Abstract
Objective
To evaluate healthy skeletal muscle pre- and post-exercise via 7 T 23Na MRI and muscle proton T2 mapping, and to evaluate diabetic muscle pre- and post-exercise via 7 T 23Na MRI.
Methods
The calves of seven healthy subjects underwent imaging pre- and post-exercise via 7 T 23Na MRI (3D fast low angle shot, TR/TE=80 ms/0.160 ms, 4 mm × 4 mm × 4 mm) and 1 week later by 1H MRI (multiple spin-echo sequence, TR/TE=3,000 ms/15–90 ms). Four type 2 diabetics also participated in the 23Na MRI protocol. Pre- and post-exercise sodium signal intensity (SI) and proton T2 relaxation values were measured/calculated for soleus (S), gastrocnemius (G), and a control, tibialis anterior (TA). Two-tailed t tests were performed.
Results
In S/G in healthy subjects post-exercise, sodium SI increased 8–13% (p<0.03), then decreased (t1/2=22 min), and 1H T2 values increased 12–17% (p<0.03), then decreased (t1/2=12–15 min). In TA, no significant changes in sodium SI or 1H T2 values were seen (−2.4 to 1%, p>0.17). In S/G in diabetics, sodium SI increased 10–11% (p<0.04), then decreased (t1/2=27–37 min) without significant change in the TA SI (−3.6%, p= 0.066).
Conclusion
It is feasible to evaluate skeletal muscle via 3D 23Na MRI at 7 T. Post-exercise muscle 1H T2 values return to baseline more rapidly than sodium SI. Diabetics may demonstrate delayed muscle sodium SI recovery compared with healthy subjects.
Keywords: Ultra high field MRI, Muscle, Sodium, T2 mapping, Diabetes
Introduction
It has been well documented that skeletal muscle proton T2 relaxation values increase following exercise, which leads to increased muscle signal intensity on T2-weighted 1H MR images [1–4]. This results predominantly from the intra-cellular accumulation of metabolites (lactate, phosphate) and osmotic shifts of water from the extracellular to the intracellular space [3, 5–7]. T2 maps of muscle can provide information on the spatial pattern of muscle activation in both normal and abnormal states [8, 9].
23Na MRI also has the potential to provide insight into muscle physiology. Within the intracellular space, skeletal muscle contains a low concentration of sodium (10–30 mM/L) and a high concentration of potassium (140 mM/L) relative to the concentrations in the extracellular space (sodium 145 mM/L, potassium 4 mM/L) [10]. This concentration gradient across the cell membrane is maintained by the Na+–K+ ATPase, which pumps out 3 Na+ ions in exchange for 2 K+ ions. When an action potential (leading to muscle contraction) is generated, there is a rapid, passive influx of sodium ions and efflux of potassium ions via other types of channels. During intense contractile activity, there is a persistent influx of sodium ions and efflux of potassium ions, which degrades the transmembrane Na+ and K+ gradients, leading to loss of membrane excitability and muscle contractility [10, 11]. Such loss of membrane excitability is believed to represent one of the main mechanisms of muscle fatigue [12].
Previous studies at 1.5 T have demonstrated the feasibility of observing increased skeletal muscle sodium signal intensity in healthy subjects after exercise [13, 14], in subjects with myotonic dystrophy [13] and in subjects with paramyotonia congenita [15, 16]. As numerous disease states, such as diabetes, starvation and hypothyroidism, have been documented to decrease Na+–K+ ATPase activity in skeletal muscle [10], 23Na MRI has the potential to play a role in imaging of these disorders as well. However, the detection of sodium signal at standard clinical field strength (1.5–3 T) can be technically challenging secondary to: (1) the low gyromagnetic ratio of sodium (16.8 MHz at 1.5 T), (2) the low tissue concentration of sodium compared with hydrogen (ca. 22,000 times less signal), and (3) the rapid biexponential T2 decay of sodium in tissue (0.5–8 ms and 15–30 ms).
Because signal-to-noise ratio (SNR) scales roughly with the magnitude of B0, ultra high field (UHF) MRI (7–9.4 T) has the potential to provide improved imaging of sodium compared with standard clinical field strength. The goals of this study were: (1) to determine the feasibility of performing 23Na MRI of skeletal muscle at 7 T and assess how skeletal muscle sodium signal intensity changes before and after exercise over several time points; (2) to compare these results with corresponding post-exercise changes in skeletal muscle proton T2 relaxation values; and (3) to assess skeletal muscle sodium signal intensity before and after exercise in a small set of subjects with impaired exercise capacity (type 2 diabetics).
Materials and methods
Phantom studies
To evaluate the relationship between sodium signal intensity and NaCl concentration, five phantoms composed of varying concentrations of NaCl (100, 150, 200, 250 and 300 mM/L) suspended in 4% agarose underwent imaging via 23Na MRI at 7 T. To evaluate the variability in signal measurements made via 23Na MRI, the phantoms were repeatedly scanned every 7 min from 0 through 35 min. To simulate the imaging protocol for human subjects (see below), after the initial image at 0 min, the phantoms were removed from the coil, repositioned and then imaged serially from 7 through 35 min. These phantoms were also included with every subject (taped to the anterior aspect of the leg) when they were imaged via 23Na MRI as a quality control measure.
B1 field homogeneity was evaluated by using the double angle method [17] to image a phantom of 15 cm in diameter composed of 300 mM/L NaCl suspended in 4% agarose.
Healthy human subjects
This study had institutional review board approval and all subjects provided written informed consent. We recruited seven healthy subjects (mean age = 29±3.6 years, 4 males, 3 females, no concurrent medical issues).
Exercise protocol
After an initial baseline image at rest, the subject got down off the MR couch, then hopped on one leg (the leg of their choice) until they reached fatigue. Mean exercise time was approximately 2±0.6 min. Subjects were then re-imaged serially every 7 min until a final time point of 35 min was reached.
23Na MRI
Imaging was performed on a 7 T whole body MRI unit (Siemens, Erlangen, Germany) using a quadrature 23Na knee coil (Rapid MR International, Columbus, OH, USA). We implemented a 3D-GRE sequence with a radial k-space acquisition (TR/TE=80 ms/0.160 ms, bandwidth (BW)= 130 Hz/pixel, signal average=10, number of projections= 512, 64 readout points, spatial resolution = 4 mm × 4 mm × 4 mm, acquisition time=6 min 50 s) using the manufacturer’s provided pulse sequence development environment (IDEA).
The x, y and z components of the readout gradient were computed by using the equations
where < represents the polar angle (0 < θ < π) and φ represents the azimuthal angle (0 < φ < 2 π) in k-space; k-space was filled from the centre to the periphery of a sphere, following cones from north to south of the sphere [16, 18]. A non-selective radiofrequency pulse with a duration of 200 μs was used and the signal was sampled during ramp-up of the readout gradient [19]. The resulting effective TE was TE = τRF/2 + τDelay = 160 µs.
1H MRI
Immediately following each 23Na MRI, subjects were imaged by 1H MRI at 7 T to delineate muscle anatomy. We utilised a 1H quadrature knee coil (Rapid MR International, Columbus, OH, USA) and performed a gradient echo sequence (3D fast low angle shot (FLASH), TR/TE= 20/4.5 ms; flip angle=10°; bandwidth =130 Hz/pixel; one signal acquired; 50 axial images with resolution 0.195 mm × 0.195 mm, 1-mm slice thickness).
To assess changes in skeletal muscle proton T2 relaxation values after exercise, healthy subjects returned 1 week later (to ensure that subjects were fully rested) and underwent H MRI at 7 T before and after performing the same identical exercise protocol described above (i.e. the same number of hops). A multiple spin-echo imaging sequence (TR=3,000 ms, TE=15, 30, 45, 60, 75, 90 ms, 0.585 mm × 0.585 mm, 1-mm slice thickness) was performed every 7 min until a final time point of 35 min to replicate the timing of the sodium MRI.
23Na and 1H MR image analysis
The scanner workstation software was used to draw 1-cm-diameter regions of interest (ROI) on proton images in flexor muscles used in the exercise, soleus (S) and medial gastrocnemius (G), and these were copied to the sodium images. An ROI was also drawn around a dorsiflexor muscle not used in the exercise, the tibialis anterior (TA), which served as an internal control.
For 23Na MRI, pre- and post-exercise sodium signal intensity (SI) was measured, and percentage change versus baseline was calculated as: [(post-exercise SI) – (resting SI)]/ (resting SI).
For 1H MRI, pre- and post-exercise muscle T2 values were calculated by fitting the six echoes to a monoexponential decay by using the equation M =M0 × e−t/T2. Half-lives (t1/2) for plots were estimated by obtaining a best-fit curve (Microsoft Excel, Redmond, WA, USA).
Statistical analysis
All statistical analyses were performed with SPSS (Chicago, Illinois). Group mean values were calculated for each muscle of interest. The coefficient of variation was calculated as the standard deviation divided by the mean signal intensity. Wilcoxon signed rank tests were utilized to determine statistical significance of measurement differences before and after exercise. Two-tailed t tests were performed to determine statistical significance of differences in half-lives.
Diabetic subjects
Finally, to determine if 23Na MRI might be able to detect alterations in muscle physiology in subjects with disease, we then recruited four subjects with longstanding type 2 diabetes (mean age=44±6.5 years, 2 males, 2 females, mean number of years since diagnosis of type 2 diabetes=7.8±4.3 years) to participate in the 23Na MRI protocol. Similar image and statistical analyses were performed.
Results
Phantom studies
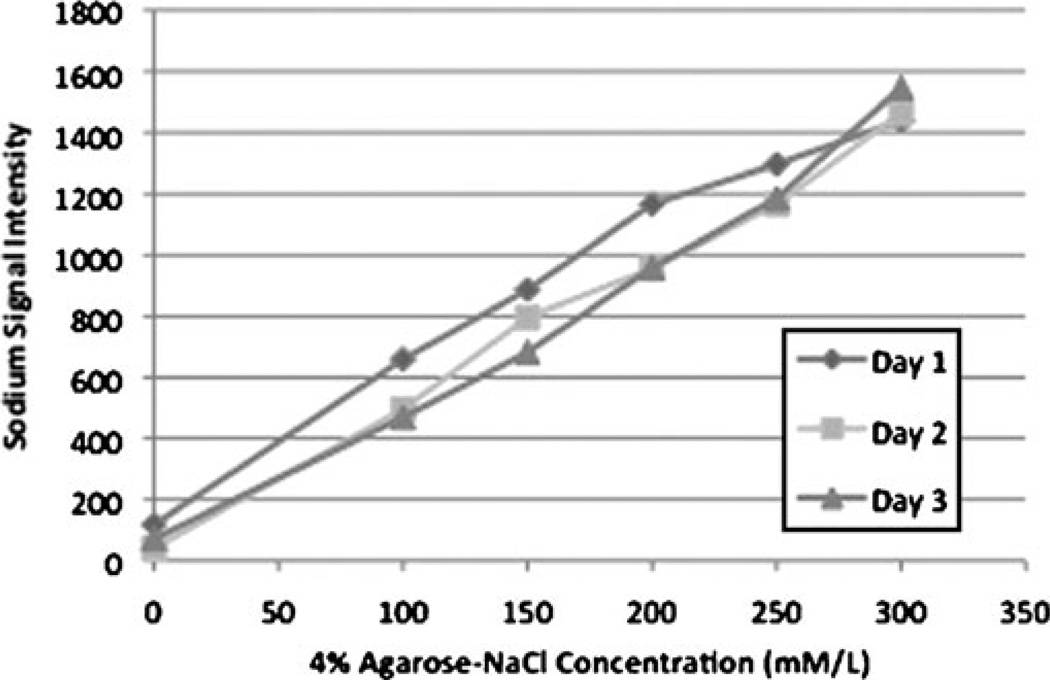
Maps of B1 field homogeneity demonstrated less than 5% variation in the B1 field along both horizontal and vertical axes when this phantom was imaged via 23Na MR at 7 T (not shown). Figure 1 is a plot of sodium signal intensity vs. phantom NaCl concentration, with background signal intensity plotted as 0 mM/L NaCl. The results of three experiments performed on different days are plotted. The plot demonstrates that there is a linear relationship between the 23Na MR signal and NaCl concentration. When phantoms were serially imaged every 7 min from 0 through 35 min, the coefficient of variation is less than 2% over time for all phantoms. Of note, the phantoms were repositioned in between the 0-min and 7-min images in order to replicate the human imaging protocol.
Fig. 1.
Plot of sodium signal intensity vs. phantom NaCl concentration (100–300 mM/L) with background signal intensity plotted as 0 mM/L NaCl. There is a linear relationship between sodium MR signal and NaCl concentration. The results of three experiments performed on different days are plotted
Muscle 23Na MRI post-exercise in healthy subjects
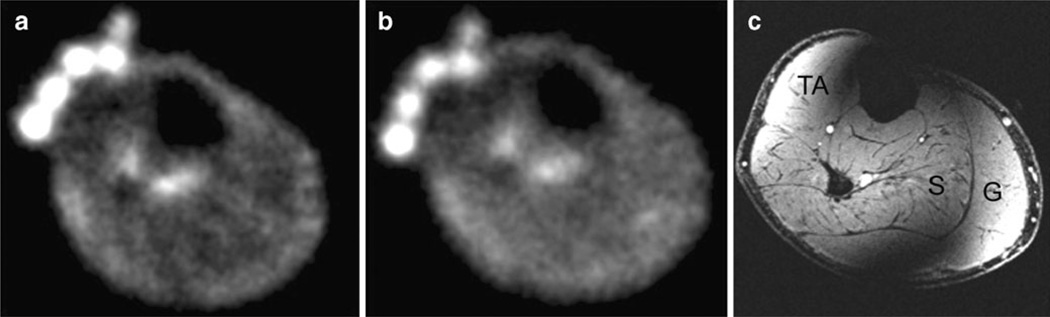
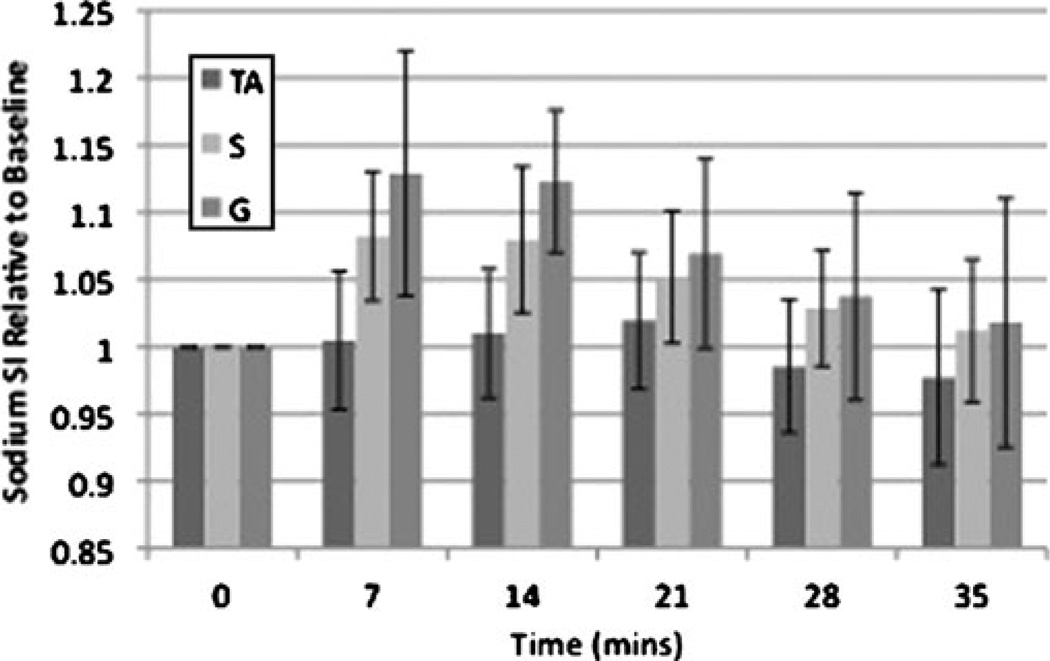
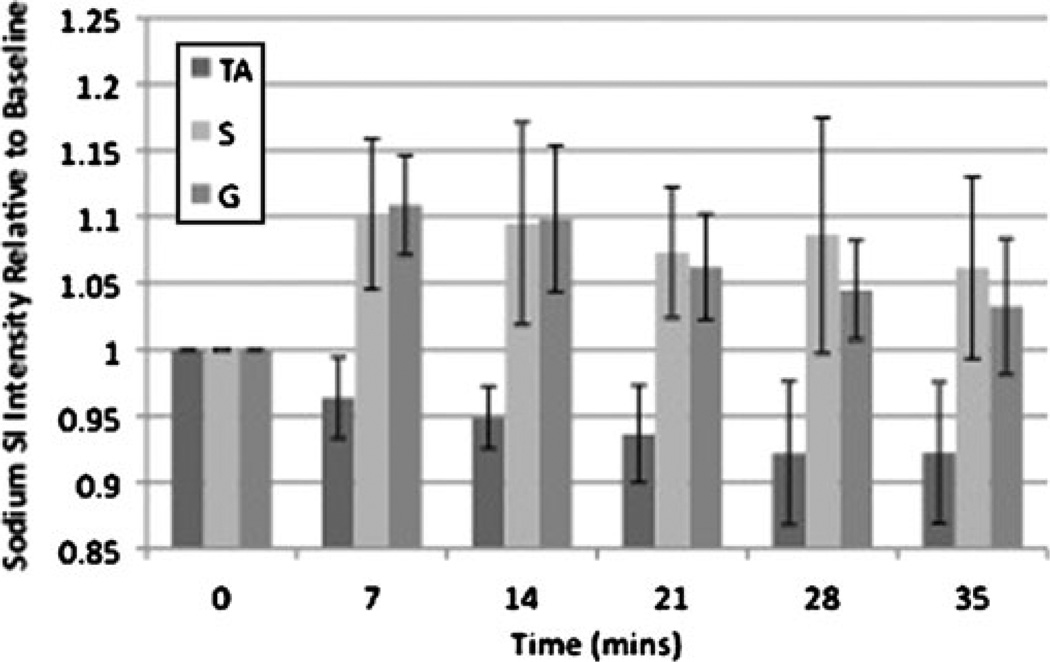
Representative resting 23Na, immediate post-exercise 23Na, and axial 1H MR images from two subjects are shown in Fig. 2. In the proton image (Fig. 2c), there is radio-frequency coil/field inhomogeneity artefact anteriorly and posteriorly. A subtle increase in signal intensity within plantar flexor muscles used in the exercise (soleus and gastrocnemius) can be seen on the post-exercise images. In healthy subjects, compared with rest, there was a statistically significant percentage increase in immediate post-exercise sodium signal intensity in S (8±4%, p= 0.028) and G (13±8%, p=0.028), but not in TA (0.5±5%, p=0.92) (Fig. 3).
Fig. 2.
a Resting sodium MR image (TR=80 ms, TE=0.160 ms, 4 mm × 4 mm × 4 mm) from the calf of a healthy volunteer. The NaCl phantoms can be seen at the anterior aspect of the leg. b Immediate post-exercise sodium MR image from the same volunteer, showing subtle increase in sodium signal intensity within the posterior compartment muscles. c Corresponding proton 3D fast low angle shot (FLASH) MR image (TR=20 ms, TE=5 ms, 0.195 mm × 0.195 mm, 1-mm slice thickness) from the same volunteer, which was performed after the sodium MRI to delineate muscle anatomy. TA tibialis anterior, S soleus, G gastrocnemius. Coil inhomogeneity artefact is seen at the anterior and posterior aspects of the leg
Fig. 3.
Bar graph demonstrating the time course of recovery for sodium signal intensity after exercise in healthy subjects. Immediately after exercise, sodium signal intensity increased in S (8±4%, p=0.028) and G (13±8%, p=0.028), but not in the dorsiflexor control muscle TA (0.55%, p=0.92). Sodium signal intensity then decreased to near baseline in an exponential fashion with a t1/2 of approximately 22 min for both S and G
Over the next 35 min, mean SI in both S and G decreased to near baseline in an exponential fashion (Fig. 3) with a t1/2 of approximately 22 min for both S and G. There was no statistically significant change in sodium signal intensity in TA (−2.4±5%, p=0.46) at 35 min.
Muscle proton T2 relaxation values post-exercise in healthy subjects
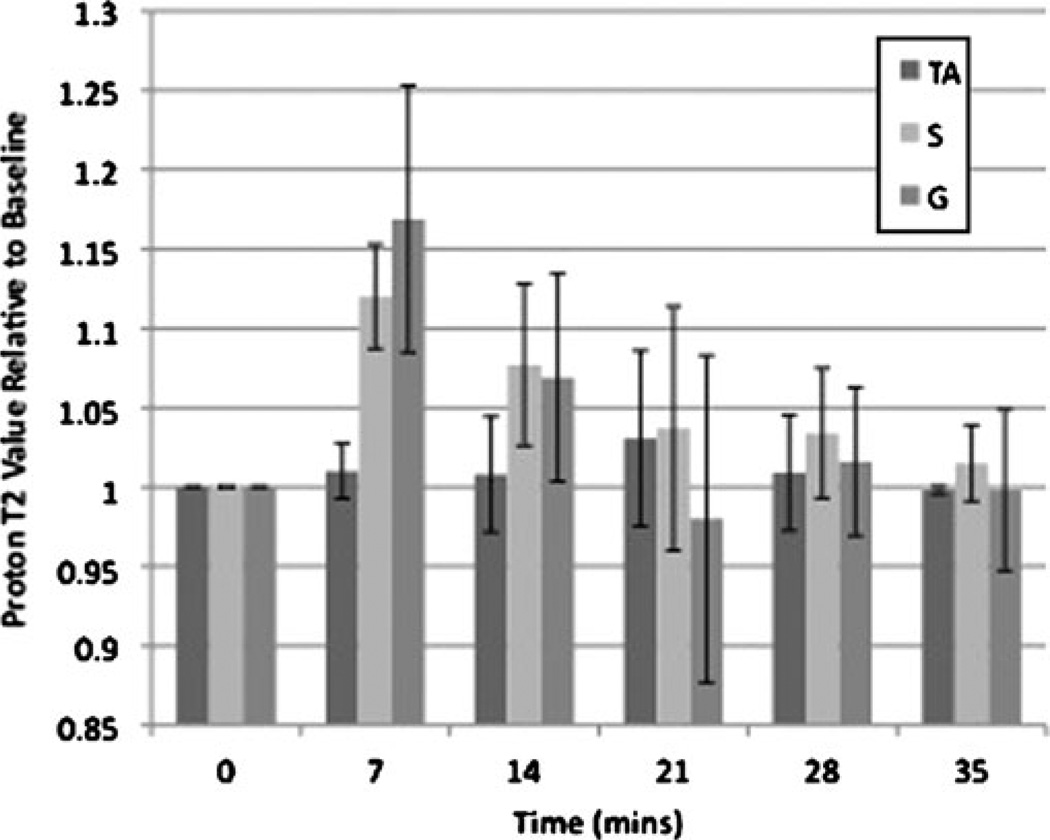
Figure 4 demonstrates the change in muscle proton T2 relaxation values before and after exercise. Mean pre-exercise proton T2 relaxation values for TA, S and G were 33.9±3.78 ms, 36.8±4.2 ms and 36.7±4.67 ms, respectively. After exercise, there was a statistically significant increase in proton T2 relaxation values for S (12±3%, p= 0.01) and G (17±8%, p=0.029), but not for TA (1±2%, p= 0.18). Proton T2 relaxation values in both S and G gradually returned to baseline in a logarithmic fashion with a t1/2 of approximately 15 min for S and 12 min for G. When compared with the half-lives for recovery of sodium signal intensity, the difference in half-lives was statistically significant (p=0.049 for S, p=0.012 for G).
Fig. 4.
Bar graph demonstrating changes in proton T2 relaxation values before and after exercise. Baseline T2 relaxation values for tibialis anterior (TA), soleus (S) and gastrocnemius (G) were 33.9± 3.78 ms, 36.8±4.2 ms and 36.7±4.67 ms, respectively. Immediately after exercise, T2 relaxation values increased in S (12±3%, p=0.01) and G (17±8%, p=0.029), but not in TA (1±2%, p=0.18). T2 relaxation values in S and G then decreased in a logarithmic fashion with half-lives (t1/2) of approximately 15 and 12 min, respectively
Muscle 23Na MRI post-exercise in subjects with diabetes
In subjects with diabetes (Fig. 5), compared with rest, there was also a statistically significant increase in immediate post-exercise sodium signal intensity in S (10±6%, p= 0.034) and G (11±4%, p=0.009), but not in TA (−3.6±4%, p=0.066). Over time, mean SI in both S and G gradually decreased to near baseline with a t1/2 of approximately 37 min for S and 27 min for G. SI in TA decreased by approximately 7±5% at 35 min; this difference compared with rest was not statistically significant (p=0.066). When compared with the half-lives for recovery of sodium signal intensity in healthy subjects (t1/2 22 min for both S and G), the difference in half-lives was statistically significant (p= 0.05 for S and G).
Fig. 5.
Bar graph demonstrating the time course of recovery for sodium signal intensity after exercise in diabetics. Immediately after exercise, sodium signal intensity increased in S (10±6%, p=0.034) and G (11±4%, p=0.009), but not in TA (−3.6±4%, p=0.066). Over time, sodium SI in S and G gradually decreased with half-lives (t1/2) of approximately 37 and 27 min, respectively
Discussion
This study demonstrates the feasibility of performing 3D 23Na MRI of human skeletal muscle at 7 T in vivo. Specifically, the results demonstrate the feasibility of monitoring physiological changes that occur in skeletal muscle after exercise via sodium MRI. In addition, this study provides evidence that changes in skeletal muscle sodium signal intensity following exercise follow a different time course than corresponding changes that occur in skeletal muscle proton T2 relaxation values. Finally, the results suggest that sodium MRI may be able to detect alterations in muscle physiology in subjects with diabetes.
Sodium imaging in this study was facilitated by imaging at 7 T (SNR scales roughly with B0; for full discussion, see Collins et al. [20]) and by the implementation of a 3D radial acquisition sequence with an ultra-short TE of 0.160 ms.
Sodium has a biexponential T2 decay with a fast component of 0.5–8 ms and a slow component of 15–30 ms, which account for approximately 60% and 40% of the signal, respectively [14]. The use of an ultra-short TE sequence will therefore decrease T2* signal loss. This echo time is shorter than those utilized in previous 23Na MR imaging studies. The 3D radial sequence is well suited for short TE imaging since no slice selection pulse is required, thus minimizing the delay between excitation and data acquisition. In addition, the k-space trajectory begins at the origin of k-space, allowing efficient sampling of low frequency, high amplitude data with tolerable streaking artefacts.
The phantom studies demonstrate that there is a linear relationship between sodium signal intensity and NaCl concentration and little variability in measurements for serial images made during the same day. Such demonstration of reproducibility of measurements is important if comparisons are going to be made between scans performed on the same day and if quantitative 23Na MR imaging is to be performed in the future.
The increase in muscle sodium signal intensity after exercise in healthy subjects is in agreement with: (1) results of studies in the physiology literature, in which skeletal muscle sodium content increases (in an intracellular fashion) after muscle stimulation [21–23], and (2) other 23Na MRI studies of skeletal muscle in human subjects in vivo [13, 14]. In the latter studies, 34±7% [13] and 16– 22% [14] increases in sodium signal intensity were observed after exercise. In a study by Weber et al. [15], no significant increase in calf muscle sodium signal intensity was observed in healthy subjects after exercise. However, these subjects performed only 30 calf raises while standing on both legs as opposed to our and previous studies in which up to 200 hops on one leg were performed. The lack of increased muscle sodium signal intensity in Weber et al.’s study may have been due to a less vigorous exercise protocol. However, their goal was to evaluate subjects with paramyotonia congenita, who have persistently depolarised muscle cells; thus the exercise protocol was likely kept to a minimum in order not to induce motor difficulties in the patient population.
The results also demonstrate an apparent approximate 3–7% decrease in sodium signal intensity in the control muscle, tibialis anterior, at the 35-min time point for both healthy subjects and diabetics. Though this difference was not statistically significant (p=0.46 for healthy subjects, p=0.066 for diabetics), the number of subjects in the study is small. The authors can only conjecture that the activation of physiologic mechanisms to maintain sodium homeostasis after exercise (i.e. mechanisms which function to decrease muscle sodium content after it rises) might nonspecifically affect the entire leg. For example, blood perfusion to the leg, which is augmented after exercise and functions in part to remove metabolites that have accumulated, might cause a small decrease in sodium content in the tibialis anterior muscle even though it was not stimulated. This is an avenue of research that is worth pursuing in the future.
The biophysical basis for the increase in sodium signal intensity in muscle after exercise is probably multifactorial. This increase in sodium signal could be due to: (1) an increase in total muscle sodium content and/or (2) alterations in sodium’s T2 relaxation values or in the proportions of slow- and fast-T2-relaxing pools of sodium.
With regard to changes in the total muscle sodium content, the sodium signal measured represents the sum of sodium contained in the intracellular (i) and extracellular (e) compartments (Total sodium content = [Na]i × Volumei+ [Na]e × Volumee), with the extracellular compartment comprising approximately 7–14% of the total skeletal muscle volume [3,24]. Furthermore, the extracellular compartment is composed of the interstitial space and the vascular space. Although the extracellular sodium concentration (ca. 140 mM/L) remains essentially constant in a patient with normal renal function and constant blood perfusion, the volumes of the intracellular and extracellular compartments increase after exercise [3], and the intracellular concentration of sodium increases during muscle cell depolarisation [21–23]. Therefore, the increase in sodium signal intensity following exercise could reflect changes in the quantity of sodium in both compartments.
With regard to alterations in muscle sodium T2 relaxation values after exercise, it is believed that the rapid-T2-relaxing pool of sodium corresponds to fast tumbling, free sodium in solution and the slow-T2-relaxing pool of sodium corresponds to sodium interacting with macromolecules [14]. Therefore, if after exercise, there is an increase in the T2 relaxation value of either pool of sodium or an increase in the proportion of slow-T2-relaxing pool of sodium, there would be less signal loss and greater signal measured for a given echo time. Future work will involve quantifying not only the contributions of the intracellular and extracellular compartments to the sodium signal, but also quantifying the contributions of slow- and fast-T2-relaxing sodium pools to the sodium signal, and determining how these contributions are altered in various disease states.
The recovery of skeletal muscle proton T2 values back to baseline (t1/2 12–15 min) was faster than the recovery of skeletal muscle sodium signal intensity back to baseline (t1/2 22 min). The difference in recovery time for these two processes likely stems from the fact that they reflect different physiological phenomena. The increase in muscle proton T2 relaxation values after exercise is related to the accumulation of intracellular metabolites (lactate, phosphate) and osmotic shifts of water from the extracellular to the intracellular space [3, 5–7]. If phosphate and lactate are cleared relatively rapidly from the cell via enzymatic processes (regeneration of adenosine triphosphate (ATP) via oxidative phosphorylation, reduction in lactate via the Cori cycle), then fluid shifts between the extracellular and intracellular compartments will in turn rapidly reverse, and muscle proton T2 relaxation values will normalise. However, normalisation of the sodium signal requires clearance of sodium from the intracellular compartment (which depends on Na+–K+ pump activity), as well as the ability of the flowing blood to remove extruded sodium ions from the extracellular space. This may simply require more time compared with the time required to clear intracellular muscle metabolites, resulting in slower recovery of the sodium signal intensity to baseline compared with the proton T2 relaxation values.
Finally, the results of this study suggest that post-exercise skeletal muscle sodium signal intensity in diabetics recovers to baseline more slowly compared with healthy subjects. One possible explanation for this observation may be related to the decreased Na+–K+ pump activity as well as the altered tissue microvasculature in diabetics. According to exercise physiology literature, Na+–K+ pump activity helps protect muscles against fatigue by maintaining Na+ and K+ concentration gradients across the cell membrane, thereby preserving muscle membrane excitability [10, 25]. Diabetics, however, have decreased Na+–K+ pump activity and decreased numbers of Na+–K+ pumps on the muscle cell membrane, which has been attributed to insulin resistance [10, 26–28]. Decreased Na+–K+ pump activity would result in a decreased ability to extrude intracellular sodium ions into the extracellular space and result in persistently elevated muscle sodium content with a slower recovery to baseline. The latter would manifest as increased signal intensity on 23Na MRI and a longer t1/2 for recovery to baseline values, as observed in this study. Furthermore, diabetics demonstrate altered tissue microvasculature and poor tissue perfusion; a decreased ability of flowing blood to remove extruded sodium ions from the extracellular space could also result in persistently elevated muscle sodium content and persistently elevated muscle sodium signal intensity. 23Na MRI might eventually be applied to patients with diabetes in order to better evaluate those who are at risk of diabetic muscle infarction—a grave complication of longstanding diabetes—as the calf is one of the most frequent sites of involvement [29, 30].
There are several limitations to this study. First, as a pilot study, the number of subjects was small. Second, although subjects performed the same exercise protocol, it is possible that subjects exercised with different intensities, which could affect the results. Third, there was no gold standard validation of measurements, as muscle biopsy would have been invasive, potentially dangerous and unethical in this patient population. Fourth, at the 130 Hz/pixel bandwidth used in this study, the total readout time is 7.69 ms; therefore, fast sodium T2 decay during this readout time will result in blurring of high frequency components on the images. Finally, based on the plot generated by phantom studies, the calculated total muscle sodium content in our healthy subjects at rest is approximately 44 mM, or about 50% greater than values reported in previous muscle sodium imaging studies [13, 14]. The greater values obtained in this study may stem from the use of a highly undersampled radial acquisition sequence. In future studies, protocols with less undersampling may have to be implemented in order to accurately perform quantitative 23Na MRI. It should be noted, however, that changes relative to baseline may ultimately represent the more important quantitative imaging biomarker.
In conclusion, this study demonstrates the feasibility of monitoring physiological changes in muscle before and after exercise via 3D 23Na MRI at 7 T. Compared with the changes that occur in muscle proton T2 relaxation values after exercise, muscle sodium signal intensity requires more time to return to baseline. Finally, the results of this study suggest that 23Na MRI might be able to detect alterations in muscle physiology after exercise in subjects with diabetes. 23Na MRI could potentially be used as a non-invasive method of gaining physiological and metabolic information about skeletal muscle.
Acknowledgements
The authors would like to acknowledge support from the RSNA (RR0806) and NIAMS/NIH (R01-AR053133-01A2).
Contributor Information
Gregory Chang, Department of Radiology, NYU, Langone Medical Center, Center for Biomedical Imaging/Hospital for Joint Diseases, 660 First Avenue, New York, NY 10016, USA, gregory.chang@nyumc.org, Tel.: +1-212-5986373, Fax: +1-212-5986125.
Ligong Wang, Department of Radiology, NYU, Langone Medical Center, Center for Biomedical Imaging/Hospital for Joint Diseases, 660 First Avenue, New York, NY 10016, USA.
Mark E. Schweitzer, Department of Diagnostic Imaging, Ottawa General Hospital, 501 Smyth Road, Ottawa, ON, Canada K1H 8L6
Ravinder R. Regatte, Department of Radiology, NYU, Langone Medical Center, Center for Biomedical Imaging/Hospital for Joint Diseases, 660 First Avenue, New York, NY 10016, USA
References
- 1.Fleckenstein JL, Canby RC, Parkey RW, Preshock RM. Acute effects of exercise on MR imaging of skeletal muscle in normal volunteers. AJR Am J Roentgenol. 1988;151:231–237. doi: 10.2214/ajr.151.2.231. [DOI] [PubMed] [Google Scholar]
- 2.Fleckenstein JL, Bertocci LA, Nunnally RL, Parkey RW, Peshock RM. Exercise-enhanced MR imaging of variation in forearm muscle anatomy and use: importance in MR spectros-copy. Am J Roentgenol. 1989;153:693–698. doi: 10.2214/ajr.153.4.693. [DOI] [PubMed] [Google Scholar]
- 3.Patten C, Meyer RA, Fleckenstein JL. T2 mapping of muscle. Semin Musculoskelet Radiol. 2003;7:297–305. doi: 10.1055/s-2004-815677. [DOI] [PubMed] [Google Scholar]
- 4.Meyer RA, Prior BM. Functional magnetic resonance imaging of muscle. Exerc Sport Sci Rev. 2000;28:89–92. [PubMed] [Google Scholar]
- 5.Damon BM, Gregory CD, Hall KL, et al. Intracellular acidification and volume increases explain R2 decreases in exercising muscle. Magn Reson Med. 2002;47:14–23. doi: 10.1002/mrm.10043. [DOI] [PubMed] [Google Scholar]
- 6.Saab G, Thompson RT, Marsh GD. Effects of exercise on muscle transverse relaxation determined by MR imaging and in vivo relaxometry. J Appl Physiol. 2000;88:226–233. doi: 10.1152/jappl.2000.88.1.226. [DOI] [PubMed] [Google Scholar]
- 7.Ploutz-Snyder LL, Nyren S, Cooper TG, Potchen EJ, Meyer RA. Different effects of exercise and edema on T2 relaxation in skeletal muscle. Magn Reson Med. 1997;37:676–82. doi: 10.1002/mrm.1910370509. [DOI] [PubMed] [Google Scholar]
- 8.Fleckenstein JL, Haller RG, Lewis SF, et al. Absence of MRI enhancement of skeletal muscle in McArdle’s disease. J Appl Physiol. 1991;71:961–969. doi: 10.1152/jappl.1991.71.3.961. [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka H, Anno I, Kuramoto K, et al. Acute effects of exercise on muscle MRI in peripheral arterial occlusive disease. Magn Reson Imaging. 1995;13:651–659. doi: 10.1016/0730-725x(95)00018-c. [DOI] [PubMed] [Google Scholar]
- 10.Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- 11.Clausen T. Na+-K+ pump stimulation improves contractility in damaged muscle fibers. Ann NY Acad Sci. 2005;1066:286–294. doi: 10.1196/annals.1363.021. [DOI] [PubMed] [Google Scholar]
- 12.McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cldisturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol. 2008;104:288–295. doi: 10.1152/japplphysiol.01037.2007. [DOI] [PubMed] [Google Scholar]
- 13.Constantinides CD, Gillen JS, Boada FE, Pomper MG, Bottomley PA. Human skeletal muscle: sodium MR imaging and quantification—potential applications in disease and exercise. Radiology. 2000;216:559–568. doi: 10.1148/radiology.216.2.r00jl46559. [DOI] [PubMed] [Google Scholar]
- 14.Bansal N, Szczepaniak L, Ternullo D, Fleckenstein JL, Malloy CR. Effect of exercise on 23Na MRI and relaxation characteristics of human calf muscle. J Magn Reson Imaging. 2000;11:532–538. doi: 10.1002/(sici)1522-2586(200005)11:5<532::aid-jmri9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Weber MA, Nielles-Vallespin S, Huttner H, et al. Evaluation of patients with paramyotonia at 23Na MR imaging during cold-induced weakness. Radiology. 2006;240:489–500. doi: 10.1148/radiol.2401050737. [DOI] [PubMed] [Google Scholar]
- 16.Nielles-Vallespin S, Weber MA, Bock M, Bongers A, Speier P, Combs SE, Wohrle J, Lehmann-Horn F, Essig M, Schad LR. 3D radial projection technique with ultrashort echo times for sodium MRI: clinical applications in human brain and skeletal muscle. Magn Reson Med. 2007;57:74–81. doi: 10.1002/mrm.21104. [DOI] [PubMed] [Google Scholar]
- 17.Stollberger R, Wach P, McKinnon G, Justich E, Ebner F. RF-field mapping in vivo. In: Proceedings of the 7th annual meeting of ISMRM, San Francisco, CA. 1988:106. [Google Scholar]
- 18.Jerecic R, Bock M, Nielles-Vallespin S, Wacker C, Bauer W, Schad LR. ECG-gated 23Na-MRI of the human heart using a 3D-radial projection technique with ultra-short echo times. MAGMA. 2004;16:297–302. doi: 10.1007/s10334-004-0038-8. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Wu Y, Chang G, et al. Rapid isotropic 3D-sodium MRI of the knee joint at in vivo at 7T. J Magn Reson Imaging. 2009;30:606–614. doi: 10.1002/jmri.21881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins CM. Radiofrequency field calculations for high field MRI. In: Robitaille PM, Berliner LJ, editors. Ultra high field magnetic resonance imaging. New York, NY: Springer; 2006. pp. 209–248. [Google Scholar]
- 21.Sjogaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol Regul Integr Comp Physiol. 1985;248:R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- 22.Fong CN, Atwood HL, Charlton MP. Intracellular sodium activity at rest and after titanic stimulation in muscles of normal and dystrophic (dy2j/dy2j)C57B1/6J mice. Exp Neurol. 1986;93:359–368. doi: 10.1016/0014-4886(86)90196-2. [DOI] [PubMed] [Google Scholar]
- 23.Juel C. Potassium and sodium shifts during in vitro isometric muscle contraction, and the time course of the ion-gradient recovery. Pflugers Arch. 1986;406:458–463. doi: 10.1007/BF00583367. [DOI] [PubMed] [Google Scholar]
- 24.Magzoub M, Zhang H, Dix JA, Verkman AS. Extracellular space volume measured by two-color pulsed dye infusion with microfiberoptic fluorescence photodetection. Biophys J. 2009;96:2382–90. doi: 10.1016/j.bpj.2008.12.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cldisturbances and Na+-K+ pump inacti-vation: implications for fatigue. J Appl Physiol. 2008;104:288–295. doi: 10.1152/japplphysiol.01037.2007. [DOI] [PubMed] [Google Scholar]
- 26.Djurhuus MS, Vaag A, Klitgaard NAH. Muscle sodium, potassium, and [3H]-ouabain binding in identical twins, discordant for type 2 diabetes. J Clin Endocrinol Metab. 2001;86:859–866. doi: 10.1210/jcem.86.2.7239. [DOI] [PubMed] [Google Scholar]
- 27.Kjeldsen K, Braendgaard H, Sidenius P, et al. Diabetes decreases Na+-K+ pump concentration in skeletal muscles, heart ventricular muscle, and peripheral nerves of rat. Diabetes. 1987;36:842–848. doi: 10.2337/diab.36.7.842. [DOI] [PubMed] [Google Scholar]
- 28.Sweeney G, Klip A. Mechanisms and consequences of Na+-K+ pump regulation by insulin and leptin. Cell Mol Biol. 2001;47:363–372. [PubMed] [Google Scholar]
- 29.Jelinek JS, Murphey MD, Aboulafia AJ, Dussault RG, Kaplan PA, Snearly WN. Muscle infarction in patients with diabetes mellitus: MR imaging findings. Radiology. 1999;211:241–247. doi: 10.1148/radiology.211.1.r99ap44241. [DOI] [PubMed] [Google Scholar]
- 30.Ly JQ, Yi EK, Beall DP. Diabetic muscle infarction. AJR Am J Roentgenol. 2003;181:1216. doi: 10.2214/ajr.181.5.1811216. [DOI] [PubMed] [Google Scholar]