Abstract
Background
Intracerebral hemorrhage (ICH) can occur in patients following acute ischemic stroke in the form of hemorrhagic transformation and results in significant longterm morbidity and mortality. Anticoagulation theoretically increases risk. We evaluated stroke patients with an indication for anticoagulation to determine the factors associated with hemorrhagic transformation.
Methods
345 patients with ICD-9 codes indicating: 1) acute ischemic stroke, and 2) an indication for anticoagulation were screened. 123 met inclusion criteria. Data were collected retrospectively. Neuroimaging was reviewed for infarct volume and evidence of ICH. Hemorrhages were classified as: hemorrhagic conversion (petechiae) versus intracerebral hematoma (a space occupying lesion); symptomatic versus asymptomatic. Using multivariable logistic regression, we determined the hypothesized factors associated with intracerebral bleeding.
Results
Age (OR= 1.50 per 10 year increment, 95% CI 1.07–2.08), infarct volume (OR= 1.10 per 10 cc’s, 95% CI 1.06–1.18), and worsening category of renal impairment by estimated GFR (OR= 1.95, 95% CI 1.04–3.66) were predictors of hemorrhagic transformation. 99 of 123 patients were anticoagulated. Hemorrhage rates of patients on and off anticoagulation did not differ (25.3% versus 20.8%; p=0.79); however, all intracerebral hematomas (n=7) and symptomatic bleeds (n=8) occurred in the anticoagulated group.
Conclusions
The risk of hemorrhagic transformation in patients with acute ischemic stroke and an indication for anticoagulation is multifactorial and most closely associated with an individual’s age, infarct volume, and eGFR.
Keywords: cerebrovascular diseases and cerebral circulation, cerebral infarction, cerebral haemorrhage, stroke, anticoagulation, renal failure
Introduction
Patients with acute ischemic stroke frequently have an indication for anticoagulation. The indication may be related to the etiology of the stroke itself (eg., atrial fibrillation), or independent, as in the case of a deep vein thrombosis. Anticoagulants theoretically increase the risk of hemorrhagic transformation of ischemic infarct (1), which is highest in the days immediately following the event (2–4). Studies on secondary stroke prevention have included analyses of intracerebral hemorrhage (ICH) rates (1,5–6,8–11); however, there are little data regarding the risk of hemorrhage in patients who have had a stroke and require acute anticoagulation for other indications.
This retrospective analysis was designed to identify the factors that predict increased risk of hemorrhagic transformation in patients with acute ischemic stroke and any indication for anticoagulation.
Design and Methods
Subjects
This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. A retrospective chart review was performed. Informed consent was not required. Adults (18 years and older) presenting to the Johns Hopkins Hospital or Bayview Medical Center with: 1) an acute ischemic stroke on head CT or diffusion weighted MRI, and 2) a condition potentially requiring treatment with anticoagulation, were included in the analysis. Patients were identified by ICD-9 codes. Charts were reviewed to confirm eligibility. Indications for anticoagulation were determined by the clinical team caring for the patient and included: atrial fibrillation, cervical arterial dissection, basilar artery thrombosis, depressed ejection fraction (<35%), mechanical aortic/mitral valve, myocardial infarction, apical thrombus, deep vein thrombosis (DVT), pulmonary embolus, high risk intracerebral/extracranial large vessel stenosis, and hypercoaguable state (eg., antiphospholipid antibody syndrome, malignancy).
Three hundred forty five patients were identified by ICD-9 codes. Their electronic patient record, bedside paper chart, and neuroimaging (head CTs and MRIs) were reviewed. Data were collected regarding patient demographics, medical profile, and stroke characteristics (see Table 1).
Table 1.
Patient characteristics- univariate analyses.
| Total (n=123) | Hemorrhage (n=30) | No Hemorrhage (n=93) | p-value* | |
|---|---|---|---|---|
| Age (years (SD)) | 63 (17) | 67 (16) | 62 (18) | 0.19 |
| Gender (N (% female)) | 66 (54%) | 18 (60%) | 48 (52%) | 0.53 |
| Race (N (% African-American)) | 54 (44%) | 16 (53%) | 38 (41%) | 0.29 |
| Stroke Type (N (% embolic)) Watershed N= 8 Large Vessel Stenosis N= 6 Lacunar N= 1 Septic Embolization N= 2 Dissection N= 18 Vasculitis N=1 Other N= 22 |
65 (53%) | 16 (53%) | 49 (53%) | 1.00 |
| NIH Stroke Scale Score (mean (SD)) | 9 (7) | 11 (6) | 8 (7) | 0.033 |
| Antiplatelet Agent on Admission (N (%)) | 43 (35%) | 13 (45%) | 30 (32%) | 0.28 |
| Anticoagulation on Admission (N (% coumadin)) | 20 (16%) | 6 (20%) | 14 (15%) | 0.57 |
| LDL (mean mg/dL (SD)) | 86 (39) | 79 (42) | 88 (38) | 0.29 |
| Renal Failure (N (% eGFR <60mL/min/1.73m2)) | 42 (34%) | 15 (50%) | 27 (29%) | 0.046 |
| eGFR (mean (SD)) | 50.5 (16) | 45.1 (19) | 52.0 (15) | 0.035 |
| Diabetes (N (%)) | 37 (30%) | 10 (33%) | 27 (29%) | 0.65 |
| Peak Systolic Blood Pressure (mean mmHg (SD)) | 176 (29) | 179 (28) | 175 (30) | 0.53 |
| Peak Diastolic Blood Pressure (mean mmHg (SD)) | 98 (16) | 97 (13) | 98 (17) | 0.74 |
| Days SBP >180mmHg (mean days (SD)) | 2 (4) | 2 (3) | 2 (4) | 0.89 |
| Indication for Anticoagulation (N (% afib)) | 46 (37%) | 14 (47%) | 32 (34%) | 0.28 |
| Anticoagulated (N (%)) | 99 (80%) | 25 (83%) | 74 (80%) | 0.79 |
| Days from stroke to initiation of anticoagulation (mean days (SD)) | 8 (12) | 8 (7) | 8 (13) | 0.93 |
| Peak INR (mean (SD)) | 2.5 (2.0) | 2.3 (1.8) | 2.5 (2.0) | 0.61 |
| Peak PTTr (mean (SD)) | 3.1 (2.4) | 2.8 (1.8) | 3.2 (2.6) | 0.46 |
| Days Supratherapeutic (mean days (SD)) | 2 (4) | 2 (3) | 2 (4) | 0.53 |
| GRE Positive (N/80 (%)) | 48 (61%) | 10 (71%) | 38 (58%) | 0.55 |
| Other Bleeding (N (%)) | 9 (7%) | 4 (13%) | 5 (5%) | 0.22 |
| tPA (N (%)) | 18 (15%) | 8 (27%) | 10 (11%) | 0.41 |
p-values evaluate differences between hemorrhage and no hemorrhage patients.
Anticoagulation
A patient was defined as “anticoagulated” if they received: warfarin, unfractionated, or low molecular weight heparin during their hospital stay. In patients who had hemorrhagic transformation of their stroke, it was recorded whether bleeding occurred before or after the initiation of treatment. In greater than two-thirds of anticoagulated patients, infusion of heparin (using our institution’s low goal unfractionated heparin nomogram; PTTr goal 1.5–2.0, no bolus) was used as a bridge to a therapeutic INR (INR goal 2.0–3.0) on warfarin therapy. INR and PTTr values were recorded, as well as the number of days a patient was supratherapeutic. Non-anticoagulated patients were typically treated with an antiplatelet agent and received subcutaneous heparin for DVT prophylaxis. This was not considered anticoagulation. Decisions regarding anticoagulant and antiplatelet use were made by the primary clinical team.
Imaging
All neuroimaging was reviewed by one investigator (EBM). A subset of images was reviewed by a second investigator (RHL) and a kappa statistic was calculated to assess inter-rater agreement of hemorrhage grading. Inter-rater reliability for classification of hemorrhages on neuroimaging was high (κ= 0.76). Images were reviewed independently from the clinical record to ensure that the reviewers remained blinded to the neurologic condition of the patient. Greater than 90% of the MRIs were performed within 24–72 hours of admission. Regions of restricted diffusion on diffusion weighted MRI (DWI) with corresponding ADC hypointensity were identified as areas of acute ischemia. Stroke volumes were calculated using the equation: (length x width x thickness x number of slices)/2. This is a validated method (12), and was chosen over standard volumetric analysis given its ease of calculation and generalizability in clinical practice. For patients with more than one lesion that restricted diffusion, both the total volume of ischemia and the volume of the largest lesion were recorded. T1-, T2-weighted, and gradient echo (GRE) images were also reviewed and the number and location of micro and macrohemorrhages noted, if applicable.
The majority of patients underwent MRI. Twenty eight had only noncontrast head CTs. On CT, areas of acute stroke were defined as acute/subacute appearing hypodensities in regions corresponding to the acute neurologic symptoms. Volume of hypodensity, was measured using the same equation as was used for MRI, with the understanding that indefinite borders make estimation of infarct size on CT less accurate.
Defining Hemorrhage
All brain imaging throughout the course of a patient’s hospitalization was reviewed for intracerebral hemorrhage. The majority of acute hemorrhages were noted on noncontrast head CT; however, T1, T2-weighted, and gradient echo MR imaging alone was used in a minority of cases (13). Hemorrhages were classified as: hemorrhagic conversion (petechiae within the area of ischemia) versus intracerebral hematoma (a space occupying lesion) (Figure 1). Symptomatic hemorrhage was defined as subjective clinical worsening with blood on subsequent head imaging. Whether a patient was symptomatic and received imaging was determined by the primary neurology team caring for the patient. A subset of patients developed significant edema as a result of large infarcts and had associated petechial hemorrhage. This clinical scenario was classified as asymptomatic, as the clinical worsening was felt to be secondary to edema and not hemorrhagic transformation. Unless otherwise specified, the use of hemorrhage throughout the rest of this paper refers to the combination of symptomatic and asymptomatic hemorrhages.
Figure 1.
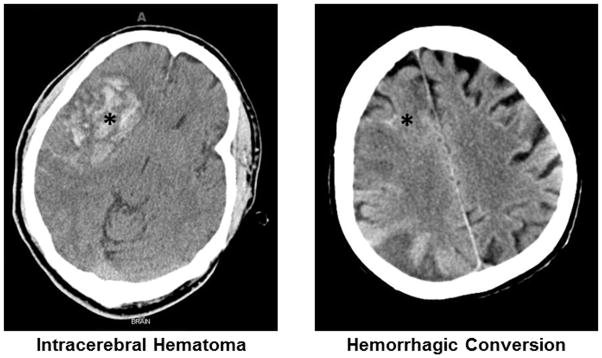
Hemorrhagic conversion: petechiae within the area of infarction. Intracerebral hematoma: a space occupying lesion.
Statistical Analysis of Variables of Interest
Univariate analysis was performed using Student’s paired t tests (for continuous variables) and Fisher’s exact tests (for categorical variables) for each of the predictors of interest. The following variables were included in analysis (Table 1): basic demographic information (age, race, and gender); information pertaining to stroke size and severity (TOAST classification, NIHSS score, and infarct volume (both total volume and largest lesion size)); information on medication/anticoagulation status (admission antiplatelet/anticoagulation use, anticoagulation status while hospitalized, indication for anticoagulation, days post-stroke to therapeutic anticoagulation, and peak INR and PTTr values); and other medical factors (LDL, renal function, diabetic status, and peak systolic and diastolic blood pressures). Renal function was defined by both linear eGFR (calculated based on admission creatinine using the Modification of Diet in Renal Disease (MDRD) Equation (14)), and category of impairment: normal (eGFR ≥60 mL/min/1.73m2); mild (eGFR 30–59 mL/min/1.73m2); and moderate (eGFR <30 mL/min/1.73m2).
Subsequent multivariable modeling included presence of intracerebral hemorrhage as the dependent variable in stepwise logistic regression. Covariates that were significant in univariate analysis or thought likely to be clinically relevant (either as primary predictors or confounders) were evaluated in this multivariable regression.
Survival analysis with Kaplan-Meier curves and a Cox proportional hazards analysis were also performed, using hemorrhage-free survival as the outcome. Time was calculated as time to hemorrhage, among those who bled, or time to last-seen-normal, for those who did not. All patients were followed through discharge. Patient records following discharge were reviewed for both follow-up appointments or readmission to Johns Hopkins Hospital or Bayview Medical Center.
As a secondary analysis, data were collected on bleeding from other sites (catheter-related, pulmonary hemorrhage, epistaxis, gastrointestinal bleed). Presence of bleeding elsewhere (defined as “other bleeding”, either alone or in combination with intracerebral bleeding), was evaluated as an outcome of interest.
Results
Final Included Cohort
Three hundred forty five patients were identified by their ICD-9 codes as potential subjects. Patients were excluded from further analysis if: they had no stroke (eg., arterial stenosis/occlusion without infarction on baseline imaging; n=86), had no need for anticoagulation (n= 20), were duplicates (n=79), were under the age of 18 (n=9), or had no available neuroimaging for review (n=20). An additional 8 patients presented with primary intracerebral hemorrhage and were therefore excluded, leaving 123 patients for further analysis.
Ninety nine (80%) of the patients were anticoagulated. Over two-thirds of the remaining unanticoagulated patients were placed on an antiplatelet agent. The most common indications for anticoagulation in the total cohort were atrial fibrillation (37.4%), deep vein thrombosis (28.5%), presence of a hypercoagulable state (19.5%), and pulmonary embolism (17.1%). Although anticoagulation was not associated with a higher risk of intracerebral hemorrhage (25.3% versus 20.8%; p=0.79), all of the intracerebral hematomas (n=7) and symptomatic bleeds (n=8) occurred in the anticoagulated group. The average number of days after stroke that a patient was started on anticoagulation varied by indication (eg., cervical dissection- 3 days, atrial fibrillation- 6 days, DVT- 10 days). The average time to hemorrhage was 25.5 days after stroke (median 4.5 days), and 4.6 days after initiation of anticoagulation (median 0 days). Those who bled prior to initiation of anticoagulation (but after the initial presenting stroke; n= 10) were included in analysis; however, the analysis was run with and without this group and it did not significantly change the results.
Univariate Analysis for Variables of Interest
Results for all variables of interest are outlined in Table 1. In univariate analyses, both total infarct size (OR 1.10 per 10cc’s, 95% CI 1.04–1.16) and largest lesion volume (OR 1.12 per 10 cc’s, 95% CI 1.06–1.18) were associated with higher risk of hemorrhage. Estimated GFR was inversely related to the rate of intracerebral hemorrhage (OR 0.98 per 1 mL/min/1.73m2 increase in eGFR; 95% CI 0.95–0.99). Of note, 18 patients were administered IV tPA. Eight (44%) had some degree of hemorrhagic transformation; but 66% of the patients with renal failure had intracerebral hemorrhage.
Multivariable Model
Infarct volume and renal function were entered into the multivariable model, along with age, gender, race, anticoagulation status, and GRE positivity on MRI. Stepwise logistic regression was performed. Age (OR 1.50 per 10 years, 95% CI 1.07–2.08), total infarct volume (OR 1.10 per 10 cc’s, 95% CI 1.06–1.18), and eGFR (both linear OR 1.03 per 1 mL/min/1.73m2 improvement, 95% CI 1.01–1.06; and worsening eGFR category (OR 1.95, 95% CI 1.04–3.66) remained statistically significant predictors of intracerebral hemorrhage. In models adjusted for age and infarct volume, those with mild renal impairment (eGFR 30–59 mL/min/1.73m2) were 2.23 times more likely to bleed, and those with moderate renal impairment (eGFR <30 mL/min/1.73m2) 3.59 times more likely, than patients with normal renal function (p-trend= 0.11). NIHSS score was also a statistically significant predictor of intracerebral hemorrhage, independent of age or eGFR (OR 1.08, 95% CI 1.02–1.15), but was no longer statistically significant when controlling for infarct volume (although volume remained significant even with NIHSS in the model).
When a survival analysis was performed, the same variables: age (HR 1.42 per 10 years, 95% CI 1.10–1.85), total infarct volume (HR 1.06 per 10 cc’s, 95% CI 1.04–1.08), and eGFR (both linear HR 1.03 per 1 mL/min/1.73m2 improvement, 95% CI 1.01–1.05; and worsening eGFR category HR 1.85, 95% CI 1.16–2.96), predicted higher hazard of intracerebral hemorrhage over time.
Other Clinically Significant Bleeding
Nine patients had clinically significant “other bleeding”; 4 of whom also had intracerebral bleeding. All 4 had an eGFR of ≤30 mL/min/1.73m2. eGFR category was a strong predictor of other bleeding. Patients were 1.9 times more likely to have either intracerebral or other bleeding (p=0.028) and 6.4 times more likely to have both (p=0.023) per worsening category.
Discussion
Following acute stroke, there is breakdown of the blood brain barrier resulting in friable intracranial vasculature. This breakdown theoretically increases the risk of intracerebral bleeding, specifically into the area of ischemia. Unfortunately, little data exist to suggest how long this friability lasts or what other factors may contribute.
Our study suggests that age, infarct volume, and even mild degrees of renal impairment are associated with increased risk for hemorrhagic transformation of acute ischemic stroke. The association with age is consistent with previous studies showing that individuals over the age of 80 years are at increased bleeding risk when administered intravenous tissue plasminogen activator (tPA) (15). Infarct volume is also intuitive, and has a basis in the literature (15). In our study, NIH stroke scale (NIHSS) was a statistically significant predictor until controlling for volume. This indicates that the NIHSS may serve as a rough surrogate for infarct size, and may be useful in the initial assessment of hemorrhage risk before imaging results can be obtained.
The role of renal impairment in ICH is more surprising. There have been recent reports to suggest that renal failure is associated with higher rates of hemorrhage in patients with atrial fibrillation (16,17) and that elevated creatinine predicts increased risk of symptomatic hemorrhage after administration of factor Xa inhibitors (18). It is also known that patients with end stage renal disease on hemodialysis are prone to both thrombosis and hemorrhage (19). One of the potential ways that renal failure increases bleeding risk is through the secondary dysfunction of platelets (“uremic platelets”) (19). Unfortunately, the underlying mechanism of the dysfunction is poorly understood, and little data exist on milder renal dysfunction.
Within our retrospective dataset we found that anticoagulation alone did not increase the risk for hemorrhagic transformation in our population. It was, however, associated with larger, more severe intracerebral hematomas (all of the larger, symptomatic bleeds occurred in this group). The lack of a significant association of intracerebral hemorrhage with anticoagulation may simply reflect that the majority of the population was placed on anticoagulation. However, these results emphasize the importance of identifying patients who are at increased risk for even asymptomatic ICH, as they have the potential to have larger bleeds when anticoagulated. We included both symptomatic and asymptomatic hemorrhages in our analysis for this reason. Additionally, Park and colleagues have shown that even “asymptomatic” ICH leads to poorer long-term neurologic outcomes (20).
Our study had a higher than previously reported total hemorrhage rate of 24%. This finding may be explained, in part, by the fact that we included patients with any indication for anticoagulation (eg., DVT, PE) along with typical causes of stroke (eg., atrial fibrillation, cervical dissection), resulting in a potentially “sicker” patient population. This may be evident by the prolonged hospital course of our population (average length of stay 19.1 days (median 9.5, range 1–134)). Additionally, we included patients with both symptomatic and asymptomatic ICH. If we included only patients with symptomatic bleeds, the hemorrhage rate in the anticoagulated group was 8.1% (6.5% overall) which is closer to rates of symptomatic intracerebral hemorrhage in the literature (21).
We defined symptomatic hemorrhages as any change in neurologic status with new hemorrhage on subsequent neuroimaging. This is the definition of symptomatic hemorrhage used in the NINDS trial for intravenous tissue plasminogen activator (21,22). We chose not to use the definition employed by the ECASS investigators (a ≥4 point increase in NIHSS with corresponding blood on neuroimaging; (23)) because the NINDS definition was more sensitive by including patients with any change in neurologic function and mirrors clinical practice.
Our study is not without limitations. Nearly half of the population had reason to require an anticoagulant. Though indications were evidence-based, the high percentage of patients receiving anticoagulation was due in part to the significant comorbidities of our patient population, and may not be an accurate representation of the majority of inpatient stroke centers. Additionally, because of the high acuity of our population, not all patients were able to undergo MRI. Volumetric analysis on head CT is not as precise as MRI, in part secondary to less discrete borders, particularly in the acute setting. This limitation was weighed against the fact that these patients had the largest strokes, highest degrees of renal failure, and highest rates of intracerebral hemorrhage, making it critical to include them in analysis. Finally, this is a retrospective analysis of a relatively small number of subjects. There are multiple factors that likely influence hemorrhagic transformation such as blood glucose, history of hypertension, smoking, and alcohol use that we were unable to account for. There may also have been other unmeasured confounders. Consequently, because the study was retrospective, it was difficult to determine why follow-up head CTs were performed in some individuals. This results in the potential risk of misclassifying a patient with symptomatic hemorrhage, or missing cases of asymptomatic hemorrhage in individuals in whom follow-up scans were not obtained. Further review of our data showed that during a single hospitalization, 11 of 12 inpatients meeting criteria for this study received some form of follow-up neuroimaging, minimizing this risk. Even with these limitations, our data strongly suggest that age, infarct volume, and renal impairment are important predictors of hemorrhagic transformation in patients with acute ischemic stroke. Validation in a large, prospectively-collected, inpatient cohort is currently underway.
Acknowledgments
Dr. Marsh is supported by an R25 Grant- NIH/NINDS Research Education Program for Residents and Fellows in Neurology and Neurosurgery (5 R25 NS065729-04).
Footnotes
Conflicts of Interest:
None.
References
- 1.Coull BM, Williams LS, Goldstein LB, Meschia JF, Heitzman D, Chaturvedi S, Johnston KC, Starkman S, Morgenstern LB, Wilterdink JL, Levine SR, Saver JL. Joint Stroke Guideline Development Committee of the American Academy of Neurology, American Stroke Association: Anticoagulants and antiplatelet agents in acute ischemic stroke: report of the Joint Stroke Guideline Development Committee of the American Academy of Neurology and the American Stroke Association (a division of the American Heart Association) Stroke. 2002;33:1934–1942. doi: 10.1161/01.str.0000028456.18614.93. [DOI] [PubMed] [Google Scholar]
- 2.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- 3.Toni D, Fiorelli M, Bastianello S, Sacchetti ML, Sette G, Argentino C, Montinaro E, Bozzao L. Hemorrhagic transformation of brain infarct: predictability in the first 5 hours from stroke onset and influence on clinical outcome. Neurology. 1996;46:341–345. doi: 10.1212/wnl.46.2.341. [DOI] [PubMed] [Google Scholar]
- 4.Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction--a prospective study. Stroke. 1986;17:179–185. doi: 10.1161/01.str.17.2.179. [DOI] [PubMed] [Google Scholar]
- 5.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators: Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 6.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349:1569–1581. [PubMed] [Google Scholar]
- 7.Berge E, Abdelnoor M, Nakstad PH, Sandset PM. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial. Lancet. 2000;355:1205–1210. doi: 10.1016/s0140-6736(00)02085-7. [DOI] [PubMed] [Google Scholar]
- 8.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG. Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators: Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 9.Mohr JP, Thompson JL, Lazar LM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HPJ, Jackson CM, Pullicino P. Warfarin-Aspirin Recurrent Stroke Study Group: A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 10.CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 11.Duke RJ, Bloch RF, Turpie AG, Trebilcock R, Bayer N. Intravenous heparin for the prevention of stroke progression in acute partial stable stroke. Ann Intern Med. 1986;105:825–828. doi: 10.7326/0003-4819-105-6-825. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman JT, Hillis AE, Jordan LC. ABC/2: estimating intracerebral haemorrhage volume and total brain volume, and predicting outcome in children. Dev Med Child Neurol. 2011;53:281–284. doi: 10.1111/j.1469-8749.2010.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley WG. MR appearance of hemorrhage in the brain. Radiology. 1993;189:15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth WT, Jr, Katz R, Tirschwell DL, Cushman M, Psaty BM. Intravenous tissue plasminogen activator and stroke in the elderly. Am J Emerg Med. 2010;28:359–363. doi: 10.1016/j.ajem.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 17.Olesen JB, Lip GYH, Kamper A-L, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. NEJM. 2012;367:625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 18.Laino C. News from the International Stroke Conference: New Analysis Shows Diastolic Blood Pressure, Renal Impairment Risk Factors for Intracranial Hemorrhage. Neurology Today. 2012;12:12, 15. [Google Scholar]
- 19.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Seminars in Thrombosis and Hemostasis. 2004;30:579–589. doi: 10.1055/s-2004-835678. [DOI] [PubMed] [Google Scholar]
- 20.Park JH, Ko Y, Kim WJ, Jang MS, Yang MH, Han MK, Oh CW, Park SH, Lee J, Lee J, Bae HJ, Gorelick PB. Is asymptomatic hemorrhagic transformation really innocuous? Neurology. 2012;78:421–426. doi: 10.1212/WNL.0b013e318245d22c. [DOI] [PubMed] [Google Scholar]
- 21.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group: Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 22.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 23.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]


