJ, Murray RM, Vallada HP, Bengel D, Müller CR, Roberts GW, Smeraldi E, Kirov G, Sham P, Lesch KP. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- David SP, Murthy NV, Rabiner EA, Munafó MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J Neurosci. 2005;25:2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B. An R and S-PLUS companion to multivariate analysis. Ed 2. London: Springer; 2007. [Google Scholar]
- Hair JF, Black BJ, Babin BJ, Anderson RE, Tatham RL. Multivariate data analysis. Ed 6. Upper Saddle River, NJ: Prentice Hall; 2006. [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: application to [C-11]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Newman TK, Higley JD, Murray EA. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proc Natl Acad Sci U S A. 2007;104:14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, Hariri AR, Bradberry CW. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer GW, Moore CF, Newman TK, Barr CS, Schneider ML. Moderate level fetal alcohol exposure and serotonin transporter gene promoter polymorphism affect neonatal temperament and limbic-hypothalamic-pituitary-adrenal axis regulation in monkeys. Biol Psychiatry. 2008;63:317–324. doi: 10.1016/j.biopsych.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Kosmatka KJ, Oakes TR, Kroenke CD, Kohama SG, Matochik JA, Ingram DK, Johnson SC. A population-average MRI-based atlas collection of the rhesus macaque. Neuroimage. 2009;45:52–59. doi: 10.1016/j.neuroimage.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu XZ, Goldman D, Huang YY, Simpson N, Arcement J, Huang Y, Ogden RT, Van Heertum RL, Arango V, Mann JJ. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Ed 1. San Diego: Academic; 2000. [Google Scholar]
- Praschak-Rieder N, Kennedy J, Wilson AA, Hussey D, Boovariwala A, Willeit M, Ginovart N, Tharmalingam S, Masellis M, Houle S, Meyer JH. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [C-11]DASB positron emission tomography study. Biol Psychiatry. 2007;62:327–331. doi: 10.1016/j.biopsych.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Reimold M, Smolka MN, Schumann G, Zimmer A, Wrase J, Mann K, Hu XZ, Goldman D, Reischl G, Solbach C, Machulla HJ, Bares R, Heinz A. Midbrain serotonin transporter binding potential measured with [C-11]DASB is affected by serotonin transporter genotype. J Neural Transm. 2007;114:635–639. doi: 10.1007/s00702-006-0609-0. [DOI] [PubMed] [Google Scholar]
- Saigal N, Pichika R, Easwaramoorthy B, Collins D, Christian BT, Shi B, Narayanan TK, Potkin SG, Mukherjee J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, F-18-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J Nucl Med. 2006;47:1697–1706. [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. A combined MRI and histology atlas of the rhesus monkey brain. Ed 1. New York: Academic; 2007. [Google Scholar]
- Schneider ML, Roughton EC, Lubach GR. Moderate alcohol consumption and psychological stress during pregnancy induce attention and neuromotor impairments in primate infants. Child Dev. 1997;68:747–759. doi: 10.1111/j.1467-8624.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Roughton EC, Koehler AJ, Lubach GR. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- Shioe K, Ichimiya T, Suhara T, Takano A, Sudo Y, Yasuno F, Hirano M, Shinohara M, Kagami M, Okubo Y, Nankai M, Kanba S. No association between genotype of the promoter region of serotonin transporter gene and serotonin transporter binding in human brain measured by PET. Synapse. 2003;48:184–188. doi: 10.1002/syn.10204. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene × environment interactions in rhesus monkeys. Ann N Y Acad Sci. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Tai C, Chatziioannou A, Siegel S, Young J, Newport D, Goble RN, Nutt RE, Cherry SR. Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Phys Med Biol. 2001;46:1845–1862. doi: 10.1088/0031-9155/46/7/308. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central serotonin transporter availability measured with [I-123]beta-CIT SPECT in relation to serotonin transporter genotype. Am J Psychiatry. 2004;161:525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]
- Wooten DW, Moraino JD, Hillmer AT, Engle JW, Dejesus OJ, Murali D, Barnhart TE, Nickles RJ, Davidson RJ, Schneider ML, Mukherjee J, Christian BT. In vivo kinetics of [F-18]MEFWAY: a comparison with [C-11]WAY100635 and [F-18]MPPF in the nonhuman primate. Synapse. 2011;65:592–600. doi: 10.1002/syn.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]