Abstract
Heme is a prosthetic group in a large number of essential proteins that have a pivotal role in oxygen transport, storage and electron shuttling. High amounts of free heme are associated with pathological states,. Recently, it has been suggested that activation of Toll-like receptor 4 (TLR4) is one of the ways in which the “danger signal” of free heme is detected. Here we examine the biochemical basis of the modulation of the TLR4 pathway by hemin (iron(III)-protoporphyrin IX) and its metabolic, oxidated derivative coprohemin (iron(III)-coproporphyrin I). High concentrations of hemin (50 µM) triggered TLR4-mediated IL-8 production in human HEK293/TLR4 cell line in the absence of the co-receptors CD14 and MD-2, the latter an essential co-receptor for TLR4 activation by endotoxin. Hemin and endotoxin have additive effects when co-administrated to HEK/TLR4 cells, suggesting that hemin and endotoxin activate TLR4 by different mechanisms. Coprohemin, in contrast to hemin, is unable to trigger TLR4-dependent activation of HEK/TLR4 cells, but instead causes dose-dependent inhibition of endotoxin-stimulated IL-8 production. The inhibitory effect of coprohemin is paralleled by reduced delivery of endotoxin to MD-2(·TLR4) that is necessary for activation of TLR4 by endotoxin. Thus, despite their similar chemical structure, hemin and coprohemin have very different effects on the TLR4 pathway, the former acting as a mild agonist of TLR4, the latter as an antagonist selectively targeting the endotoxin-MD-2 interaction.
Keywords: TLR4, MD-2, heme, hemin, coprohemin
INTRODUCTION
Heme, i.e., iron(II)-protoporphyrin IX, provides a multitude of crucial biological functions as a prosthetic moiety in proteins involved in electron transport chains and in enzymes including catalases and peroxidases.1 In addition, free heme may affect a wide spectrum of biological processes and responses.2–4 Several pathologic situations, some associated with the presence of an invading infectious agent, can lead to increased hemolysis and very high levels of free heme. This includes malaria, sickle cell disease,5 ischemia-reperfusion injury, various hemoglobinopathies, traumatic hemorrage, and muscle injury.6–8 The presence of free heme released from hemoproteins can promote inflammation and oxidative damage to cells, due in part to the formation of oxygen radicals, that catalyze the oxidation of lipids, proteins and DNA.9,10 The role of heme in inflammation can be either protective or harmful: small concentrations of heme can rapidly up-regulate heme oxygenase-1 (HO-1) and have cytoprotective effects,11 whereas accumulation of large amounts of heme that exceed the neutralizing capacity of heme-binding proteins or HO-1 may be deleterious in tissues via its pro-oxidative and pro-inflammatory actions.12 Studies of heme-induced inflammation in a mouse model have shown that heme increases vascular permeability and elicits the expression of pro-inflammatory adhesion molecules,13–15 promoting tissue infiltration of leukocytes.16
Recent studies have also suggested that heme and its derivatives (i.e., biosynthetic precursors or products of heme catabolism) can affect Toll-like Receptors (TLRs) that are involved in activation of innate immunity and production of pro-inflammatory cytokines. The malaria pigment hemozoin (HZ), an insoluble crystal formed in the food vacuole of the malaria parasite, is a polymer comprised of several β-haematin,s ([iron(III)-protoporphyrin IX]2 dimer units), linked by non-covalent bonds. Hemozoin was first reported to induce inflammation by directly engaging TLR917,18 but more recent data have shown that highly purified HZ is immunologically inert and functions as a carrier for malarial DNA, which, as a consequence of being bound to HZ, is targeted to TLR9.19
In addition, a recent study showed that hemin (iron(III) heme) activates macrophages from wild type but not from TLR4−/−, CD14−/−, or MyD88−/− mice, indicating that hemin-induced secretion of tumor necrosis factor (TNF)-α by mouse macrophages was TLR4- CD14- and MyD88-dependent manner.20 However, whereas the TLR4 antagonist E5564 and an anti-TLR4·MD-2 antibody inhibited TNF-α secretion induced by lipopolysaccharide (LPS; endotoxin), these compounds did not inhibit cell activation by hemin.20 Conversely, biologically inactive heme variants such as iron-free protoporphyrin IX inhibited TLR4-dependent TNF-α secretion induced by heme but not that induced by LPS.20 The authors of this study suggested that hemin can interact with (MD-2)·TLR4 in a CD14-dependent manner but at sites distinct from where LPS acts.20
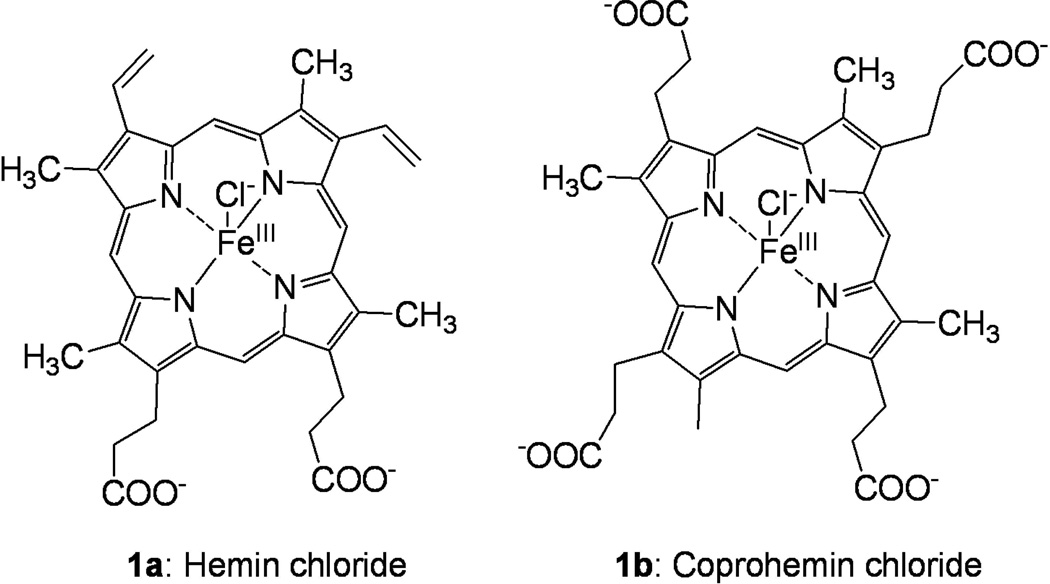
In this study, we have extended examination of the molecular determinants of TLR4-dependent cell activation by hemin (Fig. 1, compound 1a) in comparison to endotoxin (lipooligosaccharide (LOS)) from Neisseria meningitidis whose interactions with CD14, MD-2 and MD-2·TLR4 have been extensively described21–23. We show that hemin can induce TLR4-dependent cell activation in a human cell line in both a MD-2/CD14-dependent and –independent manner. In contrast, coprohemin, the iron(III)-coproporphyrin I (1b), that differs from hemin only by the presence of two proprionate rather than vinyl groups at the C-3 and C-8 positions of the tetrapyrrole ring, does not induce TLR4-dependent cell activation, but rather inhibits LPS-induced cell activation apparently by interfering with delivery of endotoxin to MD-2·TLR4. These findings extend evidence of functional interactions of heme and related compounds with the TLR4 pathway and further demonstrate that discrete structural modifications of heme can dramatically alter how these compounds influence TLR4 function.
Fig. 1.
Chemical structures of iron(III)-protoporphyrin IX chloride, hemin (1a) and iron(III)-coproporphyrin I chloride, coprohemin (1b).
MATERIALS AND METHODS
Materials
Radiolabeled lipooligosaccharide ([3H]LOS) (25,000 cpm/pmol) was metabolically labeled and isolated from an acetate auxotroph of Neisseria meningitidis serogroup B as previously described 21. Lipopolysaccharide-binding protein (LBP) and sCD14 were gifts from Xoma (Berkley, CA) and Amgen Corp. (Thousand Oaks, CA), respectively. Human serum albumin (HSA) was obtained as an endotoxin-free, 25% stock solution (Baxter Health Care, Glendale, CA). Chromatography matrices (Sephacryl HR S200 and S300) were purchased from GE Healthcare (Piscataway, NJ) and the silica-based metal chelation matrix, HisLink, was from Promega. ESF921 medium for High Five insect cells was purchased from Expressions Systems. Limulus amebocyte lysate (LAL) assay kits were purchased from Lonza Bio-Whittaker (Walkersville, MD) and used according to the manufacturer’s instructions.
Hemin (Fig. 1, compound 1a) was purchased from Sigma, and further purified by reversed-phase HPLC (C18 symmetry column, UV monitoring at 280 and 214 nm, Waters apparatus) using a gradient of acetonitrile in water. The purity of the recovered hemin was assessed by mass spectrometry analysis. High resolution mass spectra were recorded on a QSTAR Elite® LC/MS/MS system (Applied Biosystems, a hybrid quadrupole/TOF instrument), equipped with the Analyst® QS 2.0 software. This mass spectrometer, operating at negative ion mode, has a fmol detection limit. The purified hemin preparation showed peaks at mass of 615 and 632 corresponding to hemin and 632 hemin + H2O, respectively, but no masses that could correspond to LPS. The absence of contaminating endotoxin was confirmed by LAL assay: it was found that 50 µM hemin contains <100/ml pg LOS-equivalents (<1 EU,endotoxin unit/ml).
Coprohemin (Fig. 1, 1b) was synthesized by iron insertion into the commercially available coproporphyrin I (Sigma) by using the following procedure.24 Coproporphyrin I (50 mg, 0.07 mmol) was dissolved in DMF (15 ml) and heated to 60°C. FeBr2 (220 mg, 1.0 mmol) dissolved in DMF (5 ml) was added drop-wise to the mixture at 60°C. The progress of the reaction was monitored by reverse-phase HPLC (C18 symmetry column, UV monitoring at 280 and 214 nm, Waters apparatus), using a gradient of 0–100% acetonitrile (A) in water (B) in 30 min. The starting coproporphyrin eluted at 15.5 min, while coprohemin 1b eluted at 14.5 min. Solvent was evaporated in vacuo and the residue was suspended in 10 mL of 0.1 N HCl. Coprohemin 1b precipitated from the aqueous acidic solution at 0°C, and was recovered after filtration as a dark solid. The solid was dried in vacuo 18 h, resulting in 50 mg of crude coprohemin 1b, as a brown powder (85% yield). The recovered coprohemin 1b was purified by preparative HPLC and the purity (absence of endotoxin contamination) was assessed by mass spectrometry analysis and LAL assay. For the experiments described herein, stocks of purified, endotoxin free coprohemin and hemin (ca. 15 mM) were freshly prepared in 0.1 M NaOH, filtered (0.22 µm), diluted with PBS/0.1% HSA to the desired concentration and filtered (0.22 µm) once more before use.
Preparation of recombinant truncated CD14, MD-2, TLR4 ectodomain (TLR4ECD), and of MD-2·TLR4ECD complex
Preparative amounts of sMD-2 and a truncated form of CD14 (tCD14) were generated from infections of High Five (Invitrogen) insect cells with baculovirus containing the cDNA for human MD-2 inserted into pBAC3 (His6-MD-2) or for human tCD14 (amino acids 1–156) inserted into pBAC11 (tCD14-His6) as previously described.25 Conditioned medium containing MD-2 was stored at −80°C until needed. tCD14-His6 in conditioned medium was purified using Ni FF Sepharose resin (GE Healthcare) on an Explorer100 FPLC (GEHealthcare) to adsorb the His-tagged protein and an imidazole gradient to elute the adsorbed protein. Purified tCD14-His6 was stored at 4°C.25 Conditioned medium containing MD-2 associated with TLR4ECD (MD-2·TLR4ECD), was produced by transient transfection of HEK293T cells with pFLAG-CMV-TLR4ECD encoding FLAG-TLR4ECD, amino acids 24–631, and pEF-BOS-MD-2-FLAG-His as described previously.23 Media containing secreted proteins were concentrated 10–20-fold using Millipore Centricon-10 before use. Conditioned medium containing secreted MD-2·TLR4ECD proteins maintained reactivity with [3H]LOS·sCD14 for at least 6 months when stored at 4°C.
Size exclusion chromatography
Sephacryl S300 and S200 columns were used for resolution and identification of different [3H]LOS-containing protein complexes (i.e., ([3H]LOS·MD-2·TLR4ECD)2 (Mr ~190,000), [3H]LOS·sCD14 (Mr ~60,000), and ([3H]LOS·MD-2 (Mr ~25,000)). For determination of apparent Mr, these columns were calibrated with the following Mr standards:blue dextran (2 × 106, V0), thyroglobulin (650,000), ferritin (440,000), catalase (232,000), IgG (158,000), HSA (66,000), ovalbumin (44,500), myoglobin (17,500), vitamin B12 (1200, Vi).
In experiments in which the formation of [3H]LOS·MD-2 complex was monitored, chromatographic analyses were carried out using a Sephacryl HR S200 column (1.6 × 30 cm) pre-equilibrated in PBS, pH 7.4, 0.1% HSA and eluted in the same buffer at a flow-rate of 0.5 ml/min at 20–22°C using AKTA Purifier or Explorer 100 fast protein liquid chromatography (GE Healthcare). Fractions of 1 ml were collected.
In experiments in which the generation of (LOS·MD-2·TLR4ECD)2 was also monitored, the reaction mixtures were applied to a Sephacryl HR S300 column (1.6 × 70 cm) and chromatography was carried out as described above. Radioactivity ([3H]LOS) in collected fractions was analyzed by liquid scintillation spectroscopy (Beckman LS liquid scintillation counter). Total recovery of [3H]LOS was ≥ 70% in all experiments; experimental results in chromatograms are reported as % cpm of total radiolabeled LOS recovered. The composition of the various [3H]LOS-containing aggregates or LOS·protein complexes resolved by size exclusion chromatography were characterized by size and co-capture (of [3H]LOS) analyses, making use of monoclonal antibodies to CD14 and to epitope tags in MD-2 (His6-MD-2) and TLR4 ectodomain (Flag-TLR4ECD).22, 23, 25. Their identity was confirmed by co-elution with the corresponding purified [3H]LOS·protein complexes.
Production and purification of [3H]LOSagg, [3H]LOS·sCD14 and [3H]LOS·MD-2 complexes
[3H]LOSagg and [3H]LOS·sCD14 complex were prepared as previously described.21,23, 25 Briefly, [3H]LOSagg (Mr > 20 × 106) were obtained after hot phenol extraction of [3H]LOS from metabolically labeled bacteria, followed by ethanol precipitation of [3H]LOSagg, and ultracentrifugation. Monomeric [3H]LOS·CD14 complexes (Mr ~ 60,000) were prepared by treatment of [3H]LOSagg for 30 min at 37°C with substoichiometric LBP (molar ratio 200:1 LOS:LBP) and 1–1.5 molar excess sCD14 followed by gel exclusion chromatography (Sephacryl S200, 1.6 × 70 cm column) in PBS, pH 7.4, 0.03% HSA to isolate monomeric [3H]LOS·sCD14 complex. [3H]LOS·MD-2 (Mr ~25,000) was generated by treatment of [3H]LOS·sCD14 (30 min at 37°C) with High Five insect cell medium containing His6-MD-2 followed by isolation of [3H]LOS·MD-2 by S200 chromatography. Radiochemical purity of [3H]LOS·sCD14 and 3H]LOS·MD-2 was confirmed by S200 chromatography.23, 25
Effect of hemin and coprohemin on LOS transfer from [3H]LOS·sCD14 to His-tagged sMD-2)
Conditioned medium containing His6-MD-2 (corresponding to a final concentration in the incubation mixture of ca. 1.2 nM) was pre-incubated ± hemin or coprohemin in PBS, 1% HSA, 30 min at 37°C. This pre-incubation was followed by incubation for 30 min at 37°C with [3H]LOS·sCD14 (0.8 nM) to allow transfer of [3H]LOS to available His-tagged sMD-2. Products of the reactions were analyzed by size exclusion chromatography (see above) and/or by co-capture to HISLINK resin (see below).
Assay of transfer of [3H]LOS to His6-tagged proteins by co-capture to metal chelating resin
Conditioned medium containing His6-sMD-2 (1.2 nM, final concentration, as determined by the capacity of the sMD-2-containing conditioned medium to react with [3H]LOS·sCD14 to form [3H]LOS·MD-2) or no His6-sMD-2 (as a negative control) was pre-incubated in 0.3 ml PBS, 1% HSA for 30 min at 37°C ± the indicated concentrations of hemin or coprohemin. [3H]LOS·sCD14 (0.8 nM) was added followed by another incubation for 30 min at 37°C. The reaction mixture was then incubated with HISLINK resin (20 µl pre-equilibrated in PBS, 1% HSA) for 15 min at 25°C, allowing His-tagged sMD-2 to be adsorbed onto the beads. The resin was centrifuged for 2 min at 2000 × g, the supernatant was removed and the resin washed 2.x with PBS, 1% HSA using the same procedure. The [3H]LOS absorbed onto the beads or recovered in the supernatant was quantified by liquid scintillation spectroscopy.
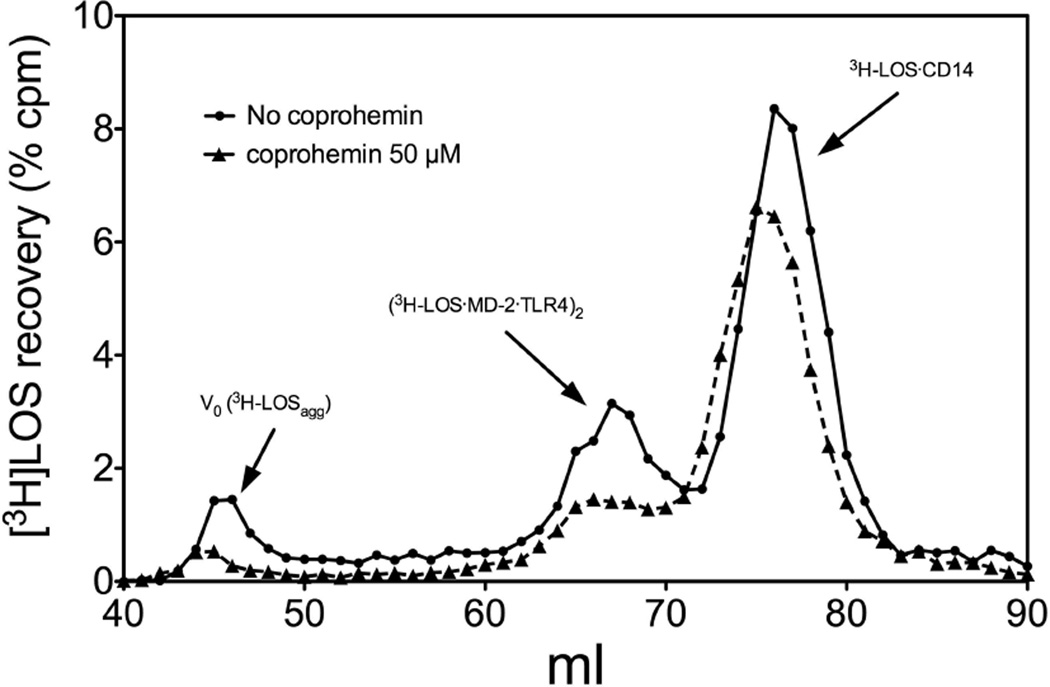
Effect of coproheme on the formation of ([3H]LOS·MD-2·TLR4ECD)2 complex from [3H]LOSagg (Fig. 6)
Fig. 6. Coproheme inhibits endotoxin transfer from [3H]LOS·sCD14 to ([3H]LOS·MD-2·TLR4ECD)2.
Incubation mixtures containing [3H]LOSagg, LBP, sCD14, and MD-2·TLR4ECD ± coproheme (50 µM) were analyzed by Sephacryl S300 chromatography as described in Materials and Methods. The chromatographic profiles shown are representative of >4 experiments. Overall recoveries of [3H]LOS were ≥ 70%.
sCD14 (0.8 nM, final concentration) was first pre-incubated with LBP (4 pM, final concentration) ± coproheme (50 µM) for 30 min at 37°C in 0.4 ml PBS, pH 7.4, 0.1% HSA. After this pre-incubation, [3H]LOSagg was added (up to 5 µl, yielding a final LOS concentration of 0.8 nM) and the mixture was incubated an additional 30 min at 37°C. This was followed by addition of conditioned HEK293T cell medium containing preformed MD-2·TLR4ECD heterodimer23 (0.200 ml, ca. 0.2 nM, final concentration, of reactive MD-2·TLR4ECD heterodimer) and an additional incubation for 15 min at 37°C. After this final incubation, analysis of the reaction products was determined by gel size exclusion chromatography (S300) as described above.
HEK cell activation assay
HEK-TLR4 cells have been previously characterized and were cultured as described.22 For cell activation assays, HEK-TLR4 or parental HEK cells were grown to confluency in 96-well plates. Cell monolayers were washed two times with warm PBS without Ca2+ and Mg2+ and incubated overnight at 37°C, 5% CO2, and 95% humidity in 200 µl DMEM supplemented with 0.1% HSA ± hemin or coprohemin (0–50 µM), and/or either [3H]LOSagg (1 nM), [3H]LOS·sCD14 (0.1 nM) or [3H]LOS·MD-2 (0.1 nM) as stimuli. Where indicated, incubation mixtures were supplemented with LBP (8 pM), sCD14 (0.8 nM), and/or sMD2-containing conditioned medium (ca. 1,2 nM). After the incubation, plates were centrifuged at 1000 rpm for 5 min and supernatants were collected. Activation of HEK cells was assessed by measuring the accumulation of extracellular IL-8 by ELISA according to supplier protocol (BD Clontech, Inc., Palo Alto, CA). Added purified LOSagg (or LPSagg), even at 1000× higher concentrations, did not activate the HEK/TLR4 cells without addition of sCD14 and MD-2.22
RESULTS
Hemin induces TLR4-dependent activation of HEK293 cells both in the presence and the absence of MD-2 and CD14
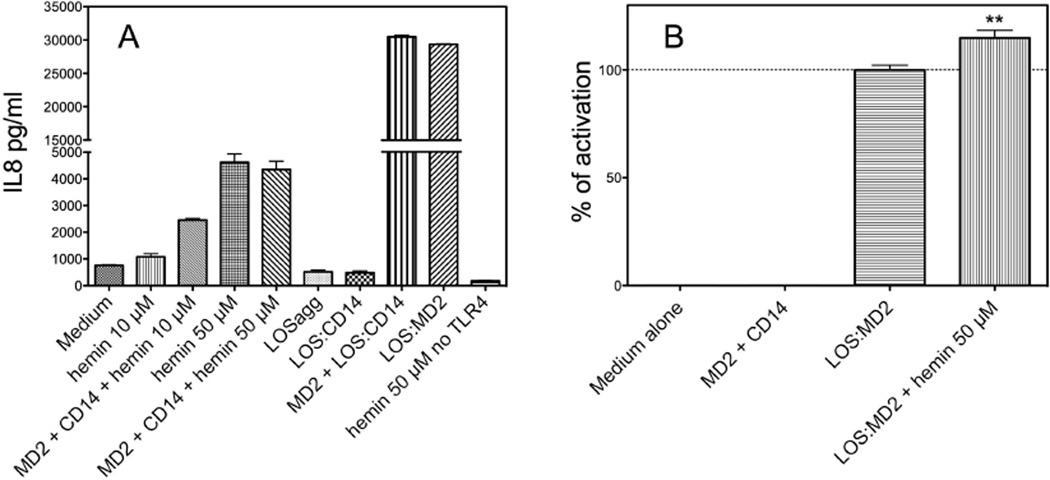
It has been recently reported that hemin induces TLR4-dependent activation of mouse macrophages.20 Activation of TLR4 by endotoxin, the most potent agonist of TLR4, requires binding of monomeric endotoxin to MD-2, a process that occurs most efficiently by transfer of endotoxin monomers from CD14 (i.e., a monomeric endotoxin·CD14 complex) to MD-2 or preformed MD-2·TLR4 heterodimer.22, 23 To determine if hemin could induce TLR4-dependent activation of HEK293 cells and if activation by hemin also required MD-2 and CD14, we tested the ability of hemin to activate transformed HEK293 cells expressing TLR4 (HEK/TLR4 cells) in the presence or absence of added soluble MD-2 ± sCD14. Figure 2A shows that hemin produced dose-dependent activation of HEK/TLR4 cells as manifest by increased extracellular accumulation of IL-8. Hemin did not activate the parental HEK293 cells (Fig. 2A), confirming that activation of HEK/TLR4 cells by hemin was TLR4-dependent. At the lower concentration of hemin tested (10 µM), TLR4-dependent cell activation was promoted by MD-2 and CD14. However, at a higher heme concentration (50 µM), cell activation by heme was the same with or without added MD-2 and sCD14 (± LBP) (Fig. 2A). In contrast, activation of HEK/TLR4 cells by endotoxin required presentation of endotoxin as a pre-formed monomeric endotoxin·MD-2 complex or as a monomeric endotoxin·sCD14 complex in combination with added soluble MD-2 (Fig. 2A).22, 26 Added LOSagg or even LOS·sCD14 alone (10 ng/ml) did not induce activation of HEK/TLR4 cells, confirming that these cells lack functionally detectable levels of MD-2 (Fig. 2).
Fig. 2. TLR4-dependent activation of HEK293 cells by hemin in the presence and absence of MD-2 and sCD14.
(A) cell activation was measured as extracellular accumulation of IL-8 after overnight incubation of HEK/TLR4 or parental HEK293 cells as described in Materials and Methods. (B) Activation of HEK/TLR4 cells by 0.3 nM LOS·MD-2 ± 50 uM hemin (as indicated in labels). IL-8 was measured by ELISA. Results shown represent the mean ± SEM of 3 independent experiments, each in triplicate. The asterisks indicate a significant difference (p< 0.01) between levels of IL-8 recovered in the extracellular medium of HEK/TLR4 cells treated with 0.3 nM LOS·MD-2 ± 50 uM hemin, as analyzed by ANOVA; Dunnett’s test.
In comparison to endotoxin (i.e., LOS·MD-2), hemin was a much weaker activator of TLR4-dependent cell activation, requiring much higher concentrations to achieve maximum TLR4-dependent cell activation (~0.1 nM for LOS·MD-2, 50 µM for hemin) and, at maximum, inducing much less extracellular accumulation of IL-8 (Fig. 2A). Addition of LOS·MD-2 and heme together, each at a dose that produced as much TLR4-dependent cell activation as possible when that agonist was added alone, produced modest but reproducible additive effects on TLR4-dependent cell activation (Fig. 2B). The absence of inhibitory effects of high concentrations of hemin on LOS·MD-2 stimulation of HEK/TLR4 cells is consistent with the hypothesis that LOS·MD-2 and hemin induce TLR4-dependent cell activation by distinct mechanisms.20
Coproheme, in contrast to heme, inhibits endotoxin-triggered activation of HEK/TLR4 cells
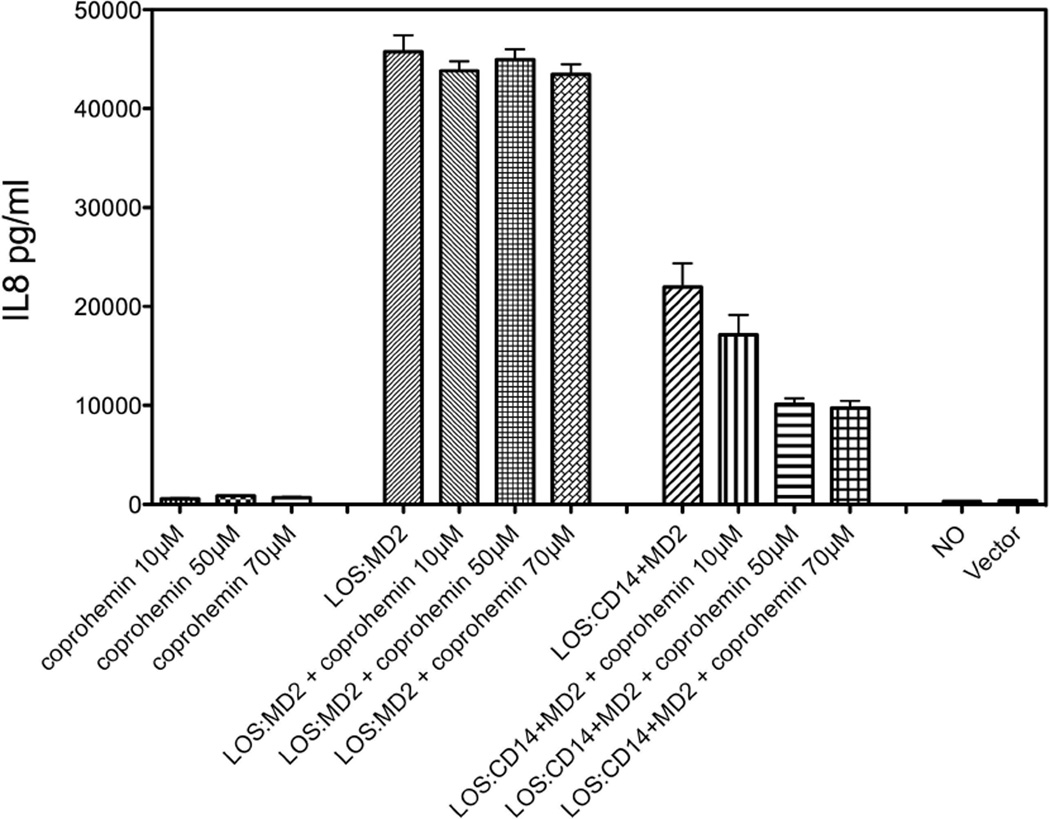
The studies of Figueiredo et al.20 indicated that the ability of hemin to induce TLR4-dependent cell activation depended on both the structure of the porphyrin ring (protoporphyrin IX, PPIX) and the coordination of PPIX with iron: PPIX that was iron-free, coordinated with a palladium ion, or had a vinyl group substituted by an ethyl group were each inactive. Coprohemin [Fe(III)-coproporphyrin I] differs from hemin by the presence of two proprionate rather than vinyl groups at the C-3 and C-8 positions of the tetrapyrrole ring (Fig. 1b). We extended our cell activation studies to test effects of coprohemin on TLR4-dependent cell activation. In contrast to hemin, added coprohemin in the same dose range (10–70 µM) did not induce TLR4-dependent cell activation (Fig. 3). At these concentrations, coprohemin also did not affect activation of HEK/TLR4 cells by purified LOS·MD-2. However, activation of HEK/TLR4 cells by added LOS·sCD14 + MD-2 was inhibited in a dose-dependent fashion by coprohemin (Fig. 3).
Fig. 3. Dose-dependent effects of coproheme and/or LOS·MD-2 (0.03 nM) or LOS·sCD14 (0.1 nM) + conditioned medium containing MD-2 (1.2 nM) on HEK/TLR4 cells.
Cell activation was measured as described in the legend to Fig. 2. Results shown represent the mean ± SEM of 3 independent experiments, each in triplicate.
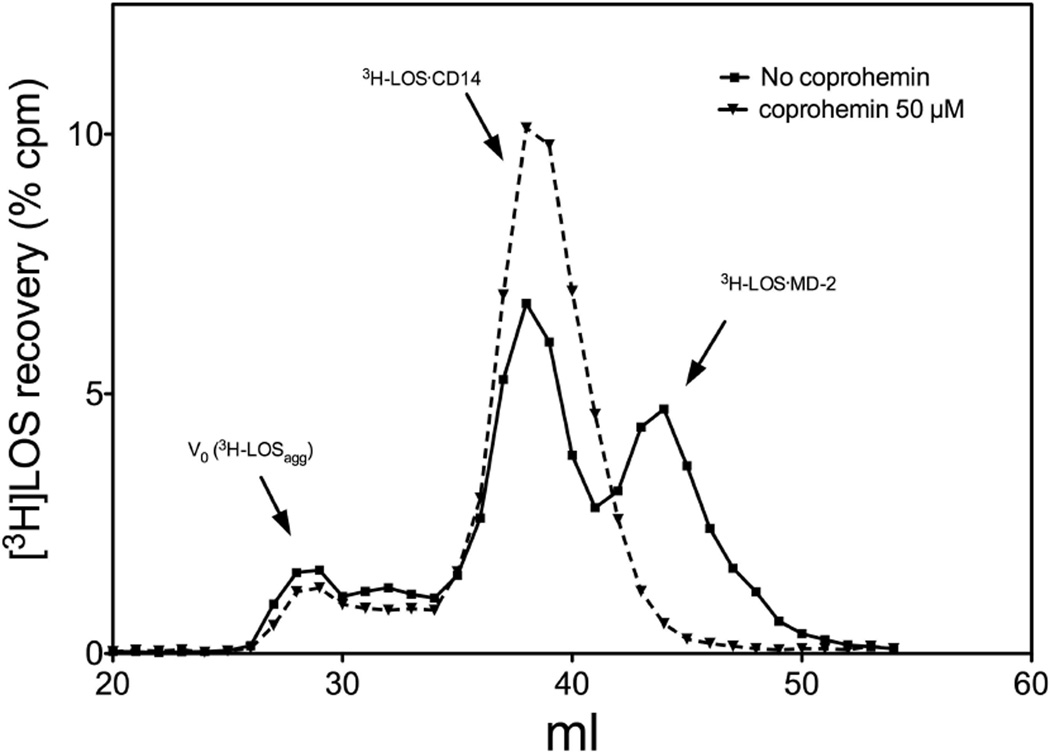
Coprohemin, in contrast to hemin, inhibits transfer of endotoxin from CD14 to MD-2
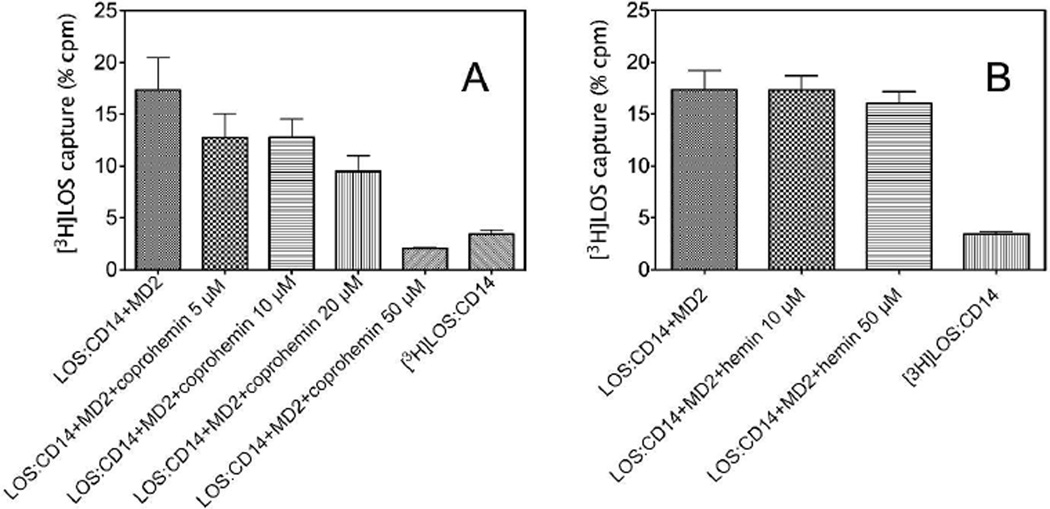
The ability of coprohemin to inhibit TLR4-dependent cell activation by LOS·sCD14 + MD-2, but not by preformed LOS·MD-2, suggested that coprohemin acted by inhibiting transfer of LOS from CD14 to MD-2. To test this hypothesis, we compared the transfer of [3H]LOS from [3H]LOS·sCD14 to His6-MD-2 in the absence or presence of coprohemin. Transfer of [3H]LOS from [3H]LOS·sCD14 to His6-MD-2 was measured in two different ways: 1) co-capture of [3H]LOS with His6-MD-2, using a metal chelating resin, HISLINK, to capture the His-tagged MD-2 (Fig. 4); and 2) gel sieving chromatography to resolve [3H]LOS·MD-2 (Mr ~25,000) from [3H]LOS·sCD14 (Mr ~60,000) (Fig. 5). Both assays showed that coprohemin inhibited transfer of [3H]LOS from [3H]LOS·sCD14 to His6-MD-2 (Fig. 4A and 5). In contrast, hemin had little or no effect on transfer of [3H]LOS from [3H]LOS·sCD14 to His6-MD-2 (Fig. 4B).
Fig. 4. Dose-dependent effects of coproheme (A) or heme (B) on [3H]LOS monomer transfer from sCD14 to His6-MD-2.
Formation of [3H]LOS·MD-2His6 complex, using [3H]LOS·CD14 as endotoxin donor, was assayed by co-capture of [3H]LOS to the HISLINK resin. Capture of [3H]LOS.sCD14 before and after incubation without His-tagged protein was < 4% (as shown). Data shown are representative of 3 experiments, each in duplicate, and are expressed as mean ± SEM.
Fig. 5. Coproheme inhibits transfer of [3H]LOS monomer from sCD14 to MD-2.
Transfer of the endotoxin from sCD14 to MD-2 was analyzed by Sephacryl S200 chromatography as described in Materials and Methods. Chromatograms shown are representative of >3 independent experiments. Overall recoveries [3H]LOS were > 70%.
To further define the specificity of coprohemin’s inhibitory action on TLR4 activation by endotoxin, we also tested the effect of coprohemin on LBP/sCD14-dependent delivery of [3H]LOS monomers from [3H]LOS aggregates (LOSagg) to MD-2·TLR4ECD. This transfer is achieved in two steps: 1) LBP/sCD14-dependent extraction and transfer of [3H]LOS monomers to sCD14 to form monomeric [3H]LOS·sCD14 complexes; and 2) transfer of [3H]LOS from [3H]LOS·sCD14 to MD-2·TLR4ECD.22, 27 Experimental conditions were chosen so that in the absence of coprohemin, most of the [3H]LOS in the added [3H]LOSagg was extracted and transferred to sCD14 (yielding [3H]LOS·sCD14) and, in addition, some [3H]LOS was transferred from [3H]LOS·sCD14 to MD-2·TLR4ECD yielding a Mr ~190,000 complex of ([3H]LOS·MD-2·TLR4ECD)2 (Fig. 6). The addition of coprohemin had little apparent effect on LBP/sCD14-dependent extraction and transfer of [3H]LOS from LOSagg to sCD14 but inhibited transfer of [3H]LOS from LOS·sCD14 to MD-2·TLR4ECD (Fig. 6).
DISCUSSION
The studies reported here confirm and extend the recent observations by Figueiredo R.T. et al. that showed TLR4- and CD14-dependent cell activation by hemin using mouse macrophages.20 We have demonstrated TLR4-dependent activation of a human HEK293/TLR4 cell line by hemin that was MD-2/CD14-dependent at hemin concentrations ~10 µM, but was MD-2/CD14-independent at higher hemin concentrations (50 µM) (Fig. 2A). The ability of hemin (50 µM) to trigger TLR4-dependent cell activation in HEK293 cells (expressing TLR4) in the absence of CD14 and MD-2 (Fig. 2A), clearly distinguishes the action of hemin from that of endotoxin (Fig. 2A).22 No contaminating endotoxin was detected in our HPLC-purified hemin preparation, either by highly sensitive mass spectral analysis or LAL assay, supporting our contention that both the MD-2 (CD14)-dependent as well as the CD14- and MD-2-independent induction of TLR4-dependent cell activation that we observed was hemin-mediated. The additive effects of LOS·MD-2 and hemin on TLR4-dependent cell activation (Fig. 2B) suggest that endotoxin·MD-2 and hemin can activate TLR4 in different ways, as originally proposed by Figueiredo et al.20 These different actions of hemin and endotoxin·MD-2 and the inability of hemin, at even relatively high concentrations, to appreciably inhibit transfer of endotoxin monomer from CD14 to MD-2 (Fig. 4B) may explain the selective ability of iron-free hemin (protoporphyrin IX) to block hemin but not endotoxin-triggered TLR4 activation.20 Whether or not the triggering of TLR4-dependent cell activation by hemin is mediated by direct heme-TLR4 interactions in the absence of MD-2 and CD14 can not yet be judged and requires further study.
Our findings also provide additional evidence that the structure of the porphyrin ring of heme is a critical determinant of the ability of heme to induce TLR4-dependent cell activation. The presence in coprohemin of two proprionate rather than vinyl groups eliminates the TLR4 agonist properties of heme and instead confers the ability to inhibit TLR4 activation by endotoxin (Fig. 3). This inhibitory effect of coprohemin is paralleled by reduced delivery of endotoxin to MD-2(·TLR4) (Fig. 4–6) that is necessary for activation of TLR4 by endotoxin.22, 23, 27 Coprohemin did not directly affect pre-formed monomeric endotoxin·MD-2 complexes (Fig. 3) nor inhibit LBP/sCD14 extraction and transfer of endotoxin monomers from endotoxin aggregates to CD14 (Fig. 6). Taken together, these findings suggest that coprohemin may selectively target MD-2, precluding transfer of endotoxin from CD14 to MD-2 and so act as a TLR4 antagonist. What properties of coprohemin (vs. hemin) favor interaction with MD-2 is not clear. One possibility is that the increased aqueous solubility of coprohemin conferred by the two additional carboxyl groups (Fig. 1) yields a higher concentration of coprohemin (vs. hemin) monomers that, like endotoxin,25 may be the preferred ligand for MD-2.22, 23 In summary, our findings provide additional evidence for the potential role of heme and its derivatives in the regulation of TLR4 function. The contrasting functional properties of hemin and coprohemin underscore the apparently specific structural requirements for induction of TLR4-dependent cell activation by heme. The contrasting functional properties of hemin and coprohemin also seem to fit the circumstances in which these compounds accumulate in vivo. Coprohemin is a product of normal heme metabolism and is present in body fluids of healthy individuals, although an increase in coproporphyrin levels is diagnostic for porphyrias.29 As such, it seems reasonable that coprohemin does not elicit any TLR4-mediated inflammatory response. In contrast, accumulation of heme in excess of normal metabolic and clearance mechanisms is more indicative of patho-physiologic circumstances. The ability of heme to act as a “danger” signal may be linked to its ability to initiate TLR4-dependent as well as TLR4-independent responses. Although the effects of these compounds on (MD-2·)TLR4 require orders of magnitude higher concentrations than that of endotoxin, concentrations of heme of ≥ 50 µM have been reported in hemolytic and hemorrhagic conditions7, 16, 30 and include circumstances in which a role for TLR4 in outcome has been strongly suggested.31
ACKNOWLEDGEMENTS
We acknowledge grants from the National Institute of Allergy and Infectious Disease (AI05732) to J.W. and the Veterans’ Administration to T.L.G. We thank Xoma Corp. (Berkeley, CA) for recombinant LBP, Amgen Corp. (Thousand Oaks, CA) for sCD14. We are grateful to DeSheng Zhang for preparation of radiolabeled LOS.
REFERENCES
- 1.Dawson JH. Probing structure-function relations in heme-containing oxygenases and peroxidases. Science. 1988;240:433–439. doi: 10.1126/science.3358128. [DOI] [PubMed] [Google Scholar]
- 2.Pfeifer K, Kim KS, Kogan S, Guarantee L. Functional dissection and sequence of yeast HAP1 activator. Cell. 1989;56:291–301. doi: 10.1016/0092-8674(89)90903-3. [DOI] [PubMed] [Google Scholar]
- 3.Lathrop JT, Timko MP. Regulation by heme of mitochondrial protein transport through a conserved amino acid motif. Science. 1993;259:522–525. doi: 10.1126/science.8424176. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Guarente L. Heme binds to a short sequence that serves a regulatory function in diverse proteins. Eur Mol Biol Organ J. 1995;14:313–320. doi: 10.1002/j.1460-2075.1995.tb07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath KA, Grande JP, Croatt AJ, Katusich ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letarte PB, Lieberman K, Nagatani K, Haworth RA, Odell GB, Duff TA. Hemin: levels in experimental subarachnoid hematoma and effects on dissociated vascular smooth-muscle cells. J Neurosurg. 1993;79:252–255. doi: 10.3171/jns.1993.79.2.0252. [DOI] [PubMed] [Google Scholar]
- 7.Nath KA, Grande JP, Croatt AJ, Likely S, Hebbel RP, Enright H. Intracellular targets in heme protein-induced renal injury. Kidney Int. 1998;53:100–111. doi: 10.1046/j.1523-1755.1998.00731.x. [DOI] [PubMed] [Google Scholar]
- 8.Alayash AI. Hemoglobin-based blood substitutes and the hazards of blood radicals. Free Radic Res. 2000;33:341–348. doi: 10.1080/10715760000300881. [DOI] [PubMed] [Google Scholar]
- 9.Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword promotion of oxidation and induction of cytoprotectants. Blood. 2000;95:3442–3450. [PubMed] [Google Scholar]
- 10.Vercellotti GM, Balla G, Balla J, Nath K, Eaton JW, Jacob HS. Heme and the vasculature: An oxidative hazard that induces antioxidant defenses in the endothelium. Artif Cells Blood Substit Immobil. 1994;22:207–213. doi: 10.3109/10731199409117415. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, Kitajima M, Makino N, Ishimura Y, Suematsu M. Induction of Heme Oxygenase-1 Suppresses Venular Leukocyte Adhesion Elicited by Oxidative Stress. Circ Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 12.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The Indispensability of Heme Oxygenase-1 in Protecting against Acute Heme Protein-Induced Toxicity in Vivo. Am J Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagener FADT, Feldman E, de Witte T, Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1 and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 14.Waegner FADT, da Silva JL, Farley T, de Witte T, Kappas A, Abraham NG. Differential Effects of Heme Oxygenase Isoforms on Heme Mediation of Endothelial Intracellular Adhesion Molecule I Expression. J Pharmacol Exp Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 15.Wagener FADT, Volk HD, Willis D, et al. Faces of the Heme-Heme Oxygenase System in Inflammation. Pharmacol Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- 16.Wagener FADT, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 17.Pichyangkul SK, Yongvanitchit U, Kum-arb H, et al. Malaria Blood Stage Parasites Activate Human Plasmacytoid Dendritic Cells and Murine Dendritic Cells through a Toll-Like Receptor 9-Dependent Pathway. J Immunol. 2004;172:4926–4933. doi: 10.4049/jimmunol.172.8.4926. [DOI] [PubMed] [Google Scholar]
- 18.Coban C, Ishii KJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. Characterization of Heme as Activator of Toll-like Receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 21.Giardina PC, Gioannini T, Buscher BA, et al. Construction of Acetate Auxotrophs of Neisseria meningitidis to Study Host-Meningococcal Endotoxin Interactions. J Biol Chem. 2001;276:5883–5891. doi: 10.1074/jbc.M009273200. [DOI] [PubMed] [Google Scholar]
- 22.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prohinar P, Re F, Widstrom R, et al. High Affinity Interactions of Monomeric Endotoxin·Protein Complexes with Toll-like Receptor 4 Ectodomain. J Biol Chem. 2007;282:1010–1017. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- 24.Wedel M, Walter A, Montforts FP. Synthesis of Metalloporphyrins and Metallochlorins for Immobilization on Electrode Surfaces. Eur J Org Chem. 2001:1681–1687. [Google Scholar]
- 25.Gioannini T, Teghanemt A, Zhang DeSheng, et al. Endotoxin-binding proteins Modulate the Susceptibility of bacterial Endotoxin to deacylation by Acyloxyacyl Hydrolase. J Biol Chem. 2007;282:7877–7884. doi: 10.1074/jbc.M605031200. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Young DW, Gusovsky F, Chow JC. Cellular Events Mediated by Lipopolysaccharide-stimulated Toll-like Receptor 4. J Biol Chem. 2000;275:20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- 27.Gioannini TL, Weiss J. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–260. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 28.Gioannini TL, Zhang DS, Teghanemt A, Weiss JP. An Essential Role for Albumin in the Interaction of Endotoxin with Lipopolysaccharide-binding Protein and sCD14 and Resultant Cell Activation. J Biol Chem. 2002;277:47818–47825. doi: 10.1074/jbc.M206404200. [DOI] [PubMed] [Google Scholar]
- 29.Sassa S. Modern diagnosis and management of the porphyrias. Br J Haematol. 2006;135:281–292. doi: 10.1111/j.1365-2141.2006.06289.x. [DOI] [PubMed] [Google Scholar]
- 30.Muller-Eberhard U, Javid J, Liem HH, Hanna M. Brief Report: Plasma Concentrations of Hemopexin, Haptoglobin and Heme in Patients with Various Hemolytic Diseases. Blood. 1968;32:811–815. [PubMed] [Google Scholar]
- 31.Oyama J, Blais C, Liu X, et al. Reduced Myocardial Ischemia-Reperfusion Injury in Toll-Like Receptor 4-Deficient Mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]